Abstract
Gastrointestinal nematodes resistant to anthelmintics have been reported in several regions of Brazil, and they may be associated with economic losses for the cattle industry. This study aimed to evaluate the resistance status of gastrointestinal nematodes from naturally infected beef cattle to several commercially available anthelmintics, as well as to test the efficacy of combinations of anthelmintics against multi-resistant gastrointestinal nematodes. Ten farms located in Rio Grande do Sul state were selected by: farmers' consent; extensive raising system; availability of calves aged from 7 to 9 months naturally infected by gastrointestinal nematodes; absence of anthelmintic treatment for 60 days before the study; and presence of 70–100 calves or more of both genders with ≥200 eggs per gram of feces (EPG) (sensitivity of 50 EPG). These calves were distributed into 10 groups (of 7–10 animals) per farm and treated with ivermectin, doramectin, eprinomectin, fenbendazole, closantel, nitroxynil, disophenol, levamisole, albendazole, or moxidectin. Feces were collected 2 days before treatment and 14 days after treatment. Additional groups of 7–10 calves were used to test six different two-drug combinations at four of the studied farms. In general terms, fenbendazole was the most effective drug, followed by levamisole, disophenol, and moxidectin. However, parasite resistance to multiple drugs was found in all herds, especially in the genera Cooperia spp., Trichostrongylus spp., and Haemonchus spp.. Some of the two-drug combinations were effective against nematode populations identified as resistant to the same compounds when used as single drugs. The most effective combinations were moxidectin + levamisole, doramectin + fenbendazole, and levamisole + closantel. In this study, parasites resistant to the main commercially available anthelmintics were found in all herds, and some combinations of two active components belonging to different chemical groups were effective against multi-drug resistant gastrointestinal nematodes.
Keywords: Endoparasites, FECRT, Bovine, Multidrug resistance
Graphical abstract
Highlights
-
•
Presence of multi-resistant populations of nematodes in all evaluated herds.
-
•
On 60% of the farms, nine of the ten active compounds tested had efficacies <90%.
-
•
All of the avermectins tested were ineffective in controlling nematode infection.
-
•
Cooperia spp. was the most resistant genus.
-
•
Increased efficacy using some drug combinations.
1. Introduction
The cattle industry is one of the largest sectors of the Brazilian economy. Brazil is the world's second largest producer of cattle, with a total herd of 217.4 million head (FAO,, Cider, 2014). Recently, the cattle industry has experienced a rise in intensity and productivity, as shown by a 50% increase in occupancy rate (animal/hectare) and a 3.4% decrease in pasture area from 1990 to 2011 (INSTITUTO FNP, 2012). Particularly in the state of Rio Grande do Sul, beef cattle production occurs predominantly on native pastures, often without considering the effects on sustainability (Beretta et al., 2002) and the environmental changes caused by increased population density and restriction of livestock movement. In addition, genetic selection for desired production characteristics has led to changes in the natural parasite/host balance, resulting in increased susceptibility of cattle to parasites (Waller, 2002).
Infections by gastrointestinal nematodes affect the well-being and productivity of hosts, causing decreased reproductive performance, a low growth rate, weight loss, and poor food conversion (Mello et al., 2006, West et al., 2009, De Graef et al., 2013). In Brazil, anthelmintics are generally used at farmers' discretion, with no restrictions to access to commercially available drugs and without any assistance from veterinarians. Thus, inadequate use of anthelmintics is not rare; indeed, animals are often treated excessively, interfering with production, accelerating selection of resistant parasites, and posing significant problems for the cattle industry (Delgado et al., 2009, Zanetti Lopes et al., 2013).
Parasite resistance has gradually become a significant problem facing cattle producers in several regions worldwide, including Brazil (de Souza et al., 2008, Demeler et al., 2009). Limited information exists regarding parasite resistance status in local cattle herds in the Brazilian state of Rio Grande do Sul; however, there is strong evidence that gastrointestinal nematodes infecting Brazilian herds have gained resistance to the main available classes of anthelmintics (Soutello et al., 2007, Cezar et al., 2010b, Borges et al., 2013, Neves et al., 2014).
This study aimed to verify the existence of populations of gastrointestinal nematodes resistant to several commercially available anthelminthic compounds by evaluating naturally infected beef cattle from herds located in the state of Rio Grande do Sul, Brazil. In addition, the efficacies of some two-drug combinations were tested to assess their potential as alternative to control the multi-drug resistant parasite populations found in the studied herds.
2. Material and methods
2.1. Farms and animals
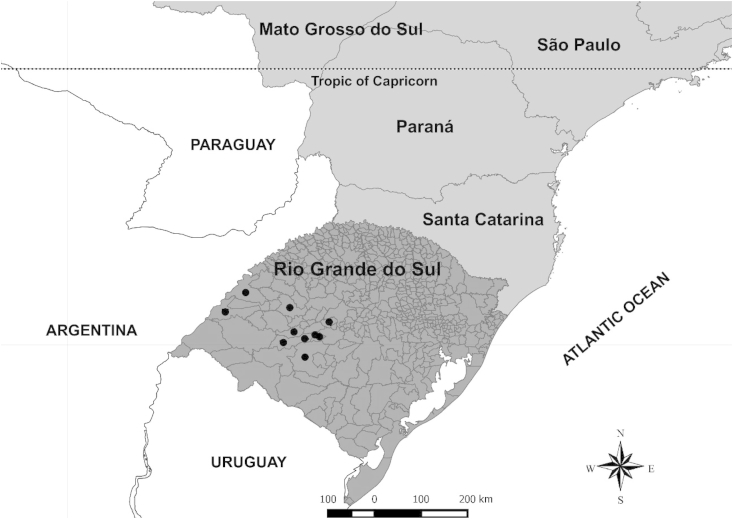
The study was conducted on ten farms located in eight counties of the Rio Grande do Sul state in southern Brazil: São Martinho da Serra, Dilermando de Aguiar (two farms), Cacequi (two farms), São Gabriel, Itaqui, São Borja, Santiago, and São Vicente do Sul (Fig. 1). Preliminarily, herds were selected based on location and previous consent by farmers. Additionally, the following technical criteria were considered: the extensive system used to raise beef cattle; the availability of Bos taurus/Bos indicus crossbred calves of both genders (aging from 7 to 9 months); the presence of 70–100 calves or more per farm with counts of ≥200 eggs per gram of feces (EPG); and the absence of anthelmintic treatment for 60 days before the experimental period. First, all calves available at each farm were included in the study; however, animals with fewer than 200 EPG before treatment were excluded prior to the formation of the experimental groups. Calves were weaned approximately six months after birth and kept in the same grazing area before and during the study on each farm. The use of animals was approved by the Committee of Ethics in Animal Experimentation of the Federal University of Santa Maria under protocol no. 3132240215.
