Abstract
While several studies have examined how particular personality traits are related to dopamine D2/3 receptor (D2/3R) availability in the striatum of humans, few studies have reported how multiple traits measured in the same persons are differentially related to D2/3R availability in different striatal sub-regions. We examined how personality traits measured with the Karolinska Scales of Personality are related to striatal D2/3R availability measured with [11C]-raclopride in 30 healthy humans. Based on previous literature, five personality traits were hypothesized to be most likely related to D2/3R availability: impulsiveness, monotony avoidance, detachment, social desirability, and socialization. We found self-reported impulsiveness was negatively correlated with D2/3R availability in the ventral striatum and globus pallidus. After controlling for age and gender, monotony avoidance was also negatively correlated with D2/3R availability in the ventral striatum and globus pallidus. Socialization was positively correlated with D2/3R availability in the ventral striatum and putamen. After controlling for age and gender, the relationship between socialization and D2/3R availability in these regions survived correction for multiple comparisons (p-threshold=.003). Thus, within the same persons, different personality traits are differentially related to in vivo D2/3R availability in different striatal sub-regions.
Keywords: Personality, Dopamine, D2/3R, PET, [11C]-raclopride
Introduction
Many attempts have been made to link personality traits – self-reported enduring patterns of perceiving, relating to, and thinking about the environment and oneself (Engler, 2013) – with the brain dopamine (DA) D2/3 receptor (D2/3R) system (Cumming, 2009). Using positron emission tomography (PET), several studies have examined the relationship between striatal D2/3R availability in healthy humans and the personality traits of impulsivity (Buckholtz et al., 2010; Kim et al., 2014; Lee et al., 2009; Oswald et al., 2007; Reeves et al., 2012; Rosa-Neto et al., 2005; Weiland et al., 2014), novelty seeking/sensation seeking (Boileau et al., 2006; Gjedde et al., 2010; Leyton et al., 2002), social attachment (Breier et al., 1998; Farde et al., 1997), and social desirability (Cervenka et al., 2010; Egerton et al., 2010; Reeves et al., 2007). However, many of these studies have not examined the relationship between these different traits and D2/3R availability within the same persons; rather they have often focused on a single trait. Moreover, not all studies have differentiated sub-regions of the striatum, for example the ventral striatum (VS) and globus pallidus (GP).
We examined in thirty healthy persons the relationship between personality traits measured with the Karolinska Scales of Personality (KSP) and DA D2/3R availability in multiple sub-regions of the striatum using [11C]-raclopride. Our a priori focus was on those traits captured by the KSP which are thought to be related to DA functioning in the striatum: impulsiveness, monotony avoidance, detachment, social desirability, and socialization. We focused our a priori personality traits based on those studies which used radioligands for D2/3R and found relationships between striatal D2/3R availability and personality traits. However, other radiotracers relevant to the DA system have been employed. For example, several studies have looked at DA synthesis capacity (Laakso et al., 2003; Lawrence and Brooks, 2014; Menza et al., 1995; Schluter et al., 2013) and DA transporter availability (Laakso et al., 2000), in relation to personality traits. It should be emphasized that although baseline striatal D2/3R availability and striatal DA synthesis capacity are related (Ito et al., 2011), they are also distinct; the former being affected by not just endogenous DA levels, but also the total density and affinity of receptors (Gunn et al., 2015). For example, using more direct estimates of endogenous DA, it has been reported that only 16% of the variance in baseline [11C]-raclopride binding in the striatum can be accounted for by endogenous DA (r(31)=−.40, p=.02) (Kegeles et al., 2014). Finally, it is noteworthy that studies have observed relationships between personality traits and baseline extrastriatal D2/3R availability (Buckholtz et al., 2010; Suhara et al., 2001).To our knowledge, this study is the first to report how all these traits, measured with the KSP, relate to D2/3R availability with [11C]-raclopride in the same persons. Moreover, to our knowledge this study employs to date the largest sample of [11C]-raclopride scans investigating the relationship between baseline D2/3R availability and personality traits. This investigation lends further support to the hypothesis that different personality traits are differentially related to the functioning of the DA D2/3R system in different striatal regions in humans.
Experimental Procedures
Participants
This analysis included PET scans previously collected from healthy persons from various studies conducted by our lab (Caravaggio et al., 2015; Graff-Guerrero et al., 2009; Graff-Guerrero et al., 2008). To decrease potential noise, in instances where subjects were scanned with multiple radiotracers besides [11C]-raclopride, only data from persons scanned with [11C]-raclopride first were used. Participants were right-handed non-smoking adults free of any major medical or psychiatric disorders as determined by clinical interview, the Mini International Neuropsychiatric Interview (Lecrubier et al., 1997), basic laboratory tests, and electrocardiography. At inclusion and before the PET scan participants were required to have a negative urine screen for drugs of abuse and/or pregnancy. All participants provided written informed consent. This study was approved by the Research Ethics Board of the Centre for Addiction and Mental Health (CAMH), Toronto.
Karolinksa Scales of Personality
On the day of the PET scan, all participants completed the KSP self-report questionnaire (Schalling et al., 1987). The KSP comprises 15 personality subscales which are scored on a four point Likert scale (1 = does not apply, 4 = applies completely). Based on previous research, we expected measurements from 5 of the subscales to be potentially related to baseline D2/3R availability measured with [11C]-raclopride: impulsiveness, monotony avoidance, detachment, social desirability, and socialization. Impulsiveness measures the degree to which people endorse acting on “the spur of the moment” (i.e. non-planning impulsivity) (Ortet et al., 2002). Monotony avoidance assesses the desire to avoid routine and seek change (Ortet et al., 2002). Detachment measures the degree to which people are involved or withdrawn from others (Farde et al., 1997; Ortet et al., 2002). Social desirability assesses the degree to which people are socially comforting, helpful, or “fake good” (Cervenka et al., 2010; Ortet et al., 2002). Socialization captures the degree to which participants had positive childhood experiences and satisfaction with current life events (Ortet et al., 2002).
