Abstract
Little is known regarding cardiac involvement (CI) by neuromuscular disorders (NMDs). The purpose of this review is to summarise and discuss the major findings concerning the types, frequency, and severity of cardiac disorders in NMDs as well as their diagnosis, treatment, and overall outcome. CI in NMDs is characterized by pathologic involvement of the myocardium or cardiac conduction system. Less commonly, additional critical anatomic structures, such as the valves, coronary arteries, endocardium, pericardium, and even the aortic root may be involved. Involvement of the myocardium manifests most frequently as hypertrophic or dilated cardiomyopathy and less frequently as restrictive cardiomyopathy, non-compaction, arrhythmogenic right-ventricular dysplasia, or Takotsubo-syndrome. Cardiac conduction defects and supraventricular and ventricular arrhythmias are common cardiac manifestations of NMDs. Arrhythmias may evolve into life-threatening ventricular tachycardias, asystole, or even sudden cardiac death. CI is common and carries great prognostic significance on the outcome of dystrophinopathies, laminopathies, desminopathies, nemaline myopathy, myotonias, metabolic myopathies, Danon disease, and Barth-syndrome. The diagnosis and treatment of CI in NMDs follows established guidelines for the management of cardiac disease, but cardiotoxic medications should be avoided. CI in NMDs is relatively common and requires complete work-up following the establishment of a neurological diagnosis. Appropriate cardiac treatment significantly improves the overall long-term outcome of NMDs.
Keywords: Muscular diseases, Neuromuscular disease, Cardiomyopathies, Cardiac arrhythmias
Introduction
Since the original description of neuromuscular disorders (NMDs), it was noted that cardiac structures could be affected alongside the myopathies.1) Among the neuropathies, cardiac involvement (CI) was first described in a patient suffering from amyloid neuropathy.2) The type, frequency, management, and outcome of CI among the various NMDs vary significantly between the different disorders of the muscles and nerves. The aim of the present review was to summarise and discuss previous and most recent findings concerning the type, prevalence, diagnosis, treatment, and outcome of CI in NMDs.
Method
Data for this review were identified by investigating the archives of MEDLINE, Current Contents, Springerlink, Wiley, EBSCO, Ovid, and Web of science by applying a sensitive search strategy using combinations of the following search terms: "myopathy", "muscle disease", "muscular dystrophy", "transmission", "myasthenia", "neuromuscular", "nerve", "neuropathy", "motor neuron disease", "plexopathy", "radiculitis", "anterior horn cell", in combination with "heart", "cardiac", "cardiologic", "myocardium", "cardiomyopathy", "noncompaction", "hypertrabeculation", "Takotsubo", "echocardiography", "electrocardiography", "cardiac magnetic resonance imaging", "late gadolinium enhancement", "heart failure", "left ventricular dysfunction", "right ventricular dysfunction", "arrhythmias", "ventricular tachycardia", and "sudden cardiac death". Further manual searches were conducted to identify other relevant articles from cross-references. Randomized (blinded or open label) clinical trials, longitudinal studies, case series, and case reports were considered. Hereditary as well as acquired NMDs were considered. Abstracts, meeting reports, and animal studies were not included. Only articles regarding humans and published in the English-speaking literature between 1966 and 2015 were considered for inclusion. Appropriate papers were studied and assessed for their applicability to be incorporated in the present review.
Results
Altogether, 224 papers met the inclusion criteria and were included in the review. The full text was available for 165 of these papers whereas only the abstracts were available for 59 papers.
Classification of cardiac involvement
CI was categorised according to various criteria. Firstly, CI was classified according to the anatomical structure affected (i.e. myocardium, conduction system, valves, coronary arteries, aortic root, endocardium, pericardium). Secondly, CI was classified according to the physiological function that was impaired (i.e. heart failure, valve stenosis/insufficiency, pulmonary hypertension, impaired autonomic innervation). Thirdly, CI was classified according to the abnormality found on further diagnostic work-up, such as systolic dysfunction, late gadolinium enhancement (LGE), mitral valve prolapse syndrome (MPS), pulmonary valve stenosis, concentric/eccentric myocardial thickening, arrhythmias, congenital anomalies.
Types of cardiac involvement
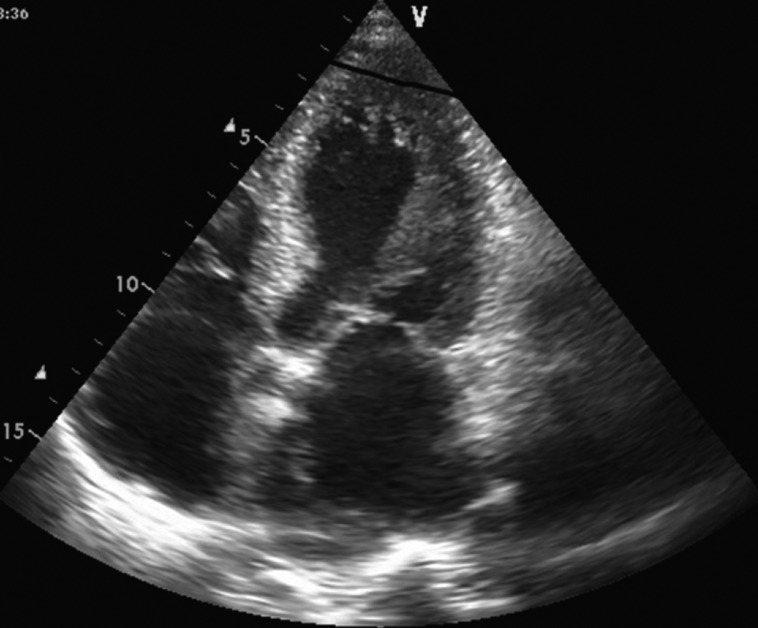
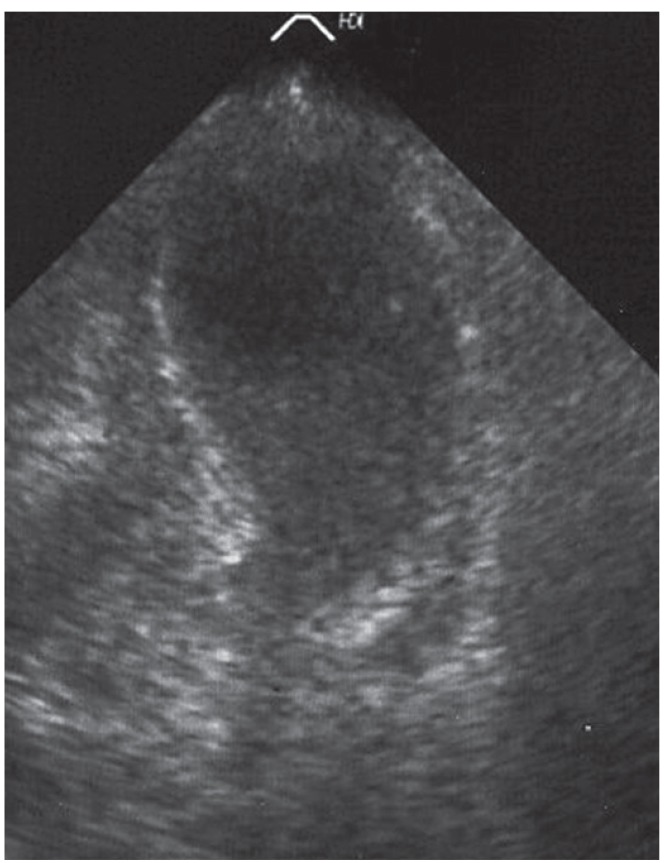
Cardiac involvement can manifest as hypertrophic cardiomyopathy (hCMP), dilated cardiomyopathy (dCMP), restrictive cardiomyopathy (rCMP), arrhythmogenic right ventricular dysplasia, histiocytoid cardiomyopathy (CMP), left ventricular hypertrabeculation/noncompaction (LVHT) (Fig. 1), or as Takotsubo syndrome (TTS) (Table 1 and Fig. 2). Involvement of the cardiac conduction system can present as spontaneous impulse generation or impulse conduction disorder. Valve disease in NMD may manifest as MPS whereas pathologic involvement of the coronary arteries can present with symptoms of coronary artery disease or coronary vascular spasms.3) NMD involvement of the aortic root may manifest as aortic root ectasia. Endocardial involvement may manifest as endocardial fibrosis exclusively affecting the endocardium or may also involve the myocardium (endocardial, subendocardial, mid-myocardial, trans-myocardial).4) Pericardial involvement by NMD can present as a pericardial effusion. Congenital abnormalities may affect any cardiac structure.
Fig. 1. Echocardiographic 4-chamber view in a patient with a mitochondrial disorder showing apical left ventricular hypertrabeculation/noncompaction.
Table 1. Definitions of cardiac abnormalities found in NMDs.
| dCMP | Combination of abnormal LV function (LVFS Z score or LVEF Z score<-2) and LV dilation (LV EDD Z score>2); or the presence of at least moderately depressed LV function, defined as LV fractional shortening Z score<-3, or LV ejection fraction Z score<-3, regardless of LV sizep |
| hCMP | Either echocardiographic evidence of localized ventricular hypertrophy or a posterior wall or septal thickness Z score>3, normal coronary angiography, normal or decreased systolic function, normal dimensions or dilation of cardiac cavities184) |
| rCMP | One or both atria enlarged relative to ventricles of normal or small size with evidence of impaired diastolic filling in the absence of significant valvular heart disease |
| LVHT | Two-layered structure of myocardium comprising a non-compacted thick layer and compacted thin layer with intertrabecular spaces perfused from the ventricular side. Systolic function may be normal or reduced |
| TTS | Segmental hypokinesia or akinesia (stunning) and reduced systolic function and compensatory hyperkinesia of segments not affected by the stunning. Most frequently, stunning affects the apical and midventricular segments of the left ventricular myocardium (classical type, apical ballooning), but rarely also the midventricular segments (midventricular type), the basal and midventricular segments (inverted type), or all segments (global type) |
| Myocardial thickening | Posterior wall thickness, septum thickness>11 mm |
| Aortic root ectasia | Aortic root diameter 41-50 mm |
| Sudden cardiac death | Unexpected death occurring<1 hour after the onset of a symptomatic cardiac event |
| Heart failure | Exertional or resting dyspnea, edema, neck vein distension, and echocardiographically EF<50% or FS<25% |
NMDs: neuromuscular disorders, dCMP: dilated cardiomyopathy, LV: left ventricular, LVFS: left ventricular fractional shortening, LVEF: left ventricular ejection fraction, EDD: end-diastolic diameter, hCMP: hypertrophic cardiomyopathy, rCMP: restrictive cardiomyopathy, LVHT: left ventricular hypertrabeculation/noncompaction, TTS: Takotsubo syndrome, EF: ejection fraction, FS: fractional shortening
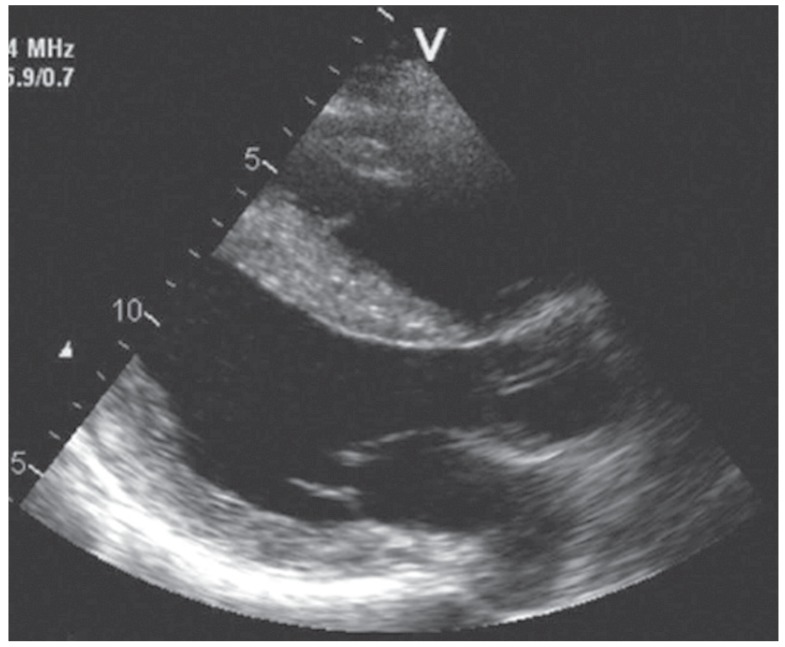
Fig. 2. Transthoracic echocardiography showing apical ballooning, akinesia, and reduced systolic function in a patient with a mitochondrial disorder.
Neuromuscular disorders with cardiac involvement
Various NMDs can affect the heart and can develop before, during, or after pathologic involvement of the skeletal muscles and/or nerves. Furthermore, CI may dominate the clinical presentation or may be an insignificant part of the clinical presentation. Particularly, NMDs with multi-organ disorder syndrome, such as myotonic dystrophies or mitochondrial disorders (MIDs) may present with CI. NMDs with CI may be classified as disorders of the nerves with CI, transmission disorders with CI, or as myopathies with CI. CI is generally much more prevalent in muscle diseases as compared to neuropathies or transmission disorders.
Disorders of the peripheral nerves with cardiac involvement
Disorders of the nerves are classified as neuronopathies (also: anterior horn cell disorders), radiculopathies, plexopathies, or neuropathies.
