Abstract
Abstract
Four hitherto unknown prenylated isoflavonoids, named derrisisoflavones H–K (1–4) and one new isoflavan, namely 6-hydroxyisosativan (5), were isolated from the ethanol extract of Derrisrobusta. Their structures were elucidated on the basis of extensive spectroscopic studies. To our knowledge, derrisisoflavones J (3) and K (4) are the first examples of hydroxyethylated isoflavonoid.
Graphical Abstract

Electronic supplementary material
The online version of this article (doi:10.1007/s13659-016-0090-x) contains supplementary material, which is available to authorized users.
Keywords: Derris robusta, Isoflavonoid, Isoflavan, Derrisisoflavone
Introduction
Derris is a genus belonging to the Leguminosae family with about 800 species that are widely distributed in tropical, subtropical areas of Asia and Africa [1]. Published studies have shown that the genus is a rich source of pterocarpans, flavonoids, particularly prenylated isoflavonoids and flavonoids [2–5] and these phytochemicals are associated with a broad spectrum of biological activities, including insecticidal, antimicrobial, cytotoxic, and antioxidant activities [3–7]. As part of a BioBioPha [http://www.chemlib.cn/] objective to assemble a large-scale natural product library valuable in the discovery of new drug leads from nature [8–10], the phytochemical investigation on the twigs and leaves of Derrisrobusta led to the isolation of four new prenylated isoflavonoids, named derrisisoflavones H–K (1–4), and a new isoflavan, namely 6-hydroxyisosativan (5). This paper describes the isolation and structural elucidation of five new compounds (Fig. 1).
Fig. 1.
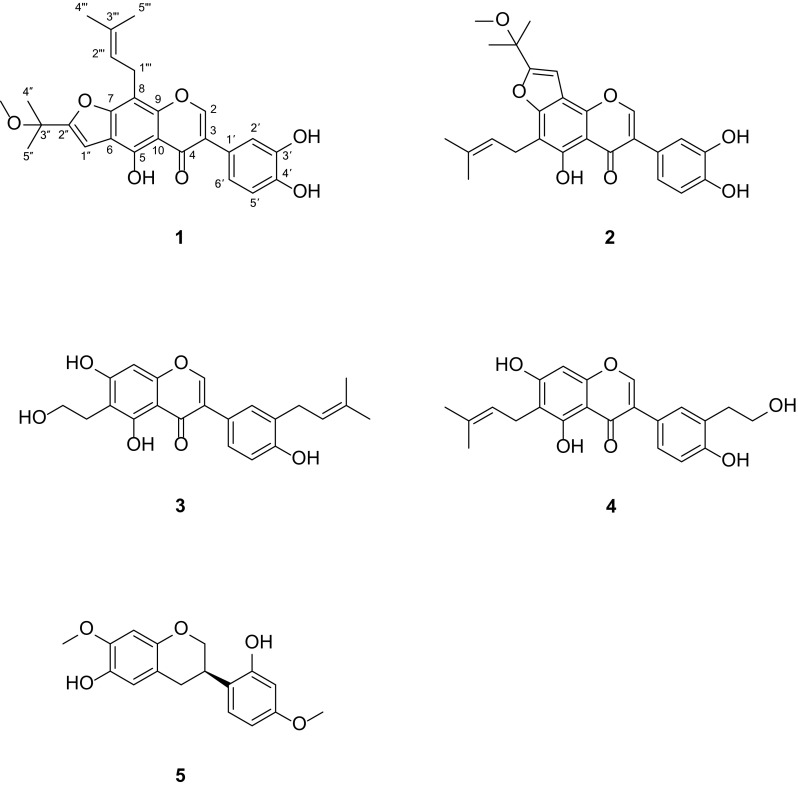
Structures of compounds 1–5
Results and Discussion
Compound 1 was obtained as a yellow amorphous powder and had a molecular formula C26H26O7 determined by its HRESIMS, showing a negative molecular ion peak at m/z 449.1608 [M − H]− (calcd. for C26H25O7, 449.1606). The UV spectrum of 1 with a set of absorption maxima at 268, 297 (sh), 359 nm suggested that it had an isoflavone skeleton as the chromophore [11]. This inference was further supported by characteristic proton singlet at δH 8.17 (H-2) and sp2 methine carbon at δC 155.6 (C-2). The 1H NMR spectrum (Table 1) showed a set of signals at δH 7.05 (br. s), 6.87 (br. d, J = 8.0 Hz) and 6.82 (d, J = 8.0 Hz) due to a 1,3,4-trisubstituted benzene ring, two aromatic or olefinic protons at δH 6.86 (s) and 5.30 (t, J = 6.8 Hz), one methylene signal at δH 3.64 (d, J = 6.8 Hz), and five methyl singlets at δH 3.11, 1.85, 1.67, 1.60 and 1.60. The 13C NMR (DEPT) spectrum (Table 2) displayed