Abstract
Background and Objectives
Numbness on the hand occurs infrequently after a transradial cardiac catheterization (TRC). The symptom resembles that of neuropathy. We, therefore, investigated the prevalence, the predicting factors and the presence of neurological abnormalities of numbness, using a nerve conduction study (NCS).
Subjects and Methods
From April to December 2013, all patients who underwent a TRC were prospectively enrolled. From among these, the patients who experienced numbness on the ipsilateral hand were instructed to describe their symptoms using a visual analogue scale; subsequently, NCSs were performed on these patients.
Results
Of the total 479 patients in the study sample, numbness occurred in nine (1.8%) following the procedure. The NCS was performed for eight out of the nine patients, four (50%) of which had an abnormal NCS result at the superficial radial nerve. A larger sheath and history of myocardial infarction (p=0.14 and 0.08 respectively) tended towards the occurrence of numbness; however, only the use of size 7 French sheaths was an independent predictor for the occurrence of numbness (odds ratio: 5.50, 95% confidence interval: 1.06-28.58, p=0.042). The symptoms disappeared for all patients but one, within four months.
Conclusion
A transient injury of the superficial radial nerve could be one reason for numbness after a TRC. A large sheath size was an independent predictor of numbness; therefore, large sized sheaths should be used with caution when performing a TRC.
Keywords: Cardiac catheterization, Numbness, Superficial radial nerve lesion, Radial neuropathy
Introduction
The first percutaneous transradial catheterization (TRC) for diagnostic coronary angiography was introduced by Campeau in 19891); however, its practice was not widely used until recently. It has been slowly gaining acceptance worldwide, along with the development of small-sized access devices. TRC is associated with a lower incidence of major access site complications, including bleeding, compared to the transfemoral approach.2) Since bleeding complications is one of the major predictors of post procedure mortality and morbidity in patients having coronary disease, a TRC may be a safer method of approach compared to the transfemoral approach3),4); however, several other rarer complications were reported in inconsiderable amounts.5) Particularly, hand numbness after the TRC, often a disabling complication, occurs infrequently, but this fact is not generally known.6) Superficial radial neuropathy, or Cheiralgia paraesthetica, is a neuropathy of the hand caused by the compression or trauma of the superficial branch of the radial nerve. Typically, the symptom area is located on the back or side of the hand at the base of the thumb, near the anatomical snuffbox,7) which resembles the symptom of numbness after the TRC. Thus, we sought to investigate the presence of nerve damage in symptomatic patients using nerve conduction studies (NCS) and, in addition, the prevalence and natural course of digital numbness after the TRC.
Subjects and Methods
Study population
From April to December 2013, patients who underwent a coronary angiography through a radial access in our hospital were prospectively and consecutively enrolled. The Institutional Review Board of the Sejong General Hospital approved this study; all patients were properly informed prior to the procedure, and gave written consent to participate in the study. A patient's risk factor survey, including hypertension, dyslipidemia, diabetes and smoking was conducted before the procedure by recording the patient's history, followed by the laboratory confirmation. A comorbidities profile was created, as the presence of congestive heart failure, previous peripheral artery disease, coronary artery disease, cerebro-vascular accident, chronic kidney disease,8) and a history of myocardial infarction was assessed. Additionally, we recorded the height and body mass index (BMI) of the patients, for the estimation of the individual vessel size.9),10)
Transradial cardiac catheterization
The radial artery puncture method and the decision regarding the proper sheath size were conducted at the physician's discretion. In order to not give a difference between the routine procedure and the study protocol, a pre-procedural needle or sheath angiography was not defined as mandatory. Hydrophilic coated sheaths were used for all procedures. Sizes 4-6 French sheaths (RADIFOCUS Introducer II, Terumo, Europe N.V, Leuven, Belgium; AVANTI, Cordis Corporation, MIAMI, FL, USA; Accu-sheath, SUNGWONMEDICAL, Chengwon, Korea) were used for the intervention; the use of a size 7 French sheath was allowed, depending on the physician's judgement of the requirements. Two different radial arterial cannulation methods were used. The first method was the Seldinger technique, with a double wall through-and-through puncture and a gradual withdrawal of the cannula using the over-the-needle cannula system. The second method was the modified Seldinger technique, with an anterior wall puncture using a short metallic access needle.11)