Neuronopathies: CI in neuronopathies is uncommon but occasional case reports exist in the literature.5) CI in neuronopathies includes dilation of the left ventricle,5) dCMP,6) or arrhythmias7) in patients with spinal muscular atrophy (SMA). The case of a patient with a SMA phenotype due to a LMNA mutation who also developed arrhythmias and systolic dysfunction has been reported.8) CI in amyotrophic lateral sclerosis (ALS) can present with dCMP,9) cardiac sympathetic hyperactivity,10) arrhythmias,11) or TTS (Table 2).12) Admittedly, the diagnosis of ALS was not definitively determined in a subset of these cases. CI in bulbar spinal muscular atrophy (Kennedy disease) manifests as electrocardiographic (ECG)-abnormalities, which can be seen in up to half of these patients.13) CI in GM2-gangliosidosis (hexosaminidase deficiency, Sandhoff) manifests as MPS or mitral regurgitation.14) CI has not been described in adrenoleukodystrophy. However, further systematic, prospective studies on the CI in neuronopathies with a definitive diagnosis are warranted before the prevalence and management of CI in neuronopathies can be adequately assessed.
Table 2. CI in neuropathies and transmission disorders.
| Neuromuscular disorder | Cardiac disease |
|---|---|
| Spinal muscular atrophy5),6),7) | Dilation of left ventricle, dCMP, ARRH |
| ALS9),10),12),11),185) | dCMP, sympathetic hyperactivity, TTS, CMP, ARRH |
| BSMA13),186) | ARRH, dCMP |
| GM2-gangliosidosis14) | MPS, mitral regurgitation |
| Neuropathy | |
| PMP2219) | LVHT, dCMP, HF |
| TTR15),16) | CMP, rCMP, ARRH, HF |
| GLA (Fabry)187) | hCMP |
| IKBKAP (HSAN-3)188) | ARRH, myocardial thickening |
| HSAN-4189) | ARRH |
| CMT1A190) | ARRH (long-QT) |
| HSMN18) | TTS |
| Refsum disease191) | dCMP, hCMP, ARRH, HF |
| LMNA8) | ARRH, HF |
| DCAF817) | CMP |
| Myasthenia20) | ARRH, myocarditis |
CI: cardiac involvement, dCMP: dilated cardiomyopathy, ARRH: arrhythmias, ALS: amyotrophic lateral sclerosis, TTS: Takotsubo syndrome, CMP: cardiomyopathy, BSMA: bulbospinal muscular atrophy, MPS: mitral valve prolapse syndrome, LVHT: left ventricular hypertrabeculation/noncompaction, HF: heart failure, TTR: transthyretin, rCMP: restrictive cardiomyopathy, GLA: galactosidase-alpha, hCMP: hypertrophic cardiomyopathy, IKBKAP: familial dysautonomia, HSAN: hereditary sensory and autonomous neuropathy, CMT1A: Charcot-Marie-Tooth type 1, HSMN: hereditary sensory-motor neuropathy
Radiculopathies: Conceivably, radiculopathies may cause cardiac disease, but in cases of radiculitis from infection with Borrelia burgdorferi or radiculopathy from amyloidosis, it is difficult to exonerate the infectious agent itself from directly causing cardiac disease as opposed to from amyloid deposition.
Plexopathy: Brachial plexus lesions from diabetes, viral infection, or trauma can theoretically affect autonomic cardiac function. However, no such reports were identified in the literature.
Neuropathies: CI in neuropathies has been described predominantly in the hereditary neuropathies and less commonly in the secondary neuropathies. Among the hereditary neuropathies, CI has been reported in hereditary transthyretin amyloidosis,15) hereditary sensory-motor neuropathy (HSMN), HSAN, Fabry disease, and Refsum disease (Table 2). CI in transthyretin amyloidosis is characterised by CMP,15) which may be independent of the neuropathy and due to primary deposition of amyloid in the myocardium, arrhythmias,16) rCMP, and heart failure. HMSN2 due to mutations in the DCAF8 can be variably accompanied by CMP.17) HSMN may also occur alongside TTS.18) Hereditary neuropathy due to a PMP22 duplication may be associated with dilatation of the ventricles, LVHT, or heart failure (Table 2).19)
Transmission disorders
CI has not comprehensively studied in patients with transmission disorders. In a study by Hofstad et al.,20) CI in myasthenia presented most commonly with arrhythmias. A small number of patients, particularly those with thymoma, can also develop myocarditis.20),21) In a study of 108 patients with myasthenia, 16% of these patients developed CI.20) In single cases, myasthenic crisis has been shown to trigger TTS.22),23),24),25)
Diseases of the muscle
Muscular dystrophies
Duchenne muscular dystrophy: In patients with Duchenne muscular dystrophy, cardiac disease occurs in nearly all cases if patients survive long enough and significantly determines the outcome. Cardiac disease in Duchenne muscular dystrophy (DMD) develops shortly after the onset of muscular manifestations. Initial manifestations of cardiac disease include abnormalities of impulse generation and conduction. Involvement of the myocardium usually becomes evident after patients have become wheelchair-bound. With disease progression, myocardial function, too, deteriorates and becomes a major outcome measure in these patients. CMP in DMD is characterised by progressive fibrosis of the myocardium, which can be most easily assessed by cardiac magnetic resonance imaging (cMRI).26),27) Progression of myocardial fibrosis is correlated with dilatation of the ventricles, decline of systolic function, and subsequent heart failure.28) A rare manifestation of CI in DMD is LVHT (Table 3).29) Various impulse generation and conduction abnormalities have also been reported in patients with DMD but it is unknown if the frequency of these abnormalities increases with worsening dCMP and progressive myocardial fibrosis. Patients may require implantation of a pacemaker because of third-degree AV-block. CI can be observed even in female carriers of the disease.30) Poor heart function in DMD is usually the cause of early death.31) Causes of sudden cardiac death (SCD) in DMD include sustained ventricular tachycardias, asystole, or acute heart failure.
Table 3. CI in myopathies.
| Myopathy | hCMP | dCMP | LVHT | PM/ICD | HF | HTX | SCD |
|---|---|---|---|---|---|---|---|
| DMD | x | x | x | x | x | x | x |
| BMD | x | x | x | x | x | x | |
| FSHMD | x | ||||||
| EDMD (LMNA) | x | x | x | x | x | x | |
| EDMD (FHL1) | x | x | |||||
| LGMD1B | x | x | x | x | x | ||
| LGMD2A | x | x | |||||
| LGMD2B | x | ||||||
| LGMD2C | x | ||||||
| LGMD2D | x | ||||||
| LGMD2E | x | x | |||||
| LGMD2I | x | x | x | ||||
| LGMD2M | x | ||||||
| CMD (COL6A) | x | x | |||||
| CMD (POMT1) | x | x | |||||
| CMD (CHKB) | x | ||||||
| CMD (FKTN) | x | ||||||
| CMD (FKRP) | x | ||||||
| MFMP (DES) | x | x | |||||
| MFMP (BAG3) | x | x | |||||
| CCD | x | x | x | ||||
| Multiminicore | x | x | |||||
| Centronuclear | x | x | |||||
| Nemaline MP | x | x | x | x | x | ||
| Distal MP (DES) | x | x | x | ||||
| Distal (MYH7) | x | x | x | ||||
| Distal (FHL1) | x | ||||||
| MD1 | x | x | x | x | x | x | |
| MIDs | x | x | x | x | x | x | x |
| Glycogenosis II | x | x | x | ||||
| Glycogenosis III | x | x | x | x | |||
| Glycogenosis IV | x | x | x | ||||
| Glycogenosis V | x | x | |||||
| Trifunctional protein | x | x | x | x | |||
| VLCADD | x | x | |||||
| Danon | x | x | x | x | x | x | x |
| Barth-syndrome | x | x | x | x | x | x | x |
CI: cardiac involvement, hCMP: hypertrophic cardiomyopathy, dCMP: dilated cardiomyopathy, LVHT: left ventricular hypertrabeculation/noncompaction, PM: pace maker, ICD: implantable cardioverter defibrillator, HF: heart failure, HTX: heart transplantation, SCD: sudden cardiac death, DMD: Duchenne muscular dystrophy, BMD: Becker muscular dystrophy, FSHMD: facioscapulohumeral muscular dystrophy, CCD: central core disease
Becker muscular dystrophy: Though BMD is due to mutations in the same gene as DMD and cardiac disease also occurs in BMD, there are several similarities and differences with respect to CI. As in DMD, the most frequent manifestations of CI are dCMP, cardiac conduction defects (CCDs), and arrhythmias (Table 3).32) Rarely, LVHT has been described in addition to dCMP in BMD patients.33) CI in BMD usually develops later during the course of the disease as in DMD.32) Only rarely may CI in BMD develop early in the natural history.34),35) In two siblings with BMD, dCMP was the initial manifestation of CI occurring at 11 years of age.34) One of the siblings died at 14 years while awaiting heart transplantation (HTX), whereas the other required implantation of a ventricular assist device.34) Few reliable data are available regarding the incidence of CI in BMD. CI in BMD is frequent, but most likely less frequent than in DMD.36) In a study of 48 BMD patients aged 26-56 years, 19% had dCMP.37) Given that patients usually die between their 40s and 80s, the prognosis is more favourable than it is with DMD patients.37)
Emery-Dreifuss muscular dystrophy: X-EDMD is due to mutations in the emerin or the FHL1 gene. CI in X-EDMD due to emerin mutations has been occasionally reported and manifests as CCDs.38) Rarely, dCMP in association with life-threatening ventricular arrhythmias has also been reported.38) A subset of patients with X-EDMD due to FHL1 mutations can present with pulmonary artery hypoplasia or SCD (Table 3).39) In another study, FHL1 patients presented with myocardial thickening, hCMP, and SCD.40) Single patients may also present with pulmonary valve stenosis.41) Autosomal dominant (AD)-EDMD is due to mutations in the LMNA gene and, like other laminopathies, frequently associated with cardiac disease. CI in AD-EDMD includes CCDs, dCMP, and heart failure.42) Rarely, hypoplasia of the aorta has been reported as a cardiac manifestation of AD-EDMD.43) Should atrial fibrillation develop in this patient population, ischemic stroke may be the initial manifestation of the muscle disease.44)
Limb girdle muscular dystrophies: Cardiac disease occurs in various types of LGMD but is particularly more common in the autosomal dominant LGMD1B laminopathy and the recessive LGMD types A (calpainopathy), B (dysferlinopathy), C (gamma-sarcoglycanopathy), D (alpha-sarcoglycanopathy), E (beta-sarcoglycanopathy), I (fukutin-related protein [FKRP]), and M (FKTN gene).45),46),47) Patients with LGMD1B may be severely affected by cardiac disease manifesting as CCDs, dCMP, or SCD.45) Some of these patients harboring duplications in the LMNA gene may even require HTX.48) Patients with LGMD2A, on the other hand, rarely develop CI.49) In isolated case reports, however, CCDs, LVHT, and heart failure have been reported.50) As with LGMD2A, CI in LGMD2B is usually mild51),52) or absent.53) Only rarely may patients present with dCMP.54) In a study of 10 patients with LGMD2C, CCDs and abnormal relaxation pattern in the tricuspid annulus were detected on tissue Doppler imaging.55) Other studies however, did not find CMP in LGMD2C.56) In a study of 32 patients with LGMD2D, two thirds developed CI before the onset of muscular manifestations.57) In a study of 6 patients with LGMD2E, 3 developed fatal dCMP.58) In LGMD2E, systolic dysfunction has been shown to progress during a follow-up of 9 years, whereas ECG parameters may remain unchanged.36) LGMD2I appears to be the LGMD subtype in which CI is most commonly observed. In a study of 23 patients with LGMD2I, almost two thirds had systolic dysfunction, although none had arrhythmias.59) In a study of 10 patients with LGMD2I all had impaired torsion.60) In another study of 7 patients with LGMD2I, 4 had myocardial fibrosis, 1 developed systolic dysfunction, and 1 had diastolic dysfunction.52) cMRI has been shown to be a sensitive method to detect CMP and heart failure in patients with LGMD2I.61) Some patients with LGMD2I may progress to the extent that they require HTX.62) In a study of 32 LGMD2I patients, systolic dysfunction was associated with increased mortality.36) In LGMD2I, systolic function may deteriorate over time while ECG-parameters do not simultaneously worsen. Similar findings were observed in patients with unclassified autosomal recessive-LGMDs.36) CI in LGMD2I may occasionally manifest as acute heart failure.63) dCMP has been reported in single cases with LGMD2M (Table 3).64) In a Dutch study of 24 patients with non-specified sarcoglycanopathy, 17% developed dCMP.65) Patients with LGMD1A, 1C, 1D, and 1E and LGMD2F, LGMD2G, and LGMD2H do not appear to be at risk of developing cardiac disease.66) Mutations in the delta-sarcoglycan gene cause isolated dCMP without skeletal muscle involvement.67)
Facio-scapulo-humeral muscular dystrophy: CI in facio-scapulohumeral musclar dystrophy (FSH-MD) is rare and has been described only in single case reports. These patients developed not only CCDs but also supraventricular and ventricular arrhythmias.68) One FSH-MD patient developed pre-excitation syndrome, supraventricular arrhythmias, and mild myocardial thickening.68) In other patients, short-PR-interval and P-wave abnormalities have been reported.69) In a study of 8 FSH-MD patients, 2 developed elevated P-waves, and three multifocal atrial premature contractions.69) In a single patient with FSH-MD hCMP was reported. In a study of 6 patients with FSH-MD, all subjects had reduced uptake of Tl-201 on Tl-201-SPECT.70) In some FSH-MD patients, sympathetic output may be increased while the parasympathetic output is decreased, as has been demonstrated by heart rate variability analysis.71)
Congenital muscular dystrophies: CMDs are due to mutations in LMNA, LAMA2, COL6A, POMT1, POMGNT1, FKTN, FKRP, LARGE, SEPN1, CHKB, or ITGA7 genes. CI has been reported in various types of CMD. Cardiac involvement, in particular, appears to occur in patients carrying mutations in the LMNA (arrhythmias, dCMP),72) COL6A (dCMP, systolic dysfunction requiring HTX),73),74) POMT1 (aortic root ectasia, systolic dysfunction),75) CHKB (dCMP, systolic dysfunction, congenital heart defects),76) and LAMA2 (dCMP)77),78) genes. Additionally, CI has been reported in patients with Fukuyama congenital muscular dystrophy (dCMP, systolic dysfunction) due to FKTN mutations,79) congenital muscular dystrophy with alpha-dystroglycan deficiency (dCMP, CCDs, mitral regurgitation),80) and in merosin-positive congenital muscular dystrophy (systolic and diastolic dysfunction).81)
Myofibrillar myopathies
Myofibrillar myopathies are caused by mutations in genes which mainly encode Z-disc proteins such as desmin, alpha β-crystalline, myotilin, ZASP, filamin-C, or BAG3.82) Patients with myofibrillar myopathy due to BAG3 mutations may present with CMP at the onset of muscular manifestations.83) In a subset of these patients, cardiac compromise may even precede the onset of muscular manifestations to the extent that HTX is required.83) Some patients carrying BAG3 mutations may present with hCMP with a restrictive filling pattern, QT-prolongation, and, potentially, a rapidly progressive course.84) Rarely, BAG3 myofibrillar myopathy can manifest with dCMP requiring HTX before the onset of muscular manifestations.83) Patients with myofibrillar myopathy and a limb-girdle phenotype due to MYOT mutations may develop CMP only in the late stages of the disease.85) Desmin gene mutations causing generalised weakness and wasting may also be associated with arrhythmias and severe rCMP.86) Patients carrying cypher/ZASP mutations may develop severe dCMP resulting in premature death.87) Patients with non-specified myofibrillar myopathy may present with dCMP and rCMP alongside atrial fibrillation and/or other supraventricular tachyarrhthmyias that respond favourably to recurrent radiofrequency ablation.88) Genetically undetermined myofibrillar myopathy may accompany hCMP, CCDs, and heart failure requiring HTX.89)
Congenital myopathies
Congenital myopathies may be classified according key histological features, such as cores, inclusion bodies, and abnormal protein accumulation, with or without central nuclei, or even based on myofibril size variation. The largest group of congenital myopathies are the core myopathies (central core disease, multiminicore disease), which represent the main non-dystrophic pediatric myopathies.90) Core myopathy due to mutations in the titin (TTN) gene may accompany CMP, atrial septal defects, and LVHT.90) A case report of multiminicore disease-associated LVHT has been described.91) CI has also been reported in multiminicore disease due to MYH7 mutations.92) Rarely, patients with central core disease and intractable dCMP may require HTX.93) A patient with centronuclear myopathy due to a dynamin-2 mutation was also diagnosed with CMP.94) At age 40, this patient subsequently developed left bundle-branch-block (LBBB), three years after which a pacemaker was implanted.94) Early-onset dCMP in centronuclear myopathy may require HTX.95) Infantile myotubular myopathy may accompany the CCDs.96) Congenital fiber-type dysproportion may be associated with CCDs requiring pacemaker implantation.97) Congenital fiber-type dysproportion due to LMNA mutations may also be associated with CCDs.98) CI in nemaline myopathy may manifest as dCMP, hCMP, non-specific CMP, and heart failure.99),100),101) Uncommonly, patients may require HTX, and SCD may also occur on rare occasion.