PET Imaging
The radiosynthesis of [11C]-raclopride and the acquisition of PET images have been described in detail elsewhere (Graff-Guerrero et al., 2008; Wilson et al., 2000). Briefly, images were acquired using a high resolution head-dedicated PET camera system (CPS-HRRT; Siemens Molecular Imaging, USA), which measures radioactivity in 207 brain slices with a thickness of 1.2mm each. The in-plane resolution was ~2.8mm full-width at half-maximum (FWHM). Transmission scans were acquired using a 137Cs (T1/2 = 30.2 yr, E = 662 KeV) single photon point source to provide attenuation correction, and the emission data were acquired in list mode. The raw data were reconstructed by filtered-back projection. The average time of injection was 1:46pm. The mean radioactivity dose was 9.75(±1.2)mCi, with a specific activity of 1127.32(±434.3)mCi/µmol, and an injected mass of 3.99(±1.8)µg. [11C]-raclopride data were acquired for 60 min and redefined into 28 frames (1–5 of 1-min duration, 6–25 of 2-min duration, and 26–28 of 5-min duration).
Image Analysis
The region of interest (ROI)-based analysis for [11C]-raclopride has been described in detail elsewhere (Graff-Guerrero et al., 2008). Using a two-step process, regional BPND estimates were extracted from ROIs defined in Montreal Neurological Institute (MNI) brain space from parametric voxelwise BPND maps, calculated for each subject. First, time activity curves (TACs) from ROIs were obtained from the dynamic PET images in native space with reference to each subjects co-registered MRI image. The co-registration of each subjects MRI to PET space was done using the normalized mutual information algorithm (Studholme et al., 1997) as implemented in SPM2 (SPM2, Wellcome Department of Cognitive Neurology, London; http://www.fil.ion.ucl.ac.uk/spm). The TACs were analyzed using the Simplified Reference Tissue Method (SRTM) (Lammertsma and Hume, 1996), using the cerebellum as the reference region, to derive a quantitative estimate of binding: binding potential relative to the non-displaceable compartment (BPND) as defined by the consensus nomenclature for in vivo imaging of reversibly binding radioligands (Innis et al., 2007). Second, the basis function implementation of the SRTM (Gunn et al., 1997) was applied to the dynamic PET images to generate parametric voxelwise BPND maps using PMOD (v2.7, PMOD Technologies, Zurich, Switzerland). These images were spatially normalized into MNI brain space by Nearest Neighbour Interpolation with a voxel size fixed in 2 × 2 × 2 mm3 using SPM2. Regional BPND estimates were then derived from ROIs defined in MNI space. The ventral striatum and dorsal striatum (dorsal caudate, hereafter caudate and dorsal putamen, hereafter putamen) were defined according to the criteria of Mawlawi et al (Mawlawi et al., 2001). The delineation of caudate and putamen was made in the coronal plane. The VS (inferiorly), caudate, and putamen (superiorly) were defined by a line joining the intersection between the outer edge of the putamen with a vertical line going through the most superior and lateral point of the internal capsule and the center of the portion of the anterior commissure (AC). This line was extended to the internal edge of the caudate. The other boundaries of the VS were visually determined by its dense gray signal and were easily distinguishable from the adjacent structures. The VS was sampled from the anterior boundary of the striatum to the level of the AC coronal plane. The caudate also was sampled from its anterior boundary to the AC coronal plane. Thus, for the VS, the sampled region included the ventral and rostral part of the striatum, with reference to AC having the brain horizontal to the AC-PC line. For the caudate, the sampled region included the dorsal part of the head of the caudate and the anterior third of the body of the caudate. The putamen was sampled from its anterior to posterior boundaries in slices posterior to the AC plane. The globus pallidus was defined according to the criteria of Tziortzi and colleagues (Tziortzi et al., 2011). The GP delineation was performed on the transverse plane, dorsal to ventral, and included both the internal and external segments.
Statistical Analysis
We examined the Pearson product-moment correlations between DA D2/3R availability in 4 subregions of the striatum with 5 personality traits measured by the KSP. These traits were selected as a priori based on previous literature. We also conducted exploratory analyses between the other personality traits measured on the KSP and DA D2/3R availability in the ROIs. Multiple comparisons in these exploratory analyses were controlled for using Bonferroni correction. Age and gender were controlled for using partial Pearson product-moment correlations. Statistical analyses were conducted using SPSS (v.12.0; SPSS, Chicago, Illinois) and GraphPad (v.5.0; GraphPad Software, La Jolla California). Normality of variables was determined using the D’Agostino-Pearson test. The significance level for all testes was set at p<0.05 (two-tailed).
Results
Thirty healthy persons (11 female; age range: 18–45, mean=32, SD=9) participated in the study. All participants provided a [11C]-raclopride scan under baseline conditions and completed the Karolinska Scales of Personality. Age was negatively correlated with [11C]-raclopride BPND in the caudate (r(28)=−.40, p=.03; 95% CI [−.66, −.04]) and putamen (r(28)=−.40, p=.03; 95% CI [−.66, −.04]), but not in the VS (r(28)=−.35, p=.06; 95% CI [−.63, .01]) or GP (r(28)=−.28, p=.14; 95% CI [−.58, .10]). Table 1 reports the simple linear regressions predicting [11C]-raclopride BPND from age for each ROI. Males had more baseline [11C]-raclopride BPND than females in the putamen (t(28)=2.20, p=.04) and GP (t(28)=3.27, p=.003). There was no significant gender difference in [11C]-raclopride BPND in the caudate (t(28)=0.91, p=.37) and VS (t(28)=2.07, p=.05). Age was not correlated with self-reported impulsiveness (r(28)=−.16, p=.41), monotony avoidance (r(28)=−.31, p=.10), detachment (r(28)=−.16, p=.39), social desirability (r(28)=.24, p=.21), or socialization (r(28)=.21, p=.27). Males and females did not differ on impulsiveness (t(28)=−.99, p=.33), monotony avoidance (t(28)=−.60, p=.55), detachment (t(28)=1.57, p=.13), social desirability (t(28)=.05, p=.96), and socialization (t(28)=−.27, p=.79).