a total of 26 carbon resonances, including five methyls, one methylene, six methines and 14 quaternary carbons. The above NMR spectroscopic features were very similar to those of 5,4′-dihydroxy-8-(3,3-dimethylallyl)-2″-methoxyisopropylfurano[4,5:6,7]isoflavone [12], and the most dramatic difference was the presence of an additional hydroxy group in 1. The hydroxy group was located at C-3′, on the basis of the HMBC correlations from the protons at δH 7.05 (br. s, H-2′) and 6.87 (br. d, J = 8.0 Hz, H-6′) to the carbon at δC 123.7 (s, C-3) (Fig. 2). The HMBC correlations from the protons at δH 3.64 (2H, d, J = 6.8 Hz, H-1′′′) to the carbons at δC 158.6 (s, C-7), 105.2 (s, C-8), and 152.4 (s, C-9) verified the location of the prenyl group [δH 1.67, 1.85 (each s), 3.64 (d, J = 6.8 Hz), and 5.30 (t, J = 6.8 Hz)] at C-8. Furthermore, the correlations from the proton at δH 6.86 (s, H-1″) to the carbons at δC 154.2 (s, C-5), 114.1 (s, C-6), and 158.6 (s, C-7) confirmed that the furan ring, derived from a prenyl group, was fused along the C-6 to C-7 bond. Consequently, the structure of 1 was determined and named as derrisisoflavone H.
Table 1.
1H NMR spectroscopic data for derrisisoflavones H–K (1–4) and 6-hydroxyisosativan (5)
| No. | 1 a | 2 b | 3 a | 4 a | 5 a |
|---|---|---|---|---|---|
| 2 | 8.17 (s) | 8.51 (s) | 7.97 (s) | 8.00 (s) | 4.17 (ddd, 10.2, 3.3, 1.9, Heq) |
| 3.90 (t, 10.2, Hax) | |||||
| 3 | 3.43 (dddd, 10.7, 10.2, 5.3, 3.3, Hax) | ||||
| 4 | 2.91 (dd, 15.9, 10.7, Hax) | ||||
| 2.73 (ddd, 15.9, 5.3, 1.9, Heq) | |||||
| 5 | 6.51 (s) | ||||
| 8 | 6.37 (s) | 6.36 (s) | 6.37 (s) | ||
| 2′ | 7.05 (br. s) | 7.05 (d, 1.6) | 7.19 (d, 2.0) | 7.25 (d, 2.3) | |
| 3′ | 6.38 (d, 2.5) | ||||
| 5′ | 6.82 (d, 8.0) | 6.81 (d, 8.0) | 6.79 (d, 8.2) | 6.82 (d, 8.2) | 6.36 (dd, 8.4, 2.5) |
| 6′ | 6.87 (br. d, 8.0) | 6.85 (dd, 8.0, 1.6) | 7.15 (dd, 8.2, 2.0) | 7.21 (dd, 8.2, 2.3) | 6.95 (d, 8.4) |
| 1″ | 6.86 (s) | 3.48 (d, 7.3) | 2.92 (t, 7.3) | 3.30 (overlapped) | |
| 2″ | 5.29 (t, 7.3) | 3.68 (t, 7.3) | 5.22 (t, 7.2) | ||
| 4″ | 1.60 (s) | 1.62 (s) | 1.65 (s) | ||
| 5″ | 1.60 (s) | 1.79 (s) | 1.77 (s) | ||
| 1′′′ | 3.64 (d, 6.8) | 7.07 (s) | 3.32 (d, 7.3) | 2.87 (t, 7.0) | |
| 2′′′ | 5.30 (t, 6.8) | 5.33 (t, 7.3) | 3.78 (t, 7.0) | ||
| 4′′′ | 1.67 (s) | 1.56 (s) | 1.72 (s) | ||
| 5′′′ | 1.85 (s) | 1.56 (s) | 1.72 (s) | ||
| 5-OH | 13.21 (s) | ||||
| 3′-OH | 9.14 (br. s) | ||||
| 4′-OH | 9.14 (br. s) | ||||
| 7-OCH3 | 3.78 (s) | ||||
| 4′-OCH3 | 3.71 (s) | ||||
| 3″-OCH3 | 3.11 (s) | ||||
| 3′′′-OCH3 | 3.00 (s) |
aMeasured in CD3OD (δ H 3.30 ppm)
bMeasured in DMSO-d 6 (δ H 2.50 ppm)
Table 2.
