Abstract
Background and Objectives
Celiac disease (CD) is a chronic autoimmune disorder induced by dietary gluten intake by individuals who are genetically sensitive. Many studies report an increased risk of cardiovascular diseases in such patients. The aim of this study is to assess aortic elasticity properties in patients with CD that may be associated with an increased risk of cardiovascular disease.
Subjects and Methods
Eighty-one patients diagnosed with CD by antibody test and biopsy and 63 healthy volunteers were included in this prospective study. Electrocardiographic and echocardiographic examinations were performed.
Results
The CD group did not have any differences in the conventional echocardiographic parameters compared to the healthy individuals. However, patients in the CD group had an increased aortic stiffness beta index (4.3±2.3 vs. 3.6±1.6, p=0.010), increased pressure strain elastic modulus (33.6±17.0 kPa vs. 28.5±16.7 kPa, p=0.037), decreased aortic distensibility (7.0±3.0×10-6 cm2/dyn vs. 8.2±3.6×10-6 cm2/dyn, p=0.037), and similar aortic strain (17.9±7.7 vs. 16.0±5.5, p=0.070) compared to the control group. Patients with CD were found to have an elevated neutrophil/lymphocyte ratio compared to the control group (2.54±0.63 vs. 2.24±0.63, p=0.012). However, gluten-free diet and neutrophil/lymphocyte ratio were not found to be associated with aortic elasticity.
Conclusion
Patients with CD had increased aortic stiffness and decreased aortic distensibility. Gluten-free diet enabled the patients with CD to have a reduction in the inflammatory parameters whereas the absence of a significant difference in the elastic properties of the aorta may suggest that the risk of cardiovascular disease persists in this patient group despite a gluten-free diet.
Keywords: Celiac disease, Elastic modulus, Aortic stiffness, Inflammation
Introduction
Celiac disease (CD) is an autoimmune disorder induced by dietary intake of gluten. After the patients are exposed to gluten, the inflammatory reaction is initiated in the small intestine while crypt hyperplasia and villous atrophy develop. The impaired small intestine function leads to the malabsorption of nutrients. Moreover, nutrient deficiency may develop due to malabsorption while increased systemic inflammatory activity may result in extra-intestinal signs.1),2)
The prevalence of CD has increased substantially over the last 50 years. The onset of CD is often assumed to be triggered during childhood and its clinical manifestations vary across age groups. Adults who develop CD may present with diarrhea, but can also have silent manifestations such as anemia. Metabolic bone disease, dermatitis herpetiformis, peripheral neuropathy, thyroid diseases, infertility can occur more frequently in patients with CD than in the general population.3)
Many trials report that cardiovascular risk is higher in patients with CD compared to the normal population.4),5),6) The main mechanisms that are inculpated for cardiovascular risk include endothelial dysfunction, hyperhomocysteinemia and an increased systemic inflammatory response.7,8,9) One trial reported that traditional cardiovascular risk factors were less common among patients with CD who also developed coronary artery disease.10) Therefore, it is important to define the new risk factors in order to identify the cardiovascular risk in patients with CD. No studies to date explore the elastic properties of the aorta among patients with CD, which is known as a cardiovascular risk predictor. In this trial, we aimed to assess the aortic strain, stiffness and elasticity in patients with CD.
Subjects and Methods
In this prospective study, we evaluated patients diagnosed with CD through both histopathology and serology (plasma anti-tissue transglutaminase and anti-endomysial antibodies) and were followed by the Gastroenterology Department of Antalya Training and Research Hospital. Eighty-one subjects with CD and 63 healthy controls were included. Patients' baseline demographic characteristics were recorded. Exclusion criteria are history of structural heart disease, coronary artery disease, permanent atrial fibrillation, acute infectious disease, collagen tissue disease, malignancies, diabetes mellitus, hypertension, and a history of inflammatory disease other than CD. Subjects consuming corticosteroids, had renal/hepatic failure, or a hematological disease were also excluded from this study.
Routine biochemical tests and complete blood count (CBC) were performed on antecubital venous sample after 12 hours of fasting. CBC including white blood cell, neutrophil and lymphocyte counts were performed using an automated CBC device (Abott Cell Dyn, Abbott Park, IL, USA). Neutrophil/lymphocyte ratio was calculated using data obtained from the CBC count. The C-reactive protein levels (normal range: 0-5 mg/L) were analyzed with an IMMAGE® 800 (Beckman Coulter Inc., Brea, CA, USA).