Table 1.
Simple linear regressions predicting [11C]-raclopride BPND from age.
| F(1, 28) | p-value | r2 | β | Predicted BPND (unstandardized beta) |
|
|---|---|---|---|---|---|
| Dorsal Caudate | 5.175 | .03 | .16 | −.395 | 4.23+(−.025)*Age |
| Dorsal Putamen | 5.212 | .03 | 1.57 | −.396 | 5.485+(−.03)*Age |
| Ventral Striatum | 3.949 | .057 | .124 | −.352 | 4.119+(−.02)*Age |
| Globus Pallidus | 2.291 | .141 | .076 | −.275 | 2.615+(−.017)*Age |
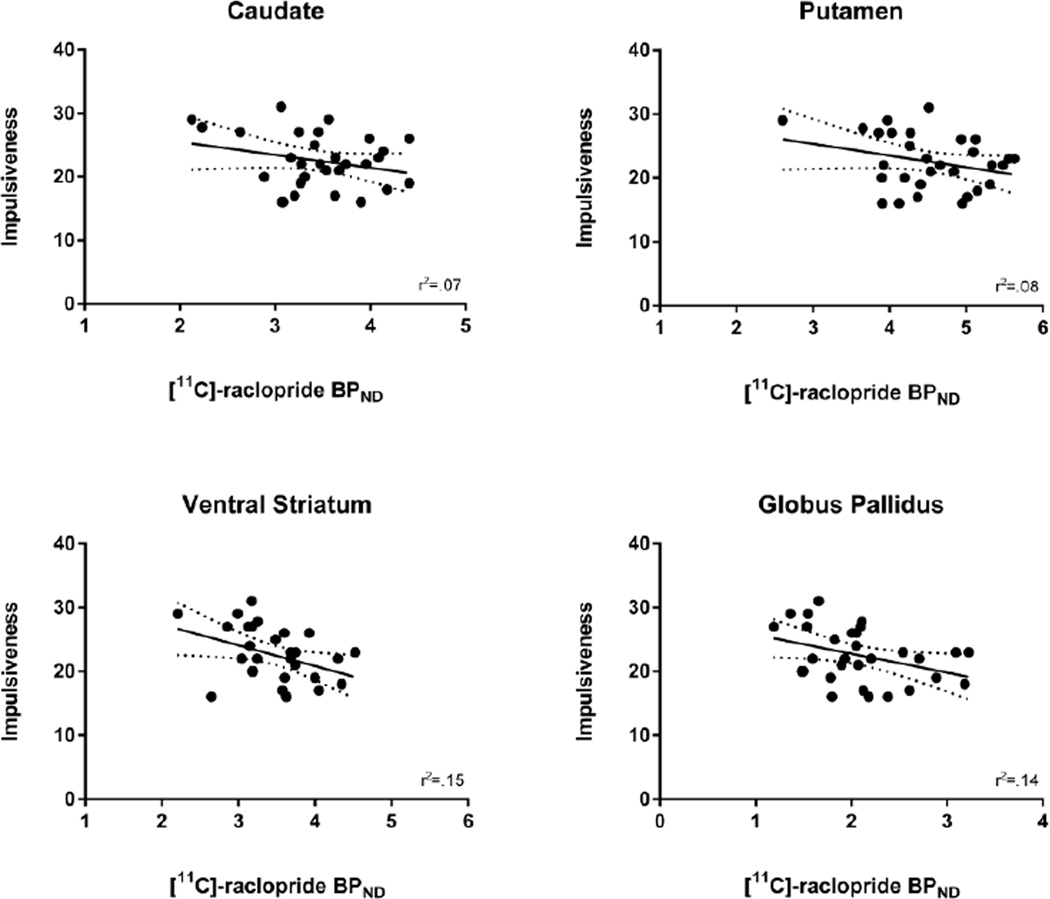
Impulsiveness was negatively correlated with [11C]-raclopride BPND in the VS (r(28)=− .39, p=.03; 95% CI [−.66, −.03]) and the GP (r(28)=−.38, p=.04; 95% CI [−.65, −.02]). These relationships became stronger after partially controlling for age and gender: VS (r(26)=−.44, p=.02), GP (r(26)=−.39, p=.04). Impulsiveness was not correlated with BPND in the caudate (r(28)=−.27, p=.16; 95% CI [−.57, .11]) or putamen (r(28)=−.30, p=.12; 95% CI [−.59, .08]), and partially controlling for age and gender did not affect these results: caudate (r(26)=−.35, p=.07), putamen (r(26)=−.34, p=.08) (see Figure 1).