After the administration of a local anesthesia, with a 2% lidocaine injection, the puncture procedure was performed and 2000 IU of unfractionated heparin was administrated for a diagnostic angiography. If a percutaneous coronary intervention was anticipated, a total of 100 IU/kg body weight of unfractionated heparin was given. An intra-arterial bolus of 0.2 mg of nitroglycerine was routinely given, with the exception of those patients intended for the ergonovine provocation test. After the procedure was finished, the sheaths were removed immediately and compression was performed using various devices (TR-Band, Terumo, Tokyo, Japan; Radi-stop, St. Jude Medical Inc., St. Paul, MN, USA; Radialis, Werkmeiser, Wanfried, Germany). All compression device application was performed according to the manufacturer's instructions. Distal pulsation of the hand, and changes in the skin color were closely monitored during the compression. The following data were collected during, or immediately following, the procedure: the size of the sheath, the amount of lidocaine, the amount of heparin, the number of puncture attempts (a needle pushed even just inside the skin was counted as a single attempt, regardless of the skin puncture times), and the distance of the puncture site (measured in centimeters) from the styloid process. One or two day's admission was considered essential for every patient.
Follow-up
All patients' symptoms were asked at the first outpatient clinic, before their complaints. All patients having neurologic symptoms (including paresthesia, allodynia, tingling, numbness, and weaknesses) mandatorily met with the rehabilitation doctor. Finally, their neurologic symptoms were assessed during their first outpatient clinic visit, and the symptoms were graded from 1 to 10 using a visual analogue scale (VAS). After a basic neurological examination, an NCS was performed on the symptomatic patients. The patients' symptom scales were recorded on a monthly or bimonthly basis until their symptoms disappeared.
Nerve conduction study protocol
The NCS was performed by the rehabilitation doctor, according to the neural distribution of the affected area, and it was carried out on both hands in symptomatic patients for a direct comparison with the unaffected side. For the superficial radial nerve (SRN) NCS, we followed the traditional form, which has been studied by stimulating the nerve in the distal forearm and recording from the base of the thumb, the anatomical snuffbox or the extensor pollicis longus. The superficial radial sensory nerve conduction study, distal latency, sensory nerve action potential amplitude and sensory nerve conduction velocity were measured. The latencies were marked at the onset of the first negative peak and the amplitudes were determined from peak to peak. The amplitudes were marked at the lowest and the highest peak (maximal peak to peak). Superficial radial sensory neuropathy was diagnosed if any of following criteria were satisfied:
Measurements were greater than 4 msec for superficial radial sensory onset latency
Measurements lower than 20 mV for the superficial radial sensory peak amplitude
Measurements slower than 50 m/s for superficial radial sensory nerve conduction velocity
Statistical analysis
Continuous variables were expressed as the mean±standard deviation, unless otherwise indicated. Discrete variables were expressed as a frequency and a percentage. Comparisons involving clinical and procedure characteristics were performed using the Fisher exact test for the categorical variables, and the Mann-Whitney U test for the continuous variables. A multivariate stepwise logistic regression analysis was used to determine the independent predictors for hand numbness; for this analysis, clinical and procedural characteristics achieving p<0.08 on the univariate analysis were entered into the model. A p of <0.05 was considered statistically significant. All statistical analyses were performed using SPSS, version 18.0 (SPSS, Chicago, IL, USA).
Results
Prevalence of hand numbness
Between April 2013 and December 2013, a total of 479 (male=316; 66%) consecutive patients were enrolled, and underwent TRC. Neurologic symptoms were found in nine patients (1.8%). All of their symptoms were confined to hand or digit numbness at the out patient department (OPD) presentation.