Fig. 1.
Location of ten beef cattle herds studied at eight counties from the state of Rio Grande do Sul in southern Brazil. The black spheres indicate the locations of the farms.
2.2. Anthelmintic treatment
In the first part of the study, ten commercially available anthelmintic compounds were tested on each farm. All treatments were administered by a veterinarian participant of the study following the manufacturer's recommendations: ivermectin 1% (0.2 mg/kg, subcutaneous, Hipramectin® HIPRA), doramectin 1% (0.2 mg/kg, subcutaneous, Dectomax® Zoetis), eprinomectin 0.5% (500 μg/kg, pour-on, Eprinex® Merial), moxidectin 1% (0.2 mg/kg, subcutaneous, Cydectin® Ford Dodge), levamisole 7.5% (3.75 mg/kg, subcutaneous, Ripercol L® Fort Dodge), albendazole 15% (3.4 mg/kg, subcutaneous, Agebendazol® Gener), nitroxynil 34% (9.7 mg/kg, subcutaneous, Dovenix Supra®, Merial), disophenol 20% (5 mg/kg, subcutaneous, Pradoverme® PRADO), fenbendazole 10% (5 mg/kg, oral, Panacur® Intervet), and closantel 10% (10 mg/kg, oral, Diantel® HIPRA).
After determining the efficacy of each single anthelmintic treatment, six combinations of two drugs were tested at four of the ten farms as a second part of this study. For this purpose, new groups of calves, selected by the criteria described before, were used. The drug combinations were based on the results of the first part of this study and selected according to the recommendations of Cezar et al., 2011, Geary et al., 2012, and Pivoto et al. (2014). The choice of two-drug combinations was made with a focus on including different modes of action and efficacy against different genera of gastrointestinal nematodes. The tested combinations were: moxidectin 1% (0.2 mg/kg, subcutaneous, Cydectin® Ford Dodge) + levamisole 7.5% (3.75 mg/kg, subcutaneous, Ripercol L® Fort Dodge), moxidectin 1% (0.2 mg/kg, subcutaneous, Cydectin® Ford Dodge) + albendazole 15% (3.4 mg/kg, subcutaneous, Agebendazol® Gener), albendazole 15% (3.4 mg/kg, subcutaneous, Agebendazol® Gener) + closantel 10% (10 mg/kg, oral, Diantel® HIPRA), doramectin 1% (0.2 mg/kg, subcutaneous, Dectomax® Zoetis) + closantel 10% (10 mg/kg, oral, Diantel® HIPRA), doramectin 1% (0.2 mg/kg, subcutaneous, Dectomax® Zoetis) + fenbendazole 10% (5 mg/kg, oral, Panacur® Intervet), and levamisole 7.5% (3.75 mg/kg, subcutaneous, Ripercol L® Fort Dodge) + closantel 10% (10 mg/kg, oral, Diantel® HIPRA). Each drug in the combination treatments was administered separately.
2.3. Experimental groups and fecal analysis
Samples were collected directly from the rectum of each calf 2 days prior to treatment (D−2) and on day 14 after treatment (D+14) according to the recommendations of Coles et al. (2006). All samples were collected in plastic bags, labeled, stored in isothermal boxes for transport to the laboratory, maintained at 10 °C for up to 12 h after collection, and processed, as recommended by McKenna (1998). All samples were maintained under controlled humidity and temperature before processing and during the larvae culture procedures.
Counting of EPG was performed by a McMaster modified technique, with a sensitivity of 50 EPG. Briefly, each sample of 4 g of homogenized feces was mixed and diluted in 56 mL of saturated solution, re-suspended, sifted, and transferred to a McMaster chamber for EPG counting by microscopic identification. Animals that had an EPG count ≥200 on D−2 were selected. These calves were distributed into 10 randomized blocks based on EPG at each farm, to balance the mean and the frequency distributions of EPG countings among the groups before the treatments. Each of the ten groups was randomly treated with a single drug in the first part of this study. At the four farms included in the second part of this study, six additional groups were treated with a combination of two anthelmintic compounds as described previously. The number of animals in each experimental group ranged from 7 to 10 depending on the available calves at each farm. The total number of calves used per farm was: 257 (farm 1), 110 (farm 2), 205 (farm 3), 108 (farm 4), 127 (farm 5), 138 (farm 6), 264 (farm 7), 184 (farm 8), 181 (farm 9), 130 (farm 10).
On each collection day, fecal samples from all calves in each experimental group were pooled, mixed with sterile wood shavings, and stored for larvae cultures (moisturized daily with sterile water under incubation for seven days at 22–27 °C and 80% humidity), according to the recommendations of Coles et al. (2006). After incubation, larvae were recovered by baermanization, after which 100 third-stage larvae in each culture were identified (by genera) following the criteria described by Van Wyk and Mayhew (2013).
2.4. Statistical analysis
On each farm, pre-treatment and post-treatment EPG counts were used to calculate the efficacy of each treatment based on the reduction in EPG. For this purpose, the approach described by Torgerson et al. (2014) was used (available at http://www.math.uzh.ch/as/index.php?id=254&L=1). The selected approach incorporated random sampling error and aggregations between individual hosts in the treatment groups to provide 95% confidence intervals, which were taken as the 2.5 and 97.5 percentiles of the resulting efficacy distribution.
The efficacy of each treatment against each genus of gastrointestinal nematodes was calculated based on the proportion of each genus of nematode in the larvae cultures at D−2 and D+14 using the following formula: PR = 100 × (1–PERfinal/PERinitial), where PR is the percentage reduction by genus; and PERinitial and PERfinal are the percentages of each genus before (D−2) and 14 days after (D+14) treatment, respectively (Coles et al., 1992, Coles et al., 2006, Neves et al., 2014).
2.5. Interpretation of the results
Anthelmintic resistance status was interpreted as recommended by Lyndal-Murphy et al. (2014) and based on the World Association for the Advancement of Veterinary Parasitology (WAAVP) guidelines on anthelmintic resistance (Coles et al., 1992), considering the EPG reduction percentage and the upper (UCL) and lower (LCL) 95% confidence limits. Therefore, each treatment was classified as effective (when the EPG reduction percentage and upper 95% confidence limit were both equal or above 95% and the lower 95% confidence limit was equal or above 90%), ineffective (parasite resistance confirmed, when the EPG reduction percentage and upper 95% confidence limit were below 95% and the lower 95% confidence limit was below 90%), or inconclusive (when none of the other criteria were fulfilled). Moreover, multi-drug resistant parasites were defined as parasite populations of gastrointestinal nematodes that were resistant to anthelmintic drugs of different chemical classes according to the recommendations of James et al. (2009).