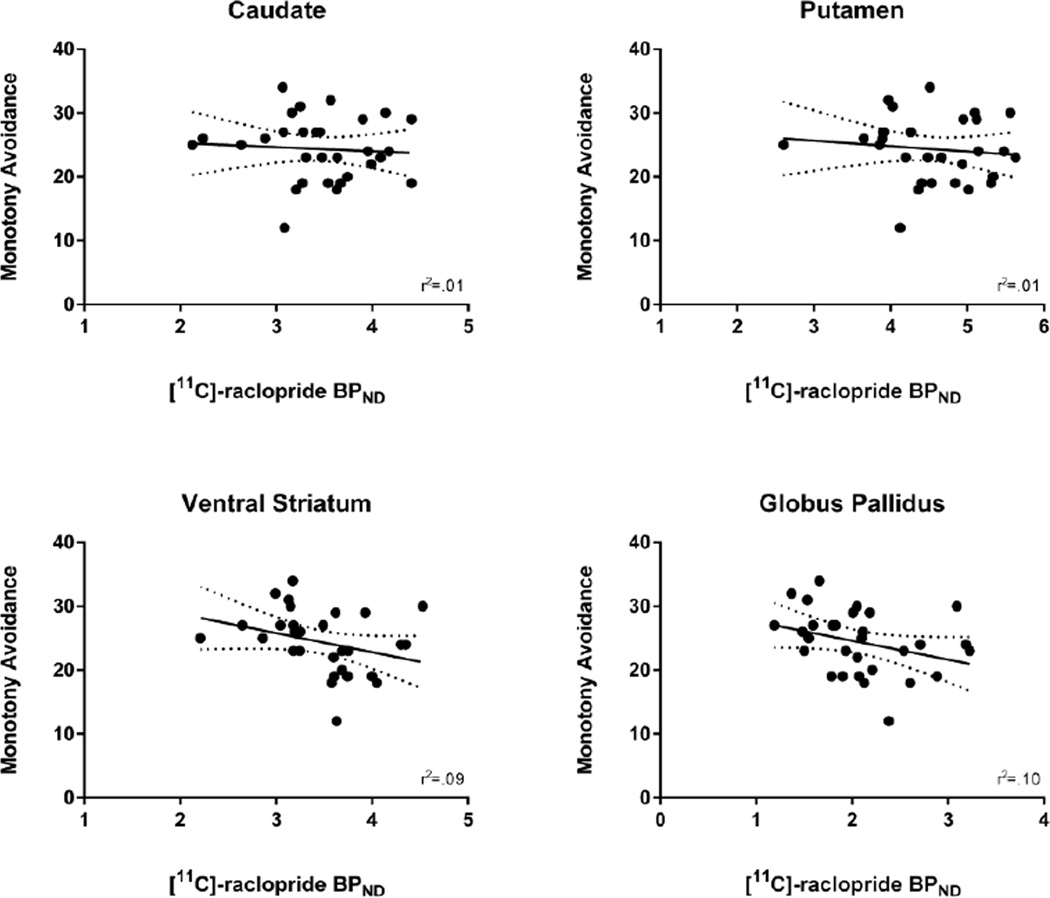
Figure 1.
Relationship between self-reported Impulsiveness on the Karolinska Scales of Personality and [11C]-raclopride BPND in each region of interest.
Monotony Avoidance was not correlated with [11C]-raclopride BPND in the caudate (r(28)=−.07, p=.70; 95% CI [−.42, .29]), putamen (r(28)=−.12, p=.54; 95% CI [−.46, .25]), VS (r(28)=−.31, p=.10; 95% CI [−.60, .06]), or GP (r(28)=−.32, p=.08; 95% CI [−.61, .04]). However, after partially controlling for age and gender monotony avoidance was negatively correlated with [11C]-raclopride BPND in the VS (r(26)=−.44, p=.02) and GP (r(26)=−.42, p=.03), but not in the caudate (r(26)=−.21, p=.29) or putamen (r(26)=−.23, p=.24) (see Figure 2).
Figure 2.
Relationship between self-reported Monotony Avoidance on the Karolinska Scales of Personality and [11C]-raclopride BPND in each region of interest.
Detachment was not correlated with [11C]-raclopride BPND in the caudate (r(28)=.18, p=.34; 95% CI [−.19, .51]), putamen (r(28)=.23, p=.23; 95% CI [−.15, .54]), VS (r(28)=.19, p=.30; 95% CI [−.18, .52]), or GP (r(28)=.22, p=.24; 95% CI [−.15, .54]). Controlling for age and gender did not change these results: caudate (r(26)=.10, p=.60), putamen (r(26)=.10, p=.63), VS (r(26)=.07, p=.73), GP (r(26)=.06, p=.75).
Social desirability was not correlated with [11C]-raclopride BPND in the caudate (r(28)=.16, p=.41; 95% CI [−.22, .49]), putamen (r(28)=.05, p=.80; 95% CI [−.32, .40]), VS (r(28)=−.005, p=.98; 95% CI [−.36, .36]), or GP (r(28)=.006, p=.97; 95% CI [−.35, .37]). Controlling for age and gender did not change these results: caudate (r(26)=.28, p=.16), putamen (r(26)=.15, p=.44), VS (r(26)=.07, p=.71), GP (r(26)=.06, p=.77).
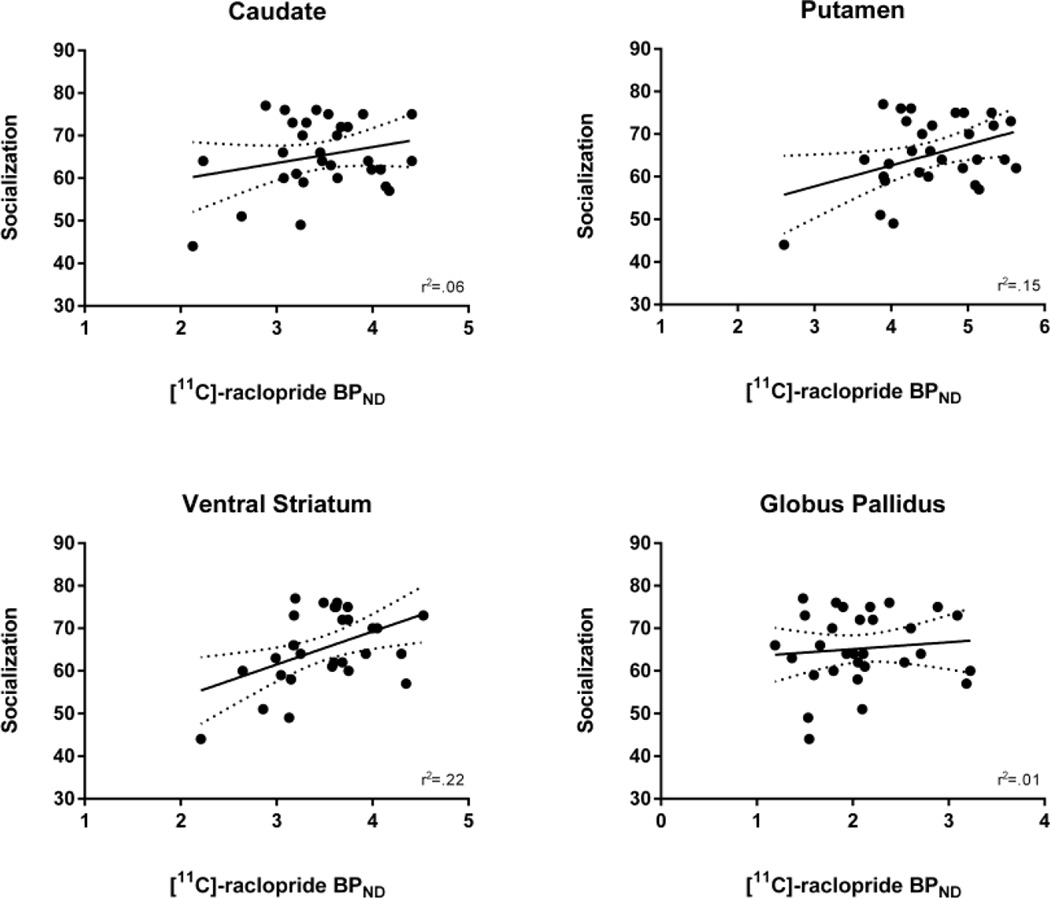
Socialization was positively correlated with [11C]-raclopride BPND in the putamen (r(28)=.39, p=.03; 95% CI [.04, .66]) and VS (r(28)=.47, p=.01; 95% CI [.12, .71]). These relationships survived statistically controlling for age and gender: putamen (r(26)=.57, p=.002), VS (r(26)=.63, p=.0001). Socialization was not correlated with [11C]-raclopride BPND in the caudate (r(28)=.25, p=.19; 95% CI [−.12, .56]) or GP (r(28)=.10, p=.59; 95% CI [−.27, .45]), and controlling for age and gender did not change these results: caudate (r(26)=.37, p=.05), GP (r(26)=.21, p=.29) (see Figure 3).
Figure 3.
Relationship between self-reported Socialization on the Karolinska Scales of Personality and [11C]-raclopride BPND in each region of interest.
The results of the exploratory correlations between [11C]-raclopride BPND in each ROI and the other ten scales on the KSP are presented in Table 2. Notably, indirect aggression was negatively correlated with [11C]-raclopride BPND in the VS (r(28)=−.40, p=.03). Controlling for age and gender, indirect aggression was negatively correlated with [11C]-raclopride BPND in both the VS (r(26)=−.40, p=.04) and the putamen (r(26)=−.40, p=.03). However, these exploratory findings did not survive correction for multiple comparisons (Bonferroni corrected p-threshold=. 001). Controlling for age and gender did not affect any other exploratory correlations (data not shown).
Table 2.