Patient characteristics
The baseline patient characteristics are shown in Table 1. The BMI, which has been proven to correlate with access vessel size in a previous study,9) was similar between the groups. All clinical comorbidities were not significantly different between the two groups, with the exception of previous myocardial infarction (the history of myocardial infarction showed a frequent trend toward the Numb+group; 11.5% vs. 33.3%; p=0.08).
Table 1. Baseline characteristics.
| Numbness negative (n=470) | Numbness positive (n=9) | p | |
|---|---|---|---|
| Age (years) | 59.0±11.6 | 52.6±9.8 | 0.10 |
| Male | 309 (65.7) | 7 (77.8) | 0.72 |
| Height (cm) | 162±8.5 | 167±7.9 | 0.10 |
| BMI (kg/m2) | 24.9±3.2 | 24.7±4.1 | 0.79 |
| Hypertension | 229 (48.7) | 4 (44) | >0.99 |
| Diabetes | 105 (22.3) | 3 (33.3) | 0.42 |
| Hypercholesterolemia | 268 (57) | 3 (33.3) | 0.18 |
| Smoking | 233 (49.6) | 6 (66.7) | 0.33 |
| CHF | 26 (5.5) | 0 | >0.99 |
| PAD | 3 (0.6) | 0 | >0.99 |
| CKD | 7 (1.5) | 0 | >0.99 |
| Previous CVA | 23 (4.9) | 0 | >0.99 |
| Previous CAD | 248 (52.8) | 6 (66.7) | 0.51 |
| Previous MI | 54 (11.5) | 3 (33.3) | 0.08 |
| Ejection fraction | 61.0±11.5 | 62.2±9.6 | 0.76 |
| Procedural parameters | |||
| Right hand | 328 (69.8) | 8 (88.9) | 0.29 |
| Modified seldinger | 141 (30.0) | 1 (11.1) | 0.20 |
| Concomitant PCI | 102 (21.8) | 3 (33.3) | 0.42 |
| ACS | 84 (17.9) | 1 (11.1) | >0.99 |
| Sheath insertion time (min) | 6.2±6.8 | 4.4±2.8 | 0.41 |
| Sheath (French) | 4.6±0.9 | 5.1±1.2 | 0.14 |
| 7 French sheath use | 24 (5.1%) | 2 (22.2%) | 0.08 |
| Lidocaine volume (cc) | 1.4±0.9 | 2.1±0.9 | 0.08 |
| Heparin inject (IU) | 2951±2247 | 3666±3535 | 0.35 |
| *Puncture location (cm) | 0.4±0.5 | 0.2±0.4 | 0.18 |
| Puncture attempts | 1.3±0.7 | 1.5±0.5 | 0.53 |
Data are expressed as mean±standard deviation or n (%). *distance from the styloid process. BMI: body mass index, CHF: congestive heart failure, PAD: peripheral artery disease, CKD: chronic kidney disease, CVA: cerebro-vascular accident, CAD: coronary artery disease, MI: myocardial infarction, PCI: percutaneous coronary intervention, ACS: acute coronary syndrome
Natural course of numbness
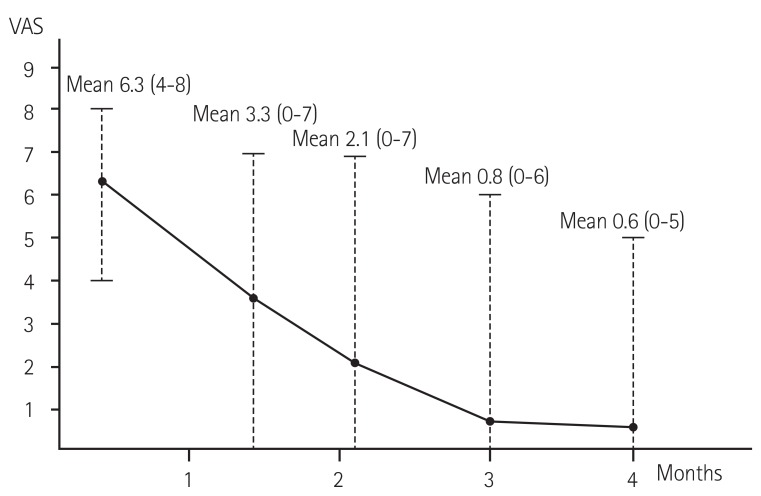
Table 2 shows the individual characteristics of the symptomatic patients and their NCS results. Nine patients had significant numbness on the day of their first OPD visit. The affected area was similar in all nine patients, presenting on the dorsal part of the thumb (near the anatomical snuffbox) or on the side of the hand at the base of the thumb (thenar area). The mean symptom value on the scale was 6 (4-8) on the day of presentation (mean 17±7 days, eight to 33 days after TRC). There was no weakness of hand or finger movement. Among the patients, four (44.4%; patient #2, #5, #6, #8 in Table 2) required medication for relief of their symptoms: three of these (#2, #6, #8) were prescribed Gabapentin; one was prescribed a short period of oral nonsteroidal anti-inflammatory