3. Results
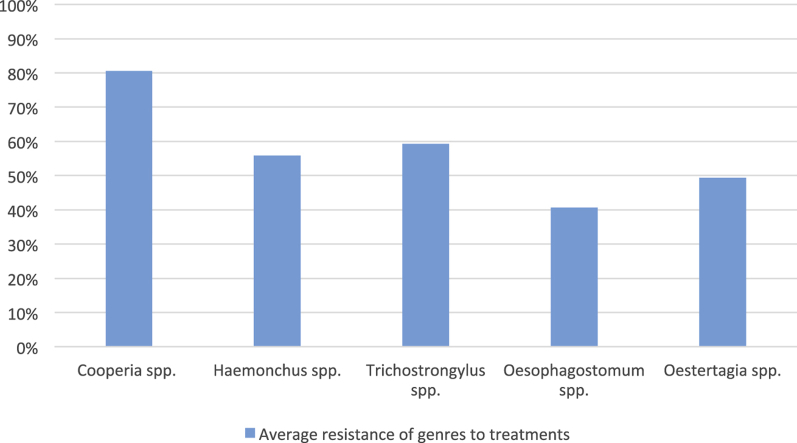
Arithmetic means, minimum EPG counts, maximum EPG counts, and the percentages of each genus of gastrointestinal nematodes found before treatment in each herd are shown in Table 1. Table 2 presents the efficacy of each treatment at each farm. Table 3 shows the percentage reduction of each genus after each treatment at each farm. The presence of gastrointestinal nematodes with resistance to multiple anthelmintic compounds was detected in all evaluated herds; on 60% (6/10) of the farms, nine of the ten active compounds tested had efficacy <90% (Table 2). Fenbendazole was the most effective compound in the studied herds, followed by levamisole, disophenol, and moxidectin. Larvae cultures from animals from all herds showed the presence of mixed infections containing the following genera: Haemonchus, Cooperia, Oesophagostomum, Trichostrongylus, and Ostertagia (Table 1). Oesophagostomum spp. were the most susceptible of the identified genera to anthelmintic compounds, whereas Cooperia spp. were the most resistant, followed by Trichostrongylus spp. and Haemonchus spp. (Table 4).
Table 1.
Arithmetic mean (AM) and standard deviation (SD), minimum (MIN) and maximum (MAX) fecal egg counts, and proportions of genera identified before the treatments (D-2) in the feces of naturally infected beef cattle from ten farms in the state of Rio Grande do Sul.
| Farms | EPG |
Genera of the gastrointestinal nematodes (%) |
||||||
|---|---|---|---|---|---|---|---|---|
| AM (SD) | MIN | MAX | Cooperia spp. | Oesophagostomum spp. | Haemonchus spp. | Ostertagia spp. | Trichostrongylus spp. | |
| 1 | 440.85 (±346.2) | 200 | 1550 | 70 | 26 | 4 | 0 | 0 |
| 2 | 249.1 (±156.5) | 200 | 2700 | 78 | 22 | 0 | 0 | 0 |
| 3 | 623.5 (±501.6) | 200 | 2850 | 60 | 8 | 0 | 4 | 28 |
| 4 | 413.6 (±225.9) | 200 | 1250 | 76 | 0 | 16 | 2 | 6 |
| 5 | 368.5 (±226.3) | 200 | 1200 | 20 | 0 | 40 | 10 | 30 |
| 6 | 776.2 (±593.6) | 200 | 3600 | 34 | 0 | 10 | 0 | 56 |
| 7 | 1657.3 (±1223.2) | 200 | 6850 | 40 | 6 | 44 | 2 | 8 |
| 8 | 806.8 (±605.3) | 200 | 2550 | 87 | 7 | 6 | 0 | 0 |
| 9 | 624.4 (±467.7) | 200 | 2350 | 68 | 14 | 8 | 0 | 0 |
| 10 | 476.7 (±296.1) | 200 | 1350 | 64 | 10 | 26 | 0 | 0 |
Table 2.
Percentage of EPG reduction (and 95% confidence interval) calculated by the fecal egg count reduction test (FECRT) fourteen days after anthelmintic treatment in beef cattle naturally infected by gastrointestinal nematodes on ten farms in the state of Rio Grande do Sul, Brazil.
| Anthelmintic treatments | Reduction of EPG after treatment on each farm |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Ivermectin 1% | 11.6 (−28.1 to 39.5) | 17.6 (−13 to 39.2) | −99.1 (−138 to −61.4) | 21.8 (−12.8 to 42.9) | 56.6 (33.8–72.8) | 17.4 (−1.87 to 33.7) | 69.6 (62.7–74.4) | 45.2 (28.1–59) | −99 (−143 to −63.8) | 61.8 (44.6–76.2) |
| Doramectin 1% | −55.6 (−55.4 to 23.8) | −28.4 (−64 -47.2) | −46 (−82.5 to −20) | 80.9 (67.2–90) | 29.7 (0.67–52.5) | −36.9 (−74.1 to −11.2) | 26.9 (15.2–34.4) | −1.82 (−25 to 23.4) | −21.6 (−47.2 to −4.21) | 42.6 (13.2–62.8) |
| Eprinomectin 0.5% | 67.8 (46.4–80.7) | 81.2 (70.4–88.6) | −9.92 (−45 to 14.8) | 50.5 (21.8–68.8) | 33.1 (−5.8 to 53.3) | 13.6 (−7.5 to 28) | 63.8 (56.6–69.7) | 21.3 (−2.8 to 38.7) | 12.4 (−6.6 to 32.1) | 69.3 (54.5–80.7) |
| Moxidectin 1% | 63.7 (41.2–77.7) | 76.7 (64.8–85.8) | 65.1 (52.5–75.9) | 78.7 (65.3–88.7) | 83.7 (71.3–92) | 80.1 (71.5–86.7) | 90.4 (87.2–93.2) | 78 (66.1–85.6) | 64.4 (49.2–74.9) | 99.2 (95.1–100) |
| Albendazole 15% | 13.4 (−20.3 to 37.7) | 60.2 (44.1–71.9) | −28.2 (−61.2 to 15.2) | 29.1 (0.51–51.9) | −4.3 (−39.5- 27.2) | 37.9 (20.4–50.5) | 57.4 (49.9–63.8) | 79.3 (70.3–86.1) | 29.3 (10.1–44.7) | 87 (74.1–94.3) |
| Levamisole 7.5% | 81.9 (65.3–91.3) | 84.7 (74.9–91.5) | 70.6 (52–82.3) | 90.2 (77.6–96.6) | 34.8 (0.8–55.9) | 46.1 (31–58.4) | 71.7 (64.6–77.5) | 93 (87.4–96.9) | 97.5 (94.2–99.2) | 97.7 (91.8–99.7) |
| Nitroxynil 34% | 71.7 (50.4–84.2) | 48.3 (22–64.8) | −13.4 (−40.3 to 10.5) | 86.1 (73–93.7) | 23.2 (−11.8-48) | 31.1 (12.6–45.9) | 72.8 (65.6–78.5) | 51.6 (37.4–63.9) | 36.7 (16.6–50.6) | 61.1 (41–76.8) |
| Disophenol 20% | 88.7 (75.3–95) | 49.3 (28.5–64.1) | 50.1 (34.2–61.1) | 60.1 (39.1–75.5) | 30 (−1.6 – 52.5) | 91.7 (84.3–95.5) | 77.6 (72.4–82.2) | 73.2 (62–81.4) | 96.3 (92.2–98.4) | 94.8 (87.5–98.5) |
| Closantel 10% | 82.8 (66.1–91.6) | 83.4 (73.6–90.5) | −1.27 (−27.9 to 17.7) | 52.4 (22.9–70.7) | 18.5 (−22.2- 43) | −13.8 (−41.9 to 9.08) | 58.2 (50.6–65.5) | 57.5 (43.2–68.8) | −28.1 (−57.3 to −3.8) | 97.5 (91.5–99.6) |
| Fenbendazole 10% | 91.4 (82.3–96.5) | 97.4 (92.8–99.5) | 85.2 (76.9–91.1) | 54.8 (29.3–71.1) | 91.5 (81.8–96.9) | 76.2 (65.4–83.8) | 88.4 (84.6–91.3) | 91.7 (85.1–95.6) | 91 (85.2–95.1) | 97.8 (92.2–99.7) |
Table 3.