Exploratory correlations between self-reported personality traits from the Karolinska Scales of Personality and [11C]-raclopride BPND in each region of interest.
| Karolinska Scales of Personality |
Dorsal Caudate |
Dorsal Putamen |
Ventral Striatum |
Globus Pallidus |
|---|---|---|---|---|
| Psychic Anxiety | .042 [−.32, .40] |
−.078 [−.43, .29] |
−.162 [−.49, .21] |
.080 [−.29, .43] |
| Somatic Anxiety | −.007 [−.37, .36] |
−.155 [−.49, .22] |
−.155 [−.49, .22] |
.102 [−.27, .45] |
| Muscular Tension | .058 [−.31, .41] |
−.139 [−.48, .23] |
−.147 [−.48, .22] |
−.050 [−.40, .32] |
| Psychasthenia | .221 [−.15, .54] |
.136 [−.23, .47] |
.212 [−.16, .53] |
.296 [−.07, .59] |
| Inhibition of Aggression | .009 [−.35, .37] |
−.173 [−.50, .20] |
−.221 [−.54, .15] |
−.070 [−.42, .30] |
| Verbal Aggression | −.103 [−.45, .27] |
−.165 [−.50, .21] |
−.217 [−.54, .16] |
−.027 [−.38, .34] |
| Indirect Aggression | −.242 [−.55, .13] |
−.409* [−.67, −.06] |
−.397* [−.66, −.04] |
−.194 [−.52, .18] |
| Irritability | .055 [−.31, .41] |
.088 [−.28, .44] |
−.080 [−.43, .29] |
.217 [−.16, .54] |
| Guilt | −.233 [−.55, .14] |
−.105 [−.44, .26] |
.008 [−.35, .37] |
.110 [−.26, .45] |
| Suspicion | .016 [−.35, .37] |
.062 [−.30, .41] |
.019 [−.34, .38] |
.180 [−.19, .50]] |
. Correlation is significant at p<0.05 (2-tailed).
Values in square brackets [] represent 95% confidence intervals
Discussion
This study examined the relationship between personality traits measured by the KSP and DA D2/3R availability in the striatum of healthy humans in vivo. Five personality traits were selected as being most likely related to D2/3R availability, and were examined as a priori based on previous literature: impulsiveness, monotony avoidance, detachment, social desirability, and socialization.
With regards to measures of impulsivity, studies have either reported, i) correlations with striatal DA release but not baseline striatal D2/3R (Buckholtz et al., 2010; Oswald et al., 2007; Weiland et al., 2014), ii) positive correlations with baseline striatal D2/3R (Kim et al., 2014; Reeves et al., 2012), or iii) negative correlations with baseline striatal D2/3R only in persons with neuropsychiatric disorders (Lee et al., 2009). Our study is the first to demonstrate a negative association between baseline D2/3R availability and self-reported impulsiveness in healthy humans, specifically in the VS and GP. Our finding is at least consistent with some findings in animals, whereby impulsivity is associated with reduced D2/3R expression in the VS (Dalley et al., 2007).
After controlling for age and gender, we observed a negative correlation between monotony avoidance and D2/3R availability in the VS and GP. Previous studies have shown that higher levels of novelty seeking predict greater DA release and sensitization in the VS of healthy persons in response to d-amphetamine (Boileau et al., 2006; Leyton et al., 2002). However, these studies did not observe correlations between baseline D2/3R availability and novelty seeking. Notably, one study has shown an inverted-U relationship between sensation seeking and D2/3R availability in the VS and putamen of healthy persons (Gjedde et al., 2010). In the current investigation, we did not observe such a non-linear relationship with monotony avoidance.