drug medication (patient #5), and one required additional local injection therapy (#6). Fig. 1 demonstrated the patients' mean and range of VAS from the initial presentation to the five months' follow-up. Of the nine symptomatic patients, two patients' symptoms completely disappeared within two months (#1 and #4) and all patients' symptoms, except one (#9), disappeared within four months. Only one patient, diagnosed with triple vessel coronary disease, complained of severe numbness consistently after the coronary artery bypass graft surgery, through to the last OPD visit (2 out of 10 on the symptom scale; required no medication; symptom sustained 475 days after TRC; patient #9). All patients' symptoms significantly decreased during the follow-up according to the VAS (6.3±1.5 at initial presentation and 0.2±0.6 at last follow up, p<0.001; Fig. 1).
Table 2. Clinical, procedural characteristics and nerve conduction study results of patient with numbness.
| ‡No. | Clinical information | Procedural parameters | §NCS for superficial radial nerve (R/L) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Sex | Height (cm) | ∥BMI (kg/m2) | Comorbidities and *risks | †Symptom scale | †Hand | Puncture methods | ¶PCI (Yes/No) | Sheath diameter (French) | §Days from TRC | #OL | Amplitude | CV | Positive study | |
| 1 | 52 | Male | 171 | 28.0 | SK, HTN, pMI | 6 | R | M | No | 5 | 12 | 1.9/1.6 | 24.5/27.3 | 63/69 | |
| 2 | 68 | Male | 170 | 24.9 | HTN, lipid | 8 | R | S | No | 4 | 16 | 2.7/2.6 | 3.6/24.0 | - | Yes |
| 3 | 66 | Female | 155 | 22.4 | Lipid | 5 | R | S | Yes | 6 | 17 | 1.6/1.7 | 22.4/43.4 | 75/80 | |
| 4 | 41 | Male | 181 | 26.8 | SK | 8 | R | S | No | 4 | 21 | 1.5/1.5 | 39.5/25.1 | 71/54 | |
| 5 | 42 | Male | 173 | 21.0 | SK, DM, lipid, pMI | 4 | R | S | Yes | 7 | 33 | 1.9/1.8 | 16.1/28.7 | 63/67 | |
| 6 | 53 | Male | 173 | 24.3 | SK, lipid | 6 | L | S | No | 4 | 8 | 2.5/2.5 | 27.9/20.3 | 52/48 | Yes |
| 7 | 45 | Female | 156 | 31.2 | DM | 5 | R | M | Yes | 7 | 13 | 3.1/1.7 | 8.9/30.3 | 42/60 | Yes |
| 8 | 56 | Male | 178 | 17.0 | SK | 7 | L | S | No | 4 | 16 | NP /1.7 | NP /21.6 | NP /NP | Yes |
| 9 | 59 | Male | 170 | 26.3 | SK, DM, HTN, pMI | 8 | R | M | No | 5 | 13 | NP | NP | NP | |
*indicated generally considered risk factors for coronary artery disease. †Symptom scale was described as 1 to 10 scale by visual analogue scale. ‡Hand: puncture site for transradial catheterization. §days from transradial catheterization to nerve conduction study. No.: patients' number, NCS: nerve conduction study, R: right, L: left, BMI: body mass index, PCI: percutaneous coronary intervention, TRC: transradial cardiac catheterization, OL: onset latency, CV: conduction velocity, SK: smoking, HTN: hypertension, pMI: previous history of myocardial infarction, DM: diabetes mellitus, S: seldinger technique, M: modefied seldinger technique, NP: not performed
Fig. 1. Changes in the patients' mean and range of symptoms since the initial presentation, with a visual analogue scale (VAS).
