Abstract
Background
Baseline prognostic biomarkers stratifying treatment strategies in first-line metastatic colorectal cancer (mCRC) are lacking. Angiopoietin-2 (Ang-2) is proposed as a potential biomarker in several cancers. We therefore decided to establish the additional prognostic value of Ang-2 for overall survival (OS) in first-line mCRC patients.
Methods
We enrolled 177 patients treated with a bevacizumab containing chemotherapy in two prospective phase II clinical trials. Patient plasma samples were collected at baseline. Enzyme-linked immunosorbent assays were used to measure Ang-2.
Results
The multivariable Cox model identified increased LDH (HR=1.60, 95%CI: 1.04–2.45, p=0.03) and Ang-2 log-transformation level (HR=1.59, 95%CI: 1.14–2.21, p=0.0065) as two significant independent OS prognostic factors. It exhibited good calibration (p=0.8) and discrimination (C-index: 0.64; 95%CI: 0.58–0.68).
Ang-2 parameter inclusion in the GERCOR reference model significantly and strongly improved its discriminative ability since the C-statistic increased significantly from 0.61 to 0.63 (bootstrap mean difference=0.07, 95%CI: 0.069–0.077). Interestingly, the addition of Ang-2 binary information with a 5 ng/mL cut-off value to the GERCOR model allowed the reclassification of intermediate-risk profile patients (41%) into two subsets of low and high-risks.
Conclusions
Our study provides robust evidence in favour of baseline Ang-2 prognostic value for OS adding to the conventional factors. Its assessment appears to be useful for the improvement in risk stratification for patients with intermediate-risk profile.
Impact
Ang-2 ability to predict OS at diagnosis could be of interest in the selection of patients eligible to intermittent or sequential therapeutic strategies dedicated to the optimization of patient’s quality of life and chemotherapy cost-effectiveness.
INTRODUCTION
Remarkable improvement of colorectal cancer patient’s survival was reported in last years, mainly due to the increasing indications of metastatic surgery and the availability of a growing number of chemotherapies and biotherapies during the course of the disease.(1) Several medical options are currently available to treat metastatic colorectal cancer patients (mCRC) in the first-line setting ranging from chemotherapy intensification using FOLFOXIRI±biotherapies (2–3) and step-up strategies based on a first prescription of 5-Fluorouracile (5FU) monotherapy±bevacizumab.(4–7) Therefore, the identification of biomarkers at diagnosis contributing to the prediction of individual mCRC patient’s prognosis will be a critical step to better individualize and stratify mCRC treatments.
Formation of new blood vessels is a major process allowing cancer progression and tumor spread. Several evidence showed that angiogenic molecular regulation is linked to the multistep oncogenesis leading to activation of an increasing number of angiogenic-related growth factors during the course of the disease.(8) The impact of bevacizumab, a Vascular Endothelial Growth Factor neutralizing monoclonal antibody (anti-VEGFA), on mCRC patient’s survival, confirmed the role of VEGF-dependent neoangiogenesis in this disease. In addition, the bevacizumab lower efficacy in advanced disease (beyond the second line of therapy) pointed out that the regulation of advanced mCRC angiogenesis might involve other angiogenic growth factors.
Several investigations were performed to determine the role of cancer-related angiogenesis in mCRC prognosis. Over the last decade, many seric potential prognostic factors were investigated in mCRC patients without any positive association with OS at baseline.(9–10)
The presence of alternative angiogenesis pathways promoting cancer progression was firstly suggested by the lack of efficacy of VEGF blockade in some tumor models in mice.(11) Further studies demonstrated that Angiopoietin-2 (Ang-2), a ligand of Tie2 receptor (12) was able to induce an anarchical blood vessel organization during cancer progression.(13–14) Preclinical studies confirmed that VEGFR and Tie2 signalling were two independent mechanisms promoting tumor angiogenesis and cancer progression.(15) Moreover, VEGF and Ang-2 were independent biomarkers at baseline to predict survival in advanced hepatocarcinoma patients treated by sorafenib in the SHARP study.(16)
In first-line mCRC, Goede V. and colleagues proposed Ang-2 as a possible prognostic biomarker for OS at diagnosis, based on a pioneering study performed in 34 patients treated with bevacizumab and chemotherapy.(17) In a cohort of 51 mCRC patients treated by FOLFIRI-3 and bevacizumab we have also recently observed an association between baseline Ang-2 plasmatic levels, OS and PFS.(18) Other exploratory studies pointed out its potential prognostic value by the description of an association between Ang-2 and OS or PFS, in small cohorts of patients.(19–20) However, the independent and additional Ang-2 prognostic value for OS, among the conventional prognostic factors and prognostic scores used in clinical practice is not yet established.
Some prognostic scores have been proposed in mCRC patients, based on clinical, biological and radiological parameters. The Clinical Risk Score of Fong (from Memorial Sloan Kettering Cancer Center), or the Nordlinger score (21–22) are used in daily practice to determine the prognosis of mCRC patients candidate for metastatic surgery. Another score usually chosen is the Kohne score, based on the Performance Status (PS), white blood cell count (WBC), number of metastatic sites, and Alkaline Phosphatase level.(23) More recently, the simplified score of the Groupe Coopérateur Multidisciplinaire en Oncologie GERCOR offered a convenient fashion to investigate mCRC patient’s prognosis, by monitoring PS and LDH status and exhibited a better discrimination ability.(24) Using these two parameters, the GERCOR score could identify three distinct groups of mCRC patients according to their risk of death: a low-risk group (median OS= 29.8 months), an intermediate-risk group (median OS= 19.5 months), and a high-risk group (median OS=13.9 months). Kohne and GERCOR scores are mainly used to estimate the prognosis of unresectable mCRC. To date, such scores are necessary tools in unresectable mCRC for the management of aggressiveness of the treatment’s strategy, its personalization, and the design optimization for future clinical trials. Nowadays, staging systems still remain rare and have to be improved.
Consequently, we decided to perform a validation study to assess the prognostic value of Ang-2 in mCRC. For this purpose, Ang-2 plasmatic levels were monitored in 177 mCRC patients enrolled in two prospective clinical trials, to confirm the potential prognostic value of Ang-2 as a biomarker of interest for the prediction of OS. We also investigate the added value of Ang-2 at baseline among conventional parameters.
MATERIALS AND METHODS
Population
Individual patient data were collected from two previous prospective cohorts, both containing bevacizumab treatment in first-line mCRC patients.