Distal myopathies
Distal myopathies may be due mutations in MYH7 (Laing), dysferlin (Miyoshi), ZASP (Griggs), VCP, FHL1, TTN (Udd), TOR1AIP, MATR3, or GNE (Nonaka). Only single case reports of patients with genetically confirmed distal myopathy have developed CI. Mutations in the MATR3 gene do not appear to have cardiac manifestations.102) Patients with distal myopathy Welander also do not appear to develop CI. Patients with distal myopathy due to MYH7 mutations may develop dCMP.103),104) An uncommon but important manifestation of CI in distal myopathy due to MYH7 mutations is LVHT.105) X-linked distal myopathy due to FHL1 mutations may accompany hCMP.106) Patients with distal myopathy due to TOR1AIP1 mutations may develop un-specified CMP.107) Distal myopathy due to mutations in desmin may manifest with CCDs requiring pacemaker implantation or, less commonly, with SCD.108) Approximately 10-20% of patients with distal myopathy due to GNE mutations may develop CI.109) Single case reports of patients with non-specified distal myopathy associated histologically with rimmed vacuoles have been reported to present with rCMP, CCDs, or SCD.110),111),112)
Myotonias
Myotonias are clinically characterised by impaired muscle relaxation. Myotonias may be divided into dystrophic and non-dystrophic myotonias. The dystrophic myotonias include myotonic dystrophy type-1 (MD1) and myotonic dystrophy type-2 (MD2).
Non-dystrophic myotonias: Non-dystrophic myotonias include the muscular channelopathies, which only rarely are associated with CI. Isolated cases of patients with hyperkalemic periodic paralysis, however, have been reported in association with CCDs and arrhythmias.113),114) In fact, CCDs appear to be more common in hypokalemic periodic paralysis than in hyperkalemic periodic paralysis.115) In single patients with myotonia congenita Becker, pre-excitation syndrome has been reported.116) CI has not been reported in paramyotonia congenita, potassium-aggravated myotonia, or normokalemic periodic paralysis. In the majority of cases with non-dystrophic myotonias, however, no cardiac abnormalities have been reported.
Dystrophic myotonias: Contrary to non-dystrophic myotonias, the heart is frequently affected in myotonic dystrophies. While both MD1 and MD2 subtypes are affected, cardiac involvement appears to be more common in MD1 with CCDs being among the most common manifestations. The most commonly reported cardiac manifestation is progressive first degree atrio-ventricular (AV)-block, although ventricular arrhythmias do occur and have a significant impact on the overall outcome. AV-conduction disturbances are associated with an increased risk of ventricular arrhythmias.117) In a study of 161 MD1-patients, atrial fibrillation/flutter occurred in 17% and strongly influenced the outcome.118) A high prevalence of ajmalin-induced Brugada ECG-pattern was recently reported in MD1 patients.119) These patients may require a pacemaker or even an implantable cardioverter defibrillator (ICD).120) In MD1-patients, SCD has been commonly reported in the literature.121) In both types of myotonic dystrophy, the myocardium may be affected in the form of dCMP or LVHT.122),123),124) Myocardial involvement may also progress to myocardial fibrosis, thereby manifesting as LGE on cMRI.125) In a study of 129 MD1 patients, CI was found in 55%.126) In this cohort, first degree AV-block was found in 23.6%, second-degree AV-block in 5.6%, right bundle branch block in 5.5%, LBBB in 3.2%, and QTc-prolongation in 7.2%, whereas atrial fibrillation occurred in 4.1%, other supraventricular tachyarrhythmias in 7.3%, and non-sustained ventricular tachycardia in 4.1%.126) In this same cohort, 20% developed systolic dysfunction and 21.7% reduced global longitudinal strain.126) Among patients with congenital myotonic dystrophy, diastolic dysfunction is known to occur.127) More frequently, however, are supraventricular arrhythmias.128) Some patients with congenital MD1 develop AV-block.129) CI in patients with DM2 manifests as conduction disturbances (Brugada-like ECG abnormalities),130) dCMP,131) or LVHT.132)
Metabolic myopathies
Mitochondrial disorders: MIDs are disorders with the highest variability in the incidence of CI. CI in MIDs may not only manifest as CMP, CCDs, or arrhythmias but also as aortic root ectasia,133) coronary artery disease,134),135) pericardial effusion in the absence of heart failure,136) valve abnormalities,137),138) or autonomic dysfunction.139) CI in MIDs most frequently develops after the onset of other systemic manifestations, and only rarely is cardiac disease the initial manifestation of a MID. Among syndromic MIDs, CI most frequently occurs in Kearns-Sayre syndrome, MELAS-syndrome, MERRF-syndrome, chronic progressive external ophthalmoplegia, neuropathy ataxia retinitis pigmentosa, Leigh-syndrome,140),141) or Leber's hereditary optic neuropathy.142),143) CI in patients with primary CoQ-deficiency may manifest as sinus-bradycardia or heart failure.144) CI has been also reported in non-syndromic MIDs.145),146) The most frequent cardiac abnormalities in MIDs are dCMP, hCMP, CCDs, and arrhythmias.147),148),149),150) LVHT is also highly prevalent in MIDs. MIDs, alongside Barth-syndrome, are the disorders with the highest incidence of LVHT. The most frequent manifestations of CI in MIDs, however, remain dCMP, CCDs, and arrhythmias. Despite the multi-organ nature of MIDs, an increasing number of MID patients with intractable dCMP undergo HTX.151),152) The prevalence of SCD in MIDs with CI is unknown, but given the high frequency of involvement of the cardiac conduction system or autonomic fibers is likely quite high. There are also a number of MID patients in whom TTS has been reported.153)
Glycogenoses: CI is common with glycogen storage disease and has been predominantly described in type II, type III, type IV, and type V variants of the disease. Glycogen storage disease type II (Pompe disease) is a rare, autosomal recessive disease in which CI manifests as hCMP, CCDs, supraventricular and ventricular arrhythmias, or heart failure.154) The course of cardiac disease varies considerably between children and adults. Early stages of cardiac involvement responds favourably to enzyme replacement therapy (ERT). CI in glycogen storage disease type-III may manifest as myocardial thickening or hCMP in most patients.155),156) Other cardiac manifestations include myocardial fibrosis, dCMP, or heart failure.157) A single patient with premature coronary artery disease has been described in the literature.158) Some of these patients require HTX.157) Glycogen storage disease IV (Andersen's disease) is due to mutations in the GBE1 gene and may manifest in the heart with dCMP leading to intractable heart failure, which is usually associated with early death during childhood.159) Glycogenosis type V (McArdle) may manifest cardiologically as obstructive hCMP160) and glycogenosis type VII (Tarui's disease) as non-specific CMP.161) Cases of two patients with undetermined vacuolar polysaccharidosis, progressive dCMP necessitating HTX at ages 13 and 14 years have been reported.162)
Lipid storage disease: Disorders of the carnitine cycle or fatty acid oxidation (beta-oxidation) frequently manifest with CI. In a study of 187 patients with beta-oxidation defects, 55% had CI, either manifesting as CMP or arrhythmias.163) Patients with mutations in the HADHB gene may manifest with LVHT and heart failure.164) Patients with very long chain acyl-CoA dehydrogenase deficiency, which manifests with hypoglycaemia, hepatomegaly, myopathy, and rhabdomyolysis, may cardiologically present with CMP or SCD at the onset of the disease.165),166) Patients with neutral lipid storage disease due to PNPLA2 mutations may develop rCMP.167),168) A subset of patients with carnitine-acyl-carnitine translocase deficiency due to a SLC25A20 mutation manifests with non-specified CMP at the onset of the disease.169) Carnitine deficiency may be accompanied by non-specified CMP.170)
Unclassified myopathies: In Barth-syndrome, approximately one-half of patients present with LVHT. Barth-syndrome, together with MIDs, is the disorders with the highest frequency of LVHT. In addition, patients with Barth-syndrome may present with dCMP or heart failure.171) Patients with Barth-syndrome may require ventricular assist devices or HTX. In patients with lysosomal Danon disease, which is due to LAMP2 mutations, hCMP, dCMP, CCDs, arrhythmias requiring prophylactic ICD implantation, and heart failure requiring HTX frequently occur.172) Rarely, these patients may develop LVHT.172) A subset of patients with Danon disease develops SCD.173) CI in Danon disease is usually severe and has strong prognostic implications, and its early diagnosis is, therefore, critical to facilitate timely HTX. In a patient with non-specific muscular dystrophy due to a novel CHKB mutation, dCMP was described.174)
Diagnosis of cardiac involvement in neuromuscular disorders
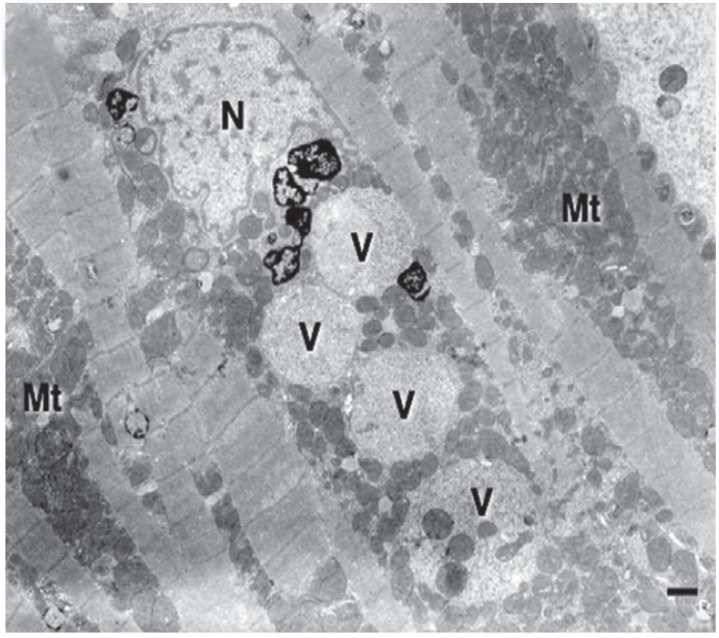
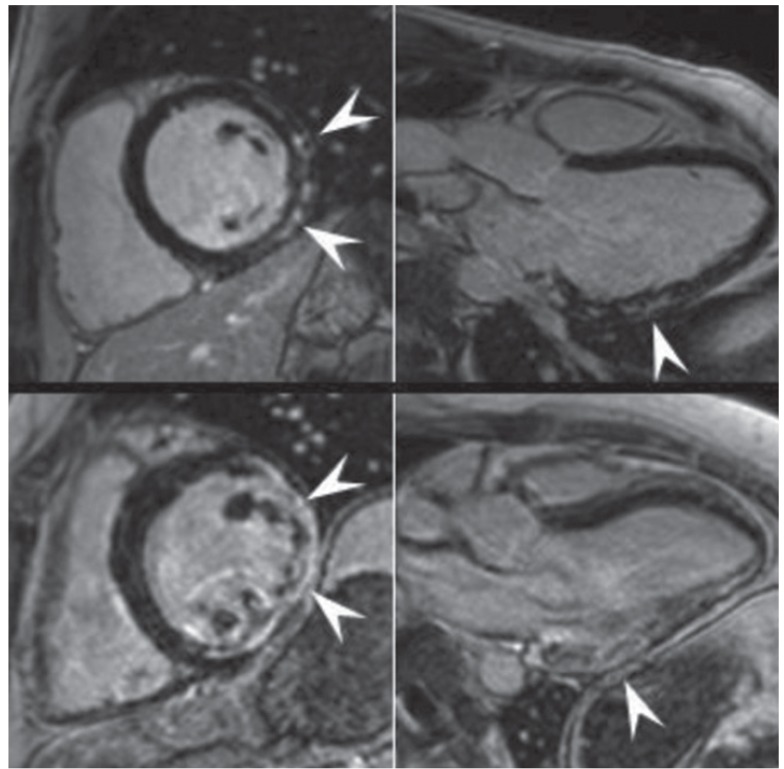