Efficacy (%) of different anthelmintic drugs against each genus of gastrointestinal nematode fourteen days after treatment in naturally infected beef cattle at ten farms in the state of Rio Grande do Sul, Brazil.
| Farm | Genus | Anthelmintic treatments and reduction percentage for each genus after treatment |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ivermectin | Doramectin | Eprinomectin | Moxidectin | Levamisole | Albendazole | Fenbendazole | Closantel | Nitroxynil | Disophenol | ||
| 1 | Coop | 0 | 0 | 0 | 100 | 0 | 1.2 | 0 | 0 | 0 | 0 |
| Haem | 0 | 100 | 0 | 0 | 100 | 0 | 100 | 100 | 100 | 100 | |
| Oesop | 100 | 100 | 0 | 0 | 100 | 100 | 0 | 100 | 100 | 0 | |
| Ostert | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 0 | 0 | 100 | |
| Trich | 100 | 100 | 100 | 100 | 0 | 72.1 | 100 | 100 | 100 | 35.8 | |
| 2 | Coop | 78.7 | 28.7 | 71.2 | 47.4 | 82.4 | 10.55 | 100 | 23 | 48.7 | 47.2 |
| Haem | 0 | 0 | 0 | 0 | – | – | – | – | 0 | – | |
| Oesop | 100 | 49.5 | 0 | 76.7 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Ostert | – | – | – | – | – | 100 | – | – | – | – | |
| Trich | 100 | 0 | 0 | 0 | 0 | 72.1 | – | 100 | 100 | 0 | |
| 3 | Coop | 10 | 16.6 | 79.2 | 40 | 40 | 30.8 | 0 | 50 | 0 | 0 |
| Haem | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – | |
| Oesop | 0 | 0 | 0 | 0 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Ostert | 0 | 0 | 0 | – | 0 | 0 | 0 | 0 | 0 | 0 | |
| Trich | 100 | 71.4 | 33 | 78.7 | 7.1 | 25.8 | 36.5 | 39.2 | 85.7 | 78.5 | |
| 4 | Coop | 26.31 | 58.4 | 34.2 | 26.4 | 23.2 | 28.9 | 34.2 | 0 | 45.1 | 0 |
| Haem | 0 | 0 | 0 | 0 | 47.9 | 87.5 | 0 | 75 | 0 | 87.5 | |
| Oesop | – | 0 | – | 0 | – | 0 | 0 | – | 0 | 100 | |
| Ostert | 0 | 100 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 100 | |
| Trich | 0 | 100 | 0 | 51.6 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 5 | Coop | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| Haem | 100 | 100 | 100 | 37.5 | 100 | 75 | 100 | 100 | 80.7 | 50 | |
| Oesop | – | – | – | – | – | 0 | – | – | – | – | |
| Ostert | 0 | 0 | 0 | 100 | 100 | 100 | 0 | 0 | 0 | 100 | |
| Trich | 16.6 | 16.6 | 100 | 100 | 100 | 66.6 | 100 | 100 | 48.7 | 0 | |
| 6 | Coop | 54.7 | 11.7 | 76.4 | 1.9 | 11.7 | 41.1 | 0 | 0 | 0 | 78.9 |
| Haem | 0 | 0 | 0 | 0 | 60 | 0 | 0 | 100 | 100 | 0 | |
| Oesop | – | 0 | 0 | 0 | – | – | – | – | – | – | |
| Ostert | – | – | – | 78.7 | – | 50 | – | – | – | – | |
| Trich | 58.7 | 78.5 | 39.2 | – | 75 | – | 70.2 | 60.7 | 71.4 | 87.2 | |
| 7 | Coop | 0 | 0 | 0 | 28.5 | 0 | 30 | 0 | 0 | 0 | 0 |
| Haem | 0 | 31.8 | 100 | 0 | 100 | 0 | 72.7 | 77.2 | 59 | 49.5 | |
| Oesop | 0 | 0 | 100 | 0 | 100 | 0 | 100 | 100 | 100 | 100 | |
| Ostert | 100 | 100 | 0 | 100 | 100 | 100 | 100 | 0 | 0 | 100 | |
| Trich | 100 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 100 | 100 | |
| 8 | Coop | 0 | 33.3 | 47.1 | 24.1 | 6.6 | 0 | 100 | 0 | 33.3 | 7.3 |
| Haem | 24.1 | 0 | 0 | 0 | 0 | 100 | 100 | 100 | 100 | 0 | |
| Oesop | 0 | 0 | 0 | 0 | 100 | 100 | 100 | 100 | 100 | 0 | |
| Ostert | – | – | – | – | – | – | – | – | – | – | |
| Trich | 0 | 0 | – | 0 | 0 | – | – | – | 0 | – | |
| 9 | Coop | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Haem | 100 | 25 | 0 | 0 | 100 | 100 | 100 | 75 | 100 | 0 | |
| Oesop | 100 | 100 | 71.4 | 25 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Ostert | – | – | – | – | – | – | – | – | – | – | |
| Trich | – | – | – | – | – | – | – | – | – | – | |
| 10 | Coop | 0 | 0 | 25.9 | NL | 3.8 | 0 | 6.2 | 0 | 0 | 0 |
| Haem | 30.7 | 61.5 | 0 | NL | 100 | 37.5 | 42.3 | 100 | 76.9 | 87.6 | |
| Oesop | 0 | 20 | 0 | NL | 100 | 37.5 | 100 | 100 | 20 | 100 | |
| Ostert | – | – | – | NL | – | – | – | 0 | – | – | |
| Trich | – | – | – | NL | – | 0 | 0 | 0 | – | – | |
Coop: Cooperia spp.; Haem: Haemonchus spp.; Oesop: Oesophagostomum spp.; Ostert: Ostertagia spp.; Trich: Trichostrongylus spp.; NL: no viable larvae after treatment (D+14).