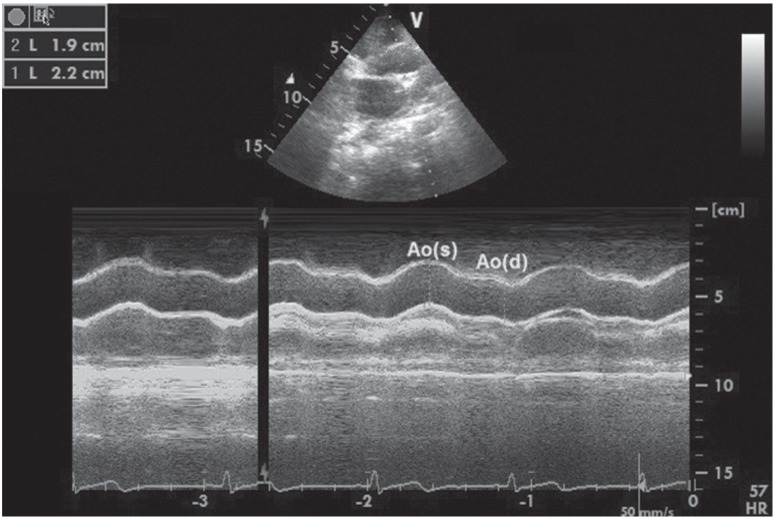
The heart rate and blood pressure were measured and 12-derivation surface electrocardiography (Nihon Kohden, Tokio, Japan) was performed. Transthoracic echocardiographic examination (EPIQ 7 Ultrasound System, Philips, Heide, Netherlands) was performed as well. The measurements were performed in line with the recommendations of the American Society of Echocardiography.11) Two cardiologists who were uninformed of the subjects'clinical conditions performed all measurements. The average of three measurements was recorded. The systolic and diastolic ascending aortic diameters were recorded in M-mode under echocardiographic and electrocardiographic guidance approximately 3 cm above the aortic valve from parasternal long axis views. The systolic aortic diameter was measured at the time of maximum anterior motion of the aorta while the diastolic diameter was measured at the start of the QRS complex in electrocardiography (Fig. 1). The following formulas were used to assess the elastic properties of the aorta.12),13)
Fig. 1. Systolic (s) and diastolic (d) ascending aortic diameters recorded in M-mode approximately 3 cm above the aortic valve from parasternal longaxis views.
Aortic strain (%)=(aortic SD [systolic diameter]-aortic DD [diastolic diameter])×100/aortic DD
Aortic stiffness beta index=natural logarithm (systolic BP [blood pressure]/diastolic BP)/([aortic SD-aortic DD]/aortic DD)
Aortic distensibility (cm2.dyne-1.10-6)=2×([aortic SD-aortic DD]/aortic DD)/(systolic BP-diastolic BP)
Pressure strain elastic modulus=(systolic BP–diastolic BP)/([aortic SD–aortic DD]/aortic DD)
Statistical analysis
Data were analyzed with SPSS software version 21.0 for Windows (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as mean±standard deviation, and categorical variables were expressed as a percentage. The χ2 test and Fisher's exact test were used to compare the categorical variables. The Shapiro-Wilk test was used to assess the distribution of the continuous variables. Student's t-test was used for variables with normal distribution and the values were presented as mean±standard deviation. Continuous variables without normal distribution were analyzed using the Mann-Whitney U test and the values obtained were presented as median (50th) values and interquartile ranges (25th and 75th). The effects of different variables on each aortic elasticity parameter were calculated in linear regression analysis. A two-tailed p <0.05 was considered statistically significant.
Results
Eighty-one patients with CD (74% female, mean age 43±11) and 63 healthy volunteers (71% female, mean age 42±5) as control group were enrolled in the study. Their baseline characteristics are listed in Table 1. There were no significant differences between the two groups with respect to hyperlipidemia, smoking, age and gender. All of the patients were in the sinus rhythm. The blood pressure values were within the normal range in the study population; however, in patients with CD, the systolic and diastolic blood pressures were higher than the control group. The self-reported gluten-free diet (GFD) adherence of patients with CD was 43%. The median time to CD diagnosis was 24 months (12-60 months). Patients with CD had higher neutrophil/lymphocyte ratios (2.54±0.63 vs. 2.24±0.63, p=0.012) from the systemic inflammatory parameters and had higher C-reactive protein values (3.9±3.4 vs. 3.0±0.8, p<0.001) compared to the control group. The analysis of patients with CD revealed that those who adhered to the GFD had lower neutrophil/lymphocyte ratios compared to those who did not adhere to the diet (2.2±0.5 vs. 2.7±0.6, p=0.005).