In all NMD patients, CI is important to recognise as it is potentially treatable and may significantly improve outcome with timely, appropriate management. Diagnosing cardiac disease in NMDs is challenging as it may be asymptomatic for a long period of time before becoming clinically evident. Diagnosing cardiac disease in NMDs is particularly difficult if cardiac disease manifests before the onset of neurological manifestations. In cardiologically asymptomatic patients with a clinically evident NMD, CI is usually diagnosed incidentally if one does not proactively search for CI. If patients are cardiologically symptomatic, appropriate diagnostic work-up must to be carried out. The basis of these investigations is a thorough history and careful clinical exam. Instrumental investigations include blood pressure measurement, routine ECG, stress testing, long-term ECG, and echocardiography. Eventually, patients may require coronary angiography, ventriculography, cMRI, stress echocardiography, tissue Doppler imaging, dipyridamole 201Tl SPECT myocardial perfusion imaging, technetium-99m sestamibi (99mTc-MIBI) scintigraphy, or endomyocardial biopsy. Some studies indicate that systolic dysfunction can be more favourably assessed by cMRI as compared to transthoracic echocardiography.175) cMRI detects abnormal distribution and intensity of LGE in 90% of DMD patients and, thus, is a sensitive diagnostic test for identifying early myocardial damage (Fig. 3).175) If the heart is the initial or primrary manifestation of the NMD, endomyocardial biopsy may be helpful to diagnose the underlying condition. Endomyocardial biopsy may show dystrophic changes, reduction or absence of dystrophin in case of a dystrophinopathy, sarcoglycan-deficiency in cases of sarcoglycanopathies, granulofilamentous material in case of a desminopathy, reactive mitochondrial changes in Danon disease, vacuolar degeneration and glycogen deposition in case of a glycogen storage disease, or proliferation and swelling of mitochondria, upregulation of heme oxygenase-1 (an adaptive enzyme to oxidative damage), abnormally shaped mitochondria, or COX-negative fibers in cases of a mitochondrial disorder (Fig. 4). In addition to cardiologic investigations, NMD patients must be evaluated by the pulmonologist and orthopaedic surgeon to rule out pulmonary or orthopaedic causes of CI. Nonetheless, it is important to actively rule out CI in a NMD as soon as the neurological diagnosis is established. Conversely, patients with cardiologic disease of unknown primary should be referred to the neurologist to exclude subclinical or mild NMD.
Fig. 3. Left ventricular endomyocardial biopsy. Electron microscopy revealing an excessive number of mitochondria of variable size with an abnormal morphology and vacuoles containing glycogen and degenerated mitochondria. N: nucleus, Bar: 1 µm (reproduced with permission applied from Nakagawa et al.192)).
Fig. 4. Late Gadolinium enhancement-cardiac magnetic resonance images in midventricular short-axis and 3-chamber-views from two muscular dystrophy patients with different stages of cardiomyopathy. Upper panel images show subepicardial late Gadolinium enhancement (LGE) as the only sign of cardiac involvement in a 22 year Becker muscular dystrophy patient. Lower panel images show transmural LGE but only mildly impaired left ventricular systolic function in a 27 year Duchenne muscular dystrophy patient with non-sustained ventricular tachycardia (reproduced with permission from Florian et al.143)).
Treatment of cardiac involvement in neuromuscular disorders
Treatment of CI in NMDs follows the general guidelines for treating specific cardiac disease and generally does not significantly vary from treatment of patients without NMD. Current treatment of CI in NMDs is symptomatic and relies on the avoidance of cardiotoxic drugs, application of cardioactive medications, as well as invasive inteventions, including placement of ventricular assist devices and HTX. If muscle function improves spontaneously or following treatment, it is important to improve overall cardiac function. Improving muscle function without addressing the cardiac aspects of NMDs may aggravate CMP and may, thus, may be harmful.176) Accordingly, the preclinical and clinical attention must assess cardiac function in NMDs.176) Treatment of cardiac disease in NMDs must also consider that patients with advanced involvement of the skeletal muscles can no longer exercise and thus are unable to participate in cardiovascular training and rehabiliation.
Restriction of cardiotoxic drugs
Among NMDs due to a metabolic defect, muscle-toxic cardiac medications, such as beta-blockers, amiodarone, milrinone, or statins should be avoided. In cases of general anesthesia in NMD patients, particularly those with malignant hyperthermia susceptibility or NMDs in which malignant hyperthermia has been previously reported, depolarising muscle relaxants and halogenated volatile anesthetics must be avoided to prevent cardiac arrest or SCD during an episode of malignant hyperthermia.177) For most of the cardiac drugs, however, it is unknown whether they have an adverse effect on nerve or muscle function. With MIDs and beta-oxidation defects, it is uncertain whether cardiac drugs have toxic effects on the mitochondrion. Application of mitochondrion-toxic drugs may not only be beneficial for cardiac disease but may also be harmful to muscle or heart.
Cardiac drugs
In case of heart failure, conservative heart failure therapy with angiotensin-converting enzyme inhibitors, AT-1-blockers, beta-blockers, diuretics, aldosterone-antagonists, must be initiated according to established guidelines. Treatment of CCDs also follows established guidelines. If supraventricular tachy-arrhythmias are also present, digitalis, beta-blockers, or amiodarone may be indicated. If atrial fibrillation occurs alongside the neurologic or muscular disease, digitoxin, beta-blockers, or amiodarone can be given. In cases of brady-arrhythmias, atropine may be a temporary option. In cases of atrial fibrillation or severe systolic dysfunction, oral anticoagulation with vitamin-K-antagonists should be considered. Patients with non-sustained ventricular tachycardia may benefit from beta-blockers. In DMD patients, steroids are beneficial for muscular manifestations but may also be effective in delaying the onset of CMP.178),179) ERT is a beneficial option for hCMP in Pompe's disease.180) A known complication of ERT in Pompe's disease, however, is SCD.180) Infantile or pediatric cases, in particular, may benefit from ERT with a favourable effect on CI.181) Administration of L-carnitine in carnitine deficiency may have a stabilising effect on CMP.170)
Invasive measures
If there is sinus-bradycardia or bradycardious atrial fibrillation in the absence of provocative drugs or if there are pauses >3.5 s, a pacemaker is indicated. If there is coronary artery stenosis, stent implantation and antithrombotic treatment is indicated. In cases of non-sustained ventricular tachycardias unresponsive to beta-blockers, an ICD should be considered. If there is drug-resistant heart failure and a LBBB, a cardiac resynchronisation therapy (CRT)-system should be considered if conventional measures are ineffective. In addition, BMD patients with dCMP, heart failure, sinus tachycardia, and mechanical dyssynchrony benefit from a CRT-system.33) If there are contraindications for an ICD or if the patient refuses ICD implantation, an external defibrillator (LifeVest®) could be an alternative. A LifeVest® can be worn even by pregnant females. Ectopic atrial activity may be treated by radiofrequency ablation.88) If there is severe valve stenosis or insufficiency, valve replacement may be necessary. In a single patient with Fukuyama-type muscular dystrophy, ventriculoplasty because of dCMP had been carried out.182) Myopathies in which implantation of an ICD had been carried out are listed in Table 3. Myopathies in which HTX had been carried out because of intractable heart failure are also listed in Table 3. NMD patients with secondary compromise due to secondary spinal or thoracic deformities frequently benefit from surgical stabilisation of the spinal column.
Preclinical options
Several preclinical therapies are undergoing development, including utrophin up-regulation, stop codon read-through therapy, viral gene therapy, cell-based therapy, or exon skipping.176) Some of these therapies are undergoing clinical trials, but these have predominantly focused on correction of skeletal muscle compromise.176) However, myopathy cannot be treated without simultaneous treatment of CI. Therapies focused on the muscle must also consider and include the myocardium.
Prognosis
In the majority of the cases, the prognosis of cardiac disease in NMDs is fair if the diagnosis is established early, if therapy is adequate, if patients are compliant, and if cardiac abnormalities respond favourably to treatment. However, in the setting of severe ventricular arrhythmias and if the patient is not supplied with an ICD, the ICD is insufficiently shocking, and cardio-pulmonary resuscitation is insufficient, the outcome may be fatal. The prognosis may also be unfavourable in cases of intractable heart failure not amenable to adequate drug treatment, in cases of an unavailable donor heart, or contraindications for a CRT-system or transplantation. In cases of atrial fibrillation, severe heart failure, or embolic events from noncompaction, and contraindications to oral anticoagulation with vitamin-K-antagonists, insufficient anticoagulation or not yet established anticoagulation, thromboembolic events may worsen prognosis and outcome of affected patients.
Discussion
The present review demonstrates that CI predominantly occurs in myopathies and only rarely in neuropathies or transmission disorders. Myopathies most frequently developing CI include the dystrophinopathies, laminopathies, desminopathies, nemaline myopathy, myotonic dystrophies, metabolic myopathies, Danon disease, and Barth-syndrome. It also reveals that CI most frequently includes CMPs (Fig. 5), impulse generation, or CCDs. CI needs to be proactively investigated as it may be subclinical or go unrecognised by the patient. This may be one reason that the prevalence of CI appears low in many myopathies. Another reason may be the overall rarity of the various NMDs. The low prevalence of CI can be also explained by the few systemic cardiac investigations carried out to diagnose CI. This review, in addition, shows that CI in NMDs must be recognised and appropriately treated as early as possible as it significantly determines the outcome of these patients. The earlier that CI in a patient with a NMD is recognised, the better the outcome. An appropriate method to detect early myocardial involvement in NMDs is cMRI. As soon as a NMD is diagnosed, the affected patient must be screened for CI. This is of paramount importance and mandatory irrespective of whether CI is subclinical or symptomatic. The NMDs with a particularly high risk of SCD (Table 3) require close and careful cardiac follow-up so that the therapeutic window is not overlooked.
Fig. 5. Two-dimensional echocardiogram, parasternal long-axis view showing marked concentric hypertrophy with a non-dilated ventricle in a male with mitochondrial hCMP due to the mutation m.3303T>C (reproduced with applied permission from Palecek et al.193)). hCMP: hypertrophic cardiomyopathy.