We did not observe a relationship between detachment scores and D2/3R availability in the dorsal striatum like that reported in previous studies (Breier et al., 1998; Farde et al., 1997). However, our null findings are consistent with those of Schneier et al. who found no relationship between level of detachment and striatal D2/3R availability in healthy persons and persons with generalized anxiety disorder (Schneier et al., 2009). Moreover, we did not observe a relationship between social desirability and D2/3R in any striatal region, despite a correlation being observed using a scale which is a revised version of the KSP (Cervenka et al., 2010). Further work is necessary to elucidate the nature of these discrepancies.
We observed a positive correlation between self-reported socialization and D2/3R availability in the putamen and VS. This seems consistent with previous work showing a positive correlation between social support/status in healthy persons and striatal D2/3R availability (Martinez et al., 2010). Further work is necessary to elucidate the relationship between personality traits related to social, intersubjective behaviors and D2/3R receptor availability.
There are several limitations to the current investigation. First, for our a priori personality traits we did not explicitly control for multiple comparisons. In total, we made 20 comparisons (5 personality traits*4 ROIs). Using Bonferroni correction, the corrected p-value for significance would be p=.003 (α=.05/20). Notably, using this criterion the relationship between socialization and BPND in both the VS and putamen survived after controlling for age and gender (p=.0001 and p=.002, respectively). While we believe enough literature exists examining the relationship between D2/3R availability and personality traits to validate our a priori approach, this is admittedly a potential limitation. Second, newer reversions of the KSP exist which were not employed in this study (Gustavsson et al., 2000). Moreover, it will be important to corroborate these findings with other personality scales which capture similar traits (e.g. the NEO-P-IR and the temperament and character inventory). Third, this study only looked at baseline receptor availability. It will be important to further elucidate how functional PET measures – i.e. estimates of DA release and endogenous DA levels – relate to these personality traits (Suridjan et al., 2012). Fourth, to our knowledge, no formal validation of the GP ROI for [11C]-raclopride has been published. However, since the HRRT camera offers the resolution to resolve the VS, we argue that we can also appropriately delineate the GP. Notably, BPND in the GP appears distinct from other regions. For example, [11C]-raclopride BPND in the GP was not found to be related to age, as it is for the other striatal ROIs (Nakajima et al., 2015). We contend that this is a valid ROI with [11C]-raclopride and note that for the current investigation, GP BPND was found to be differentially related to personality traits compared to nearby structures, such as the putamen. However, test-retest values for this structure using [11C]-raclopride are warranted by future studies. Due to their high cost, PET imaging studies often employ small sample sizes. This engenders low statistical power and the potential for false negatives. While attempts have been made to accommodate for these problems statistically (Ko et al., 2011), acquiring representative samples in PET studies nevertheless remains pertinent. The vast majority of PET studies looking at the relationship between personality traits and D2/3R have done so retrospectively, or as a secondary aim. This is true for the current investigation. Thus, across studies it is unclear how representative of the general healthy population these subjects may be – as opposed to, for example, selected based on the purpose of matching to neuropsychiatric participants. This potentially biased sampling coupled with small sample sizes is a limitation which remains to be addressed by the field in general (Button et al., 2013). Finally, the current investigation only examined the relationship between personality traits and DA D2/3R availability in healthy people. It will be of interest to examine the relationship between D2/3R availability and personality traits in persons with neuropsychiatric disorders (Martinez et al., 2004) – e.g. in persons with schizophrenia and ultra-high risk for psychosis (Fresan et al., 2014; Kim et al., 2011).
Our study adds to the literature examining how differences in personality traits may relate to in vivo biomarkers of brain DA system functioning.
Acknowledgments
This study was funded by Canadian Institutes of Health Research (MOP-114989) and U.S. National Institute of Health (RO1MH084886-01A2).
Dr. Nakajima reports having received grants from Japan Society for the Promotion of Science and Inokashira Hospital Research Fund and speaker’s honoraria from GlaxoSmith Kline, Janssen Pharmaceutical, Pfizer, and Yoshitomiyakuhin within the past 3 years. Dr. Graff- Guerrerro currently receives research support from the following external funding agencies: Canadian Institutes of Health Research, the U.S. National Institute of Health, and the Mexico Instituto de Ciencia y Tecnologıa para la Capital del Conocimiento en el Distrito Federal (ICyTDF). He has also received professional services compensation from Abbott Laboratories, Gedeon-Richter Plc, and Lundbeck; grant support from Janssen; and speaker compensation from Eli Lilly.
Role of Funding Source
These organizations had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The other authors report no biomedical financial interests or potential conflicts of interest relevant to the current study.
Contributors
Graff-Guerrero, Gerretsen, Wilson, and Caravaggio designed the study and wrote the protocol. Caravaggio and Chung analyzed the PET images. Caravaggio, Chung, Gerretsen, Fervaha, Nakajima, Iwata, Plitman, Wilson, and Graff-Guerrero managed the literature searches and analyses. Caravaggio and Fervaha undertook the statistical analyses. Caravaggio wrote the first draft of the manuscript, which was subsequently edited by all the authors.