Nerve conduction study results
Among the nine symptomatic patients, the exact planned procedure was carried out on six. Only one patient carried out the NCS on the affected side, due to severe dermatitis. He was diagnosed with only right superficial radial neuropathy, followed by the cut value of onset latency and amplitude (patient #8). One patient failed to achieve a result for the conduction velocity recording (not recordable); thus, the determination of neuropathy was made by the result of the onset latency and amplitude comparison (patient #2). Except for one patient who refused the test, half (n=4) of the patients achieved a positive result, according to our nerve conduction velocity neuropathy criteria.
Procedural data
There was no severe access site arterial anomaly interrupting the wire or catheter entrance in the study population (including no access site change or withdraw). The right hand was the preferred access site in both groups, without significance. The puncture method was not different between the groups; a percutaneous coronary intervention was similarly performed (Numb negative: 21.8% vs. Numb positive: 33.3%; p=0.42), and there were 84 (17.9%) and 1 (11.1%) acute coronary syndromes in the groups (p>0.99). The total sheath insertion time was approximately five minutes in both groups (Numb negative: 6.2±6.8 vs. Numb positive: 4.4±2.8 minutes; p=0.41). The puncture times were not different between the groups (1.3±0.7 vs. 1.5±0.5; p=0.53), but the lidocaine volume had a larger trend (Numb negative: 1.4±0.9 vs. Numb positive: 2.1±0.9 cc; p=0.08) and the location of puncture site had a lower trend towards the Numb+group (Numb negative: 0.4±0.9 vs. Numb positive: 0.2±0.4 cm; p=0.18). 26 patients (24 of whom were male) were treated with a size 7 French sheath due to an anticipated complex procedure, or a need for a kissing balloon inflation. There was a tendency toward a larger sheath in the Numb+group (Numb negative: 4.6±0.9 vs. Numb positive: 5.1±1.2 French; p=0.14).
Predictor for numbness
The univariate and multivariate analyses of the predictors for post-procedural numbness is displayed in Table 3. The use of a size 7 French sheath was a predictor of post-procedural numbness, and there was a greater trend toward previously diagnosed myocardial infarctions in the numbness group in the univariate analysis. In multivariate regression analysis, only the use of size 7 French sheaths (odds ratio: 5.50, 95% confidence interval: 1.06-28.58, p=0.042) was significantly associated with post-procedural numbness.
Table 3. Factor analysis for hand numbness.