Survival in NMD patients can be prolonged by oral anticoagulation, heart failure therapy, pacemaker implantation, ICD implantation, or HTX. An increasingly common treatment in drug-resistant heart failure or dCMP is HTX. HTX has the disadvantage of the inevitable use of of steroids and immunosuppressants. Some of these immunosuppressants (e.g. cyclosporine) may secondarily induce myopathy and may, thus, worsen the pre-existing NMD. In addition, neurotoxic drugs, such as chemotherapeutics, may secondarily deteriorate neuropathy. Survival of NMDs with CI is not only dependent on the underlying NMD and the type of cardiac disease, but also on the effectiveness of treatment and avoidance of drugs with a cardio-toxic or myo-toxic effect. Thus, the outcome of patients with NMD and CI is dependent on the medication prescribed, which should be kept to only that which is required. Outcome in patients with LVHT is better if LVHT is isolated compared to LVHT patients with concomitant dCMP or hCMP.183)
In conclusion, CI in NMD is more commonly observed in myopathies, such as the dystrophinopathies, laminopathies, desminopathies, nemaline myopathy, myotonic dystrophies, metabolic myopathies, Danon disease, and Barth-syndrome. Management of CI in NMDs is an evolving field, which has gained increasing scientific attention, has practical implications, and needs to be carefully addressed as its appropriate management can prolong life-expectancy and considerably determines the outcome in most of these patients.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Rubin HJ, Lowbeer L. Progressive muscular dystrophy with involvement of heart muscle. Proc Staff Meet Tulsa Okla Hillcrest Meml Hosp. 1947;4:141–156. [PubMed] [Google Scholar]
- 2.Coelho E. Heart changes in the familial type of paramyloidosis with peripheral neuropathy. Z Kreislaufforsch. 1963;52:1066–1078. [PubMed] [Google Scholar]
- 3.Hertzman PA, Maddoux GL, Sternberg EM, et al. Repeated coronary artery spasm in a young woman with the eosinophilia-myalgia syndrome. JAMA. 1992;267:2932–2934. [PubMed] [Google Scholar]
- 4.Rakocević-Stojanović V, Pavlović S, Seferović P, et al. Pathohistological changes in endomyocardial biopsy specimens in patients with myotonic dystrophy. Panminerva Med. 1999;41:27–30. [PubMed] [Google Scholar]
- 5.Palladino A, Passamano L, Taglia A, et al. Cardiac involvement in patients with spinal muscular atrophies. Acta Myol. 2011;30:175–178. [PMC free article] [PubMed] [Google Scholar]
- 6.Yasuma F, Kuru S, Konagaya M. Dilated cardiomyopathy in Kugelberg-Welander disease: coexisting sleep disordered breathing and its treatment with continuous positive airway pressure. Intern Med. 2004;43:951–954. doi: 10.2169/internalmedicine.43.951. [DOI] [PubMed] [Google Scholar]
- 7.Elkohen M, Vaksmann G, Elkohen MR, Francart C, Foucher C, Rey C. Cardiac involvement in Kugelberg-Welander disease. A prospective study of 8 cases. Arch Mal Coeur Vaiss. 1996;89:611–617. [PubMed] [Google Scholar]
- 8.Iwahara N, Hisahara S, Hayashi T, Kawamata J, Shimohama S. A novel lamin A/C gene mutation causing spinal muscular atrophy phenotype with cardiac involvement: report of one case. BMC Neurol. 2015;15:13. doi: 10.1186/s12883-015-0269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Namazi MH, Khaheshi I, Haybar H, Esmaeeli S. Cardiac failure as an unusual presentation in a patient with history of amyotrophic lateral sclerosis. Case Rep Neurol Med. 2014;2014:986139. doi: 10.1155/2014/986139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka Y, Yamada M, Koumura A, et al. Cardiac sympathetic function in the patients with amyotrophic lateral sclerosis: analysis using cardiac [123I] MIBG scintigraphy. J Neurol. 2013;260:2380–2386. doi: 10.1007/s00415-013-7005-0. [DOI] [PubMed] [Google Scholar]
- 11.Shemisa K, Kaelber D, Parikh SA, Mackall JA. Autonomic etiology of heart block in amyotrophic lateral sclerosis: a case report. J Med Case Rep. 2014;8:224. doi: 10.1186/1752-1947-8-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massari FM, Tonella T, Tarsia P, Kirani S, Blasi F, Magrini F. Tako-tsubo syndrome in a young man with amyotrophic lateral sclerosis. A case report. G Ital Cardiol (Rome) 2011;12:388–391. doi: 10.1714/643.7506. [DOI] [PubMed] [Google Scholar]
- 13.Araki A, Katsuno M, Suzuki K, et al. Brugada syndrome in spinal and bulbar muscular atrophy. Neurology. 2014;82:1813–1821. doi: 10.1212/WNL.0000000000000434. [DOI] [PubMed] [Google Scholar]
- 14.Sakpichaisakul K, Taeranawich P, Nitiapinyasakul A, Sirisopikun T. Identification of Sandhoff disease in a Thai family: clinical and biochemical characterization. J Med Assoc Thai. 2010;93:1088–1092. [PubMed] [Google Scholar]
- 15.Carr AS, Pelayo-Negro AL, Jaunmuktane Z, et al. Transthyretin V122I amyloidosis with clinical and histological evidence of amyloid neuropathy and myopathy. Neuromuscul Disord. 2015;25:511–515. doi: 10.1016/j.nmd.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Longhi S, Quarta CC, Milandri A, et al. Atrial fibrillation in amyloidotic cardiomyopathy: prevalence, incidence, risk factors and prognostic role. Amyloid. 2015;22:147–155. doi: 10.3109/13506129.2015.1028616. [DOI] [PubMed] [Google Scholar]
- 17.Klein CJ, Wu Y, Vogel P, et al. Ubiquitin ligase defect by DCAF8 mutation causes HMSN2 with giant axons. Neurology. 2014;82:873–878. doi: 10.1212/WNL.0000000000000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uechi Y, Higa K. Recurrent takotsubo cardiomyopathy within a short span of time in a patient with hereditary motor and sensory neuropathy. Intern Med. 2008;47:1609–1613. doi: 10.2169/internalmedicine.47.1186. [DOI] [PubMed] [Google Scholar]
- 19.Corrado G, Checcarelli N, Santarone M, Stollberger C, Finsterer J. Left ventricular hypertrabeculation/noncompaction with PMP22 duplication-based Charcot-Marie-Tooth disease type 1A. Cardiology. 2006;105:142–145. doi: 10.1159/000091152. [DOI] [PubMed] [Google Scholar]
- 20.Hofstad H, Ohm OJ, Mørk SJ, Aarli JA. Heart disease in myasthenia gravis. Acta Neurol Scand. 1984;70:176–184. doi: 10.1111/j.1600-0404.1984.tb00817.x. [DOI] [PubMed] [Google Scholar]
- 21.Tanahashi N, Sato H, Nogawa S, Satoh T, Kawamura M, Shimoda M. A case report of giant cell myocarditis and myositis observed during the clinical course of invasive thymoma associated with myasthenia gravis. Keio J Med. 2004;53:30–42. [PubMed] [Google Scholar]
- 22.Thanaviratananich S, Katirji B, Alshekhlee A. Broken heart syndrome during myasthenic crisis. J Clin Neuromuscul Dis. 2014;15:90–95. doi: 10.1097/CND.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 23.Mayor-Gomez S, Lacruz F, Ezpeleta D. Myasthenic crisis and Takotsubo syndrome: a non-chance relationship. Rev Neurol. 2012;55:725–728. [PubMed] [Google Scholar]
- 24.Bansal V, Kansal MM, Rowin J. Broken heart syndrome in myasthenia gravis. Muscle Nerve. 2011;44:990–993. doi: 10.1002/mus.22220. [DOI] [PubMed] [Google Scholar]
- 25.Beydoun SR, Wang J, Levine RL, Farvid A. Emotional stress as a trigger of myasthenic crisis and concomitant takotsubo cardiomyopathy: a case report. J Med Case Rep. 2010;4:393. doi: 10.1186/1752-1947-4-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang SM, Shugh S, Mazur W, et al. Myocardial fibrosis and left ventricular dysfunction in Duchenne muscular dystrophy carriers using cardiac magnetic resonance imaging. Pediatr Cardiol. 2015;36:1495–1501. doi: 10.1007/s00246-015-1192-7. [DOI] [PubMed] [Google Scholar]
- 27.Brunklaus A, Parish E, Muntoni F, et al. The value of cardiac MRI versus echocardiography in the pre-operative assessment of patients with Duchenne muscular dystrophy. Eur J Paediatr Neurol. 2015;19:395–401. doi: 10.1016/j.ejpn.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Tandon A, Villa CR, Hor KN, et al. Myocardial fibrosis burden predicts left ventricular ejection fraction and is associated with age and steroid treatment duration in duchenne muscular dystrophy. J Am Heart Assoc. 2015;(4):pii: e001338. doi: 10.1161/JAHA.114.001338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finsterer J, Gelpi E, Stöllberger C. Left ventricular hypertrabeculation/noncompaction as a cardiac manifestation of Duchenne muscular dystrophy under non-invasive positive-pressure ventilation. Acta Cardiol. 2005;60:445–448. doi: 10.2143/AC.60.4.2004996. [DOI] [PubMed] [Google Scholar]
- 30.Florian A, Rösch S, Bietenbeck M, et al. Cardiac involvement in female Duchenne and Becker muscular dystrophy carriers in comparison to their first-degree male relatives: a comparative cardiovascular magnetic resonance study. Eur Heart J Cardiovasc Imaging. 2015 doi: 10.1093/ehjci/jev161. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Birnkrant DJ, Ararat E, Mhanna MJ. Cardiac phenotype determines survival in Duchenne muscular dystrophy. Pediatr Pulmonol. 2015 doi: 10.1002/ppul.23215. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Vidal-Pérez R, Diaz-Villanueva J, Arzanauskaite M, Rojas R, Carreras F. Cardiac involvement in Becker muscular dystrophy: role of cardiovascular magnetic resonance. Eur Heart J Cardiovasc Imaging. 2013;14:1038. doi: 10.1093/ehjci/jet118. [DOI] [PubMed] [Google Scholar]
- 33.Andrikopoulos G, Kourouklis S, Trika C, et al. Cardiac resynchronization therapy in becker muscular dystrophy. Hellenic J Cardiol. 2013;54:227–229. [PubMed] [Google Scholar]
- 34.Tsuda T, Fitzgerald K, Scavena M, et al. Early-progressive dilated cardiomyopathy in a family with Becker muscular dystrophy related to a novel frameshift mutation in the dystrophin gene exon 27. J Hum Genet. 2015;60:151–155. doi: 10.1038/jhg.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo X, Dai Y, Cui L, Fang Q. A novel dystrophin deletion mutation in a becker muscular dystrophy patient with early-onset dilated cardiomyopathy. Can J Cardiol. 2014;30:956.e1–956.e3. doi: 10.1016/j.cjca.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Petri H, Sveen ML, Thune JJ, et al. Progression of cardiac involvement in patients with limb-girdle type 2 and Becker muscular dystrophies: a 9-year follow-up study. Int J Cardiol. 2015;182:403–411. doi: 10.1016/j.ijcard.2014.12.090. [DOI] [PubMed] [Google Scholar]
- 37.van den Bergen JC, Schade van Westrum SM, Dekker L, et al. Clinical characterisation of Becker muscular dystrophy patients predicts favourable outcome in exon-skipping therapy. J Neurol Neurosurg Psychiatry. 2014;85:92–98. doi: 10.1136/jnnp-2012-304729. [DOI] [PubMed] [Google Scholar]
- 38.Finsterer J, Stöllberger C, Sehnal E, Rehder H, Laccone F. Dilated, arrhythmogenic cardiomyopathy in emery-dreifuss muscular dystrophy due to the emerin splice-site mutation c.449 + 1G>A. Cardiology. 2015;130:48–51. doi: 10.1159/000368222. [DOI] [PubMed] [Google Scholar]
- 39.Pen AE, Nyegaard M, Fang M, et al. A novel single nucleotide splice site mutation in FHL1 confirms an Emery-Dreifuss plus phenotype with pulmonary artery hypoplasia and facial dysmorphology. Eur J Med Genet. 2015;58:222–229. doi: 10.1016/j.ejmg.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Gossios TD, Lopes LR, Elliott PM. Left ventricular hypertrophy caused by a novel nonsense mutation in FHL1. Eur J Med Genet. 2013;56:251–255. doi: 10.1016/j.ejmg.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 41.Tiffin HR, Jenkins ZA, Gray MJ, et al. Dysregulation of FHL1 spliceforms due to an indel mutation produces an Emery-Dreifuss muscular dystrophy plus phenotype. Neurogenetics. 2013;14:113–121. doi: 10.1007/s10048-013-0359-8. [DOI] [PubMed] [Google Scholar]
- 42.Wessely R, Seidl S, Schömig A. Cardiac involvement in Emery-Dreifuss muscular dystrophy. Clin Genet. 2005;67:220–223. doi: 10.1111/j.1399-0004.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 43.Coutance G, Labombarda F, Cauderlier E, et al. Hypoplasia of the aorta in a patient diagnosed with LMNA gene mutation. Congenit Heart Dis. 2013;8:E127–E129. doi: 10.1111/j.1747-0803.2012.00695.x. [DOI] [PubMed] [Google Scholar]
- 44.Redondo-Vergé L, Yaou RB, Fernández-Recio M, Dinca L, Richard P, Bonne G. Cardioembolic stroke prompting diagnosis of LMNA-associated Emery-Dreifuss muscular dystrophy. Muscle Nerve. 2011;44:587–589. doi: 10.1002/mus.22179. [DOI] [PubMed] [Google Scholar]