References
- Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, Benkelfat C. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry. 2006;63:1386–1395. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- Breier A, Kestler L, Adler C, Elman I, Wiesenfeld N, Malhotra A, Pickar D. Dopamine D2 receptor density and personal detachment in healthy subjects. Am J Psychiatry. 1998;155:1440–1442. doi: 10.1176/ajp.155.10.1440. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Kessler RM, Zald DH. Dopaminergic Network Differences in Human Impulsivity. Science (New York, NY) 2010;329:532–532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafo MR. Power failure: why small sample size undermines the reliability of neuroscience. Nature reviews. Neuroscience. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Caravaggio F, Raitsin S, Gerretsen P, Nakajima S, Wilson A, Graff-Guerrero A. Ventral striatum binding of a dopamine D2/3 receptor agonist but not antagonist predicts normal body mass index. Biol Psychiatry. 2015;77:196–202. doi: 10.1016/j.biopsych.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervenka S, Gustavsson JP, Halldin C, Farde L. Association between striatal and extrastriatal dopamine D2-receptor binding and social desirability. Neuroimage. 2010;50:323–328. doi: 10.1016/j.neuroimage.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Cumming P. Imaging Dopamine. Cambridge University Press; 2009. [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton A, Rees E, Bose SK, Lappin JM, Stokes PR, Turkheimer FE, Reeves SJ. Truth, lies or self-deception? Striatal D(2/3) receptor availability predicts individual differences in social conformity. Neuroimage. 2010;53:777–781. doi: 10.1016/j.neuroimage.2010.06.031. [DOI] [PubMed] [Google Scholar]
- Engler B. Personality Theories. Cengage Learning. 2013 [Google Scholar]
- Farde L, Gustavsson JP, Jonsson E. D2 dopamine receptors and personality traits. Nature. 1997 Feb 13;385(6617):590. doi: 10.1038/385590a0. 1997. [DOI] [PubMed] [Google Scholar]
- Fresan A, Leon-Ortiz P, Robles-Garcia R, Azcarraga M, Guizar D, Reyes-Madrigal F, Tovilla-Zarate CA, de la Fuente-Sandoval C. Personality features in ultra-high risk for psychosis: A comparative study with schizophrenia and control subjects using the Temperament and Character Inventory-Revised (TCI-R) J Psychiatr Res. 2014;23:00354–00359. doi: 10.1016/j.jpsychires.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Kumakura Y, Cumming P, Linnet J, Moller A. Inverted-U-shaped correlation between dopamine receptor availability in striatum and sensation seeking. Proc Natl Acad Sci U S A. 2010;107:3870–3875. doi: 10.1073/pnas.0912319107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Guerrero A, Mamo D, Shammi CM, Mizrahi R, Marcon H, Barsoum P, Rusjan P, Houle S, Wilson AA, Kapur S. The effect of antipsychotics on the high-affinity state of D2 and D3 receptors: a positron emission tomography study With [11C]-(+)-PHNO. Arch Gen Psychiatry. 2009;66:606–615. doi: 10.1001/archgenpsychiatry.2009.43. [DOI] [PubMed] [Google Scholar]
- Graff-Guerrero A, Willeit M, Ginovart N, Mamo D, Mizrahi R, Rusjan P, Vitcu I, Seeman P, Wilson AA, Kapur S. Brain region binding of the D2/3 agonist [11C]-(+)-PHNO and the D2/3 antagonist [11C]raclopride in healthy humans. Hum Brain Mapp. 2008;29:400–410. doi: 10.1002/hbm.20392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–287. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- Gunn RN, Slifstein M, Searle GE, Price JC. Quantitative imaging of protein targets in the human brain with PET. Physics in medicine and biology. 2015;60:R363–R411. doi: 10.1088/0031-9155/60/22/R363. [DOI] [PubMed] [Google Scholar]
- Gustavsson JP, Bergman H, Edman G, Ekselius L, von Knorring L, Linder J. Swedish universities Scales of Personality (SSP): construction, internal consistency and normative data. Acta Psychiatr Scand. 2000;102:217–225. doi: 10.1034/j.1600-0447.2000.102003217.x. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Ito H, Kodaka F, Takahashi H, Takano H, Arakawa R, Shimada H, Suhara T. Relation between presynaptic and postsynaptic dopaminergic functions measured by positron emission tomography: implication of dopaminergic tone. The Journal of Neuroscience. 2011;31:7886–7890. doi: 10.1523/JNEUROSCI.6024-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegeles LS, Martinez D, Slifstein M, Laruelle M, Abi-Dargham A. Baseline [11C]raclopride Binding Potential is Inversely Related to D2/3 Receptor Stimulation by Endogenous Dopamine. Neuropsychopharmacology. 2014;39:S112–S290. [Google Scholar]
- Kim JH, Son YD, Kim HK, Lee SY, Cho SE, Kim YB, Cho ZH. Association of harm avoidance with dopamine D2/3 receptor availability in striatal subdivisions: a high resolution PET study. Biol Psychol. 2011;87:164–167. doi: 10.1016/j.biopsycho.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Kim JH, Son YD, Kim HK, Lee SY, Kim YB, Cho ZH. Dopamine D2/3 receptor availability and human cognitive impulsivity: a high-resolution positron emission tomography imaging study with [11C]raclopride. Acta Neuropsychiatr. 2014;26:35–42. doi: 10.1017/neu.2013.29. [DOI] [PubMed] [Google Scholar]
- Ko JH, Reilhac A, Ray N, Rusjan P, Bloomfield P, Pellecchia G, Houle S, Strafella AP. Analysis of variance in neuroreceptor ligand imaging studies. PloS one. 2011;6:e23298. doi: 10.1371/journal.pone.0023298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso A, Vilkman H, Kajander J, Bergman J, paranta M, Solin O, Hietala J. Prediction of detached personality in healthy subjects by low dopamine transporter binding. Am J Psychiatry. 2000;157:290–292. doi: 10.1176/appi.ajp.157.2.290. [DOI] [PubMed] [Google Scholar]