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | HR | p | |
| Acute coronary syndrome | 0.07-4.64 | 0.60 | |||
| Height | 0.98-1.17 | 0.10 | |||
| Previous MI | 0.93-15.85 | 0.06 | 0.95-16.58 | 3.96 | 0.06 |
| Sheath diameter (5 French)* | 0.23-0.71 | 0.77 | |||
| Sheath diameter (6 French)* | 0.11-0.94 | 0.97 | |||
| Sheath diameter (7 French)* | 0.99-32.66 | 0.04 | 1.06-28.58 | 5.5 | 0.042 |
| Lidocaine volume (cc) | 0.92-3.24 | 0.09 | |||
| Location of puncture site† | 0.08-1.89 | 0.24 | |||
*each group of sheath diameter was compared to 4 Fr. sheath. †location of puncture site was analysed distance from styloid process. OR: odds ratio, CI: confidence interval, HR: hazard ratio, MI: myocardial infarction
Discussion
Principal findings
The current prospective registry indicated that the hand numbness occurred in more than a few cases after a TRC (1.8%) and that it was associated with the use of large access sheaths for intervention. Numbness after a TRC was firstly documented as an SRN injury, through the NCS, and the natural course of the numbness seems to be relatively benign. However, long lasting symptoms can infrequently develop after a TRC, and we suggest that complex procedures requiring a size 7 French sheath should not be performed through a radial access.
Superficial radial nerve injury and nerve conduction study
The SRN is fragile, in regard to potential injuries, due to its superficial position and the crossing of the lateral surface of the distal radius.12) A radial nerve could be injured by the TRC because the radial artery and radial nerve lay side by side near the styloid process of the radius, which is the most widely used puncture site.13) The concept of nerve damage after a TRC was accepted for this symptom in previous studies, because the symptom of digital numbness resembles that of nerve injury. This was believed to be related to a median or radial nerve injury, however it is not well analyzed because of its self-limiting, benign nature after presentation.14) The patient's symptoms, in our study, similarly occurred at the dorsal and the side of the thumb in all patients, which is the area supplied by the superficial radial nerve.7) Importantly, only 50% of the patients who performed the NCS achieved a positive result of superficial radial neuropathy. We assume the reason behind this finding is the sensitivity of the traditional method of NCS. We followed the traditional method of NCS, which has been studied by stimulating the nerve in the distal forearm and recording from the base of the thumb, the anatomical snuffbox or the extensor pollicis longus. However, with the presence of two branches of the SRN, Park et al.13) insisted that nerve conduction studies should be performed on both the medial and lateral branches in order to determine the presence and location of the SRN lesions near the wrist. Recently, a more reliable method was introduced for measuring the sensory conduction of the SRN and its terminal branches.15) Considering that the injuries can only partially involve the main nerve trunk or cause impairment at either the medial or lateral terminal branches, the NCS results could not be conducted as positive using the traditional method of NCS.
Prevalence and natural course of numbness
The prevalence of numbness was 1.8% in our study. All symptomatic patients had more than a moderate degree of the pain according to the VAS (the mean symptom scale value was 6 -from 4 to 8- on the day of presentation) and their symptoms often required medication for relief. However, all patients' symptoms (according to the VAS) decreased from initial presentation to the follow-up, which follows the natural course of superficial radial neuropathy. The numbness after the TRC was regarded as a benign and minor occurrence, since many physicians usually experience gradual improvement in the patients' symptoms.14) In contrast, there are several cases of a severe form of neuropathy associated with TRC; these patients had severe symptoms, including complex regional pain syndrome, which was treated as a sympathetic blockage.6),16),17) Traditionally, superficial radial neuropathy is considered to be a relatively benign disease regardless of the cause of injury, which could be improved by a local steroid injection or simple medication; however, similarly to our registry data, a minority of patients have remnant symptoms despite invasive treatment.7) It is not surprising that one of our symptomatic patients has had long lasting symptoms, even past the one year observational period.