Table 1. Demographic characteristics of the study populations.
| Variable | Celiac disease (n=81) |
Control group (n=63) |
p |
|---|---|---|---|
| Age (years) | 43.2±11.1 | 42.2±5.2 | 0.329 |
| Female, n(%) | 60 (74) | 45 (71) | 0.426 |
| Smoking, n(%) | 26 (32) | 24 (38) | 0.316 |
| BMI (kg/m2) | 23.3 (22-25) | 23.5 (22-24) | 0.168 |
| Systolic BP (mmHg) | 125.8±9.1 | 120.9±8.8 | 0.004 |
| Diastolic BP (mmHg) | 75.1±9.3 | 85.0±7.3 | <0.001 |
| TC (mg/dL) | 190.9±44.7 | 196.8±44.6 | 0.322 |
| LDL (mg/dL) | 118.1 (95-144) | 108.8 (83-141) | 0.087 |
| HDL (mg/dL) | 42.5±11.0 | 43.1±8.6 | 0.177 |
| Trigliseride (mg/dL) | 124.8±63.0 | 140.6±77.4 | 0.965 |
| Uric acid (mg/dL) | 4.0±1.5 | 3.5±1.1 | 0.370 |
| CRP (mg/L) | 3.9±3.4 | 3.0±0.8 | <0.001 |
| N/L ratio | 2.54±0.63 | 2.24±0.63 | 0.012 |
| Glucose (mg/dL) | 86.5±12.4 | 91.5±8.6 | 0.126 |
Data are expressed as mean±standard deviation for normally distributed data, median (50th) values and interquantile ranges (25th and 75th) for continuous variables without normal distribution, and percentage for categorical variables. BMI:body mass index, BP: blood pressure, TC: total cholesterol, LDL: low density lipoprotein, HDL: high density lipoprotein, CRP: C-reactive proteine, N/L:neutrophil/lympocyte
Among the conventional echocardiographic parameters; left ventricular ejection fraction, interventricular septum thickness, posterior wall thickness, left atrial diameter, left ventricular end-diastolic diameter, left ventricular end-systolic diameter, systolic pulmonary artery pressure averages were within the normal ranges, and the two groups did not have significant differences. Further, the groups did not have significant differences regarding E/Ea value as an indicator of diastolic dysfunction (Table 2).
Table 2. Conventionally echocardiographic parameters of the study populations.
| Variable | Celiac disease (n=81) |
Control group (n=63) |
p |
|---|---|---|---|
| LVEF (%) | 65.1±2.2 | 65.2±2.2 | 0.893 |
| LA diameter (mm) | 34.0±4.1 | 33.1±4.1 | 0.105 |
| LVEDD (mm) | 43.1±4.4 | 44.8±3.5 | 0.072 |
| LVESD (mm) | 24.2±3.2 | 23.8±4.0 | 0.708 |
| E/A ratio | 1.3±0.4 | 1.2±0.3 | 0.587 |
| E/Ea | 0.66±0.23 | 0.68±0.30 | 0.754 |
| EDT (ms) | 205±37.6 | 200±39.1 | 0.146 |
| IVRT (ms) | 80.3±14.4 | 86.5±17.7 | 0.937 |
| LVH presence, n(%) | 3 (3) | 2 (3) | 1.000 |
Data are expressed as mean±standard deviation for normally distributed data and percentage for categorical variables. LVEF: left ventricular ejection fraction, LA: left atrium, LVEDD: left ventricular end diastolic diameter, LVESD: left ventricular end systolic diameter, E: mitral inflow early diastolic velocity, A: mitral inflow late diastolic velocity, Ea: annular early diastolic velocity, EDT: early deceleration time, IVRT: isovolumetric relaxation time, LVH: left ventricular hypertrophy
