Abstract
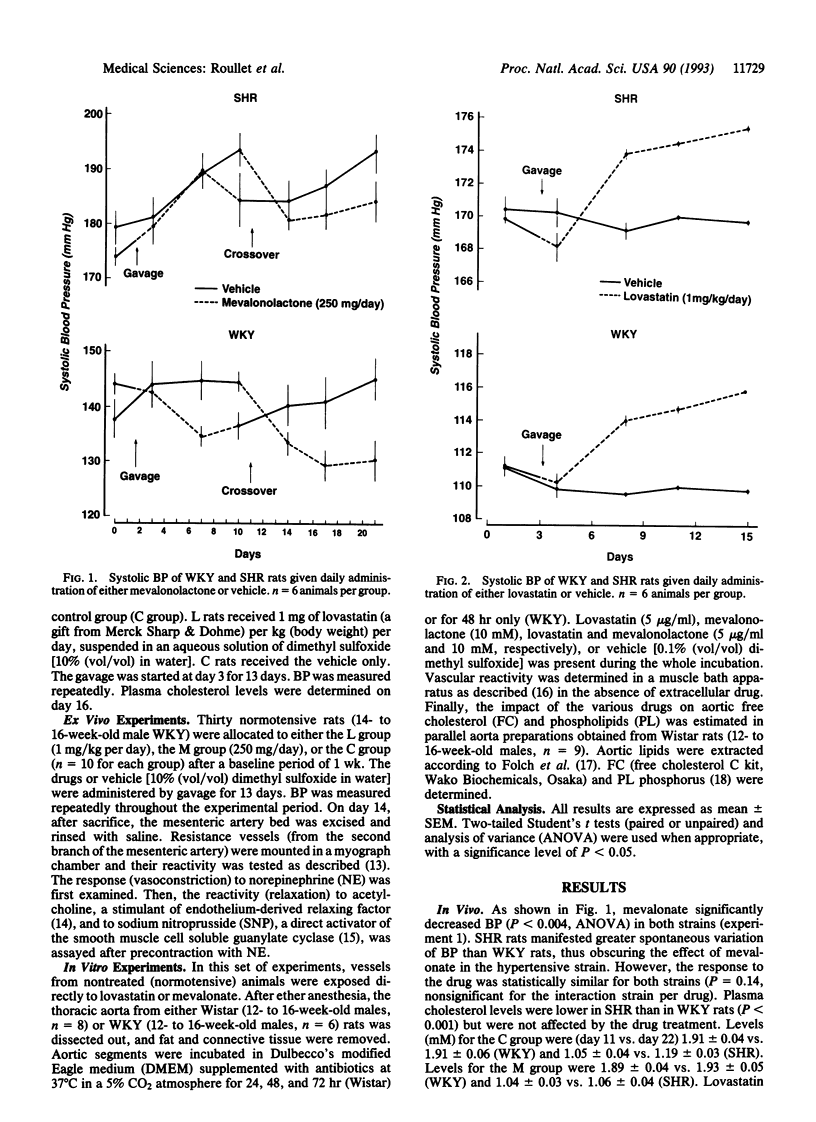
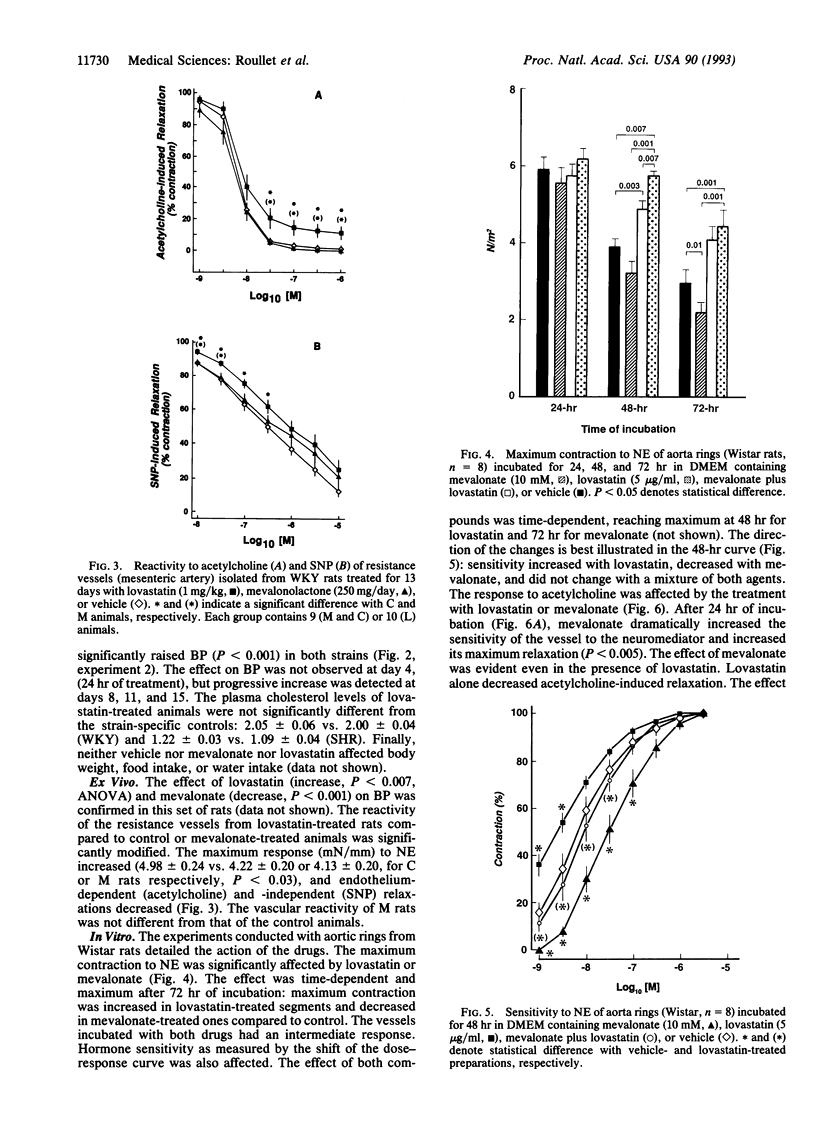
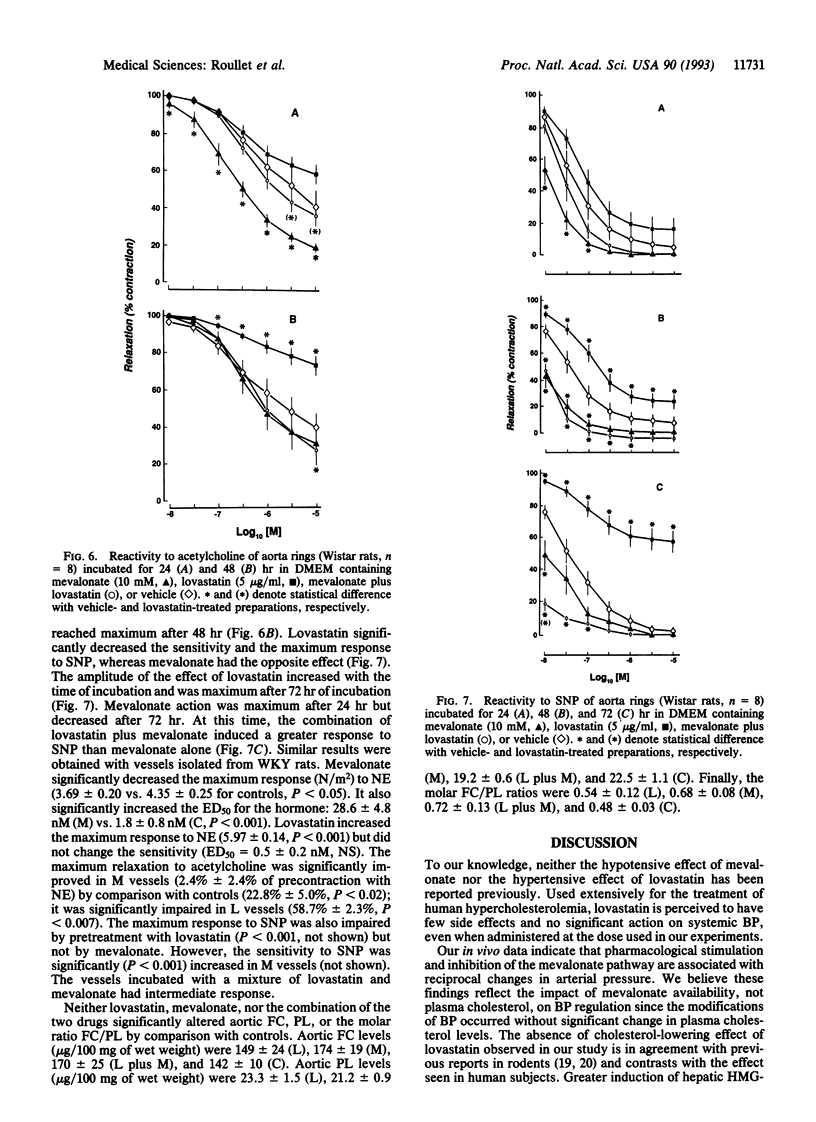
Data delineating the relationship between disorders of cholesterol metabolism and elevated blood pressure (BP) do not exist. We postulated that mevalonate, the metabolic precursor of endogenous cholesterol and the direct product of 3-hydroxy-3-methylglutaryl-CoA reductase, was a contributing factor for the maintenance of vascular tone and systemic BP. We conducted in vivo, ex vivo, and in vitro experiments in normotensive and hypertensive rats, where exogenous mevalonate and lovastatin, a competitive inhibitor of 3-hydroxy-3-methylglutaryl-CoA reductase, were used, respectively, to increase or limit mevalonate availability. Mevalonate decreased BP in the whole animal without significant change in plasma cholesterol. Incubation of aortas with mevalonate attenuated their reactivity to norepinephrine and increased their response to endothelium-dependent and -independent relaxing factors. Lovastatin, in contrast, had the opposite effect in vivo and in vitro: it increased BP, enhanced vascular response to norepinephrine, and impaired endothelium-dependent and -independent relaxations. Neither agent modified cholesterol vascular content. Alteration of vascular reactivity was also observed in resistance vessels from animals pretreated with lovastatin. Our findings suggest that mevalonate availability is an unrecognized metabolic contributor to vascular tone and BP. They imply that (i) metabolites of the mevalonate pathway other than cholesterol could potentially control vascular functions and cardiovascular hemodynamics, (ii) elevated arterial pressure could be in part the consequence of primary disorders of this pathway, and (iii) pharmacological inhibition of mevalonate production as a means to lower plasma cholesterol may have an adverse impact on other cardiovascular risk factors, such as BP.