- 45.Maggi L, D'Amico A, Pini A, et al. LMNA-associated myopathies: the Italian experience in a large cohort of patients. Neurology. 2014;83:1634–1644. doi: 10.1212/WNL.0000000000000934. [DOI] [PubMed] [Google Scholar]
- 46.Sveen ML, Thune JJ, Køber L, Vissing J. Cardiac involvement in patients with limb-girdle muscular dystrophy type 2 and Becker muscular dystrophy. Arch Neurol. 2008;65:1196–1201. doi: 10.1001/archneur.65.9.1196. [DOI] [PubMed] [Google Scholar]
- 47.Groh WJ. Arrhythmias in the muscular dystrophies. Heart Rhythm. 2012;9:1890–1895. doi: 10.1016/j.hrthm.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 48.Volpi L, Ricci G, Passino C, et al. Prevalent cardiac phenotype resulting in heart transplantation in a novel LMNA gene duplication. Neuromuscul Disord. 2010;20:512–516. doi: 10.1016/j.nmd.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 49.Quick S, Schaefer J, Waessnig N, et al. Evaluation of heart involvement in calpainopathy (LGMD2A) using cardiovascular magnetic resonance. Muscle Nerve. 2015;52:661–663. doi: 10.1002/mus.24717. [DOI] [PubMed] [Google Scholar]
- 50.Okere A, Reddy SS, Gupta S, Shinnar M. A cardiomyopathy in a patient with limb girdle muscular dystrophy type 2A. Circ Heart Fail. 2013;6:e12–e13. doi: 10.1161/CIRCHEARTFAILURE.112.971424. [DOI] [PubMed] [Google Scholar]
- 51.Nishikawa A, Mori-Yoshimura M, Segawa K, et al. Respiratory and cardiac function in Japanese patients with dysferlinopathy. Muscle Nerve. 2015 doi: 10.1002/mus.24741. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 52.Rosales XQ, Moser SJ, Tran T, et al. Cardiovascular magnetic resonance of cardiomyopathy in limb girdle muscular dystrophy 2B and 2I. J Cardiovasc Magn Reson. 2011;13:39. doi: 10.1186/1532-429X-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takahashi T, Aoki M, Suzuki N, et al. Clinical features and a mutation with late onset of limb girdle muscular dystrophy 2B. J Neurol Neurosurg Psychiatry. 2013;84:433–440. doi: 10.1136/jnnp-2011-301339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuru S, Yasuma F, Wakayama T, et al. A patient with limb girdle muscular dystrophy type 2B (LGMD2B) manifesting cardiomyopathy. Rinsho Shinkeigaku. 2004;44:375–378. [PubMed] [Google Scholar]
- 55.Calvo F, Teijeira S, Fernandez JM, et al. Evaluation of heart involvement in gamma-sarcoglycanopathy (LGMD2C). A study of ten patients. Neuromuscul Disord. 2000;10:560–566. doi: 10.1016/s0960-8966(00)00147-4. [DOI] [PubMed] [Google Scholar]
- 56.Merlini L, Kaplan JC, Navarro C, et al. Homogeneous phenotype of the gypsy limb-girdle MD with the gamma-sarcoglycan C283Y mutation. Neurology. 2000;54:1075–1079. doi: 10.1212/wnl.54.5.1075. [DOI] [PubMed] [Google Scholar]
- 57.Semplicini C, Vissing J, Dahlqvist JR, et al. Clinical and genetic spectrum in limb-girdle muscular dystrophy type 2E. Neurology. 2015;84:1772–1781. doi: 10.1212/WNL.0000000000001519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fanin M, Melacini P, Boito C, Pegoraro E, Angelini C. LGMD2E patients risk developing dilated cardiomyopathy. Neuromuscul Disord. 2003;13:303–309. doi: 10.1016/s0960-8966(02)00280-8. [DOI] [PubMed] [Google Scholar]
- 59.Wahbi K, Meune C, Hamouda el H, et al. Cardiac assessment of limb-girdle muscular dystrophy 2I patients: an echography, Holter ECG and magnetic resonance imaging study. Neuromuscul Disord. 2008;18:650–655. doi: 10.1016/j.nmd.2008.06.365. [DOI] [PubMed] [Google Scholar]
- 60.Hollingsworth KG, Willis TA, Bates MG, et al. Subepicardial dysfunction leads to global left ventricular systolic impairment in patients with limb girdle muscular dystrophy 2I. Eur J Heart Fail. 2013;15:986–994. doi: 10.1093/eurjhf/hft057. [DOI] [PubMed] [Google Scholar]
- 61.Gaul C, Deschauer M, Tempelmann C, et al. Cardiac involvement in limb-girdle muscular dystrophy 2I: conventional cardiac diagnostic and cardiovascular magnetic resonance. J Neurol. 2006;253:1317–1322. doi: 10.1007/s00415-006-0213-0. [DOI] [PubMed] [Google Scholar]
- 62.D'Amico A, Petrini S, Parisi F, et al. Heart transplantation in a child with LGMD2I presenting as isolated dilated cardiomyopathy. Neuromuscul Disord. 2008;18:153–155. doi: 10.1016/j.nmd.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 63.Schottlaender LV, Petzold A, Wood N, Houlden H. Diagnostic clues and manifesting carriers in fukutin-related protein (FKRP) limb-girdle muscular dystrophy. J Neurol Sci. 2015;348:266–268. doi: 10.1016/j.jns.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 64.Matsui M, Endo T, Matsumura T, Saito T, Fujimura H. A case of limb-girdle muscular dystrophy 2M diagnosed by the occurence of dilated cardiomyopathy. Rinsho Shinkeigaku. 2015;55:585–588. doi: 10.5692/clinicalneurol.cn-000686. [DOI] [PubMed] [Google Scholar]
- 65.Schade van Westrum SM, Dekker LR, de Voogt WG, et al. Cardiac involvement in Dutch patients with sarcoglycanopathy: a cross-sectional cohort and follow-up study. Muscle Nerve. 2014;50:909–913. doi: 10.1002/mus.24233. [DOI] [PubMed] [Google Scholar]
- 66.Dinçer P, Bönnemann CG, Erdir Aker O, et al. A homozygous nonsense mutation in delta-sarcoglycan exon 3 in a case of LGMD2F. Neuromuscul Disord. 2000;10:247–250. doi: 10.1016/s0960-8966(00)00100-0. [DOI] [PubMed] [Google Scholar]
- 67.Tsubata S, Bowles KR, Vatta M, et al. Mutations in the human delta-sarcoglycan gene in familial and sporadic dilated cardiomyopathy. J Clin Invest. 2000;106:655–662. doi: 10.1172/JCI9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Finsterer J, Stöllberger C, Gatterer E, Jakubiczka S. Intermittent pre-excitation-syndrome in facio-scapulo-humeral muscular dystrophy. Korean Circ J. 2014;44:348–350. doi: 10.4070/kcj.2014.44.5.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakayama T, Komiya T, Tyou E, Watanabe S, Kawai M. Cardiac deformity and dysfunction in facioscapulohumeral dystrophy--electrocardiogram, ECG gate cardiac MRI studies. Rinsho Shinkeigaku. 1999;39:610–614. [PubMed] [Google Scholar]
- 70.Faustmann PM, Farahati J, Rupilius B, Dux R, Koch MC, Reiners C. Cardiac involvement in facio-scapulo-humeral muscular dystrophy: a family study using Thallium-201 single-photon-emission-computed tomography. J Neurol Sci. 1996;144:59–63. doi: 10.1016/s0022-510x(96)00145-1. [DOI] [PubMed] [Google Scholar]
- 71.Della Marca G, Frusciante R, Scatena M, et al. Heart rate variability in facioscapulohumeral muscular dystrophy. Funct Neurol. 2010;25:211–216. [PubMed] [Google Scholar]
- 72.Pasqualin LM, Reed UC, Costa TV, et al. Congenital muscular dystrophy with dropped head linked to the LMNA gene in a Brazilian cohort. Pediatr Neurol. 2014;50:400–406. doi: 10.1016/j.pediatrneurol.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 73.Flöck A, Kornblum C, Hammerstingl C, Claeys KG, Gembruch U, Merz WM. Progressive cardiac dysfunction in Bethlem myopathy during pregnancy. Obstet Gynecol. 2014;123(2 Pt 2 Suppl 2):436–438. doi: 10.1097/AOG.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 74.Plonka C, Wearden PD, Morell VO, Miller SA, Webber SA, Feingold B. Successful heart transplantation from a donor with Ullrich congenital muscular dystrophy. Am J Transplant. 2013;13:1915–1917. doi: 10.1111/ajt.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martinez HR, Craigen WJ, Ummat M, Adesina AM, Lotze TE, Jefferies JL. Novel cardiovascular findings in association with a POMT2 mutation: three siblings with α-dystroglycanopathy. Eur J Hum Genet. 2014;22:486–491. doi: 10.1038/ejhg.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haliloglu G, Talim B, Sel CG, Topaloglu H. Clinical characteristics of megaconial congenital muscular dystrophy due to choline kinase beta gene defects in a series of 15 patients. J Inherit Metab Dis. 2015;38:1099–1108. doi: 10.1007/s10545-015-9856-2. [DOI] [PubMed] [Google Scholar]
- 77.Marques J, Duarte ST, Costa S, et al. Atypical phenotype in two patients with LAMA2 mutations. Neuromuscul Disord. 2014;24:419–424. doi: 10.1016/j.nmd.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 78.Carboni N, Marrosu G, Porcu M, et al. Dilated cardiomyopathy with conduction defects in a patient with partial merosin deficiency due to mutations in the laminin-α2-chain gene: a chance association or a novel phenotype. Muscle Nerve. 2011;44:826–828. doi: 10.1002/mus.22228. [DOI] [PubMed] [Google Scholar]
- 79.Matsuda H, Arai M, Okamoto H. Total intravenous anesthesia for a patient with Fukuyama congenital muscular dystrophy undergoing scoliosis surgery. Masui. 2014;63:650–653. [PubMed] [Google Scholar]
- 80.Pane M, Messina S, Vasco G, et al. Respiratory and cardiac function in congenital muscular dystrophies with alpha dystroglycan deficiency. Neuromuscul Disord. 2012;22:685–689. doi: 10.1016/j.nmd.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ceviz N, Alehan F, Alehan D, et al. Assessment of left ventricular systolic and diastolic functions in children with merosin-positive congenital muscular dystrophy. Int J Cardiol. 2003;87:129–133. discussion 133-4. doi: 10.1016/s0167-5273(02)00320-0. [DOI] [PubMed] [Google Scholar]
- 82.Vattemi G, Neri M, Piffer S, et al. Clinical, morphological and genetic studies in a cohort of 21 patients with myofibrillar myopathy. Acta Myol. 2011;30:121–126. [PMC free article] [PubMed] [Google Scholar]
- 83.Konersman CG, Bordini BJ, Scharer G, et al. BAG3 myofibrillar myopathy presenting with cardiomyopathy. Neuromuscul Disord. 2015;25:418–422. doi: 10.1016/j.nmd.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 84.Lee HC, Cherk SW, Chan SK, et al. BAG3-related myofibrillar myopathy in a Chinese family. Clin Genet. 2012;81:394–398. doi: 10.1111/j.1399-0004.2011.01659.x. [DOI] [PubMed] [Google Scholar]
- 85.Liewluck T, Kintarak J, Sangruchi T, Selcen D, Kulkantrakorn K. Myofibrillar myopathy with limb-girdle phenotype in a Thai patient. J Med Assoc Thai. 2009;92:290–295. [PubMed] [Google Scholar]
- 86.Piñol-Ripoll G, Shatunov A, Cabello A, et al. Severe infantile-onset cardiomyopathy associated with a homozygous deletion in desmin. Neuromuscul Disord. 2009;19:418–422. doi: 10.1016/j.nmd.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zheng M, Cheng H, Li X, et al. Cardiac-specific ablation of Cypher leads to a severe form of dilated cardiomyopathy with premature death. Hum Mol Genet. 2009;18:701–713. doi: 10.1093/hmg/ddn400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stöllberger C, Gatterer E, Finsterer J, Kuck KH, Tilz RR. Repeated radiofrequency ablation of atrial tachycardia in restrictive cardiomyopathy secondary to myofibrillar myopathy. J Cardiovasc Electrophysiol. 2014;25:905–907. doi: 10.1111/jce.12436. [DOI] [PubMed] [Google Scholar]
- 89.El-Menyar AA, Al-Suwaidi J, Gehani AA, Bener A. Clinical and histologic studies of a Qatari family with myofibrillar myopathy. Saudi Med J. 2004;25:1723–1726. [PubMed] [Google Scholar]
- 90.Chauveau C, Bonnemann CG, Julien C, et al. Recessive TTN truncating mutations define novel forms of core myopathy with heart disease. Hum Mol Genet. 2014;23:980–991. doi: 10.1093/hmg/ddt494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Şimşek Z, Açar G, Akçakoyun M, Esen Ö, Emiroğlu Y, Esen AM. Left ventricular noncompaction in a patient with multiminicore disease. J Cardiovasc Med (Hagerstown) 2012;13:660–662. doi: 10.2459/JCM.0b013e32833cdcd0. [DOI] [PubMed] [Google Scholar]
- 92.Cullup T, Lamont PJ, Cirak S, et al. Mutations in MYH7 cause Multi-minicore Disease (MmD) with variable cardiac involvement. Neuromuscul Disord. 2012;22:1096–1104. doi: 10.1016/j.nmd.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 93.Hachenberg T, Brüssel T, Lawin P, Konertz W, Scheld HH. Heart transplantation in a patient with central core disease. J Cardiothorac Vasc Anesth. 1992;6:386–387. doi: 10.1016/1053-0770(92)90183-8. [DOI] [PubMed] [Google Scholar]
- 94.Gal A, Inczedy-Farkas G, Pal E, et al. The coexistence of dynamin 2 mutation and multiple mitochondrial DNA (mtDNA) deletions in the background of severe cardiomyopathy and centronuclear myopathy. Clin Neuropathol. 2015;34:89–95. doi: 10.5414/NP300789. [DOI] [PubMed] [Google Scholar]