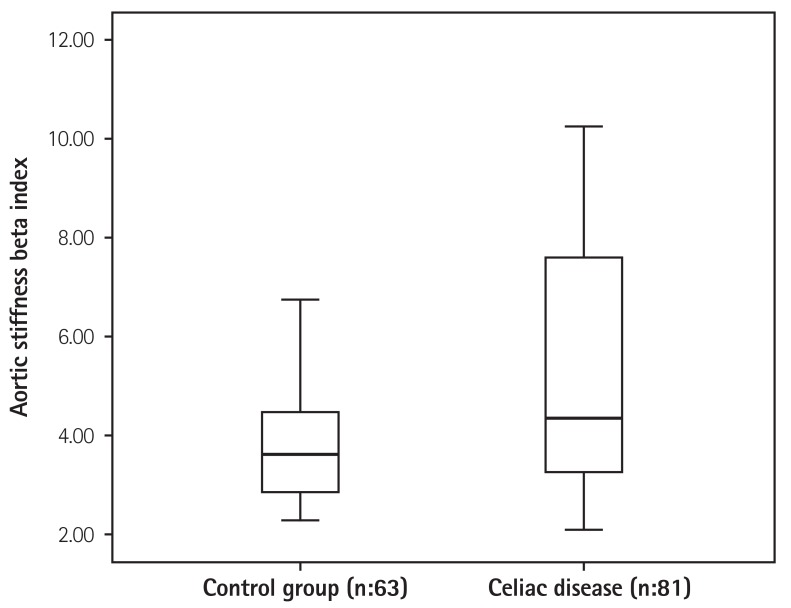
Patients with CD were found to have significantly decreased aortic diastolic and systolic diameters compared to the control group. Aortic elastic properties of both groups were compared. There were no significant differences in aortic strain between the patients with CD and the control group (17.9±7.7 vs. 16.0±5.5, p=0.070). Pressure strain elastic modulus was found to be higher in patients with CD than in the control group (33.6±17.0 kPa vs. 28.5±16.7 kPa, p=0.037). Aortic stiffness beta index was higher in patients with CD than in the control group (4.3±2.3 vs. 3.6±1.6, p=0.010) (Fig. 2). Aortic distensibility was observed to be lower in patients with CD (7.0±3.0×10-6 cm2/dyn vs. 8.2±3.6×10-6 cm2/dyn, p=0.037) (Table 3). The analysis of patients with CD alone showed no significant difference in the aortic elastic properties between those who followed a GFD and those who did not (Table 4).
Fig. 2. Aortic stiffness beta index values in patients with celiac disease and in the control group.
Table 3. Aortic elastic properties of the study populations.
| Variable | Celiac disease (n=81) |
Control group (n=63) |
p |
|---|---|---|---|
| Aortic DD (mm) | 25.1±3.5 | 27.3±2.7 | <0.001 |
| Aortic SD (mm) | 29.0±3.2 | 31.5±2.9 | 0.001 |
| Aortik strain (%) | 17.9±7.7 | 16.0±5.5 | 0.070 |
| Aortik distensibility (10-6 cm2/dyn) |
7.0±3.0 | 8.2±3.6 | 0.037 |
| Aortik stiffness β index | 4.3±2.3 | 3.6±1.6 | 0.010 |
| Elastic modulus (kPa) | 33.6±17.0 | 28.5±16.7 | 0.037 |
Data are expressed as mean±standard deviation for normally distributed data. DD: diastolic diameter, SD: systolic diameter
Table 4. Gluten-free diet, aortic elastic properties and inflammatory parameters.
| Variable | GFD (+) (n=44) |
GFD (-) (n=37) |
p |
|---|---|---|---|
| Aortic DD (mm) | 24.5±2.7 | 24.5±3.5 | 0.986 |
| Aortic SD (mm) | 28.7±2.7 | 29.1±2.8 | 0.763 |
| Aortik strain (%) | 17.7±7.4 | 19.2±8.0 | 0.451 |
| Aortik distensibilite (10-6 cm2/dyn) |
6.9±2.7 | 7.5±3.2 | 0.454 |
| Aortik stiffness β index | 4.7±2.0 | 5.7±2.5 | 0.058 |
| Elastic modulus (kPa) | 34.7±17.0 | 32.6±17.2 | 0.441 |
| CRP (mg/L) | 3.0±3.2 | 4.5±3.6 | 0.715 |
| N/L ratio | 2.2±0.5 | 2.7±0.6 | 0.005 |
Data are expressed as mean±standard deviation for normally distributed data. Aortik stiffness β index: (BETA=ln [systolic/diastolic pressure]×2 blood viscosity/pulse pressure×PWV2). GFD: gluten-free diet, DD: diastolic diameter, SD: systolic diameter, CRP: C-reactive proteine, N/L: neutrophil/lymphocyte
The linear regression analysis on parameters affecting the aortic elastic properties revealed that there was a significant association between the presence of CD and aortic systolic and diastolic diameters, aortic stiffness beta index and aortic strain (Table 5).