Table 4.
Efficacy (mean (%) and standard deviation) of anthelmintic drugs against each genus of gastrointestinal nematode fourteen days after treatment in naturally infected beef cattle from ten farms in the state of Rio Grande do Sul, Brazil.
| Compounds | Genera |
||||
|---|---|---|---|---|---|
| Cooperia spp. | Haemonchus spp. | Oesophagostomum spp. | Ostertagia spp. | Trichostrongylus spp. | |
| Ivermectin 1% | 19.4 (±27.5) | 23 (±41.6) | 42.8 (±53.4) | 40 (±54.7) | 46.9 (±47.9) |
| Doramectin 1% | 14.8 (±19.8) | 31 (±41.2) | 29.9 (±42.9) | 60 (±54.7) | 45.8 (±45.8) |
| Eprinomectin 0.5% | 33.4 (±42.9) | 20 (±42.1) | 21.4 (±40.4) | 40 (±54.7) | 38.9 (±44.7) |
| Moxidectin 1% | 29.8 (±31.5) | 4.1 (±12.5) | 12.7 (±27.3) | 60 (±54.7) | 63.6 (±42.5) |
| Levamisole 7.5% | 16.7 (±26.4) | 67.5 (±43.0) | 100 (0) | 66.6 (±51.6) | 22.7 (±40.5) |
| Albendazole 15% | 14.2 (±16.5) | 50 (±48.0) | 59.7 (±49.1) | 60 (±54.7) | 26.8 (±31.7) |
| Fenbendazole 10% | 34.0 (±46.6) | 57.2 (±46.9) | 75 (±46.2) | 60 (±48.9) | 43.8 (±46.2) |
| Closantel 10% | 7.30 (±16.6) | 80.8 (±32.5) | 100 (0) | 0 (0) | 37.4 (±44.6) |
| Nitroxynil 34% | 12.7 (±20.8) | 61.6 (±44.5) | 77.5 (±42.0) | 40 (±54.7) | 38.2 (±43.3) |
| Disophenol 20% | 13.3 (±27.3) | 46.8 (±42.6) | 75 (±46.2) | 80 (±44.7) | 43 (±44.8) |
| Overall efficacy means (%) | 19.5 | 44.2 | 59.4 | 50.6 | 40.7 |
Treatment of the animals with avermectin compounds did not result in satisfactory EPG reduction in any herd. Moxidectin was fully effective at one farm, but unsatisfying reductions in EPG counts were observed in the other nine herds. With regard to the benzimidazoles employed in this study, albendazole was ineffective against gastrointestinal parasites at nine farms and showed an inconclusive result at the farm 10. Fenbendazole was effective at farms 2 and 10 and resulted in lower, but not negligible, EPG reductions of approximately 90% at farms 1, 5, 7, 8, and 9. In the same way, levamisole exhibited outstanding efficacy greater than 95% at farms 9 and 10; however, levamisole produced no similar reduction in EPG count at the other six tested farms.
Considering the narrow spectrum compounds tested, closantel had unsatisfying results at 90% (9/10) of the farms. However, closantel was effective against Haemonchus spp. at farms 1, 5, 6, 8, and 10, while it showed no action against Cooperia spp. Phenolic-substituted compounds disophenol and nitroxynil showed differing efficacy. Nitroxynil was ineffective at all farms when EPG reduction was considered, mainly because it had little effect on Cooperia spp., but it was effective against Haemonchus spp. on farms 1, 6, 8, and 9. Disophenol was not effective at six farms, showed inconclusive results at three farms (1, 6, and 10), and had an efficacy of 96.3% at farm 9. Disophenol was effective against Haemonchus spp. at farm 1 and Ostertagia spp. at farms 1, 4, 5, and 7.
The efficacy of each two-drug combination is presented in Table 5. Some combinations were highly effective, surpassing 95% efficacy. The most effective treatment was moxidectin 1% + levamisole 7.5%, followed by doramectin 1% + fenbendazole 10%, which presented some inconclusive results with efficacy of approximately 90%. Table 6 shows the effect of each anthelmintic combination on each genus of gastrointestinal nematodes at each farm. Table 7 shows the mean efficacy of each anthelmintic combination against gastrointestinal nematode genera found at all farms. In general, the same genera identified as resistant to single drugs were found to be resistant to two-drug combinations. However, some groups showed large reductions in EPG counts after treatment with anthelmintic combinations, resulting in a lack of viable larvae after treatment (D+14) (Table 6, Table 7).
Table 5.
Percentage of EPG reduction (and 95% confidence interval) calculated by the fecal egg count reduction test (FECRT) fourteen days after treatment with anthelmintic combinations in beef cattle naturally infected by gastrointestinal nematodes at farms 1, 3, 7, and 8 in the state of Rio Grande do Sul, Brazil.
| Anthelmintic combination | Reduction of EPG after treatment on each farm |
|||
|---|---|---|---|---|
| 1 | 3 | 7 | 8 | |
| Moxidectin 1% + Levamisole 7.5% | 98.1 (93.5–99.8) | 96.5 (93.1–98.6) | 99.1 (94.6–100) | 98.9 (95.2–100) |
| Moxidectin 1% + Albendazole 15% | 87 (76.8–93.7) | 93.1 (87.7–96.3) | 78 (64–87.3) | 95.7 (89.4–98.9) |
| Albendazole 15% + Closantel 10% | 63 (47.4–74) | 61.7 (50–72.2) | 64.7 (44–78.2) | 51.4 (28.6–67.8) |
| Doramectin 1% + Closantel 10% | 67.1 (49.6–78.5) | 54.9 (40.5–65.5) | 71.9 (56.2–82.5) | 66.3 (46.1–77.9) |
| Doramectin 1% + Fenbendazole 10% | 87.9 (77.4–93.7) | 93.1 (89.2–96.4) | 89.2 (78.1–95.4) | 98 (91.2–99.8) |
| Levamisole 7.5% + Closantel 10% | 94.3 (88.7–97.9) | 87.2 (79.9–92.4) | 81.2 (67–90) | 91.8 (83.3–96.4) |
Table 6.