- 95.Al-Ruwaishid A, Vajsar J, Tein I, Benson L, Jay V. Centronuclear myopathy and cardiomyopathy requiring heart transplant. Brain Dev. 2003;25:62–66. doi: 10.1016/s0387-7604(02)00151-1. [DOI] [PubMed] [Google Scholar]
- 96.Hikita T, Wakita S, Mori Y, et al. Severe infantile myotubular myopathy with complete atrioventricular block. Pediatr Int. 2008;50:698–700. doi: 10.1111/j.1442-200X.2008.02719.x. [DOI] [PubMed] [Google Scholar]
- 97.Fujita K, Nakano S, Yamamoto H, Ito H, Ito H, Goto Y, Kusaka H. An adult case of congenital fiber type disproportion (CFTD) with cardiomyopathy. Rinsho Shinkeigaku. 2005;45:380–382. [PubMed] [Google Scholar]
- 98.Kajino S, Ishihara K, Goto K, et al. Congenital fiber type disproportion myopathy caused by LMNA mutations. J Neurol Sci. 2014;340:94–98. doi: 10.1016/j.jns.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 99.Gatayama R, Ueno K, Nakamura H, et al. Nemaline myopathy with dilated cardiomyopathy in childhood. Pediatrics. 2013;131:e1986–e1990. doi: 10.1542/peds.2012-1139. [DOI] [PubMed] [Google Scholar]
- 100.Sarullo FM, Vitale G, Di Franco A, et al. Nemaline myopathy and heart failure: role of ivabradine; a case report. BMC Cardiovasc Disord. 2015;15:5. doi: 10.1186/1471-2261-15-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mir A, Lemler M, Ramaciotti C, Blalock S, Ikemba C. Hypertrophic cardiomyopathy in a neonate associated with nemaline myopathy. Congenit Heart Dis. 2012;7:E37–E41. doi: 10.1111/j.1747-0803.2011.00588.x. [DOI] [PubMed] [Google Scholar]
- 102.Müller TJ, Kraya T, Stoltenburg-Didinger G, et al. Phenotype of matrin-3-related distal myopathy in 16 German patients. Ann Neurol. 2014;76:669–680. doi: 10.1002/ana.24255. [DOI] [PubMed] [Google Scholar]
- 103.Naddaf E, Waclawik AJ. Two families with MYH7 distal myopathy associated with cardiomyopathy and core formations. J Clin Neuromuscul Dis. 2015;16:164–169. doi: 10.1097/CND.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 104.Lefter S, Hardiman O, McLaughlin RL, Murphy SM, Farrell M, Ryan AM. A novel MYH7 Leu1453pro mutation resulting in Laing distal myopathy in an Irish family. Neuromuscul Disord. 2015;25:155–160. doi: 10.1016/j.nmd.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 105.Finsterer J, Stöllberger C, Brandau O, Laccone F, Bichler K, Laing NG. Novel MYH7 mutation associated with mild myopathy but life-threatening ventricular arrhythmias and noncompaction. Int J Cardiol. 2014;173:532–535. doi: 10.1016/j.ijcard.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 106.D'Arcy C, Kanellakis V, Forbes R, et al. X-linked recessive distal myopathy with hypertrophic cardiomyopathy caused by a novel mutation in the FHL1 gene. J Child Neurol. 2015;30:1211–1217. doi: 10.1177/0883073814549807. [DOI] [PubMed] [Google Scholar]
- 107.Kayman-Kurekci G, Talim B, Korkusuz P, et al. Mutation in TOR1AIP1 encoding LAP1B in a form of muscular dystrophy: a novel gene related to nuclear envelopathies. Neuromuscul Disord. 2014;24:624–633. doi: 10.1016/j.nmd.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 108.Nalini A, Gayathri N, Richard P, Cobo AM, Urtizberea JA. New mutation of the desmin gene identified in an extended Indian pedigree presenting with distal myopathy and cardiac disease. Neurol India. 2013;61:622–626. doi: 10.4103/0028-3886.125269. [DOI] [PubMed] [Google Scholar]
- 109.Nishino I, Noguchi S, Murayama K, et al. Molecular pathomechanism of distal myopathy with rimmed vacuoles. Rinsho Shinkeigaku. 2005;45:943–945. [PubMed] [Google Scholar]
- 110.Ishiwata S, Nishiyama S, Seki A, Kojima S. Restrictive cardiomyopathy with complete atrioventricular block and distal myopathy with rimmed vacuoles. Jpn Circ J. 1993;57:928–933. doi: 10.1253/jcj.57.928. [DOI] [PubMed] [Google Scholar]
- 111.Kimpara T, Imamura T, Tsuda T, Sato K, Tsuburaya K. Distal myopathy with rimmed vacuoles and sudden death--report of two siblings. Rinsho Shinkeigaku. 1993;33:886–890. [PubMed] [Google Scholar]
- 112.Krendel DA, Gilchrist JM, Bossen EH. Distal vacuolar myopathy with complete heart block. Arch Neurol. 1988;45:698–699. doi: 10.1001/archneur.1988.00520300118032. [DOI] [PubMed] [Google Scholar]
- 113.Maffé S, Signorotti F, Perucca A, et al. Atypical arrhythmic complications in familial hypokalemic periodic paralysis. J Cardiovasc Med (Hagerstown) 2009;10:68–71. doi: 10.2459/jcm.0b013e3283189564. [DOI] [PubMed] [Google Scholar]
- 114.Green DS, Hayward LJ, George AL, Jr, Cannon SC. A proposed mutation, Val781Ile, associated with hyperkalemic periodic paralysis and cardiac dysrhythmia is a benign polymorphism. Ann Neurol. 1997;42:253–256. doi: 10.1002/ana.410420219. [DOI] [PubMed] [Google Scholar]
- 115.Stunnenberg BC, Deinum J, Links TP, Wilde AA, Franssen H, Drost G. Cardiac arrhythmias in hypokalemic periodic paralysis: Hypokalemia as only cause? Muscle Nerve. 2014;50:327–332. doi: 10.1002/mus.24225. [DOI] [PubMed] [Google Scholar]
- 116.Caballero PE. Becker myotonia congenita associated with Wolff-Parkinson-White syndrome. Neurologist. 2011;17:38–40. doi: 10.1097/NRL.0b013e3181d35c93. [DOI] [PubMed] [Google Scholar]
- 117.Benhayon D, Lugo R, Patel R, Carballeira L, Elman L, Cooper JM. Long-term arrhythmia follow-up of patients with myotonic dystrophy. J Cardiovasc Electrophysiol. 2015;26:305–310. doi: 10.1111/jce.12604. [DOI] [PubMed] [Google Scholar]
- 118.Brembilla-Perrot B, Schwartz J, Huttin O, et al. Atrial flutter or fibrillation is the most frequent and life-threatening arrhythmia in myotonic dystrophy. Pacing Clin Electrophysiol. 2014;37:329–335. doi: 10.1111/pace.12260. [DOI] [PubMed] [Google Scholar]
- 119.Pambrun T, Bortone A, Bois P, et al. Unmasked Brugada pattern by ajmaline challenge in patients with myotonic dystrophy type 1. Ann Noninvasive Electrocardiol. 2015;20:28–36. doi: 10.1111/anec.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Russo V, Di Meo F, Rago A, et al. Paroxysmal atrial fibrillation in myotonic dystrophy type 1 patients: P wave duration and dispersion analysis. Eur Rev Med Pharmacol Sci. 2015;19:1241–1248. [PubMed] [Google Scholar]
- 121.Finsterer J, Stöllberger C, Gencik M, Höftberger R, Rahimi J, Mokocki J. Syncope and hyperCKemia as minimal manifestations of short CTG repeat expansions in myotonic dystrophy type 1. Rev Port Cardiol. 2015;34:361.e1–361.e4. doi: 10.1016/j.repc.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 122.Finsterer J, Rudnik-Schöneborn S. Myotonic dystrophies: clinical presentation, pathogenesis, diagnostics and therapy. Fortschr Neurol Psychiatr. 2015;83:9–17. doi: 10.1055/s-0034-1385734. [DOI] [PubMed] [Google Scholar]
- 123.Kilic T, Vural A, Ural D, et al. Cardiac resynchronization therapy in a case of myotonic dystrophy (Steinert's disease) and dilated cardiomyopathy. Pacing Clin Electrophysiol. 2007;30:916–920. doi: 10.1111/j.1540-8159.2007.00782.x. [DOI] [PubMed] [Google Scholar]
- 124.Finsterer J, Stölberger C, Kopsa W. Noncompaction in myotonic dystrophy type 1 on cardiac MRI. Cardiology. 2005;103:167–168. doi: 10.1159/000084588. [DOI] [PubMed] [Google Scholar]
- 125.Petri H, Ahtarovski KA, Vejlstrup N, et al. Myocardial fibrosis in patients with myotonic dystrophy type 1: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2014;16:59. doi: 10.1186/s12968-014-0059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Petri H, Witting N, Ersbøll MK, et al. High prevalence of cardiac involvement in patients with myotonic dystrophy type 1: a crosssectional study. Int J Cardiol. 2014;174:31–36. doi: 10.1016/j.ijcard.2014.03.088. [DOI] [PubMed] [Google Scholar]
- 127.Bu'Lock FA, Sood M, De Giovanni JV, Green SH. Left ventricular diastolic function in congenital myotonic dystrophy. Arch Dis Child. 1999;80:267–270. doi: 10.1136/adc.80.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Viitasalo MT, Kala R, Karli P, Eisalo A. Ambulatory electrocardiographic recording in mild or moderate myotonic dystrophy and myotonia congenita (Thomsen's disease) J Neurol Sci. 1983;62:181–190. doi: 10.1016/0022-510x(83)90198-3. [DOI] [PubMed] [Google Scholar]
- 129.Kim HN, Cho YK, Cho JH, Yang EM, Song ES, Choi YY. Transient complete atrioventricular block in a preterm neonate with congenital myotonic dystrophy: case report. J Korean Med Sci. 2014;29:879–883. doi: 10.3346/jkms.2014.29.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rudnik-Schöneborn S, Schaupp M, Lindner A, et al. Brugada-like cardiac disease in myotonic dystrophy type 2: report of two unrelated patients. Eur J Neurol. 2011;18:191–194. doi: 10.1111/j.1468-1331.2010.03077.x. [DOI] [PubMed] [Google Scholar]
- 131.Lee TM, Maurer MS, Karbassi I, Braastad C, Batish SD, Chung WK. Severe dilated cardiomyopathy in a patient with myotonic dystrophy type 2 and homozygous repeat expansion in ZNF9. Congest Heart Fail. 2012;18:183–186. doi: 10.1111/j.1751-7133.2011.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wahbi K, Meune C, Bassez G, et al. Left ventricular non-compaction in a patient with myotonic dystrophy type 2. Neuromuscul Disord. 2008;18:331–333. doi: 10.1016/j.nmd.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 133.Brunetti-Pierri N, Pignatelli R, Fouladi N, et al. Dilation of the aortic root in mitochondrial disease patients. Mol Genet Metab. 2011;103:167–170. doi: 10.1016/j.ymgme.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 134.Finsterer J. Is atherosclerosis a mitochondrial disorder? Vasa. 2007;36:229–240. doi: 10.1024/0301-1526.36.4.229. [DOI] [PubMed] [Google Scholar]
- 135.Dominic EA, Ramezani A, Anker SD, Verma M, Mehta N, Rao M. Mitochondrial cytopathies and cardiovascular disease. Heart. 2014;100:611–618. doi: 10.1136/heartjnl-2013-304657. [DOI] [PubMed] [Google Scholar]
- 136.Yajima N, Yazaki Y, Yoshida K, et al. A case of mitochondrial cardiomyopathy with pericardial effusion evaluated by (99m)Tc-MIBI myocardial scintigraphy. J Nucl Cardiol. 2009;16:989–994. doi: 10.1007/s12350-009-9149-y. [DOI] [PubMed] [Google Scholar]
- 137.Barisić N, Kleiner IM, Malcić I, Papa J, Boranić M. Spinal dysraphism associated with congenital heart disorder in a girl with MELAS syndrome and point mutation at mitochondrial DNA nucleotide 3271. Croat Med J. 2002;43:37–41. [PubMed] [Google Scholar]
- 138.Barragán-Campos HM, Barrera-Ramírez CF, Iturralde Torres P, et al. Kearns-Sayre syndromes an absolute indication for prophylactic implantation of definitive pacemaker? Arch Inst Cardiol Mex. 1999;69:559–565. [PubMed] [Google Scholar]
- 139.Aimo A, Giannoni A, Piepoli MF, et al. Myocardial damage in a mitochondrial myopathy patient with increased ergoreceptor sensitivity and sympatho-vagal imbalance. Int J Cardiol. 2014;176:1396–1398. doi: 10.1016/j.ijcard.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 140.Brecht M, Richardson M, Taranath A, Grist S, Thorburn D, Bratkovic D. Leigh syndrome daused by the MT-ND5 m.13513G>A mutation: a case present ing wi th WPW-l ike conduct ion defect , cardiomyopathy, hypertension and hyponatraemia. JIMD Rep. 2015;19:95–100. doi: 10.1007/8904_2014_375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Limongelli G, Tome-Esteban M, Dejthevaporn C, Rahman S, Hanna MG, Elliott PM. Prevalence and natural history of heart disease in adults with primary mitochondrial respiratory chain disease. Eur J Heart Fail. 2010;12:114–121. doi: 10.1093/eurjhf/hfp186. [DOI] [PubMed] [Google Scholar]
- 142.Watanabe Y, Odaka M, Hirata K. Case of Leber's hereditary optic neuropathy with mitochondrial DNA 11778 mutation exhibiting cerebellar ataxia, dilated cardiomyopathy and peripheral neuropathy. Brain Nerve. 2009;61:309–312. [PubMed] [Google Scholar]
- 143.Florian A, Ludwig A, Stubbe-Dräger B, et al. Characteristic cardiac phenotypes are detected by cardiovascular magnetic resonance in patients with different clinical phenotypes and genotypes of mitochondrial myopathy. J Cardiovasc Magn Reson. 2015;17:40. doi: 10.1186/s12968-015-0145-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brea-Calvo G, Haack TB, Karall D, et al. COQ4 mutations cause a broad spectrum of mitochondrial disorders associated with CoQ10 deficiency. Am J Hum Genet. 2015;96:309–317. doi: 10.1016/j.ajhg.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kopajtich R, Nicholls TJ, Rorbach J, et al. Mutations in GTPBP3 cause a mitochondrial translation defect associated with hypertrophic cardiomyopathy, lactic acidosis, and encephalopathy. Am J Hum Genet. 2014;95:708–720. doi: 10.1016/j.ajhg.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Distelmaier F, Haack TB, Catarino CB, et al. MRPL44 mutations cause a slowly progressive multisystem disease with childhood-onset hypertrophic cardiomyopathy. Neurogenetics. 2015;16:319–323. doi: 10.1007/s10048-015-0444-2. [DOI] [PubMed] [Google Scholar]