The “cohort set-1”, was a pilot, single-arm, multicenter, phase II trial conducted to characterize the response rate and toxicity profile of a FOLFIRI-3/bevacizumab association as initial treatment for mCRC. Sixty-one patients were enrolled between october 2007 and october 2009, and levels of plasma Ang-2 were measured in 51 patients by enzyme-linked immunosorbent assay at baseline. Tumor responses were assessed every eight weeks by spiral-computed tomography until progression. As the main objective was to assess the tumor response rate, surgery of metastases was allowed after 6 cycles of treatment, and the precise timing left at discretion of investigators.(18) This phase II study was funded by the university hospital of Besançon, and registered on ClinicalTrials.gov (study NCT00544011, approved by french ethical committee in 2007, February 1st).
The “cohort set-2” was conducted from 2007 to 2010 to evaluate the role of contrast-enhanced ultrasound with gas-encapsulated microbubbles, to assess anti-angiogenic efficacy of bevacizumab in first-line metastatic colorectal cancers with unresectable liver mCRC metastases.(25) Of note, all patients had unresectable liver metastases. Tumor responses were assessed every twelve weeks or less until progression. This study was funded by the French National Institute of Cancer and registered on ClinicalTrials.gov (study NCT00489697, approved by french ethical committee in 2006, November 11th).
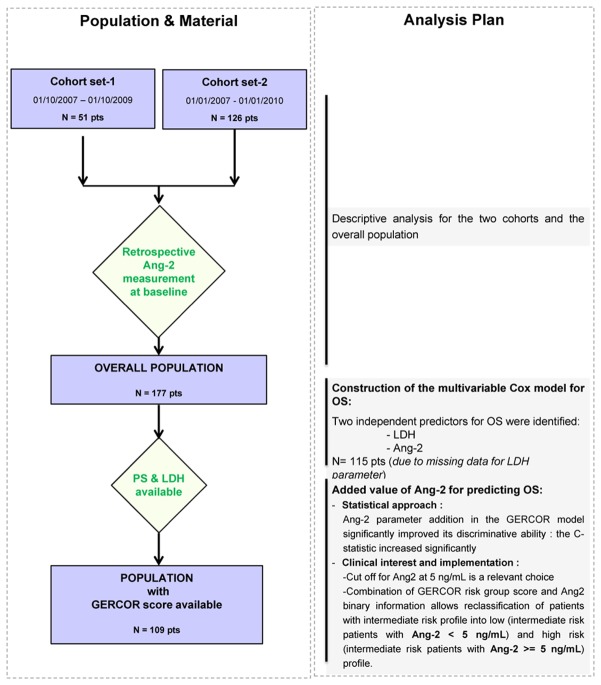
In both studies all patients gave written informed consent, and were followed until death. Data were anonymized. Population selection during the study is summarized in the flow chart (Figure 1).
Figure 1. Flow chart and Analysis plan of the study.
Population selection during the study is summarized in the flow chart.
In the left panel the population selection during the study is described.
In the right panel the derived analysis and their synthetic results are summarized.
Data extraction
The following data were collected for each patient in the two cohorts: center and patient identification, age, sex, performance status, primary tumor site (colon, rectum), site of metastases (liver-limited, liver and other, other), time of metastasis diagnostic (synchronous, metachronous), primary tumor resection, LDH level at baseline (normal value was considered if LDH were below 350 UI/L), lymphocyte and leucocyte counts, survival status, and date of last news or death. Of note, ALP level was not available in “cohort set-2”.
Ang-2 plasma sample measurement procedure is precisely described in supplementary materials (Supp methods).
Statistical analysis
We provided the mean (SD) values and frequency (percentages) for the description of continuous and categorical variables, respectively. The means and the proportion were compared using Student’s t test and the chi-squared test (or Fisher’s exact test, if appropriate), respectively. Due to the skewed Angiopoietin-2 distribution we used for its description the median, and the interquartile range for the dispersion measurement, as recommended.(26) Wilcoxon rank-sum test was performed for Ang-2 distribution comparison among the cohort set.
OS was calculated from the date of study enrolment to the date of death from any cause. Alive patients were censored at the last follow-up. OS was estimated using Kaplan Meier method and described using median or rate at specific time points with 95%CI. Follow-up was calculated using reverse Kaplan-Meier estimation.(27) A Cox proportional hazard model was performed to estimate the hazard ratio (HR) and 95% confidence intervals for the factors associated with OS. The association of parameters at enrolment with OS was first assessed using univariate Cox analysis and then included (for those with p<0.05) in a final multivariate Cox regression model with stepwise backward elimination. When used in continuous in the Cox modelisation, Ang-2 variable had to be normalized by logarithmic transformation, considering its skewed distribution. Hazard proportionality was checked by plotting log-minus-log survival curves.
Accuracy of the model was checked regarding two parameters: discrimination and calibration.(28) The predictive value and the discrimination ability of the model was evaluated with Harrell’s Concordance (C)-index. One thousand random samples of the population were used to derive 95%CI for the Harrell’s Concordance statistic. Calibration and goodness of fit of the model were assessed by using the extension of Hosmer-Lemeshow test for survival analysis and p-value greater than 0.1 was considered as an indicator for acceptable agreement.
Internal validity of the model was assessed by a bootstrap sample procedure. Several approaches have been proposed to assess the performance in samples of the same population (internal validation).(29)
Sensitivity analyses were performed for univariate and multivariate Cox models with a stratified approach on the cohort set parameter that allowed to consider the two cohort heterogeneities in the Cox modelisation.
We further focused on the improvement of one reference prognostic model for OS (GERCOR model) performance after the inclusion of Ang-2 measurement, comparing two sets of predictions for death: one based on a Cox proportional hazard model without Ang-2 parameter and one based on a model with Ang-2 parameter. The discrimination ability and incremental value of Ang-2 level to the GERCOR score was evaluated with the use of C statistic. This analysis was repeated 1,000 times using bootstrap samples to derive 95%CIs for the difference in the C statistic between models. We also used net continuous reclassification improvement (NRI) and integrated discrimination improvement (IDI) to quantify the performance and the net benefit of the addition of Ang-2 to the reference model for the prediction of 48 months death probability.(30–32) Continuous NRI has several limitations but would give a consistent message and is therefore a descriptive marker.(33) Of note, cNRI does not consider the magnitude of the change, but only the direction. This is done by the IDI. When significantly greater than 0, IDI and cNRI are in favor of a net benefit of the addition of the marker of interest to the reference model considered.
We finally investigated the possibility to provide a simple implementation of Ang-2 monitoring in clinical practice with the determination of a cut-off value by contrasting Ang-2 level distributions in boxplot among healthy volunteers and mCRC patients.