Efficacy (%) of anthelmintic combinations against each genus of gastrointestinal nematode fourteen days after treatment in naturally infected beef cattle at farms 1, 3, 7, and 8 in the state of Rio Grande do Sul, Brazil.
| Farm | Genus | Anthelmintic treatment and percentage reduction of each genus |
|||||
|---|---|---|---|---|---|---|---|
| Moxi + Leva | Moxi + Albe | Albe + Clos | Dora + Clos | Dora + Fenb | Leva + Clos | ||
| 1 | Coop | NL | 0 | 0 | 0 | 42.8 | 0 |
| Haem | NL | 100 | 100 | 100 | 0 | 100 | |
| Oesop | NL | 100 | 100 | 100 | 100 | 100 | |
| Ostert | NL | – | – | – | – | – | |
| Trich | NL | – | – | – | – | – | |
| 3 | Coop | 100 | 23 | 0 | 0 | 0 | 0 |
| Haem | 100 | 0 | 100 | 0 | 100 | 100 | |
| Oesop | 100 | 41.1 | 100 | 100 | 100 | 100 | |
| Ostert | 100 | 0 | 87.6 | 100 | 100 | 25.6 | |
| Trich | 0 | 47 | 100 | 29.3 | 100 | 0 | |
| 7 | Coop | 100 | 16.2 | 35.1 | 0 | 0 | 100 |
| Haem | 100 | 100 | 100 | 100 | 100 | 100 | |
| Oesop | 0 | 100 | 0 | 0 | 100 | 0 | |
| Ostert | – | – | – | – | – | 100 | |
| Trich | – | 0 | – | – | – | – | |
| 8 | Coop | NL | 0 | 0 | 0 | 100 | 0 |
| Haem | NL | 100 | 100 | 100 | 100 | 0 | |
| Oesop | NL | 100 | – | – | 100 | 100 | |
| Ostert | NL | – | – | – | – | – | |
| Trich | NL | – | – | – | – | – | |
Moxi + Leva = moxidectin 1% (0.2 mg/kg, subcutaneous) + levamisole 7.5% (3.75 mg/kg, subcutaneous), Moxi + Albe = moxidectin 1% (0.2 mg/kg, subcutaneous) + albendazole 15% (3.4 mg/kg, subcutaneous), Albe + Clos = albendazole 15% (3.4 mg/kg, subcutaneous) + closantel 10% (10 mg/kg, oral), Dora + Clos = doramectin 1% (0.2 mg/kg, subcutaneous) + closantel 10% (10 mg/kg, oral), Dora + Fenb = doramectin 1% (0.2 mg/kg, subcutaneous) + fenbendazole 10% (5 mg/kg, oral), Leva + Clos = levamisole 7.5% (3.75 mg/kg, subcutaneous) + closantel 10% (10 mg/kg, oral). Coop: Cooperia spp.; Haem: Haemonchus spp.; Oesop: Oesophagostomum spp.; Ostert: Ostertagia spp.; Trich: Trichostrongylus spp.; NL: no viable larvae after treatment (D+14).
Table 7.
Efficacy (mean (%) and standard deviation) of anthelmintic combinations against each genus of gastrointestinal nematode fourteen days after treatment in naturally infected beef cattle from farms 1, 3, 7, and 8 in the state of Rio Grande do Sul, Brazil.
| Anthelmintic combination | Genera |
||||
|---|---|---|---|---|---|
| Cooperia spp. | Haemonchus spp. | Oesophagostomum spp. | Ostertagia spp. | Trichostrongylus spp. | |
| Moxidectin 1% + Levamisole 7.5% | 100 (0) | 100 (0) | 75 (±70.7) | 100 (0) | 0 (0) |
| Moxidectin 1% + Albendazole 15% | 9.8 (±11.6) | 75 (±50) | 85.2 (±29.4) | 0 (0) | 23.58 (±33.2) |
| Albendazole 15% + Closantel 10% | 8.7 (±17.5) | 100 (0) | 75 (±57.7) | 87.6 (0) | 100 (0) |
| Doramectin 1% + Closantel 10% | 0 (0) | 75 (±50) | 75 (±57.7) | 100 (0) | 29.4 (0) |
| Doramectin 1% + Fenbendazole 10% | 35.7 (±47.3) | 100 (±50) | 100 (0) | 100 (0) | 100 (0) |
| Levamisole 7.5% + Closantel 10% | 25 (±50) | 6.6 (±50) | 75 (±50) | 25.7 (±52.6) | 0 (0) |
| Overall efficacy means (%) | 29.8 | 76.1 | 80.8 | 68.8 | 44.7 |
4. Discussion
Resistance of gastrointestinal nematodes infecting cattle to some classes of anthelmintic compounds has been demonstrated in Brazilian herds in the states of Santa Catariana, São Paulo, and Mato Grosso do Sul by Souza et al., 2008, Condi et al., 2009, and Almeida et al. (2013), respectively. However, the results of the present study indicate a worrying situation in relation to the control of gastrointestinal nematodes infections in cattle herds from Rio Grande do Sul because of the high level of multi-drug resistance of the parasite populations found in all farms studied. The broad detection of parasite resistance to several anthelmintics recognized as good quality commercial drugs suggests that parasite populations have developed resistance to the main classes of anthelmintic drugs available in Brazil.
Macrocyclic lactones (MLs), especially avermectins, were not effective in any of the herds assessed in this study, with the exception of moxidectin at one farm. Similar results were found in other cattle herds by Mello et al., 2006, Cezar et al., 2010b, and Lagunes et al. (2015). Mello et al. (2006) and Demeler et al. (2009) reported that MLs are the most commonly used class of compounds for the control of gastrointestinal helminths in ruminants because of their broad spectrum and endectocide activity, which encourage excessive use and have led to resistance. In the farms evaluated here, no detailed information was obtained regarding the history of each drug at each farm because of a lack of available data. Drug use on Brazilian farms is often not based on established criteria, while trademarks and compound names are not well recognized by the farmers. As an exception, ivermectin is well recognized and the most widely used anthelmintic, followed by other avermectins, benzimidazoles, levamisole, and cydectin. Other compounds are eventually used when the farmer suspects that conventional drugs are failing. Commercial availability, endectocide action, and price are generally considered most important criteria influencing the choice of drugs by farmers.