Table 5. Multivariate linear regression for change in aortic elasticity parameters among adults with and without Celiac disease.
| Aortik stiffness β index | Elastic modulus | Aortik distensibility | Aortik strain | Aortic DD | Aortic SD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | β | p | β | p | β | p | β | p | β | p | β | p |
| Celiac disease | 0.333 | 0.001 | 0.126 | 0.202 | -0.041 | 0.671 | 0.223 | 0.017 | -0.372 | <0.001 | -0.348 | <0.001 |
| Age (years) | -0.010 | 0.921 | 0.252 | 0.014 | -0.305 | 0.003 | -0.324 | 0.001 | 0.380 | <0.001 | 0.300 | 0.002 |
| Smoking | -0.051 | 0.587 | 0.038 | 0.696 | -0.072 | 0.452 | -0.050 | 0.586 | 0.114 | 0.179 | 0.092 | 0.304 |
| BMI | -0.036 | 0.714 | -0.007 | 0.943 | -0.018 | 0.863 | -0.031 | 0.751 | -0.019 | 0.835 | -0.045 | 0.639 |
| CRP | -0.012 | 0.897 | 0.043 | 0.652 | 0.018 | 0.188 | 0.039 | 0.669 | 0.031 | 0.709 | 0.028 | 0.751 |
| N/L ratio | -0.015 | 0.876 | -0.101 | 0.299 | 0.087 | 0.365 | 0.015 | 0.873 | -0.059 | 0.480 | -0.079 | 0.375 |
| E/Ea ratio | 0.037 | 0.693 | 0.066 | 0.493 | -0.002 | 0.982 | -0.107 | 0.241 | 0.098 | 0.241 | 0.065 | 0.465 |
| LVH presence | -0.157 | 0.088 | -0.034 | 0.717 | -0.011 | 0.908 | -0.019 | 0.828 | 0.115 | 0.165 | 0.129 | 0.140 |
| LVEF | 0.083 | 0.350 | -0.024 | 0.792 | 0.083 | 0.361 | 0.068 | 0.436 | -0.063 | 0.430 | -0.059 | 0.484 |
β: standardized regression coefficient. DD:diastolic diameter, SD:systolic diameter, BMI: body mass index, CRP: C-reactive proteine, N/L: neutrophil/lymphocyte, E: mitral inflow early diastolic velocity, Ea: annular early diastolic velocity, LVH: left ventricular hypertrophy, LVEF: left ventricular ejection fraction
Discussion
In this study, we observed that the aortic stiffness beta index increased as aortic distensibility decreased in patients with CD. Patients with CD who followed a GFD had lower neutrophil/lymphocyte ratios than those who did not follow the GFD. There was no significant association between GFD, neutrophil/lymphocyte ratio and aortic elastic properties. These findings suggest that increased aortic stiffness in patients with CD is multifactorial and thus, cannot be explained by inflammation alone. Further, this phenomenon is not influenced by a GFD.
CD is a chronic small intestinal immune-mediated enteropathy that occurs in genetically predisposed people in response to the dietary intake of gluten.14) Chronic inflammation of the small intestine, villous atrophy and secondary nutrient malabsorption develop in patients suffering from CD. The prevalence of CD is reported to range from 0.5 to 3 %.15),16) Malabsorption symptoms (diarrhea, vomiting, steatorrhea, deficiency of fat-soluble minerals, iron and folate deficiencies) are more pronounced in the classic type of CD, which is more common among children. However Atypical CD, is the most common form among adolescents and adults, in which extra-intestinal symptoms, such as laboratory abnormalities (especially iron deficiency anemia) and osteopenia are prevalent.17)
The pronounced symptoms may vary across different geographical regions. Ehsani-Ardakani et al.18) found that the most common CD symptom in European countries is associated with the upper abdominal area (abdominal pain and dyspepsia). In contrast, the lower gastrointestinal symptoms (diarrhea, bloating), as well as anemia are more prevalent in the Middle Eastern countries. CD may either be asymptomatic or presented with various clinical manifestations; therefore, the exact prevalence of the disease may be greater than reported.