13C NMR spectroscopic data for derrisisoflavones H–K (1–4) and 6-hydroxyisosativan (5)
| No. | 1 a | 2 b | 3 a | 4 a | 5 a |
|---|---|---|---|---|---|
| 2 | 155.6 (d) | 154.1 (d) | 154.5 (d) | 154.6 (d) | 71.0 (t) |
| 3 | 123.7 (s) | 123.5 (s) | 125.0 (s) | 124.7 (s) | 33.1 (d) |
| 4 | 184.2 (s) | 181.4 (s) | 182.4 (s) | 182.3 (s) | 31.4 (t) |
| 5 | 154.2 (s) | 154.6 (s) | 161.3 (s) | 160.5 (s) | 116.4 (d) |
| 6 | 114.1 (s) | 107.6 (s) | 109.9 (s) | 113.1 (s) | 141.0 (s) |
| 7 | 158.6 (s) | 157.0 (s) | 164.2 (s) | 163.7 (s) | 148.1 (s) |
| 8 | 105.2 (s) | 107.9 (s) | 94.2 (d) | 93.9 (d) | 101.4 (d) |
| 9 | 152.4 (s) | 147.7 (s) | 157.9 (s) | 157.6 (s) | 148.8 (s) |
| 10 | 107.6 (s) | 107.4 (s) | 106.2 (s) | 106.1 (s) | 115.0 (s) |
| 1′ | 123.7 (s) | 121.5 (s) | 123.4 (s) | 123.4 (s) | 121.4 (s) |
| 2′ | 117.5 (d) | 116.8 (d) | 131.4 (d) | 132.8 (d) | 157.2 (s) |
| 3′ | 146.2 (s) | 145.0 (s) | 129.5 (s) | 126.7 (s) | 102.3 (d) |
| 4′ | 146.8 (s) | 145.8 (s) | 156.5 (s) | 157.0 (s) | 160.8 (s) |
| 5′ | 116.3 (d) | 115.5 (d) | 115.8 (d) | 116.1 (d) | 105.6 (d) |
| 6′ | 121.8 (d) | 120.3 (d) | 128.7 (d) | 129.5 (d) | 128.8 (d) |
| 1″ | 102.7 (d) | 21.6 (t) | 26.8 (t) | 22.3 (t) | |
| 2″ | 161.3 (s) | 120.9 (d) | 61.9 (t) | 123.4 (d) | |
| 3″ | 74.7 (s) | 132.1 (s) | 132.1 (s) | ||
| 4″ | 25.5 (q) | 25.5 (q) | 26.0 (q) | ||
| 5″ | 25.5 (q) | 17.6 (q) | 17.9 (q) | ||
| 1′′′ | 22.9 (t) | 101.2 (d) | 29.3 (t) | 35.1 (t) | |
| 2′′′ | 122.2 (d) | 159.3 (s) | 123.9 (d) | 63.0 (t) | |
| 3′′′ | 133.8 (s) | 72.8 (s) | 133.1 (s) | ||
| 4′′′ | 25.9 (q) | 24.9 (q) | 25.9 (q) | ||
| 5′′′ | 18.0 (q) | 24.9 (q) | 17.9 (q) | ||
| 7-OCH3 | 56.3 (q) | ||||
| 4′-OCH3 | 55.5 (q) | ||||
| 3″-OCH3 | 51.4 (q) | ||||
| 3′′′-OCH3 | 50.4 (q) |
aMeasured in CD3OD (δ C 49.0 ppm)
bMeasured in DMSO-d 6 (δ C 39.5 ppm)
Fig. 2.
Selected HMBC ( ) correlations of derrisisoflavone H (1)
) correlations of derrisisoflavone H (1)
Compound 2, was purified as a yellow amorphous powder and had the same molecular formula as 1 based on its HRESIMS (neg.): m/z 449.1607 [M − H]− (calcd. for C26H25O7, 449.1606). The NMR spectroscopic data of 2 (Tables 1, 2) were very similar to those of 1. The structural discrepancy was only from the switch positions of prenyl group and furan ring on ring A. The positions were verified by the HMBC correlations from the protons at δH 3.48 (2H, d, J = 7.3 Hz, H-1″) and 13.21 (s, 5-OH) to the carbon at δC 154.6 (s, C-5), and from the protons at δH 7.07 (s, H-1′′′) and 8.51 (s, H-2) to the carbon at δC 147.7 (s, C-9), respectively. Accordingly, the structure of 2 was elucidated as shown and given the name derrisisoflavone I.