- 147.Brisca G, Fiorillo C, Nesti C, et al. Early onset cardiomyopathy associated with the mitochondrial tRNALeu((UUR)) 3271T>C MELAS mutation. Biochem Biophys Res Commun. 2015;458:601–604. doi: 10.1016/j.bbrc.2015.01.157. [DOI] [PubMed] [Google Scholar]
- 148.Wortmann SB, Champion MP, van den Heuvel L, et al. Mitochondrial DNA m.3242G > A mutation, an under diagnosed cause of hypertrophic cardiomyopathy and renal tubular dysfunction. Eur J Med Genet. 2012;55:552–556. doi: 10.1016/j.ejmg.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 149.Wang SB, Weng WC, Lee NC, Hwu WL, Fan PC, Lee WT. Mutation of mitochondrial DNA G13513A presenting with Leigh syndrome, Wolff-Parkinson-White syndrome and cardiomyopathy. Pediatr Neonatol. 2008;49:145–149. doi: 10.1016/S1875-9572(08)60030-3. [DOI] [PubMed] [Google Scholar]
- 150.Menotti F, Brega A, Diegoli M, Grasso M, Modena MG, Arbustini E. A novel mtDNA point mutation in tRNA(Val) is associated with hypertrophic cardiomyopathy and MELAS. Ital Heart J. 2004;5:460–465. [PubMed] [Google Scholar]
- 151.Homan DJ, Niyazov DM, Fisher PW, et al. Heart transplantation for a patient with Kearns-Sayre syndrome and end-stage heart failure. Congest Heart Fail. 2011;17:102–104. doi: 10.1111/j.1751-7133.2011.00211.x. [DOI] [PubMed] [Google Scholar]
- 152.Malfatti E, Laforêt P, Jardel C, et al. High risk of severe cardiac adverse events in patients with mitochondrial m.3243A>G mutation. Neurology. 2013;80:100–105. doi: 10.1212/WNL.0b013e31827b1a2f. [DOI] [PubMed] [Google Scholar]
- 153.Finsterer J, Stöllberger C, Sehnal E, Valentin A, Huber J, Schmiedel J. Apical ballooning (Takotsubo syndrome) in mitochondrial disorder during mechanical ventilation. J Cardiovasc Med (Hagerstown) 2007;8:859–863. doi: 10.2459/JCM.0b013e3280103d1b. [DOI] [PubMed] [Google Scholar]
- 154.Sacconi S, Wahbi K, Theodore G, et al. Atrio-ventricular block requiring pacemaker in patients with late onset Pompe disease. Neuromuscul Disord. 2014;24:648–650. doi: 10.1016/j.nmd.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 155.Mogahed EA, Girgis MY, Sobhy R, Elhabashy H, Abdelaziz OM, El-Karaksy H. Skeletal and cardiac muscle involvement in children with glycogen storage disease type III. Eur J Pediatr. 2015;174:1545–1548. doi: 10.1007/s00431-015-2546-0. [DOI] [PubMed] [Google Scholar]
- 156.Sentner CP, Caliskan K, Vletter WB, Smit GP. Heart failure due to severe hypertrophic cardiomyopathy reversed by low calorie, high protein dietary adjustments in a glycogen storage disease type IIIa patient. JIMD Rep. 2012;5:13–16. doi: 10.1007/8904_2011_111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Austin SL, Proia AD, Spencer-Manzon MJ, Butany J, Wechsler SB, Kishnani PS. Cardiac pathology in glycogen storage disease type III. JIMD Rep. 2012;6:65–72. doi: 10.1007/8904_2011_118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.LaBarbera M, Milechman G, Dulbecco F. Premature coronary artery disease in a patient with glycogen storage disease III. J Invasive Cardiol. 2010;22:E156–E158. [PubMed] [Google Scholar]
- 159.Magoulas PL, El-Hattab AW. In: Glycogen Storage Disease Type IV. GeneReviews® [Internet] Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K, editors. Seattle (WA): University of Washington, Seattle; 1993-2015. [2013 Jan 03]. Available from http://www.ncbi.nlm.nih.gov/books/NBK115333/ [Google Scholar]
- 160.Moustafa S, Patton DJ, Connelly MS. Unforeseen cardiac involvement in McArdle's disease. Heart Lung Circ. 2013;22:769–771. doi: 10.1016/j.hlc.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 161.Amit R, Bashan N, Abarbanel JM, Shapira Y, Sofer S, Moses S. Fatal familial infantile glycogen storage disease: multisystem phosphofructokinase deficiency. Muscle Nerve. 1992;15:455–458. doi: 10.1002/mus.880150406. [DOI] [PubMed] [Google Scholar]
- 162.Schoser B, Bruno C, Schneider HC, et al. Unclassified polysaccharidosis of the heart and skeletal muscle in siblings. Mol Genet Metab. 2008;95:52–58. doi: 10.1016/j.ymgme.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Baruteau J, Sachs P, Broué P, et al. Clinical and biological features at diagnosis in mitochondrial fatty acid beta-oxidation defects: a French pediatric study of 187 patients. J Inherit Metab Dis. 2013;36:795–803. doi: 10.1007/s10545-012-9542-6. [DOI] [PubMed] [Google Scholar]
- 164.Ojala T, Nupponen I, Saloranta C, et al. Fetal left ventricular noncompaction cardiomyopathy and fatal outcome due to complete deficiency of mitochondrial trifunctional protein. Eur J Pediatr. 2015;174:1689–1692. doi: 10.1007/s00431-015-2574-9. [DOI] [PubMed] [Google Scholar]
- 165.Zhang RN, Li YF, Qiu WJ, et al. Clinical features and mutations in seven Chinese patients with very long chain acyl-CoA dehydrogenase deficiency. World J Pediatr. 2014;10:119–125. doi: 10.1007/s12519-014-0480-2. [DOI] [PubMed] [Google Scholar]
- 166.Eminoglu TF, Tumer L, Okur I, Ezgu FS, Biberoglu G, Hasanoglu A. Very long-chain acyl CoA dehydrogenase deficiency which was accepted as infanticide. Forensic Sci Int. 2011;210:e1–e3. doi: 10.1016/j.forsciint.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 167.Missaglia S, Tasca E, Angelini C, Moro L, Tavian D. Novel missense mutations in PNPLA2 causing late onset and clinical heterogeneity of neutral lipid storage disease with myopathy in three siblings. Mol Genet Metab. 2015;115:110–117. doi: 10.1016/j.ymgme.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 168.Kaneko K, Kuroda H, Izumi R, et al. A novel mutation in PNPLA2 causes neutral lipid storage disease with myopathy and triglyceride deposit cardiomyovasculopathy: a case report and literature review. Neuromuscul Disord. 2014;24:634–641. doi: 10.1016/j.nmd.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 169.Iacobazzi V, Invernizzi F, Baratta S, et al. Molecular and functional analysis of SLC25A20 mutations causing carnitine-acylcarnitine translocase deficiency. Hum Mutat. 2004;24:312–320. doi: 10.1002/humu.20085. [DOI] [PubMed] [Google Scholar]
- 170.Yilmaz BS, Kor D, Mungan NO, Erdem S, Ceylaner S. Primary systemic carnitine deficiency: a Turkish case with a novel homozygous SLC22A5 mutation and 14 years follow-up. J Pediatr Endocrinol Metab. 2015;28:1179–1181. doi: 10.1515/jpem-2014-0528. [DOI] [PubMed] [Google Scholar]
- 171.Ronvelia D, Greenwood J, Platt J, Hakim S, Zaragoza MV. Intrafamilial variability for novel TAZ gene mutation: barth syndrome with dilated cardiomyopathy and heart failure in an infant and left ventricular noncompaction in his great-uncle. Mol Genet Metab. 2012;107:428–432. doi: 10.1016/j.ymgme.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Van Der Starre P, Deuse T, Pritts C, Brun C, Vogel H, Oyer P. Late profound muscle weakness following heart transplantation due to Danon disease. Muscle Nerve. 2013;47:135–137. doi: 10.1002/mus.23517. [DOI] [PubMed] [Google Scholar]
- 173.Miani D, Taylor M, Mestroni L, et al. Sudden death associated with danon disease in women. Am J Cardiol. 2012;109:406–411. doi: 10.1016/j.amjcard.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 174.Oliveira J, Negrão L, Fineza I, et al. New splicing mutation in the choline kinase beta (CHKB) gene causing a muscular dystrophy detected by whole-exome sequencing. J Hum Genet. 2015;60:305–312. doi: 10.1038/jhg.2015.20. [DOI] [PubMed] [Google Scholar]
- 175.Dittrich S, Tuerk M, Haaker G, et al. Cardiomyopathy in duchenne muscular dystrophy: current value of clinical, electrophysiological and imaging findings in children and teenagers. Klin Padiatr. 2015;227:225–231. doi: 10.1055/s-0034-1398689. [DOI] [PubMed] [Google Scholar]
- 176.van Westering TL, Betts CA, Wood MJ. Current understanding of molecular pathology and treatment of cardiomyopathy in duchenne muscular dystrophy. Molecules. 2015;20:8823–8855. doi: 10.3390/molecules20058823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wochna K, Jurczyk AP, Krajewski W, Berent J. Sudden death due to malignant hyperthermia during general anesthesia. Arch Med Sadowej Kryminol. 2013;63:11–14. 7–10. [PubMed] [Google Scholar]
- 178.Finsterer J, Cripe L. Treatment of dystrophin cardiomyopathies. Nat Rev Cardiol. 2014;11:168–179. doi: 10.1038/nrcardio.2013.213. [DOI] [PubMed] [Google Scholar]
- 179.Dec GW. Steroid therapy effectively delays Duchenne's cardiomyopathy. J Am Coll Cardiol. 2013;61:955–956. doi: 10.1016/j.jacc.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 180.But WM, Lee SH, Chan AO, Lau GT. Enzyme replacement therapy for infantile Pompe disease during the critical period and identification of a novel mutation. Hong Kong Med J. 2009;15:474–477. [PubMed] [Google Scholar]
- 181.Prater SN, Banugaria SG, DeArmey SM, et al. The emerging phenotype of long-term survivors with infantile Pompe disease. Genet Med. 2012;14:800–810. doi: 10.1038/gim.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yoda M, Tanabe H, Nishino I, Suma H. Left ventriculoplasty for dilated cardiomyopathy in Fukuyama-type muscular dystrophy. Eur J Cardiothorac Surg. 2011;40:514–516. doi: 10.1016/j.ejcts.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 183.Jefferies JL, Wilkinson JD, Sleeper LA, et al. Cardiomyopathy phenotypes and outcomes for children with left ventricular myocardial noncompaction: results from the pediatric cardiomyopathy registry. J Card Fail. 2015;21:877–884. doi: 10.1016/j.cardfail.2015.06.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Authors/Task Force members. Elliott PM, Anastasakis A, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC) Eur Heart J. 2014;35:2733–2779. doi: 10.1093/eurheartj/ehu284. [DOI] [PubMed] [Google Scholar]
- 185.Gdynia HJ, Kurt A, Endruhn S, Ludolph AC, Sperfeld AD. Cardiomyopathy in motor neuron diseases. J Neurol Neurosurg Psychiatry. 2006;77:671–673. doi: 10.1136/jnnp.2005.078600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hattori T, Ikeda S, Yoshida K, Yanagisawa N, Furihata K, Yoshida K. A patient with Kennedy-Alter-Sung syndrome showing cardiomyopathy. Rinsho Shinkeigaku. 1995;35:1246–1249. [PubMed] [Google Scholar]
- 187.Al-Thihli K, Ebrahim H, Hughes DA, et al. A variant of unknown significance in the GLA gene causing diagnostic uncertainty in a young female with isolated hypertrophic cardiomyopathy. Gene. 2012;497:320–322. doi: 10.1016/j.gene.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 188.Nussinovitch U, Katz U, Nussinovitch M, Nussinovitch N. Beat-to-beat QT interval dynamics and variability in familial dysautonomia. Pediatr Cardiol. 2010;31:80–84. doi: 10.1007/s00246-009-9575-2. [DOI] [PubMed] [Google Scholar]
- 189.Ergül Y, Ekici B, Keskin S. Cardiac arrest after anesthetic management in a patient with hereditary sensory autonomic neuropathy type IV. Saudi J Anaesth. 2011;5:93–95. doi: 10.4103/1658-354X.76486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Losito L, De Rinaldis M, Gennaro L, et al. Charcot-Marie-Tooth type 1a in a child with Long QT syndrome. Eur J Paediatr Neurol. 2009;13:459–462. doi: 10.1016/j.ejpn.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 191.Millaire A, Warembourg A, Leys D, et al. Refsum's disease. Apropos of 2 cases disclosed by myocardiopathy. Ann Cardiol Angeiol (Paris) 1990;39:173–178. [PubMed] [Google Scholar]
- 192.Nakagawa H, Okayama S, Kamon D, et al. Refractory high output heart failure in a patient with primary mitochondrial respiratory chain disease. Intern Med. 2014;53:315–319. doi: 10.2169/internalmedicine.53.1386. [DOI] [PubMed] [Google Scholar]
- 193.Palecek T, Tesarova M, Kuchynka P, et al. Hypertrophic cardiomyopathy due to the mitochondrial DNA mutation m.3303C>T diagnosed in an adult male. Int Heart J. 2012;53:383–387. doi: 10.1536/ihj.53.383. [DOI] [PubMed] [Google Scholar]