The analyses were conducted using SAS 9.2 (Statistical Analysis System, Cary, NC, USA) and R 3.0.2 (R foundation for Statistical Computing). All statistical tests were 2-sided, and probability values <0.05 were regarded as significant.
RESULTS
Population
The characteristics of the 177 eligible patients are summarized in table 1. Fifty-one samples were available from “cohort set-1”, and 126 from “cohort set-2”. Patient’s characteristics of these two cohorts are similar, except for sex and liver metastatic involvement. Ang-2 values were also different in the two cohorts. Of note, the statistical unbalance in metastatic sites between the two cohorts was awaited since liver metastasis was an inclusion criteria in the “cohort set-2”. The rate of PS 2 was 2% in the “cohort set-1”, instead of 5% in the “cohort set-2”, we consequently differentiated the patients PS 0 from the patients PS > 0. There were 23 (45%), and 45 (36%) patients with liver exclusive metastatic sites. Surgery of the primary tumor was performed in 28 (57%) and 82 (65%) patients, in the cohorts set-1 and set-2, respectively. LDH values at baseline were available in 33 and 82 patients and increased upper limit normal values in 13 (39%) and 46 (56%) patients of cohort set-1 and set-2, respectively.
Table 1.
Baseline Characteristics of Patients, according to the cohort set
| Characteristics | Overall population (N =177) | Cohort set-1 (N =51) | Cohort set-2 | P† | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| N | N | N | |||||
| Age — years ‡ | 51 | 126 | |||||
| <= 65 | 177 | 93 (53%) | 27 (53%) | 66 (52%) | |||
| >65 | 84 (47%) | 24 (47%) | 60 (48%) | p = 0.9461 | |||
|
| |||||||
| Patient male sex — no. (%)‡ | 177 | 74 (42%) | 51 | 28 (55%) | 126 | 46 (37%) | p = 0.0246 |
|
| |||||||
| Performance status OMS ‡ | 167 | ||||||
| 0— no. (%) | 91 (55%) | 48 | 27 (56%) | 119 | 64 (54%) | ||
| >0— no. (%) | 76 (45%) | 21 (44%) | 55 (46%) | p = 0.7719 | |||
|
| |||||||
| Primary Tumor site ‡ | 177 | 51 | 126 | ||||
| Colon — no. (%) | 120 (68%) | 32 (63%) | 88 (70%) | ||||
| Rectum — no. (%) | 57 (32%) | 19 (37%) | 38 (30%) | p = 0.3602 | |||
|
| |||||||
| Metastases localisation ‡ | 177 | 51 | 126 | ||||
| Liver and other — no. (%) | 98 (55%) | 17 (33%) | 81 (64%) | ||||
| Liver alone — no. (%) | 68 (39%) | 23 (45%) | 45 (36%) | ||||
| Other alone — no. (%) | 11 (6%) | 11 (22%) | 0 (0%) | p <0.0001 | |||
|
| |||||||
| Timing of metastases ‡ | 173 | 48 | 125 | ||||
| Synchrone — no. (%) | 119 (69%) | 30 (63%) | 89 (71%) | ||||
| Metachrone — no. (%) | 54 (31%) | 18 (37%) | 36 (29%) | p = 0.2688 | |||
|
| |||||||
| Surgery of the primary tumor — no. (%)‡ | 175 | 110 (63%) | 49 | 28 (57%) | 126 | 82 (65%) | p = 0.3293 |
|
| |||||||
| Leucocyte (×106/mL)* | 137 | 8.1 ± 3.2 | 49 | 8.1 ± 3.5 | 88 | 8.2 ± 3.0 | p = 0.8926 |
|
| |||||||
| Lymphocyte (×106/mL)* | 132 | 1.5 ± 0.7 | 45 | 1.7 ± 0.7 | 87 | 1.4 ± 0.6 | p = 0.0550 |
|
| |||||||
| LDH ‡ | 115 | 33 | 82 | ||||
| <=350 (ULN) | 56 (49%) | 20 (61%) | 36 (44%) | ||||
| >350 (ULN) | 59 (51%) | 13 (39%) | 46 (56%) | p = 0.1050 | |||
|
| |||||||
| Ang-2 (ng/mL) ** | 177 | 4.249 (2.683–7.153) | 51 | 2.793 (2.103–4.330) | 126 | 4.728 (3.298–7.798) | p <0.0001 |
|
| |||||||
| Death event ‡ | 177 | 51 | 36 (71%) | 126 | 126 (100%) | p <.0001 | |
|
| |||||||
|
Median F-up time in months 95%CI All patients were F-up until death |
57.9 (53.1–60.3) | Max time observed = 64.1 | |||||
Plus–minus values are means ±SD and the unpaired t-test was used for the comparison of variable among groups.
Median and Inter-quartile range are described and the Wilcoxon rank sum test was used for the comparison of variable among groups.
P values are for the comparison between cohort set-1 and cohort set-2 populations
Chi-square or Fisher exact tests were used for the comparison of categorical variables,.
Abbreviations: PS, performance status ULN: Upper Limit of Normal
Bold characters represent significative results.
Ang-2 plasma level biomarker and prediction of OS
The association of clinical, biological, and radiological parameters with risk of death in univariate and multivariate Cox regression analysis with stepwise backward elimination was performed and results are shown in table 2. We identified six variables as prognostic factors for OS in the univariate analysis: Ang-2 log-value (HR=1.91; 95%CI: 1.492; 2.448; p< 0.0001), metastatic localisations (liver and other: HR=1.82; 95%CI: 1.305–2.527 - other: HR=0.69; 95%CI: 0.326–1.442; p=0.0003 - liver alone as the reference), absence of resection of the primary site (HR=1.43 95%CI: 1.035–1.984; p=0.0304), synchronous metastases (HR=1.46; 95%CI: 1.035–2.054; p=0.0308), high LDH level (HR=2.03; 95%CI: 1.373–2.988; p=0.0004) and leucocyte count (HR=1.12; 95%CI: 1.056–1.188; p=0.0002) (table 2).
Table 2.
