Abstract
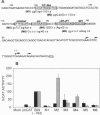
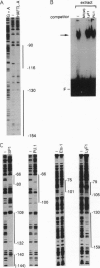
The B-cell-specific B29 and mb1 genes code for covalently linked proteins (B29 or Ig beta and mb1 or Ig alpha, respectively) associated with membrane immunoglobulins in the antigen receptor complex on B cells. We have functionally analyzed the upstream region of the B29 gene and have identified a 164-bp region which comprises the minimal promoter responsible for B-cell-specific transcription. Linker scanning mutagenesis of this minimal promoter has established that both the previously identified octamer motif and a DNA motif that binds an unknown protein factor are critical for B29 gene expression in a pre-B-cell and B-cell line. Further mutations showed that binding motifs for Ets, microB/LyF1, and Sp1 also significantly contributed to the overall activity of the minimal B29 promoter. However, the relative contribution of certain motifs to promoter activity was different in a pre-B versus a B-cell line. The microB/LyF1 motif was necessary for full promoter activity in the pre-B cells but was not required in the B cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agura E. D., Howard M., Collins S. J. Identification and sequence analysis of the promoter for the leukocyte integrin beta-subunit (CD18): a retinoic acid-inducible gene. Blood. 1992 Feb 1;79(3):602–609. [PubMed] [Google Scholar]
- Bhat N. K., Fisher R. J., Fujiwara S., Ascione R., Papas T. S. Temporal and tissue-specific expression of mouse ets genes. Proc Natl Acad Sci U S A. 1987 May;84(10):3161–3165. doi: 10.1073/pnas.84.10.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier J. C. Signal transduction by T- and B-cell antigen receptors: converging structures and concepts. Curr Opin Immunol. 1992 Jun;4(3):257–264. doi: 10.1016/0952-7915(92)90074-o. [DOI] [PubMed] [Google Scholar]
- Campbell K. S., Cambier J. C. B lymphocyte antigen receptors (mIg) are non-covalently associated with a disulfide linked, inducibly phosphorylated glycoprotein complex. EMBO J. 1990 Feb;9(2):441–448. doi: 10.1002/j.1460-2075.1990.tb08129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. S., Hager E. J., Friedrich R. J., Cambier J. C. IgM antigen receptor complex contains phosphoprotein products of B29 and mb-1 genes. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3982–3986. doi: 10.1073/pnas.88.9.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. R., Friedrich R. J., Campbell K. S., Cambier J. C. Human pre-B and B cell membrane mu-chains are noncovalently associated with a disulfide-linked complex containing a product of the B29 gene. J Immunol. 1992 Nov 1;149(9):2857–2863. [PubMed] [Google Scholar]
- Corcoran L. M., Karvelas M., Nossal G. J., Ye Z. S., Jacks T., Baltimore D. Oct-2, although not required for early B-cell development, is critical for later B-cell maturation and for postnatal survival. Genes Dev. 1993 Apr;7(4):570–582. doi: 10.1101/gad.7.4.570. [DOI] [PubMed] [Google Scholar]
- Dalton S., Treisman R. Characterization of SAP-1, a protein recruited by serum response factor to the c-fos serum response element. Cell. 1992 Feb 7;68(3):597–612. doi: 10.1016/0092-8674(92)90194-h. [DOI] [PubMed] [Google Scholar]
- Ernst P., Hahm K., Smale S. T. Both LyF-1 and an Ets protein interact with a critical promoter element in the murine terminal transferase gene. Mol Cell Biol. 1993 May;13(5):2982–2992. doi: 10.1128/mcb.13.5.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldhaus A. L., Mbangkollo D., Arvin K. L., Klug C. A., Singh H. BLyF, a novel cell-type- and stage-specific regulator of the B-lymphocyte gene mb-1. Mol Cell Biol. 1992 Mar;12(3):1126–1133. doi: 10.1128/mcb.12.3.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Baltimore D. Cell-type specificity of immunoglobulin gene expression is regulated by at least three DNA sequence elements. Cell. 1985 Jul;41(3):885–897. doi: 10.1016/s0092-8674(85)80069-6. [DOI] [PubMed] [Google Scholar]
- Gégonne A., Bosselut R., Bailly R. A., Ghysdael J. Synergistic activation of the HTLV1 LTR Ets-responsive region by transcription factors Ets1 and Sp1. EMBO J. 1993 Mar;12(3):1169–1178. doi: 10.1002/j.1460-2075.1993.tb05758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagman J., Grosschedl R. An inhibitory carboxyl-terminal domain in Ets-1 and Ets-2 mediates differential binding of ETS family factors to promoter sequences of the mb-1 gene. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8889–8893. doi: 10.1073/pnas.89.19.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagman J., Travis A., Grosschedl R. A novel lineage-specific nuclear factor regulates mb-1 gene transcription at the early stages of B cell differentiation. EMBO J. 1991 Nov;10(11):3409–3417. doi: 10.1002/j.1460-2075.1991.tb04905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S., Gregersen P. K., Chiorazzi N. The human Ig-beta cDNA sequence, a homologue of murine B29, is identical in B cell and plasma cell lines producing all the human Ig isotypes. J Immunol. 1993 Jan 15;150(2):491–498. [PubMed] [Google Scholar]