Compound 3, was isolated as a white amorphous powder, with a molecular formula of C22H22O6 according to its HRESIMS (pos.): m/z 405.1305 [M + Na]+ (calcd. for C22H22O6Na, 405.1309). The general features of NMR spectra (Tables 1, 2) of 3 were similar to those of lupalbigenin, a diprenylated isoflavone [13], except for the signals of a hydroxyethyl moiety [δH 2.92 (t, J = 7.3 Hz), 3.68 (t, J = 7.3 Hz); δC 26.8 (t), 61.9 (t)] instead of one of prenyl group in the latter. The hydroxyethyl group was linked to C-6 on the basis of the following HMBC correlations: from the protons at δH 2.92 (t, J = 7.3 Hz, H-1″) to the carbons at δC 161.3 (s, C-5), 109.9 (s, C-6) and 164.2 (s, C-7), and from the protons at δH 7.97 (s, H-2) and 6.37 (s, H-8) to the carbon at δC 157.9 (s, C-9). Similarly, the connection of the prenyl group to C-3′ was established by the correlation from the proton at δH 7.19 (d, J = 2.0 Hz, H-2′) to the carbon at 29.3 (t, C-1′′′). Therefore, the structure of 3 was characterized and named as derrisisoflavone J.
Compound 4 was afforded as a white amorphous powder and possessed the same molecular formula as 3 according to its HRESIMS (pos.): m/z 405.1307 [M + Na]+ (calcd. for C22H22O6Na, 405.1309). The NMR data (Tables 1, 2) were very similar to those of 3, which allowed us to infer that their structural discrepancy may result from the different substitution patterns of the hydroxyethyl and prenyl groups. This deduction was confirmed by the HMBC correlations from the protons at 3.30 (overlapped, H-1″) to δC 160.5 (s, C-5), 113.1 (s, C-6) and 163.7 (s, C-7), and from the proton at δH 7.25 (d, J = 2.3 Hz, H-2′) to δC 35.1 (t, C-1′′′). Therefore, the structure of 4 was established as shown and given the name derrisisoflavone K.
Compound 5, a white amorphous powder, had a molecular formula of C17H18O5 by its HRESIMS (pos.): m/z 325.1031 [M + Na]+ (calcd. for C17H18O5Na, 325.1046). Its 1H NMR spectrum (Table 1) displayed an ABX-type aromatic proton system [δH 6.38 (d, J = 2.5 Hz), 6.36 (dd, J = 8.4, 2.5 Hz), and 6.95 (d, J = 8.4 Hz)], two aromatic singlets at δH 6.51 and 6.37, two methoxy signals at δH 3.78 and 3.71, and a set of signals [δH 4.17 (ddd, J = 10.2, 3.3, 1.9 Hz, Heq-2), 3.90 (t, J = 10.2 Hz, Hax-2), 3.43 (dddd, J = 10.7, 10.2, 5.3, 3.3 Hz, Hax-3), 2.91 (dd, J = 15.9, 10.7 Hz, Hax-4), and 2.73 (ddd, J = 15.9, 5.3, 1.9 Hz, Heq-4)] due to ring C protons of an isoflavan. The above NMR signals were similar to those of isosativan (also called 7-O-methylvestitol) [14], and a prominent difference was two aromatic singlets at δH 6.51 and 6.37 instead of one of ABX-type system of isosativan. By careful analysis of the MS and NMR data, the isoflavan was inferred as a hydroxylated derivative of isosativan. The additional hydroxy group was located at C-6 by the HMBC correlations from the proton singlet at δH 6.51 (H-5) to the carbon at δC 31.4 (t, C-4), and from the methoxy signal at δH 3.78 (7-OMe) to the carbons at δC 148.1 (s, C-7) and 101.4 (d, C-8). Thereupon, the structure of 5 was established and named 6-hydroxyisosativan. The absolute configuration at C-3 was postulated as being R-form in the light of a negative specific rotation value (−11.7, MeOH), consistent with those of (3R)-vestitol derivatives [15].