Univariate and multivariate analyses (*)
|
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of patients | Number of deaths | Univariate analysis | Multivariate analysis | Multivariate analysis with bootstrap procedure | |||||
|
| |||||||||
| HR | 95%CI | P | HR | 95%CI | P | 95% percentile CI | |||
| Age — years | |||||||||
| <= 65 | 93 | 84 | 1 | - | - | ||||
| >65 | 84 | 78 | 1.243 | [0.912; 1.694] | 0.1689 | ||||
|
| |||||||||
| Sex | |||||||||
| Male | 74 | 65 | 1 | ||||||
| Female | 103 | 97 | 1.310 | [0.955; 1.798] | 0.0941 | ||||
|
| |||||||||
| Performance status OMS | |||||||||
| 0 | 91 | 80 | 1 | ||||||
| >0 | 76 | 73 | 1.274 | [0.927; 1.751] | 0.1355 | ||||
|
| |||||||||
| Primary Tumor site | |||||||||
| Colon | 120 | 113 | 1 | ||||||
| Rectum | 57 | 49 | 0.855 | [0.610; 1.196] | 0.3597 | ||||
|
| |||||||||
| Timing to metastasis | |||||||||
| Metachronous | 119 | 111 | 1 | ||||||
| Synchronous | 54 | 47 | 1.458 | [1.035; 2.054] | 0.0308 | ||||
|
| |||||||||
| Metastases localisation | |||||||||
| Liver alone | 68 | 59 | 1 | ||||||
| Liver and other | 98 | 95 | 1.816 | [1.305; 2.527] | |||||
| Other alone | 11 | 8 | 0.686 | [0.326; 1.442] | 0.0003 | ||||
|
| |||||||||
| Surgery of the primary tumor | |||||||||
| Yes | 110 | 101 | 1 | ||||||
| No | 65 | 59 | 1.433 | [1.035; 1.984] | 0.0304 | ||||
|
| |||||||||
| Leucocyte (×106/mL)* | 137 | 122 | 1.120 | [1.056; 1.188] | 0.0002 | ||||
|
| |||||||||
| Lymphocyte (×106/mL)* | 132 | 120 | 0.795 | [0.589; 1.073] | 0.1339 | ||||
|
| |||||||||
| LDH ‡ | |||||||||
| <=350 (ULN) | 56 | 49 | 1 | 1 | |||||
| >350 (ULN) | 59 | 58 | 2.026 | [1.373; 2.988] | 0.0004 | 1.598 | [1.040; 2.454] | 0.0323 | [1.039; 2.628] |
|
| |||||||||
| Log_Ang-2 (pg/mL) | 177 | 162 | 1.911 | [1.492; 2.448] | <.0001 | 1.587 | [1.138; 2.213] | 0.0065 | [1.161; 2.263] |
CI denotes confidence interval.
Abbreviations: PS, performance status ULN: Upper Limit of Normal
Bold characters represent significative results.
Two independent predictors of OS were identified by the multivariate analysis: LDH high level (HR=1.60; 95%CI: 1.04–2.45; p=0.03) and log Ang-2 high level (HR=1.59; 95%CI: 1.14–2.21; p=0.0065) (table 2).
Final Multivariate model performance assessment
Accuracy of the model was checked regarding two parameters: discrimination and calibration, which measure the ability to separate patients with different prognosis and to provide unbiased survival predictions in groups of similar patients, respectively.
Our final multivariable Cox model exhibited good calibration (Hosmer–Lemeshow with deciles p=0.8) and acceptable discrimination (C-statistic 0.64; 95%CI: 0.58–0.68).
Internal validation of the final model
With the replicated datasets (n=1000) derived from the bootstrap sample procedure, uncertainties around hazard ratio estimates can be measured.
Bootstrapping results for the internal validation reflect the robustness of the final model (HR 95%CI percentile for LDH: 1.039 to 2.628 and HR 95%CI percentile for Ang-2 (log value): 1.161 to 2.263).
Sensitivity analysis
Considering the differences observed between the two cohorts (table 1), we performed a sensitivity analysis to validate the robustness of our final model with a stratified approach in the Cox modelisation.
With this approach similar results were obtained for the univariate analysis except for timing to metastases parameters, which were reported to be non-statistically significant (Supp data table 1). The multivariate analysis confirmed that Ang-2 and LDH were two independent predictors for OS (HR=1.800; 95%IC: 1.081–2.998; p=0.0239 and HR=1.548; 95%IC: 1.028–2.331; p=0.0365, respectively).
Added value of the Ang-2 parameter for predicting Overall Survival
Then, we decided to investigate the performance improvement in OS discrimination after the inclusion of Ang-2 measurement in a reference prognostic model.
Currently, in our situation two main staging systems are available: Kohne and GERCOR scores. In our population the Kohne model cannot be calculated. However, GERCOR score displayed a better discriminative ability (C=0.64) than Kohne model (C=0.55).(24) We then used the GERCOR model as the reference score.
Ang-2 was first identified as a factor independently associated with OS (HR=1.568 95%CI=1.111–2.213; p=0.0104). Then, the inclusion of the Ang-2 parameter in the GERCOR reference model significantly improved its discriminative ability since the C-statistic increased significantly from 0.61 to 0.63 (bootstrap mean difference=0.07, 95% CI: 0.069–0.077).
The integrated discrimination improvement was 0.03 (p=0.07). Similarly, the addition of Ang-2 measurement to the reference model showed a favourable trend to adequately reclassified patients at lower risk for death and those at higher risk, as reported by a continuous net reclassification improvement of 0.26 (95%CI: −0.23 to 0.75). Indeed for patients without death at 48 months Ang-2 measurement moved risk prediction in the correct (downward) direction in 11/18=61%. Conversely, patients with death indicate a correct, upward, change in risk assessment when using the Ang-2 measurement (47/91=52%). The risk reclassification analysis (IDI and NRI) results are greater than 0 and statistically border line reflecting a favourable tendency for Ang-2 parameter.
Proposal for an implementation of Ang-2 monitoring in clinical practice
After the statistical investigation and determination of the baseline Ang-2 added value for predicting OS among the conventional factors, we investigated the possibility to provide a simple implementation of Ang-2 monitoring in clinical practice.
Cut-off value of Ang-2 fixed at 5 ng/mL
Simple implementation of Ang-2 monitoring in clinical practice is first guided by the determination for a relevant cut-off in order to categorize patients into groups with low and high Ang-2 level at baseline.
A preliminary set of experiments was done by dosing the Ang-2 value in 41 healthy volunteers, blood donors in the Etablissement Français du Sang (EFS, Bourgogne Franche-Comté). These volunteers were major (more than 55 years old), having signed an informed consent and were randomly chosen. We hypothesized that this population have no active angiogenesis. Among these healthy donors, 40 (98%) had mean values lower than 5 ng/mL. This observation was in agreement with the results previously published by Goede et al where levels of Ang-2 were inferior to 5 ng/mL in the 33 healthy volunteers.