- Hermanson G. G., Briskin M., Sigman D., Wall R. Immunoglobulin enhancer and promoter motifs 5' of the B29 B-cell-specific gene. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7341–7345. doi: 10.1073/pnas.86.19.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanson G. G., Eisenberg D., Kincade P. W., Wall R. B29: a member of the immunoglobulin gene superfamily exclusively expressed on beta-lineage cells. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6890–6894. doi: 10.1073/pnas.85.18.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipskind R. A., Rao V. N., Mueller C. G., Reddy E. S., Nordheim A. Ets-related protein Elk-1 is homologous to the c-fos regulatory factor p62TCF. Nature. 1991 Dec 19;354(6354):531–534. doi: 10.1038/354531a0. [DOI] [PubMed] [Google Scholar]
- Hombach J., Lottspeich F., Reth M. Identification of the genes encoding the IgM-alpha and Ig-beta components of the IgM antigen receptor complex by amino-terminal sequencing. Eur J Immunol. 1990 Dec;20(12):2795–2799. doi: 10.1002/eji.1830201239. [DOI] [PubMed] [Google Scholar]
- Hombach J., Tsubata T., Leclercq L., Stappert H., Reth M. Molecular components of the B-cell antigen receptor complex of the IgM class. Nature. 1990 Feb 22;343(6260):760–762. doi: 10.1038/343760a0. [DOI] [PubMed] [Google Scholar]
- Hombach J., Tsubata T., Leclercq L., Stappert H., Reth M. Molecular components of the B-cell antigen receptor complex of the IgM class. Nature. 1990 Feb 22;343(6260):760–762. doi: 10.1038/343760a0. [DOI] [PubMed] [Google Scholar]
- Ishihara K., Wood W. J., Jr, Damore M., Hermanson G. G., Wall R., Kincade P. W. B29 gene products complex with immunoglobulins on B lymphocytes. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):633–637. doi: 10.1073/pnas.89.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. G., Carayannopoulos L., Capra J. D., Tucker P. W., Hanke J. H. The ubiquitous octamer-binding protein(s) is sufficient for transcription of immunoglobulin genes. Mol Cell Biol. 1990 Mar;10(3):982–990. doi: 10.1128/mcb.10.3.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwamura S., Koyama T., Matsuo T., Steinmetz M., Kimoto M., Sakaguchi N. Structure of the murine mb-1 gene encoding a putative sIgM-associated molecule. J Immunol. 1990 Jul 1;145(1):337–343. [PubMed] [Google Scholar]
- Kemler I., Bucher E., Seipel K., Müller-Immerglück M. M., Schaffner W. Promoters with the octamer DNA motif (ATGCAAAT) can be ubiquitous or cell type-specific depending on binding affinity of the octamer site and Oct-factor concentration. Nucleic Acids Res. 1991 Jan 25;19(2):237–242. doi: 10.1093/nar/19.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley C., Winoto A. Cloning of GT box-binding proteins: a novel Sp1 multigene family regulating T-cell receptor gene expression. Mol Cell Biol. 1992 Oct;12(10):4251–4261. doi: 10.1128/mcb.12.10.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A. The macrophage and B cell-specific transcription factor PU.1 is related to the ets oncogene. Cell. 1990 Apr 6;61(1):113–124. doi: 10.1016/0092-8674(90)90219-5. [DOI] [PubMed] [Google Scholar]
- Kudo A., Melchers F. A second gene, VpreB in the lambda 5 locus of the mouse, which appears to be selectively expressed in pre-B lymphocytes. EMBO J. 1987 Aug;6(8):2267–2272. doi: 10.1002/j.1460-2075.1987.tb02500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo A., Sakaguchi N., Melchers F. Organization of the murine Ig-related lambda 5 gene transcribed selectively in pre-B lymphocytes. EMBO J. 1987 Jan;6(1):103–107. doi: 10.1002/j.1460-2075.1987.tb04725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau N. R., St John T. P., Weissman I. L., Wolf S. C., Silverstone A. E., Baltimore D. Cloning of terminal transferase cDNA by antibody screening. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5836–5840. doi: 10.1073/pnas.81.18.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBowitz J. H., Kobayashi T., Staudt L., Baltimore D., Sharp P. A. Octamer-binding proteins from B or HeLa cells stimulate transcription of the immunoglobulin heavy-chain promoter in vitro. Genes Dev. 1988 Oct;2(10):1227–1237. doi: 10.1101/gad.2.10.1227. [DOI] [PubMed] [Google Scholar]