Experimental Section
General Experimental Procedures
Optical rotation was measured on a Jasco P-1020 automatic digital polarimeter. UV data were obtained from HPLC online analysis. NMR spectra were carried out on a Bruker AV-400, Bruker DRX-500 or Bruker AV-600 instrument with deuterated solvent signals used as internal standards. ESI and HRESIMS were performed with a Shimadzu LC-IT-TOF mass spectrometer equipped with an ESI interface (Shimadzu, Kyoto, Japan). Silica gel 200–300 mesh (Qingdao Marine Chemical Inc., Qingdao, China), Chromatorex C-18 (40–75 μm, Fuji Silysia Chemical Ltd., Japan) and Sephadex LH-20 (Amersham Biosciences, Uppsala, Sweden) were used for normal pressure column chromatography (CC). Fractions were monitored and analyzed by TLC, in combination with Agilent 1200 series HPLC system equipped by Extend-C18 column (5 μm, 4.6 × 150 mm).
Plant Material
The twigs and leaves of D. robusta were collected from the Pu’er region of Yunnan Province, People’s Republic of China, in May 2011, and identified by Mr. Yu Chen of Kunming Institute of Botany, Chinese Academy of Sciences. A voucher specimen (BBP0350022DR) was deposited at BioBioPha Co., Ltd.
Extraction and Isolation
The air-dried and powdered twigs and leaves (12.0 kg) of D. robusta were extracted with EtOH-H2O (95:5, v/v; 3 × 20 L, each 4 days) at room temperature, and the combined filtrates were concentrated under reduced pressure to give crude extract (ca. 870 g), which was further fractionated by silica gel CC successively eluted with a gradient of increasing acetone in petroleum ether (PE) (10:1 → 0:1, v/v) and then MeOH to obtain nine fractions A–I. Fraction D (PE/acetone, 5:1, v/v) was subjected to silica gel CC (CHCl3/MeOH, 100:0 → 100:1, v/v) and Sephadex LH-20 (CHCl3/MeOH, 1:1, v/v) to give 5 (14 mg). Fraction E (PE/acetone, 4:1, v/v) was isolated on silica gel CC (CHCl3/MeOH, 100:1 → 5:1, v/v), RP-18 (30 % MeOH/H2O, v/v), and Sephadex LH-20 (MeOH) to yield 1 (7 mg), 2 (11 mg), and 4 (14 mg). Fraction H (PE/acetone, 1:1) was purified by silica gel CC (CHCl3/MeOH, 10:1 → 2:1, v/v) and repeated Sephadex LH-20 (MeOH) to afford 3 (10 mg). The retention times (tR) of 1–5 on an analytical HPLC Extend-C18 column (20 % → 100 % MeOH in H2O over 8.0 min followed by 100 % MeOH to 13.0 min, 1.0 ml/min, 25 °C) were 10.32, 10.12, 9.13, 9.16, and 7.76 min, respectively.
Derrisisoflavone H (1)
Yellow amorphous powder; UV (MeOH) λmax (log ε): 268 (4.75), 297 (sh) (4.23), 359 (3.60) nm; 1H NMR data: see Table 1; 13C NMR data: see Table 2; ESIMS (neg.): m/z 449 [M − H]−; HRESIMS (neg.): 449.1608 [M − H]− (calcd. for C26H25O7, 449.1606).
Derrisisoflavone I (2)
Yellow amorphous powder; UV (MeOH) λmax (log ε): 263 (4.75), 302 (sh) (4.35), 357 (sh) (3.68) nm; 1H NMR data: see Table 1; 13C NMR data: see Table 2; ESIMS (neg.): m/z 449 [M − H]−; HRESIMS (neg.): m/z 449.1607 [M − H]− (calcd. for C26H25O7, 449.1606).
Derrisisoflavone J (3)
White amorphous powder; UV (MeOH) λmax (log ε): 213 (4.59), 267 (4.56), 338 (sh) (3.59) nm; 1H NMR data: see Table 1; 13C NMR data: see Table 2; ESIMS (pos.): m/z 405 [M + Na]+; HRESIMS (pos.): m/z 405.1305 [M + Na]+ (calcd. for C22H22O6Na, 405.1309).
Derrisisoflavone K (4)
White amorphous powder; UV (MeOH) λmax (log ε): 214 (4.59), 268 (4.57), 336 (sh) (3.67) nm; 1H NMR data: see Table 1; 13C NMR data: see Table 2; ESIMS (pos.): m/z 405 [M + Na]+; HRESIMS (pos.): m/z 405.1307 [M + Na]+ (calcd. for C22H22O6Na, 405.1309).