In our study population, the median value of Ang-2 was 4.045 ng/mL for the 109 patients included in the final analysis. Of note, similar results were observed when the median value was used as a cut-off. A value of 5 ng/mL was then chosen considering results provided by previous publications (17, 20) regarding that 98% of healthy volunteers have baseline plasmatic levels of Ang-2 below 5ng/mL. In consequence, in order to investigate a cut-off value for clinical use, a level of 5 ng/mL seemed to be a relevant choice (Figure 2).
Figure 2. Distribution of Ang-2 measurement value among Healthy Volunteers (n=41) and mCRC patients (n=109) involved in the final analysis.
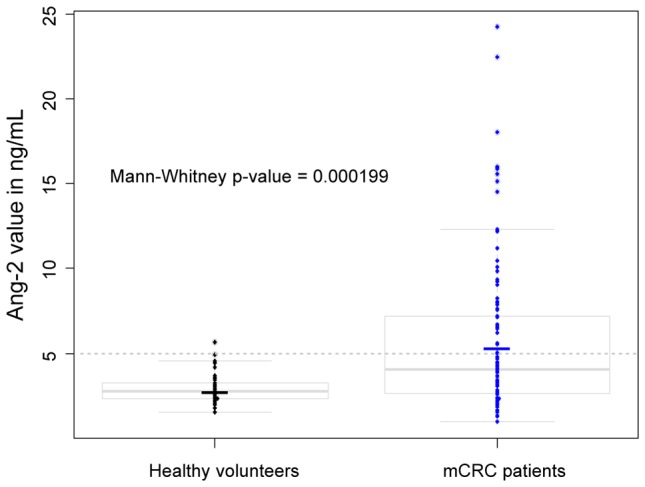
In our study population, the median value of Ang-2 was 4.045 ng/mL for the 109 patients included in the final analysis. In consequence, in order to investigate a cutoff value for clinical use, a level of 5 ng/mL seemed to be a relevant choice.
Phenotypic characterisation of patients with high and low Ang-2 levels
The determination of 5 ng/mL as a cut-off value allowed us to classify patients into two groups: low (<5 ng/mL) and high (>=5 ng/mL) Ang-2 level group at baseline. Among the 109 patients involved in the final analysis, 40 (36.7%) had levels of Ang-2 above 5 ng/mL. The clinico-biological characteristics according to Ang-2 value are summarized in table 3. Increased Ang-2 level was associated with enhanced LDH levels (77%, p< 0.0001), metastatic sites (63% liver and other, p=0.04515), and synchronous metastases (92%, p< 0.0001). Patients with high Ang-2 levels exhibited more surgery of the primary tumor, increased leucocytes count and lymphopenia.
Table 3.
Baseline characteristics of the patients involved in the multivariate analysis according to the Ang-2 level (n=109)
|
|
||||
|---|---|---|---|---|
| n | Ang-2 < 5 ng/mL (n=69) | Ang-2 >=5 ng/mL (n=40) | p† | |
| Age — years ‡ | ||||
| <= 65 | 56 | 31 (45) | 25 (63) | |
| >65 | 53 | 38 (55) | 15(37) | 0.11 |
|
| ||||
| Sex‡ | ||||
| Male | 45 | 39 (57) | 25 (63) | |
| Female | 64 | 30 (43) | 15 (37) | 0.54 |
|
| ||||
| Performance status OMS‡ | ||||
| 0 | 57 | 41 (59) | 16 (40) | |
| >0 | 52 | 28(41) | 24 (60) | 0.07 |
|
| ||||
| Primary Tumor site‡ | ||||
| Colon | 73 | 46(67) | 27 (68) | |
| Rectum | 36 | 23(33) | 13 (32) | 0.93 |
|
| ||||
| Timing to metastasis ‡ | ||||
| Synchronous | 74 | 39 (57) | 35 (92) | |
| Metachronous | 32 | 29 (43) | 3 (8) | < 0.0001 |
|
| ||||
| Metastases localisation‡ | ||||
| Liver alone | 43 | 28 (41) | 15 (37) | |
| Liver and other | 58 | 33 (48) | 25 (63) | |
| Other alone | 8 | 8 (12) | 0 (0) | 0.04515 |
|
| ||||
| Surgery of the primary tumor ‡ | ||||
| No | 73 | 16 (24) | 18 (46) | |
| Yes | 34 | 52 (76) | 21 (54) | 0.0189 |
|
| ||||
| Leucocyte (×106/mL)* | 89 | 7.3713±2.3972 | 9.4180± 3.8269 | 0.0026 |
|
| ||||
| Lymphocyte (×106/mL)* | 85 | 1.6589±0.6355 | 1.2187±0.5816 | 0.0017 |
|
| ||||
| LDH ‡ | ||||
| <=350 (ULN) | 52 | 43 (62) | 9 (23) | |
| >350 (ULN) | 57 | 26 (38) | 31 (77) | < 0.0001 |
|
| ||||
| GERCOR score‡ | ||||
| 0 Low-risk | 32 | 29 (42) | 3 (7) | |
| 1 Intermediate-risk | 45 | 26 (38) | 19 (48) | |
| 2 High-risk | 32 | 14 (20) | 18 (45) | 0.0002 |
Plus–minus values are means ±SD and the unpaired t-test was used for the comparison of variable among groups.
P values are for the comparison between cohort set 1 and cohort set 2 populations
Chi-square or Fisher exact tests were used for the comparison of categorical variables,.
Abbreviations: PS, performance status
Bold characters represent significative results.
As expected, we noted a significant difference for the GERCOR prognostic score distribution among groups of patients with low and high Ang-2 levels (42%-38%-20% vs 7%-48%-45%; p=0.0002 for low, intermediate and high risk GERCOR group, respectively).
Kaplan-Meier curves for OS by combining Ang-2 binary information and conventional score
Considering the added value of Ang-2 measurement for OS risk stratification among conventional factors, previously described, we investigated the interest for a combination of Ang-2 simple binary information and GERCOR prognostic score in clinical practice.