- Laakso A, Wallius E, Kajander J, Bergman J, Eskola O, Solin O, Ilonen T, Salokangas RK, Syvalahti E, Hietala J. Personality traits and striatal dopamine synthesis capacity in healthy subjects. Am J Psychiatry. 2003;160:904–910. doi: 10.1176/appi.ajp.160.5.904. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Lawrence AD, Brooks DJ. Ventral striatal dopamine synthesis capacity is associated with individual differences in behavioral disinhibition. Front Behav Neurosci. 2014;8:86. doi: 10.3389/fnbeh.2014.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrubier Y, Sheehan DV, Weiller E, Amorim P, Bonora I, Harnett Sheehan K, Janavs J, Dunbar GC. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. European Psychiatry. 1997;12:224–231. [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M, Boileau I, Benkelfat C, Diksic M, Baker G, Dagher A. Amphetamine-induced increases in extracellular dopamine, drug wanting, and novelty seeking: a PET/[11C]raclopride study in healthy men. Neuropsychopharmacology. 2002;27:1027–1035. doi: 10.1016/S0893-133X(02)00366-4. [DOI] [PubMed] [Google Scholar]
- Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y, Perez A, Frankle WG, Cooper T, Kleber HD, Fischman MW, Laruelle M. Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology. 2004;29:1190–1202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- Martinez D, Orlowska D, Narendran R, Slifstein M, Liu F, Kumar D, Broft A, Van Heertum R, Kleber HD. Dopamine type 2/3 receptor availability in the striatum and social status in human volunteers. Biol Psychiatry. 2010;67:275–278. doi: 10.1016/j.biopsych.2009.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Menza MA, Mark MH, Burn DJ, Brooks DJ. Personality correlates of [18F]dopa striatal uptake: results of positron-emission tomography in Parkinson's disease. The Journal of neuropsychiatry and clinical neurosciences. 1995;7:176–179. doi: 10.1176/jnp.7.2.176. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Caravaggio F, Boileau I, Chung JK, Plitman E, Gerretsen P, Wilson AA, Houle S, Mamo DC, Graff-Guerrero A. Lack of Age-Dependent Decrease in Dopamine D3 Receptor Availability: A [11C]-(+)-PHNO and [11C]-Raclopride Positron Emission Tomography Study. Journal of Cerebral Blood Flow & Metabolism. 2015;35:1812–1818. doi: 10.1038/jcbfm.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortet G, Ibáñez MI, Llerena A, Torrubia R. The underlying traits of the Karolinska Scales of Personality (KSP) European Journal of Psychological Assessment. 2002;18:139. [Google Scholar]
- Oswald LM, Wong DF, Zhou Y, Kumar A, Brasic J, Alexander M, Ye W, Kuwabara H, Hilton J, Wand GS. Impulsivity and chronic stress are associated with amphetamine-induced striatal dopamine release. Neuroimage. 2007;36:153–166. doi: 10.1016/j.neuroimage.2007.01.055. [DOI] [PubMed] [Google Scholar]
- Reeves SJ, Mehta MA, Montgomery AJ, Amiras D, Egerton A, Howard RJ, Grasby PM. Striatal dopamine (D2) receptor availability predicts socially desirable responding. Neuroimage. 2007;34:1782–1789. doi: 10.1016/j.neuroimage.2006.10.042. [DOI] [PubMed] [Google Scholar]
- Reeves SJ, Polling C, Stokes PR, Lappin JM, Shotbolt PP, Mehta MA, Howes OD, Egerton A. Limbic striatal dopamine D2/3 receptor availability is associated with non-planning impulsivity in healthy adults after exclusion of potential dissimulators. Psychiatry Res. 2012;202:60–64. doi: 10.1016/j.pscychresns.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Rosa-Neto P, Lou HC, Cumming P, Pryds O, Karrebaek H, Lunding J, Gjedde A. Methylphenidate-evoked changes in striatal dopamine correlate with inattention and impulsivity in adolescents with attention deficit hyperactivity disorder. Neuroimage. 2005;25:868–876. doi: 10.1016/j.neuroimage.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Schalling D, Asberg M, Edman G, Oreland L. Markers for vulnerability to psychopathology: temperament traits associated with platelet MAO activity. Acta Psychiatr Scand. 1987;76:172–182. doi: 10.1111/j.1600-0447.1987.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Schluter T, Winz O, Henkel K, Prinz S, Rademacher L, Schmaljohann J, Dautzenberg K, Cumming P, Kumakura Y, Rex S, Mottaghy FM, Grunder G, Vernaleken I. The impact of dopamine on aggression: an [18F]-FDOPA PET Study in healthy males. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:16889–16896. doi: 10.1523/JNEUROSCI.1398-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneier FR, Abi-Dargham A, Martinez D, Slifstein M, Hwang D-R, Liebowitz MR, Laruelle M. Dopamine Transporters, D(2) Receptors, and Dopamine Release in Generalized Social Anxiety Disorder. Depression and anxiety. 2009;26:411–418. doi: 10.1002/da.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studholme C, Hill DL, Hawkes DJ. Automated three-dimensional registration of magnetic resonance and positron emission tomography brain images by multiresolution optimization of voxel similarity measures. Med Phys. 1997;24:25–35. doi: 10.1118/1.598130. [DOI] [PubMed] [Google Scholar]
- Suhara T, Yasuno F, Sudo Y, Yamamoto M, Inoue M, Okubo Y, Suzuki K. Dopamine D2 receptors in the insular cortex and the personality trait of novelty seeking. Neuroimage. 2001;13:891–895. doi: 10.1006/nimg.2001.0761. [DOI] [PubMed] [Google Scholar]
- Suridjan I, Boileau I, Bagby M, Rusjan PM, Wilson AA, Houle S, Mizrahi R. Dopamine response to psychosocial stress in humans and its relationship to individual differences in personality traits. J Psychiatr Res. 2012;46:890–897. doi: 10.1016/j.jpsychires.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M, Laruelle M, Rabiner EA, Gunn RN. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. NeuroImage. 2011;54:264–277. doi: 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- Weiland BJ, Heitzeg MM, Zald D, Cummiford C, Love T, Zucker RA, Zubieta JK. Relationship between impulsivity, prefrontal anticipatory activation, and striatal dopamine release during rewarded task performance. Psychiatry Res. 2014;223:244–252. doi: 10.1016/j.pscychresns.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AA, Garcia A, Jin L, Houle S. Radiotracer synthesis from [(11)C]-iodomethane: a remarkably simple captive solvent method. Nucl Med Biol. 2000;27:529–532. doi: 10.1016/s0969-8051(00)00132-3. [DOI] [PubMed] [Google Scholar]