Patients with CD may present with gastrointestinal symptoms, as well as with extra-intestinal symptoms, the cause of which cannot be explained precisely. Furthermore, CD is reported to be concomitant with other autoimmune diseases, such as type 1 diabetes mellitus, Hashimoto's thyroiditis, and collagen tissue diseases.19),20),21) The majority of the population-based studies have reported that patients with CD are have an increased risk of cardiovascular disease compared to the normal population and that the most common cause of death is cardiovascular diseases.4),5),6)
However, most of the cardiovascular events in these patients cannot be explained by traditional risk factors. Emilsson et al.10) demonstrated that patients with CD, who had a history of myocardial infarction had a better risk profile regarding the traditional cardiovascular risk factors compared to those without CD. Therefore, it is important to define new risk factors that are associated with the increased cardiovascular risk in this patient group. It is known from previous studies that the increased aortic stiffness and chronic systemic inflammation is associated with an increased cardiovascular risk.22),23) In our study, aortic stiffness was found to increase in patients with CD, although they were similar with respect to the traditional risk factors. Hence, measurement of aortic elastic properties as a non-traditional risk factor in patients with CD may help identify the cardiovascular risk.
Ongoing inflammation may have an effect on the increased aortic stiffness etiology in patients with CD. El Gamal et al.24) investigated patients with systemic lupus erythematous, an autoimmune disorder like CD, and asserted that increased aortic stiffness was associated with disease activity and inflammation rather than the atherosclerotic process. In another study, Barbulescu et al.25) found that increased aortic stiffness was associated with levels of the inflammatory markers: C-reactive protein and interleukin-6. In our study, the neutrophil/lymphocyte ratio of patients with CD was found to be higher than in the control group, while those who adhered to a GFD had lower neutrophil/lymphocyte ratios than those who did not adhere.
These findings may suggest that chronic systemic inflammation decreases with diet in these patients. Persistent inflammation may be an important factor for increased cardiovascular risk in patients who do not adhere to a GFD. However, GFD, neutrophil/lymphocyte ratio and C-reactive protein levels, as well as aortic elastic properties did not have significant associations. This suggests that the impairment of the aortic elasticity in patients with CD is multifactorial and cannot be explained by inflammation alone.
Hyperhomocysteinemia may also play a role in the increased cardiovascular risk in patients with CD. Folate deficiency secondary to malabsorption may lead to the impairment of homocysteine metabolism and to hyperhomocysteinemia.2),26) Elevated homocysteine affects the cardiovascular system in various ways. It increases oxidative stress causing cardiomyocyte dysfunction and apoptosis, decreases the response of myofilaments to calcium declining the cardiomyocyte contractibility, increases oxidative stress in vessels, and finally, decreases nitric oxide synthesis and availability causing endothelial dysfunction.27),28) Furthermore, elevated homocysteine was found to destroy elastin fibers and cause proliferation in vascular smooth muscles and thus decrease vessel elasticity.
Nestel et al.29) demonstrated that elevated homocysteine due to an increase in methionine caused an acute increase in aortic stiffness (in 5 hours). To date, there are contradictory data regarding the effect of a GFD on homocysteine levels in patients with CD.8),26) Gefel et al.26) reported that a GFD enabled a decrease in the homocysteine levels. On the other hand, De Marchi et al.8) reported that there was no significant change in homocysteine levels in patients observing a GFD, although C-reactive protein levels declined with a GFD. In our study, elevated homocysteine levels may play a major role in increased aortic stiffness in patients with CD because significant changes were observed in aortic elastic properties with a GFD, although the inflammatory activity decreased. However, we could not assess this association since we could not analyze homocysteine levels of our patients.