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bialecki R. A., Tulenko T. N. Acute exposure to cholesterol increases arterial nitroprusside- and endothelium-mediated relaxation. Am J Physiol. 1993 Jan;264(1 Pt 1):C32–C39. doi: 10.1152/ajpcell.1993.264.1.C32. [DOI] [PubMed] [Google Scholar]
- Broderick R., Bialecki R., Tulenko T. N. Cholesterol-induced changes in rabbit arterial smooth muscle sensitivity to adrenergic stimulation. Am J Physiol. 1989 Jul;257(1 Pt 2):H170–H178. doi: 10.1152/ajpheart.1989.257.1.H170. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J Lipid Res. 1980 Jul;21(5):505–517. [PubMed] [Google Scholar]
- Bukoski R. D., Xue H., McCarron D. A. Vascular changes during calcium loading in experimental hypertension. Pharmacol Res Commun. 1988 Sep;20 (Suppl 3):55–70. doi: 10.1016/s0031-6989(88)80106-1. [DOI] [PubMed] [Google Scholar]
- Chowienczyk P. J., Watts G. F., Cockcroft J. R., Ritter J. M. Impaired endothelium-dependent vasodilation of forearm resistance vessels in hypercholesterolaemia. Lancet. 1992 Dec 12;340(8833):1430–1432. doi: 10.1016/0140-6736(92)92621-l. [DOI] [PubMed] [Google Scholar]
- Creager M. A., Cooke J. P., Mendelsohn M. E., Gallagher S. J., Coleman S. M., Loscalzo J., Dzau V. J. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest. 1990 Jul;86(1):228–234. doi: 10.1172/JCI114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo A., Tsujita Y., Kuroda M., Tanzawa K. Effects of ML-236B on cholesterol metabolism in mice and rats: lack of hypocholesterolemic activity in normal animals. Biochim Biophys Acta. 1979 Nov 21;575(2):266–276. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Freiman P. C., Mitchell G. G., Heistad D. D., Armstrong M. L., Harrison D. G. Atherosclerosis impairs endothelium-dependent vascular relaxation to acetylcholine and thrombin in primates. Circ Res. 1986 Jun;58(6):783–789. doi: 10.1161/01.res.58.6.783. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gebhard R. L., Prigge W. F., Andreini J. P., Freeman M. L. Effect of probucol on blood cholesterol and basal and lovastatin-induced 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in mice. J Lab Clin Med. 1991 Apr;117(4):299–304. [PubMed] [Google Scholar]
- Gleason M. M., Medow M. S., Tulenko T. N. Excess membrane cholesterol alters calcium movements, cytosolic calcium levels, and membrane fluidity in arterial smooth muscle cells. Circ Res. 1991 Jul;69(1):216–227. doi: 10.1161/01.res.69.1.216. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Regulation of the mevalonate pathway. Nature. 1990 Feb 1;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Jayakody L., Senaratne M., Thomson A., Kappagoda T. Endothelium-dependent relaxation in experimental atherosclerosis in the rabbit. Circ Res. 1987 Feb;60(2):251–264. doi: 10.1161/01.res.60.2.251. [DOI] [PubMed] [Google Scholar]
- Kawahara Y., Kawata M., Sunako M., Araki S., Koide M., Tsuda T., Fukuzaki H., Takai Y. Small GTP-binding proteins in bovine aortic smooth muscle. Jpn Circ J. 1991 Oct;55(10):1036–1043. doi: 10.1253/jcj.55.1036. [DOI] [PubMed] [Google Scholar]
- MacMahon S. W., Macdonald G. J., Blacket R. B. Plasma lipoprotein levels in treated and untreated hypertensive men and women. The National Heart Foundation of Australia Risk Factor Prevalence Study. Arteriosclerosis. 1985 Jul-Aug;5(4):391–396. doi: 10.1161/01.atv.5.4.391. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Kawaguchi H., Togashi H., Minami M., Saito H., Yasuda H. Effect of coenzyme Q10 on structural alterations in the renal membrane of stroke-prone spontaneously hypertensive rats. Biochem Med Metab Biol. 1991 Apr;45(2):216–226. doi: 10.1016/0885-4505(91)90024-f. [DOI] [PubMed] [Google Scholar]
- Strickberger S. A., Russek L. N., Phair R. D. Evidence for increased aortic plasma membrane calcium transport caused by experimental atherosclerosis in rabbits. Circ Res. 1988 Jan;62(1):75–80. doi: 10.1161/01.res.62.1.75. [DOI] [PubMed] [Google Scholar]
- Tulenko T., Broderick R. The role of cholesterol in arterial wall function and its alteration in vascular disease. Prog Clin Biol Res. 1986;219:75–101. [PubMed] [Google Scholar]
- Verbeuren T. J., Jordaens F. H., Zonnekeyn L. L., Van Hove C. E., Coene M. C., Herman A. G. Effect of hypercholesterolemia on vascular reactivity in the rabbit. I. Endothelium-dependent and endothelium-independent contractions and relaxations in isolated arteries of control and hypercholesterolemic rabbits. Circ Res. 1986 Apr;58(4):552–564. doi: 10.1161/01.res.58.4.552. [DOI] [PubMed] [Google Scholar]
- Walsh M. R., Teal S. W., Gamble W. Biosynthesis of cholesterol by the bovine aorta and the mechanism of action of alpha-para-chlorophenoxyisobutyric acid. Arch Biochem Biophys. 1969 Mar;130(1):7–18. doi: 10.1016/0003-9861(69)90003-4. [DOI] [PubMed] [Google Scholar]
- Xue H., Bukoski R. D., McCarron D. A., Bennett W. M. Induction of contraction in isolated rat aorta by cyclosporine. Transplantation. 1987 May;43(5):715–718. doi: 10.1097/00007890-198705000-00022. [DOI] [PubMed] [Google Scholar]
- Yuen P. S., Garbers D. L. Guanylyl cyclase-linked receptors. Annu Rev Neurosci. 1992;15:193–225. doi: 10.1146/annurev.ne.15.030192.001205. [DOI] [PubMed] [Google Scholar]