Proportionally to the other genera of gastrointestinal nematodes found in the tested herds, Cooperia spp. larvae showed lower susceptibility to MLs (Table 3, Table 4). Resistance of Cooperia spp. to MLs is not rare; however, treatment failure is often not perceived by farmers because of the low pathogenicity of some species of Cooperia (except, for example, C. oncophora and C. punctata) (Cezar et al., 2010b, Fazzio et al., 2014, Zanetti Lopes et al., 2014). Nevertheless, massive infections by Cooperia spp. can lead to loss of appetite, diarrhea, and decreased weight gain (Demeler et al., 2009). Despite the presence of resistant populations of Cooperia spp. in the studied herds, clinical signs were not apparent in calves. Moreover, larvae of the genera Trichostrongylus, Haemonchus, Ostertagia, and Oesophagostomum were identified as resistant after treatment with MLs; however, these genera were not present in samples from all farms (Table 3).
Levamisole, an imidazothiazole derivative, was a good alternative for the treatment of gastrointestinal nematodes at some farms, in line with reports by Duarte et al. (2012) and Gasbarre (2014). While farmers reported knowledge of levamisole in the present study, it was not frequently used, indicating low selection pressure. This condition may have contributed to the good efficacy of levamisole at some farms. A similar result was found by Molento et al. (2013) regarding sheep in Brazil, where reintroduction of levamisole in a flock that had not been exposed to it for 10 years resulted in efficacy of more than 95%. However, in the present study, with the exception of Oesophagostomum spp., other genera were not fully controlled by levamisole, corroborating the data obtained by de Souza et al. (2008) and Neves et al. (2014).
Phenolic substitutes nitroxynil and disophenol are narrow spectrum anthelmintics that are not recommended in the presence of infections by Cooperia spp., Trichostrongylus spp., or Ostertagia spp.; however, they are indicated to control Haemonchus spp., which is associated with a decrease in food consumption, weight loss, and loss of productivity in cattle (McKellar and Jackson, 2004, Gasbarre, 2014). Nitroxynil and Disophenol were ineffective in reducing the EPG in most herds, mainly because of the presence of genera of gastrointestinal nematodes that were not sensitive to these compounds. Some strains of Oesophagostumum spp. and Ostertagia spp. were susceptible to nitroxynil and disophenol; however, resistance of Haemonchus spp. to nitroxynil and disophenol was detected in some herds. Nitroxynil was effective against Haemonchus spp. at farms 1, 6, 8, and 9, whereas disophenol was effective against Haemonchus spp. at farm 1 and Ostertagia spp. at farms 1, 4, 5, and 7. These results show that phenolic-substituted drugs have limited applicability in the studied cattle herds.
Benzimidazoles (BZs), including albendazole and fenbendazole, are broad-spectrum drugs widely used as anthelmintics in ruminants worldwide (De Graef et al., 2013). Yazwinski et al., 2009, Cezar et al., 2010b, and Demeler et al. (2009) reported efficacies >95% for these anthelmintics in large ruminants. However, in the present study, albendazole had efficacy <90% at all tested farms, while fenbendazole was highly effective at only 2 farms. Frequent use of BZs at the studied farms may have resulted in the establishment of benzimidazole-resistant parasite populations. Considering the location of the farms, these data suggest that parasite resistance to BZs may be spreading in Rio Grande do Sul, similar to the situation observed for avermectins in several Brazilian herds. The resistance of Cooperia spp., Haemonchus spp., and Trichostrongylus spp. to BZs at most farms was similar to the results reported by Yazwinski et al. (2009).
Closantel presents a narrow spectrum of action against gastrointestinal nematodes of ruminants. In Brazil, closantel (Diantel®) is recommended mainly to control Haemonchus spp. infections in sheep and cattle. Thus, closantel can be considered as a treatment for controlling gastrointestinal nematodes in certain conditions (Costa et al., 1996). Although closantel was not previously used in any of the studied cattle herds, it did not control infection by gastrointestinal nematodes at 90% of the tested farms. Cooperia spp. (the least sensitive genus), Trichostrongylus spp., and Ostertagia spp. were not susceptible to closantel in most cases. Closantel was effective against Haemonchus spp. at farms 1, 5, 6, 8, and 10, but Haemonchus spp. were resistant to closantel on farms 3, 4, 7, and 9. While this is the first report of gastrointestinal nematode resistance to closantel in cattle herds of the state of Rio Grande do Sul, resistance to this compound has been reported by Costa et al., 1986, Costa et al., 1996 in the state of São Paulo. Furthermore, closantel resistance is very common in sheep, as reported at several studies, due to its intensive use on small ruminants (Cezar et al., 2010a, Sczesny-Moraes et al., 2010, Verissimo et al., 2012).
Multi-drug resistance occurs when multiple classes of anthelmintics no longer control certain parasitic populations that originally consisted of a large majority (more than 95%) of susceptible genotypes (Taylor et al., 2009). Multi-drug resistance is very common among the main types of gastrointestinal nematodes that infect sheep and goats; indeed, multi-drug resistance is an emerging issue in cattle around the world, including those raised in Brazil and a number of European countries (Rangel et al., 2005, Geurden et al., 2015). The low efficacy of each single drug and the presence of multi-drug resistant gastrointestinal nematodes infecting cattle are major problems that prevent adequate anthelmintic control at the farms evaluated in this study. Thus, more sustainable strategies of anthelmintic control in ruminants are required to overcome the problem of multi-drug resistance (Cezar et al., 2011, Geary et al., 2012).
Given that the main classes of anthelmintics did not reduce the EPG of treated calves, combinations of active compounds were administered as an alternative treatment approach (Bartram et al., 2012). Similar to the results of a study performed by Cezar et al. (2011) in sheep, two-drug combinations of anthelmintics were tested on cattle herds in the present work based on previous tests of the efficacy of single drugs. Therefore, previous knowledge regarding parasite resistance was used as a tool to inform the choice of potentially efficacious combinations of drugs. The use of combinations of two anthelmintic compounds with good efficacy as single drugs could be an effective means of delaying the development of drug resistance in parasites. However, this study was focused on situations in which two effective drugs were unavailable to farmers. Thus, combinations of two anthelmintics that were not fully effective as single drugs, had different modes of action, had broad spectra of action (when possible), and were effective against different genera of gastrointestinal nematodes were tested.
Some of the anthelmintic combinations were effective against multi-drug-resistant parasite populations, reaching EPG reduction percentages ≥95% (Table 5, Table 6). Despite the unsatisfying efficacies of moxidectin and levamisole as single drugs, the combination of moxidectin 1% + levamisole 7.5% was effective in all four evaluated herds. The combination of doramectin 1% + fenbendazole 10% was highly effective at farm 8. Acceptable efficacy was shown by some combinations: moxidectin 1% + albendazole 15% at farms 3 and 8, doramectin 1% + fenbendazole 10% at farm 3, and levamisole 7.5% + closantel 10% at farm 1. The success of this practice can be justified by the fact that combinations of drugs belonging to unrelated chemical groups (with different mechanisms of action) can effectively control parasite genotypes which are not simultaneously resistant to both anthelmintic compounds (Geerts and Gryseels, 2000, Hu et al., 2010). Many of the tested combinations were not effective, probably because of the presence of genotypes of gastrointestinal nematodes resistant to both drugs used in the combinations.