6-Hydroxyisosativan (5)
White amorphous powder; UV (MeOH) λmax (log ε): 226 (sh) (4.22), 287 (3.89) nm; −11.7 (c 0.2, MeOH); 1H NMR data: see Table 1; 13C NMR data: see Table 2; ESIMS (pos.): m/z 325 [M + Na]+; HRESIMS (pos.): m/z 325.1031 [M + Na]+ (calcd. for C17H18O5Na, 325.1046).
Electronic supplementary material
Acknowledgments
This work was financially supported by the “Large-scale Compound Library” project of National Development and Reform Commission of China.
Compliance with Ethical Standards
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Editorial Committee of Flora Reipublicae Popularis Sinicae Flora Reipublicae Popularis Sinicae. Academic Press: Beijing. 1994;40:194–195. [Google Scholar]
- 2.Li XM, Mao MF, Ren FC, Jiang XJ, Hai P, Wang F. Nat. Prod. Bioprospect. 2015;5:287–291. doi: 10.1007/s13659-015-0078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morel S, Helesbeux JJ, Séraphin D, Derbré S, Gatto J, Aumond MC, Abatuci Y, Grellier P, Beniddir MA, Le Pape P, Pagniez F, Litaudon M, Landreau A, Richomme P. Phytochem. Lett. 2013;6:498–503. doi: 10.1016/j.phytol.2013.06.002. [DOI] [Google Scholar]
- 4.Sekine T, Inagaki M, Ikegami F, Fujii Y, Ruangrungsi N. Phytochemistry. 1999;52:87–94. doi: 10.1016/S0031-9422(99)00103-X. [DOI] [Google Scholar]
- 5.Mahabusarakam W, Deachathai S, Phongpaichit S, Jansakul C, Taylor WC. Phytochemistry. 2004;65:1185–1191. doi: 10.1016/j.phytochem.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Decharchoochart P, Suthiwong J, Samatiwat P, Kukongviriyapan V, Yenjai C. J. Nat. Med. 2014;68:730–736. doi: 10.1007/s11418-014-0851-y. [DOI] [PubMed] [Google Scholar]
- 7.Rao SA, Srinivas PV, Tiwari AK, Vanka UMS, Rao RVS, Dasari KR, Rao MJ. J. Chromatogr. B. 2007;855:166–172. doi: 10.1016/j.jchromb.2007.04.048. [DOI] [PubMed] [Google Scholar]
- 8.Wang F, Gao Y, Zhang L, Liu JK. Org. Lett. 2010;12:2354–2357. doi: 10.1021/ol1007247. [DOI] [PubMed] [Google Scholar]
- 9.Wang F, Li YJ, Ren FC, Wei GZ, Liu JK. Chem. Pharm. Bull. 2011;59:484–487. doi: 10.1248/cpb.59.484. [DOI] [PubMed] [Google Scholar]
- 10.Wang F, Li XL, Wei GZ, Ren FC, Liu JK. Nat. Prod. Bioprospect. 2013;3:238–242. doi: 10.1007/s13659-013-0062-3. [DOI] [Google Scholar]
- 11.Wang GR, Tang WZ, Yao QQ, Zhong H, Liu YJ. J. Nat. Med. 2010;64:358–361. doi: 10.1007/s11418-010-0407-8. [DOI] [PubMed] [Google Scholar]
- 12.Li XL, Wang NL, Wong MS, Chen ASC, Yao XS. Chem. Pharm. Bull. 2006;54:570–573. doi: 10.1248/cpb.54.570. [DOI] [PubMed] [Google Scholar]
- 13.Pistelli L, Spera K, Flamini G, Mele S, Morelli I. Phytochemistry. 1996;42:1455–1458. doi: 10.1016/0031-9422(96)00128-8. [DOI] [Google Scholar]
- 14.Piccinelli AL, Fernandez MC, Cuesta-Rubio O, Hernández IM, De Simone F, Rastrelli L. J. Agric. Food Chem. 2005;53:9010–9016. doi: 10.1021/jf0518756. [DOI] [PubMed] [Google Scholar]
- 15.Yahara S, Ogata T, Saijo R, Konishi R, Yamahara J, Miyahara K, Nohara T. Chem. Pharm. Bull. 1989;37:979–987. doi: 10.1248/cpb.37.979. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