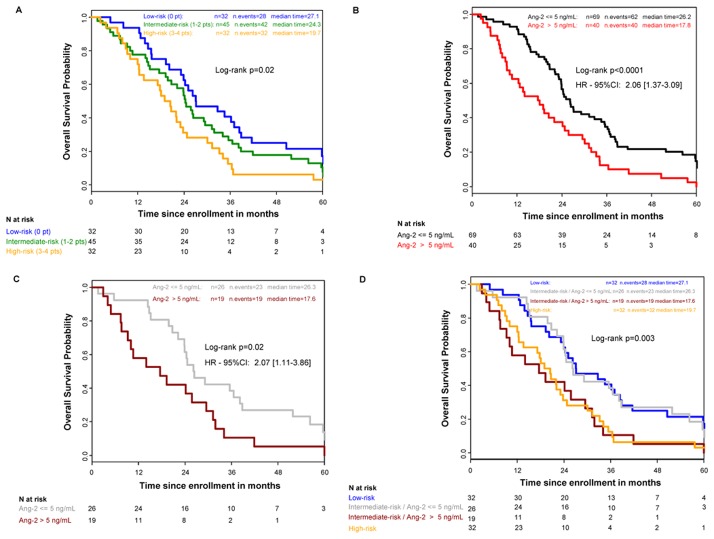
In our population, 30 (59%) and 79 (63%) patients are eligible for the GERCOR score calculation among the 51 and 126 patients in the cohort set-1 and 2, respectively (Fisher-exact test p= 0.7332). In total, data were available to assess the GERCOR prognostic score in 109 patients. This risk model identified 32 patients at “low-risk”, 45 patients at “intermediate-risk”, and 32 patients at “high-risk” level. In our study, the median OS was 27.1 months 95%CI: 20.2–38.4 for the low-risk, 24.3 months 95%CI: 19.3–29.5 for the intermediate-risk and 19.7 months 95%CI: 12.3–23.8 for the high-risk group (figure 3, panel A, global p-logrank=0.02).
Figure 3. Kaplan–Meier Curves for Overall Survival.
Panel A shows the classic approach based on the GERCOR’s score in which LDH and OMS are key parameters.
Panel B shows the stratification according to the level of Ang-2.
Panel C shows the stratification according to the level of Ang-2 in the 45 (41%) patients classified in intermediate risk with GERCOR’s score.
Panel D shows that the determination of Ang-2 in the intermediate risk patients with GERCOR’s group (n=45) reclassified these patients in two groups with similar profiles than low and high risk GERCOR’s groups.
According to the Ang-2 value, the median OS was significantly better in patients with low levels of Ang-2 than in patients with high levels of Ang-2 (median OS=26.2 months, 95%CI: 23.2–34.5 vs 17.8 months, 95%CI: 10.1–24.1, respectively, HR=2.06; 95%CI: 1.37–3.09; p<0.0001) (figure 3, panel B).
Finally, the Ang-2 binary status was combined with the GERCOR score. In low and high-risk patients, the Ang-2 value only triggers a trend in better discrimination of prognosis, without statistical significance (p=0.27 and 0.45, respectively). However, in the intermediate risk GERCOR group (n=45, 41%), the consideration of Ang-2 binary status allowed us to split the population in two subsets of patients. Indeed, patients with low Ang-2 level had a significant better prognosis than those with high Ang-2 level (26.3 95%CI: 22.2–38.2 vs 17.6 months 95%CI: 7.6–29.5 p=0.02; HR=2.07 95%IC=1.11–3.86) (figure 3, panel C).
Interestingly, patients with intermediate risk and Ang-2< 5 ng/mL (n=26, 58%) had a similar risk profile than the GERCOR low risk patients (OS=26.3 and 27.1 months, respectively). Similarly, those with Ang-2> 5 ng/mL (n=19, 42%) had a similar risk profile than the GERCOR high-risk patients (OS=17.6 and 19.7 months, respectively, figure 3, panel D).
DISCUSSION
The present results, consistently with previous studies, confirm that Angiopoietin-2 is a biomarker of interest for OS prediction in non-previously treated mCRC patients.(17–18) The main interest of Ang-2 as a biomarker is its ability to predict OS when measured at diagnosis, before treatment initiation. Low levels of Ang-2 identify a significant population (n=69; 63%) displaying an encouraging median OS of 26.2 months (HR=2.06; 95%CI: 1.37–3.09; p<0.0001).
Identification of patients with a favourable prognosis at baseline might be of clinical interest to better individualize cancer therapies. Such prognostic biomarkers might enable the appropriate selection of patients eligible to intermittent chemotherapy or sequential therapeutic strategies dedicated to the optimization of patient’s quality of life and chemotherapy cost-effectiveness.(5–6, 34)
In our cohort, the GERCOR model allows the identification of 32 (29%) patients of good prognosis, with a median OS of 27.1 months. When considering the population exhibiting both low Ang-2 level and low GERCOR risk, no difference was established in OS, as well as for elevation of Ang-2 level and high GERCOR risk.
However, considering the intermediate GERCOR subgroup risk (n=45, 41% of patients in the present study) the binary Ang-2 status succeeded in reclassifying the prognostic risk into a low (n=26, 58%) and a high risk (n=19, 42%), corresponding to those of GERCOR model. In this case, the soluble Ang-2 value appears to be of particular interest since i) the intermediate subgroup represents a consequent number of patients, and ii) the relevance of an intermediate group in clinical practice is not clear leading to considerable confusion for their management.
There are some limitations in our study. First, all patients of these cohorts were treated with bevacizumab and chemotherapy, excluding the possibility to analyse the predictive value of Ang-2. This question was recently addressed by Llovet et al in the SHARP study.(16) Ang-2 was monitored in 491 hepatocellular carcinoma patients treated by sorafenib or placebo. Baseline plasma Ang-2 was correlated to OS both in sorafenib and placebo groups, ruling out a predictive value of Ang-2 in the context of this anti-angiogenic therapy. More recently the predictive impact of Ang-2 has also been assessed in pancreatic cancer. In that study, three seric factors have been proposed as predictive for efficacy in 328 patients treated by gemcitabine with bevacizumab or placebo, including Ang-2, Stomal-cell Derived Factor-1 (SDF1) and VEGF-D.(35) Another limitation of our study is the absence of PAL recording in the “cohort set-2”. Nevertheless, we did not observe any association between PAL and Ang-2 in the “cohort set-1” Moreover, the GERCOR analysis, performed on 803 mCRC patients treated by FOLFOX or FOLFIRI in the first-line setting demonstrated that LDH, number of metastatic sites and PS were the only independent prognostic factors.(24)
From a statistical point of view, the assessment of models performance measures such as discrimination, calibration, and internal validation strengthen the present investigation. While the model developed here has good calibration, discrimination and robust internal validation (reproducibility), these results have to be replicated and confirmed in a prospectively recruited validation cohort, in order to ensure wider transportability and generalizability of our results.