Diagnosis was established by mucosa of the proximal small intestine biopsy. Presence of circulating antibodies against gliadin, endomysium and tissue transglutaminase further support diagnoses.14) It is essential in the treatment to exclude gluten from diet. In a study of 20 newly diagnosed CD patients, initially impaired endothelium-dependent flow mediated dilation improved and carotid intima-media thickness receded after 8 weeks of a GFD.8) Lim et al.30) reported that blood pressure was restored to normal levels in a patient with CD. The majority of symptoms and clinical findings improve quickly and antibody titers decrease with GFD adherence. However, improvement of the aortic elastic properties could not be demonstrated in our study although the inflammation was lowered with GFD. This may suggest that the increased cardiovascular risk persists in patients despite a GFD.
Study limitations
Concerning the possible limitations of the present study, it was a single-center study and included a small number of patients. The current homocysteine values and antibody titers could not be obtained from all patients at the time of the echocardiographic examination. Therefore, the association between the aortic elastic properties, homocysteine and antibody titers could not be explored. The study protocol did not include long-term follow-up, hence the effect of aortic elastic properties on prognosis could not be assessed.
Conclusion
Aortic stiffness increased while aortic elasticity decreased in patients with CD. Assessment of aortic elastic properties as non-traditional cardiovascular risk factors may contribute to the identification of cardiovascular risk in patients with CD.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Green PH, Cellier C. Celiac disesase. N Engl J Med. 2007;357:1731–1743. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 2.Hallert C, Grant C, Grehn S, et al. Evidence of poor vitamin status in coeliac patients on a gluten-free diet for 10 years. Aliment Pharmacol Ther. 2002;16:1333–1339. doi: 10.1046/j.1365-2036.2002.01283.x. [DOI] [PubMed] [Google Scholar]
- 3.Cekin AH, Cekin Y, Sezer C. Celiac disease prevalence in patients with iron deficiency anemia. Turk J Gastroenterol. 2012;23:490–495. [PubMed] [Google Scholar]
- 4.Ludvigsson JF, de Faire U, Ekbom A, Montgomery SM. Vascular diasease in a population-based cohort of individuals hospitalised with coeliac disease. Heart. 2007;93:1111–1115. doi: 10.1136/hrt.2006.097097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei L, Spiers E, Reynolds N, Walsh S, Fahey T, MacDonald TM. The association between coeliac disease and cardiovascular disease. Aliment Pharmacol Ther. 2008;27:514–519. doi: 10.1111/j.1365-2036.2007.03594.x. [DOI] [PubMed] [Google Scholar]
- 6.Peters U, Askling J, Gridley G, Ekbom A, Linet M. Causes of death in patients with celiac disease in a population-based Swedish cohort. Arch Intern Med. 2003;163:1566–1572. doi: 10.1001/archinte.163.13.1566. [DOI] [PubMed] [Google Scholar]
- 7.Emilsson L, Smith JG, West J, Melander O, Ludvigsson JF. Increased risk of atrial fibrillation in patients with coeliac disease: a nationwide cohort study. Eur Heart J. 2011;32:2430–2437. doi: 10.1093/eurheartj/ehr167. [DOI] [PubMed] [Google Scholar]
- 8.De Marchi S, Chiarioni G, Prior M, Arosio E. Young adults with coeliac disease may be at increased risk of early atherosclerosis. Aliment Pharmacol Ther. 2013;38:162–169. doi: 10.1111/apt.12360. [DOI] [PubMed] [Google Scholar]
- 9.Ludvigsson JF, James S, Askling J, Stenestrand U, Ingelsson E. Nationwide cohort study of risk of ischemic heart disease in patients with celiac disease. Circulation. 2011;123:483–490. doi: 10.1161/CIRCULATIONAHA.110.965624. [DOI] [PubMed] [Google Scholar]
- 10.Emilsson L, Carlsson R, Holmqvist M, James S, Ludvigsson JF. The characterisation and risk factors of ischaemic heart disease in patients with coeliac disease. Aliment Pharmacol Ther. 2013;37:905–914. doi: 10.1111/apt.12271. [DOI] [PubMed] [Google Scholar]