- Libermann T. A., Lenardo M., Baltimore D. Involvement of a second lymphoid-specific enhancer element in the regulation of immunoglobulin heavy-chain gene expression. Mol Cell Biol. 1990 Jun;10(6):3155–3162. doi: 10.1128/mcb.10.6.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo K., Landau N. R., Smale S. T. LyF-1, a transcriptional regulator that interacts with a novel class of promoters for lymphocyte-specific genes. Mol Cell Biol. 1991 Oct;11(10):5229–5243. doi: 10.1128/mcb.11.10.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Fujii H., Gerster T., Roeder R. G. A novel B cell-derived coactivator potentiates the activation of immunoglobulin promoters by octamer-binding transcription factors. Cell. 1992 Oct 16;71(2):231–241. doi: 10.1016/0092-8674(92)90352-d. [DOI] [PubMed] [Google Scholar]
- Nelsen B., Kadesch T., Sen R. Complex regulation of the immunoglobulin mu heavy-chain gene enhancer: microB, a new determinant of enhancer function. Mol Cell Biol. 1990 Jun;10(6):3145–3154. doi: 10.1128/mcb.10.6.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelsen B., Tian G., Erman B., Gregoire J., Maki R., Graves B., Sen R. Regulation of lymphoid-specific immunoglobulin mu heavy chain gene enhancer by ETS-domain proteins. Science. 1993 Jul 2;261(5117):82–86. doi: 10.1126/science.8316859. [DOI] [PubMed] [Google Scholar]
- Pongubala J. M., Nagulapalli S., Klemsz M. J., McKercher S. R., Maki R. A., Atchison M. L. PU.1 recruits a second nuclear factor to a site important for immunoglobulin kappa 3' enhancer activity. Mol Cell Biol. 1992 Jan;12(1):368–378. doi: 10.1128/mcb.12.1.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieckmann P., Wilson G. L., Thevenin C., Hong J. X., Kehrl J. H. Analysis of cis-acting elements present in the CD20/B1 antigen promoter. J Immunol. 1991 Dec 1;147(11):3994–3999. [PubMed] [Google Scholar]
- Roman C., Matera A. G., Cooper C., Artandi S., Blain S., Ward D. C., Calame K. mTFE3, an X-linked transcriptional activator containing basic helix-loop-helix and zipper domains, utilizes the zipper to stabilize both DNA binding and multimerization. Mol Cell Biol. 1992 Feb;12(2):817–827. doi: 10.1128/mcb.12.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley C. S., Farokhzad O. C., Arnaout M. A. Identification of cell-specific and developmentally regulated nuclear factors that direct myeloid and lymphoid expression of the CD11a gene. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5364–5368. doi: 10.1073/pnas.90.11.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale S. T., Baltimore D. The "initiator" as a transcription control element. Cell. 1989 Apr 7;57(1):103–113. doi: 10.1016/0092-8674(89)90176-1. [DOI] [PubMed] [Google Scholar]
- Thompson C. B., Wang C. Y., Ho I. C., Bohjanen P. R., Petryniak B., June C. H., Miesfeldt S., Zhang L., Nabel G. J., Karpinski B. cis-acting sequences required for inducible interleukin-2 enhancer function bind a novel Ets-related protein, Elf-1. Mol Cell Biol. 1992 Mar;12(3):1043–1053. doi: 10.1128/mcb.12.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis A., Hagman J., Grosschedl R. Heterogeneously initiated transcription from the pre-B- and B-cell-specific mb-1 promoter: analysis of the requirement for upstream factor-binding sites and initiation site sequences. Mol Cell Biol. 1991 Nov;11(11):5756–5766. doi: 10.1128/mcb.11.11.5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Noesel C. J., Brouns G. S., van Schijndel G. M., Bende R. J., Mason D. Y., Borst J., van Lier R. A. Comparison of human B cell antigen receptor complexes: membrane-expressed forms of immunoglobulin (Ig)M, IgD, and IgG are associated with structurally related heterodimers. J Exp Med. 1992 Jun 1;175(6):1511–1519. doi: 10.1084/jem.175.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkitaraman A. R., Williams G. T., Dariavach P., Neuberger M. S. The B-cell antigen receptor of the five immunoglobulin classes. Nature. 1991 Aug 29;352(6338):777–781. doi: 10.1038/352777a0. [DOI] [PubMed] [Google Scholar]
- Wilson G. L., Najfeld V., Kozlow E., Menniger J., Ward D., Kehrl J. H. Genomic structure and chromosomal mapping of the human CD22 gene. J Immunol. 1993 Jun 1;150(11):5013–5024. [PubMed] [Google Scholar]
- Zhou L. J., Ord D. C., Omori S. A., Tedder T. F. Structure of the genes encoding the CD19 antigen of human and mouse B lymphocytes. Immunogenetics. 1992;35(2):102–111. doi: 10.1007/BF00189519. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]