Predictor for superficial radial neuropathy
In many procedural parameters, including the sheath insertion duration, lidocaine volume, heparin amount, puncture methods and puncture times, only the use of the large sized sheath was a predictor of SRN injury in our study. An earlier radial inner diameter and sheath outer diameter comparison study demonstrated the feasibility of a size 7 French sheath intervention system for the TRC in the majority of patients,18) and a sheathless technique could make size 7 or 8 French guiding catheters possible for complex procedures for a TRC.19) Thus, although TRC generally limits the arterial sheath to a size 6 French, the percutaneous intervention (using a size 7 or 8 French system) could be permitted in certain circumstances by the transradial committee.20) We didn't regulate the procedural details for this prospective study, to find the real practice outcome. Indeed, some interventionists in our center believed that up-sizing the transradial sheath is better for a complex procedure rather than getting an additional access in the femoral artery. However, there is convincing evidence that the complications (represented as a radial artery spasm) and occlusions were closely associated with larger sheath use, even the size 6 French sheath compared with the size 5 French sheath.21),22) Although the numbness can be caused by various mechanisms, there was a paucity of previous studies on this matter. Some authors suggest that the nerve injury may be due to the repeated needle entry during the TRC.23) The other hypothesis regarding nerve injury is nerve ischemia, resulting from radial artery occlusion, prolonged compression of the puncture site and hematoma formation.14) Our study demonstrated that the number of punctures was not a predictor of neuropathy. However, considering that a longer duration of compression time is usually required for large sheath intervention, the compression-related theory may be a more reliable explanation for the cause of nerve injury.
Study limitation
There were several limitations to this study. First, the procedural aspect was totally followed at the physician's discretion. However, we eliminated the 'learning curve effect', as all physicians had more than three years' experience in TRC, and no trainees carried out the procedures. Second, specific compression devices were individually applied following each device's instructions, and were not regulated by the researchers. Third, the total sample size was small, and the nerve conduction studies were selectively performed according to the presence of the patient's symptoms; therefore there could have existed individual differences in pain thresholds.
Conclusions
A transient injury of the superficial radial nerve could be one reason for numbness after a TRC. A large sheath size was an independent predictor of numbness. Therefore, a large sized sheath should be used with caution when performing TRC.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Campeau L. Percutaneous radial artery approach for coronary angiography. Cathet Cardiovasc Diagn. 1989;16:3–7. doi: 10.1002/ccd.1810160103. [DOI] [PubMed] [Google Scholar]
- 2.Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am Heart J. 2009;157:132–140. doi: 10.1016/j.ahj.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 3.Chase AJ, Fretz EB, Warburton WP, et al. Association of the arterial access site at angioplasty with transfusion and mortality: the M.O.R.T.A.L study (Mortality benefit Of Reduced Transfusion after percutaneous coronary intervention via the Arm or Leg) Heart. 2008;94:1019–1025. doi: 10.1136/hrt.2007.136390. [DOI] [PubMed] [Google Scholar]
- 4.Rao SV, Cohen MG, Kandzari DE, Bertrand OF, Gilchrist IC. The transradial approach to percutaneous coronary intervention: historical perspective, current concepts, and future directions. J Am Coll Cardiol. 2010;55:2187–2195. doi: 10.1016/j.jacc.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 5.Kanei Y, Kwan T, Nakra NC, et al. Transradial cardiac catheterization: a review of access site complications. Catheter Cardiovasc Interv. 2011;78:840–846. doi: 10.1002/ccd.22978. [DOI] [PubMed] [Google Scholar]