One scheming observation is the association between Ang-2 level and presence of the primary tumor. Surgery of the primary tumor was linked to a decreased probability to observe plasmatic Ang-2 in mCRC patients (76% Ang-2< 5 ng/mL vs 54% Ang-2> 5 ng/mL). The prognostic value of primary tumor resection still remains a matter of debates. It appears to be significantly associated with OS in our univariate analysis, but not in the multivariate one. Many clinical and biological investigations support a potential role of the presence of the primary carcinoma in mCRC prognosis.(36–39) Data reported in two phase III clinical trials CAIRO and CAIRO II, and a recent meta-analysis suggested a prognostic association of primary tumor removal with OS in mCRC patients.(38, 40–42) The potential association of primary colorectal cancer on Ang-2 production might account for its potential detrimental effect on prognosis. Prospective studies should monitor Ang-2 production before and following primary tumor resection.
The biological basis underlying the adverse role of Ang-2 also deserves further investigations. There are some evidences that VEGF is early expressed during cancer progression (43) Ang-2 could be overexpressed in latter stages of the disease, as suggested by Abajo et al.(44) In line with this hypothesis, the production of Ang-2 within the tumor microenvironment could depend on VEGF.(44) Moreover, Ang-2 expression was shown to be correlated with colorectal cancer stages and progression.(44–45) Nevertheless the precise role of Ang-2 cancer progression need to be further clarified.
In conclusion, the assessment of the Ang-2 value at baseline could guide clinicians in stratifying risk for death in first-line mCRC patients and in providing a basis for early and adapted therapeutic interventions. The determination of Ang-2 at baseline should allow death risk stratification that could also be useful in the design optimization for future clinical trials.
Supplementary Material
Supplementary Table 1: Cox Univariate analyses for OS prediction with and without the stratified approach (sensitivity analysis)
Red characters represent significative results
Acknowledgments
Financial support:
No external funding
We thank Jeremy Balland for providing us data for Healthy Volonteers.
Footnotes
Conflict of interest:
T. Lecomte, O. Bouché: Roche – A. Goncalves: Roche (consulting and member of advisory board)
References
- 1.Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;2722:3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loupakis F, Cremolini C, Salvatore L, Masi G, Sensi E, Schirripa M, et al. FOLFOXIRI plus bevacizumab as first-line treatment in BRAF mutant metastatic colorectal cancer. Eur J Cancer. 2014;501:57–63. doi: 10.1016/j.ejca.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 3.Masi G, Loupakis F, Salvatore L, Fornaro L, Cremolini C, Cupini S, et al. Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil, and folinate) as first-line treatment for metastatic colorectal cancer: a phase 2 trial. Lancet Oncol. 2010;119:845–852. doi: 10.1016/S1470-2045(10)70175-3. [DOI] [PubMed] [Google Scholar]
- 4.Tebbutt NC, Wilson K, Gebski VJ, Cummins MM, Zannino D, van Hazel GA, et al. Capecitabine, bevacizumab, and mitomycin in first-line treatment of metastatic colorectal cancer: results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J Clin Oncol. 2010;2819:3191–3198. doi: 10.1200/JCO.2009.27.7723. [DOI] [PubMed] [Google Scholar]
- 5.Ducreux M, Malka D, Mendiboure J, Etienne PL, Texereau P, Auby D, et al. Sequential versus combination chemotherapy for the treatment of advanced colorectal cancer (FFCD 2000-05): an open-label, randomised, phase 3 trial. Lancet Oncol. 2011;1211:1032–1044. doi: 10.1016/S1470-2045(11)70199-1. [DOI] [PubMed] [Google Scholar]
- 6.Seymour MT, Maughan TS, Ledermann JA, Topham C, James R, Gwyther SJ, et al. Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): a randomised controlled trial. Lancet. 2007;3709582:143–152. doi: 10.1016/S0140-6736(07)61087-3. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham D, Lang I, Marcuello E, Lorusso V, Ocvirk J, Shin DB, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol. 2013;1411:1077–1085. doi: 10.1016/S1470-2045(13)70154-2. [DOI] [PubMed] [Google Scholar]
- 8.Rak J, Filmus J, Kerbel RS. Reciprocal paracrine interactions between tumour cells and endothelial cells: the ‘angiogenesis progression’ hypothesis. Eur J Cancer. 1996;32A14:2438–2450. doi: 10.1016/s0959-8049(96)00396-6. [DOI] [PubMed] [Google Scholar]
- 9.Jubb AM, Hurwitz HI, Bai W, Holmgren EB, Tobin P, Guerrero AS, et al. Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol. 2006;242:217–227. doi: 10.1200/JCO.2005.01.5388. [DOI] [PubMed] [Google Scholar]
- 10.Kopetz S, Hoff PM, Morris JS, Wolff RA, Eng C, Glover KY, et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol. 2010;283:453–459. doi: 10.1200/JCO.2009.24.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Millauer B, Longhi MP, Plate KH, Shawver LK, Risau W, Ullrich A, et al. Dominant-negative inhibition of Flk-1 suppresses the growth of many tumor types in vivo. Cancer Res. 1996;567:1615–1620. [PubMed] [Google Scholar]
- 12.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;2775322:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 13.Reiss Y, Knedla A, Tal AO, Schmidt MH, Jugold M, Kiessling F, et al. Switching of vascular phenotypes within a murine breast cancer model induced by angiopoietin-2. J Pathol. 2009;2174:571–580. doi: 10.1002/path.2484. [DOI] [PubMed] [Google Scholar]
- 14.Falcon BL, Hashizume H, Koumoutsakos P, Chou J, Bready JV, Coxon A, et al. Contrasting actions of selective inhibitors of angiopoietin-1 and angiopoietin-2 on the normalization of tumor blood vessels. Am J Pathol. 2009;1755:2159–2170. doi: 10.2353/ajpath.2009.090391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siemeister G, Schirner M, Weindel K, Reusch P, Menrad A, Marme D, et al. Two independent mechanisms essential for tumor angiogenesis: inhibition of human melanoma xenograft growth by interfering with either the vascular endothelial growth factor receptor pathway or the Tie-2 pathway. Cancer Res. 1999;5913:3185–3191. [PubMed] [Google Scholar]