- 11.Lang RM, Bierig M, Devereux RB, et al. Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantification in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 13.Lacombe F, Dart A, Dewar E, Jennings G, Cameron J, Laufer E. Arterial elastic properties in man: a comparison of echo-Doppler indices of aortic stiffness. Eur Heart J. 1992;13:1040–1045. doi: 10.1093/oxfordjournals.eurheartj.a060311. [DOI] [PubMed] [Google Scholar]
- 14.Ludvigsson JF, Lefner DA, Bai JC, et al. The Oslo definitions for celiac disease and related terms. Gut. 2013;62:43–52. doi: 10.1136/gutjnl-2011-301346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green PH, Celliere C. Celiac disease. N Engl J Med. 2007;357:1731–1743. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- 16.Green PH, Jabri B. Coeliac disease. Lancet. 2003;362:383–391. doi: 10.1016/S0140-6736(03)14027-5. [DOI] [PubMed] [Google Scholar]
- 17.Norsa L, Shamir R, Zevit N. Gluten-free diet in celiac disease: protective or providing additive risk factors fort he development of cardiovascular disease? Nutr Ther Metab. 2012;30:1–9. [Google Scholar]
- 18.Ehsani-Ardakani MJ, Rostami Nejad M, Villanacci V, et al. Gastrointestinal and non-gastrointestinal presentation in patients with celiac disease. Arch Iran Med. 2013;16:78–82. [PubMed] [Google Scholar]
- 19.Park SW, Park SJ, Shin JI. Another possible underlying mechanism for the positive association between celiac disease and systemic lupus erythematosus: the role of interleukin 21. J Rheumatol. 2013;40:1619. doi: 10.3899/jrheum.130426. [DOI] [PubMed] [Google Scholar]
- 20.van den Driessche A, Eenkhoorn V, Van Gaal L, De Block C. Type I diabetes and autoimmune polyglandular syndrome: a clinical review. Neth J Med. 2009;67:376–387. [PubMed] [Google Scholar]
- 21.Fasano A. Systemic autoimmune disorders in celiac disease. Curr Opin Gastroenterol. 2006;22:674–679. doi: 10.1097/01.mog.0000245543.72537.9e. [DOI] [PubMed] [Google Scholar]
- 22.Yingchoncharoen T, Limpijankit T, Jongjirasiri S, Laothamatas J, Yamwong S, Sritara P. Arterial stiffness contributes to coronary artery disease risk prediction beyond the traditional risk score (RAMA-EGAT score) Heart Asia. 2012;4:77–82. doi: 10.1136/heartasia-2011-010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuchikura S, Shoji T, Kimoto E, et al. Central versus peripheral arterial stiffness in association with coronary, cerebral and peripheral arterial disease. Atherosclerosis. 2010;211:480–485. doi: 10.1016/j.atherosclerosis.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 24.El Gamal YM, Elmasry OA, El Hadidi IS, Soliman OK. Proximal aortic stiffness is increased in systemic lupus erythematosus activity in children and adolescents. ISRN Pediatr. 2013:765253. doi: 10.1155/2013/765253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbulescu AL, Vreju F, Cojocaru-Gofita IR, Musetescu AE, Ciurea PL. Impaired arterial stiffness in systemic lupus ertythematosus-correlations with inflammation markers. Curr Health Sci J. 2012;38:61–65. [PMC free article] [PubMed] [Google Scholar]
- 26.Gefel D, Doncheva M, Ben-Valid E, el Wahab-Daraushe A, Lugassy G, Sela BA. Recurrent stroke in a young patient with celiac disease and hyperhomocysteinemia. Isr Med Assoc J. 2002;4:222–223. [PubMed] [Google Scholar]
- 27.Wang X, Cui L, Joseph J, et al. Homocysteine induces cardiomyocyte dysfunction and apoptosis through p38MAPK-mediated increase in oxidant stress. J Mol Cell Cardiol. 2012;52:753–760. doi: 10.1016/j.yjmcc.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao P, Wang SQ, Wang S, et al. p38 Mitogen-activated protein kinase mediated a negative inotropic effect in cardiac myocytes. Circ Res. 2002;90:190–196. doi: 10.1161/hh0202.104220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nestel PJ, Chronopoulos A, Cehun M. Arterial stiffness is rapidly induces by raising the plasma homocysteine concentration with methionine. Atherosclerosis. 2003;171:83–86. doi: 10.1016/j.atherosclerosis.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Lim PO, Tzemos N, Farquharson CA, et al. Reversible hypertension following coeliac disease treatment: the role moderate hyperhomocysteinaemia and vascular endothelial dysfunction. J Hum Hypertens. 2002;16:411–415. doi: 10.1038/sj.jhh.1001404. [DOI] [PubMed] [Google Scholar]