- 6.Cho EJ, Yang JH, Song YB. Type II complex regional pain syndrome of the hand resulting from repeated arterial punctures during transradial coronary intervention. Catheter Cardiovasc Interv. 2013;82:E465–E468. doi: 10.1002/ccd.24853. [DOI] [PubMed] [Google Scholar]
- 7.Braidwood AS. Superficial radial neuropathy. J Bone Joint Surg Br. 1975;57:380–383. [PubMed] [Google Scholar]
- 8.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed B, Lischke S, De Sarno M, Holterman LA, Straight F, Dauerman HL. Gender related differences in predictors of vascular complications: role of vessel size and BMI. J Thromb Thrombolysis. 2013;36:84–90. doi: 10.1007/s11239-012-0847-y. [DOI] [PubMed] [Google Scholar]
- 10.Velasco A, Ono C, Nugent K, Tarwater P, Kumar A. Ultrasonic evaluation of the radial artery diameter in a local population from Texas. J Invasive Cardiol. 2012;24:339–341. [PubMed] [Google Scholar]
- 11.Pancholy SB, Sanghvi KA, Patel TM. Radial artery access technique evaluation trial: randomized comparison of Seldinger versus modified Seldinger technique for arterial access for transradial catheterization. Catheter Cardiovasc Interv. 2012;80:288–291. doi: 10.1002/ccd.23445. [DOI] [PubMed] [Google Scholar]
- 12.Spindler HA, Felsenthal G. Radial sensory conduction in the hand. Arch Phys Med Rehabil. 1986;67:821–823. [PubMed] [Google Scholar]
- 13.Park BK, Bun HR, Hwang M, Hong J, Kim DH. Medial and lateral branches of the superficial radial nerve: cadaver and nerve conduction studies. Clin Neurophysiol. 2010;121:228–232. doi: 10.1016/j.clinph.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Kanei Y, Kwan T, Nakra NC, et al. Transradial cardiac catheterization: a review of access site complications. Catheter Cardiovasc Interv. 2011;78:840–846. doi: 10.1002/ccd.22978. [DOI] [PubMed] [Google Scholar]
- 15.Cappellari AM, Bona AR, Lukasova K. Electrophysiological study of medial and lateral branches of the superficial radial nerve. Muscle Nerve. 2013;47:105–107. doi: 10.1002/mus.23483. [DOI] [PubMed] [Google Scholar]
- 16.Papadimos TJ, Hofmann JP. Radial artery thrombosis, palmar arch systolic blood velocities, and chronic regional pain syndrome 1 following transradial cardiac catheterization. Catheter Cardiovasc Interv. 2002;57:537–540. doi: 10.1002/ccd.10367. [DOI] [PubMed] [Google Scholar]
- 17.Sasano N, Tsuda T, Sasano H, Ito S, Sobue K, Katsuya H. A case of complex regional pain syndrome type II after transradial coronary intervention. J Anesth. 2004;18:310–312. doi: 10.1007/s00540-004-0266-0. [DOI] [PubMed] [Google Scholar]
- 18.Saito S, Ikei H, Hosokawa G, Tanaka S. Influence of the ratio between radial artery inner diameter and sheath outer diameter on radial artery flow after transradial coronary intervention. Catheter Cardiovasc Interv. 1999;46:173–178. doi: 10.1002/(SICI)1522-726X(199902)46:2<173::AID-CCD12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 19.From AM, Gulati R, Prasad A, Rihal CS. Sheathless transradial intervention using standard guide catheters. Catheter Cardiovasc Interv. 2010;76:911–916. doi: 10.1002/ccd.22742. [DOI] [PubMed] [Google Scholar]
- 20.Caputo RP, Tremmel JA, Rao S, et al. Transradial arterial access for coronary and peripheral procedures: executive summary by the Transradial Committee of the SCAI. Catheter Cardiovasc Interv. 2011;78:823–839. doi: 10.1002/ccd.23052. [DOI] [PubMed] [Google Scholar]
- 21.Kiemeneij F. Prevention and management of radial artery spasm. J Invasive Cardiol. 2006;18:159–160. [PubMed] [Google Scholar]
- 22.Uhlemann M, Möbius-Winkler S, Mende M, et al. The Leipzig prospective vascular ultrasound registry in radial artery catheterization: impact of sheath size on vascular complications. JACC Cardiovasc Interv. 2012;5:36–43. doi: 10.1016/j.jcin.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Stella PR, Kiemeneij F, Laarman GJ, Odekerken D, Slagboom T, van der Wieken R. Incidence and outcome of radial artery occlusion following transradial artery coronary angioplasty. Cathet Cardiovasc Diagn. 1997;40:156–158. doi: 10.1002/(sici)1097-0304(199702)40:2<156::aid-ccd7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]