- 16.Llovet JM, Pena CE, Lathia CD, Shan M, Meinhardt G, Bruix J. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2012;188:2290–2300. doi: 10.1158/1078-0432.CCR-11-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goede V, Coutelle O, Neuneier J, Reinacher-Schick A, Schnell R, Koslowsky TC, et al. Identification of serum angiopoietin-2 as a biomarker for clinical outcome of colorectal cancer patients treated with bevacizumab-containing therapy. Br J Cancer. 2010;1039:1407–1414. doi: 10.1038/sj.bjc.6605925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S, Dobi E, Jary M, Monnien F, Curtit E, Nguyen T, et al. Bifractionated CPT-11 with LV5FU2 infusion (FOLFIRI-3) in combination with bevacizumab: clinical outcomes in first-line metastatic colorectal cancers according to plasma angiopoietin–2 levels. BMC Cancer. 2013;13:611. doi: 10.1186/1471-2407-13-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauerschlag DO, Hilpert F, Meier W, Rau J, Meinhold-Heerlein I, Maass N, et al. Evaluation of potentially predictive markers for anti-angiogenic therapy with sunitinib in recurrent ovarian cancer patients. Transl Oncol. 2013;63:305–310. doi: 10.1593/tlo.13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulz P, Fischer C, Detjen KM, Rieke S, Hilfenhaus G, von Marschall Z, et al. Angiopoietin-2 drives lymphatic metastasis of pancreatic cancer. FASEB J. 2011;2510:3325–3335. doi: 10.1096/fj.11-182287. [DOI] [PubMed] [Google Scholar]
- 21.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;2303:309–318. doi: 10.1097/00000658-199909000-00004. discussion 318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;777:1254–1262. [PubMed] [Google Scholar]
- 23.Kohne CH, Cunningham D, Di Costanzo F, Glimelius B, Blijham G, Aranda E, et al. Clinical determinants of survival in patients with 5-fluorouracil-based treatment for metastatic colorectal cancer: results of a multivariate analysis of 3825 patients. Ann Oncol. 2002;132:308–317. doi: 10.1093/annonc/mdf034. [DOI] [PubMed] [Google Scholar]
- 24.Chibaudel B, Bonnetain F, Tournigand C, Bengrine-Lefevre L, Teixeira L, Artru P, et al. Simplified prognostic model in patients with oxaliplatin-based or irinotecan-based first-line chemotherapy for metastatic colorectal cancer: a GERCOR study. Oncologist. 2011;169:1228–1238. doi: 10.1634/theoncologist.2011-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goujon G, PP, Ladam-Marcu V, Labbe-Devilliers C, Bouché O, Tranquart F, Lecomte T. JFHOD. 2010. Évaluation précoce et quantitative de l’effet pharmacodynamique du bevacizumab au moyen de l’échographie de contraste dans les métastases hépatiques de cancer colorectal. [Google Scholar]
- 26.Schuster DPPW, Castro M, Saffitz JE, Shannon W. Translational and Experimental Clinical Research. Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 27.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;174:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 28.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;154:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 29.Efron Bootstrap Methods: Another Look at the Jackknife. Ann Stat. 1979;7:1–26. [Google Scholar]
- 30.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;2313:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 31.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;272:157–172. doi: 10.1002/sim.2929. discussion 207-112. [DOI] [PubMed] [Google Scholar]
- 32.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;301:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leening MJ, Vedder MM, Witteman JC, Pencina MJ, Steyerberg EW. Net reclassification improvement: computation, interpretation, and controversies: a literature review and clinician’s guide. Ann Intern Med. 2014;1602:122–131. doi: 10.7326/M13-1522. [DOI] [PubMed] [Google Scholar]
- 34.Labianca R, Sobrero A, Isa L, Cortesi E, Barni S, Nicolella D, et al. Intermittent versus continuous chemotherapy in advanced colorectal cancer: a randomised ‘GISCAD’ trial. Ann Oncol. 2011;225:1236–1242. doi: 10.1093/annonc/mdq580. [DOI] [PubMed] [Google Scholar]
- 35.Nixon AB, Pang H, Starr MD, Friedman PN, Bertagnolli MM, Kindler HL, et al. Prognostic and predictive blood-based biomarkers in patients with advanced pancreatic cancer: results from CALGB80303 (Alliance) Clin Cancer Res. 2013;1924:6957–6966. doi: 10.1158/1078-0432.CCR-13-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karoui M, Roudot-Thoraval F, Mesli F, Mitry E, Aparicio T, Des Guetz G, et al. Primary colectomy in patients with stage IV colon cancer and unresectable distant metastases improves overall survival: results of a multicentric study. Dis Colon Rectum. 2011;548:930–938. doi: 10.1097/DCR.0b013e31821cced0. [DOI] [PubMed] [Google Scholar]
- 37.Poultsides GA, Servais EL, Saltz LB, Patil S, Kemeny NE, Guillem JG, et al. Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J Clin Oncol. 2009;2720:3379–3384. doi: 10.1200/JCO.2008.20.9817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Venderbosch S, de Wilt JH, Teerenstra S, Loosveld OJ, van Bochove A, Sinnige HA, et al. Prognostic value of resection of primary tumor in patients with stage IV colorectal cancer: retrospective analysis of two randomized studies and a review of the literature. Ann Surg Oncol. 2011;1812:3252–3260. doi: 10.1245/s10434-011-1951-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stillwell AP, Buettner PG, Ho YH. Meta-analysis of survival of patients with stage IV colorectal cancer managed with surgical resection versus chemotherapy alone. World J Surg. 2010;344:797–807. doi: 10.1007/s00268-009-0366-y. [DOI] [PubMed] [Google Scholar]
- 40.Koopman M, Antonini NF, Douma J, Wals J, Honkoop AH, Erdkamp FL, et al. Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trial. Lancet. 2007;3709582:135–142. doi: 10.1016/S0140-6736(07)61086-1. [DOI] [PubMed] [Google Scholar]
- 41.Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;3606:563–572. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]
- 42.Ferrand F, Malka D, Bourredjem A, Allonier C, Bouche O, Louafi S, et al. Impact of primary tumour resection on survival of patients with colorectal cancer and synchronous metastases treated by chemotherapy: results from the multicenter, randomised trial Federation Francophone de Cancerologie Digestive 9601. Eur J Cancer. 2013;491:90–97. doi: 10.1016/j.ejca.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 43.Brahimi-Horn MC, Chiche J, Pouyssegur J. Hypoxia and cancer. J Mol Med (Berl) 2007;8512:1301–1307. doi: 10.1007/s00109-007-0281-3. [DOI] [PubMed] [Google Scholar]
- 44.Abajo A, Bitarte N, Zarate R, Boni V, Lopez I, Gonzalez-Huarriz M, et al. Identification of colorectal cancer metastasis markers by an angiogenesis-related cytokine-antibody array. World J Gastroenterol. 2012;187:637–645. doi: 10.3748/wjg.v18.i7.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Volkova E, Willis JA, Wells JE, Robinson BA, Dachs GU, Currie MJ. Association of angiopoietin-2, C-reactive protein and markers of obesity and insulin resistance with survival outcome in colorectal cancer. Br J Cancer. 2011;1041:51–59. doi: 10.1038/sj.bjc.6606005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Cox Univariate analyses for OS prediction with and without the stratified approach (sensitivity analysis)
Red characters represent significative results