The results of this study showed the presence of gastrointestinal nematodes resistant to the main commercially available anthelmintic drugs on cattle farms evaluated in the state of Rio Grande do Sul, Brazil. In critical situations of parasite resistance, in which no options of effective drugs are commercially available, combinations of two anthelmintic compounds with different mechanisms of action and unsatisfying efficacy as single drugs can effectively control multi-drug-resistant gastrointestinal nematodes. However, such combinations should be evaluated under the particular conditions unique to each farm.
Conflicts of interest
The authors of this manuscript have no financial or personal relationships with other people or organizations that could inappropriately influence or bias the content of the paper.
Acknowledgments
The authors are grateful for the availability and collaboration of producers and their employees to carry out this work.
References
- Almeida G.D., Feliza D.C., Heckler R.P., Borges D.G.L., Onizuka M.K.V., Tavares L.E.R., Paiva F., Borges F.A. Beef Cattle, Mato Grosso do Sul, Brazil. 2013. Ivermectin and moxidectin resistance characterization by larval migration inhibition test in field isolates of Cooperia spp; pp. 59–65. (Vet. Parasitol. 191). [DOI] [PubMed] [Google Scholar]
- Bartram D.J., Leathwick D.M., Taylor M.A., Geurden T., Maeder S.J. The role of combination anthelmintic formulations in the sustainable control of sheep nematodes. Vet. Parasitol. 2012;186:151–158. doi: 10.1016/j.vetpar.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Beretta V., Lobato J.F.P., Netto C.G.M. Produtividade e Eficiência Biológica de Sistemas de Produção de Gado de Corte de Ciclo Completo no Rio Grande de Sul. Rev. Bras. Zoot. 2002;31:991–1001. [Google Scholar]
- Borges F.A., Almeida G.D., Heckler R.P., Lemes, Onizuka M.K.V., Borges D.G.L. Anthelmintic resistance impact on tropical beef cattle productivity: effect on weight gain of weaned calves. Trop. Anim. Health Prod. 2013;45:723–727. doi: 10.1007/s11250-012-0280-4. [DOI] [PubMed] [Google Scholar]
- Cezar A.S., Ribas H.O., Pivoto F.L., Sangioni L.A., Vogel F.S.F. Combinação de drogas antiparasitárias como uma alternativa para o controle de nematódeos gastrintestinais multirresistentes em ovinos. Pesq. Vet. Bras. 2011;31:151–157. [Google Scholar]
- Cezar A.S., Toscan G., Camillo G., Sangioni L.A., Ribas H.O., Vogel F.S.F. Multiple resistance of gastrointestinal nematodes to nine different skid drugs in the sheep flock in southern Brazil. Vet. Parasitol. 2010;173:157–160. doi: 10.1016/j.vetpar.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Cezar A.S., Vogel F.S.F., Sangioni L.A., Antonello A.M., Camillo G., Toscan G., Araujo L.O. Anthelminthic action of different formulations of lactones macrocíclicas on resistant strains of nematodes of cattle. Pesq. Vet. Bras. 2010;30:523–528. [Google Scholar]
- Cider . 2014. System IBGE Data Retrieval.http://www.sidra.ibge.gov.br/bda/pecua/%20default.asp?t=2&Z=t&o=24&u1=1&u2=1&u3=1&u4=1&u5=1&u6=1&u7=1 Available at: (accessed 26.02.15.) [Google Scholar]
- Coles G.C., Bauer C., Borgsteede F.H., Geerts S., Klei T.R., Taylor M.A., Waller P.J. World Association for the Advancement of Veterinary Parasitology (WAAVP) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 1992;44:35–44. doi: 10.1016/0304-4017(92)90141-u. [DOI] [PubMed] [Google Scholar]
- Coles G.C., Jackson F., Pomroy W.E., Prichard R.K., Samson-Himmelstjerna G.V., Silvestre A., Taylor M.A., Vercruysse J. The detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 2006;136:167–185. doi: 10.1016/j.vetpar.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Condi G.K., Soutello R.G.V., Amarante A.F.T. Moxidectin-resistant nematodes in cattle in Brazil. Vet. Parasitol. 2009;161:213–217. doi: 10.1016/j.vetpar.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Costa A.J., Arantes G.J., Vasconcelos O.T., Barbosa O.F., Morais F.R., Paulillo A.C. Espectro de ação do closantel, a 2,5mg/kg, contra nematoides parasitos de bovinos. Rev. Bras. Parasitol. Vet. 1996;5:11–14. [Google Scholar]
- Costa A.J., Rocha U.F., Melito I., Vidotto O. Atividade anti-helmíntica do closantel, nas doses de 10 e 25mg/kg, via oral, contra nematoides gastrintestinais de bovinos naturalmente infectados. Semina. 1986;7(especial):28–33. [Google Scholar]
- De Graef J., Claerebout E., Geldhof P. Anthelmintic resistance of gastrointestinal nematodes cattle. Vlaams Diergeneeskd. Tijdschr. 2013;82:113–123. [Google Scholar]
- Delgado F.E.F., Lima W.D.S., da Cunha A.P., Bello A.C.P.P., Domingues L.N., Wanderley R.P.B., Leite P.V.B., Leite R.C. Verminoses dos bovinos: percepção de pecuaristas em Minas Gerais, Brasil. Rev. Bras. Parasitol. Vet. 2009;18:29–33. doi: 10.4322/rbpv.01803005. [DOI] [PubMed] [Google Scholar]
- Demeler J., Van Zeveren A.M., Kleinschmidt N., Vercruysse J., Höglund J., Koopmann R., Cabaret J., Claerebout E., Areskog M., von Samson-Himmelstjerna G. Monitoring the efficacy of ivermectin and albendazole against gastrointestinal nematodes of intestinal cattle in Northern Europe. Vet. Parasitol. 2009;160:109–115. doi: 10.1016/j.vetpar.2008.10.030. [DOI] [PubMed] [Google Scholar]
- Duarte E.R., Silva R.B., Vasconcelos V.O., Nogueira F.A., Oliveira N.J.F. Diagnostic of the control and sensitivity profile of nematodes from sheep to albendazole and levamisole in northern Minas Gerais, Brazil. Pesq. Vet. Bras. 2012;32:147–152. [Google Scholar]
- FAO, Food and Agriculture Organization. Livestock densities. Available at: http://www.fao.org/Ag/againfo/resources/en/glw/GLW_dens.html (accessed 12.09.14.).
- Fazzio L.E., Sánchez R.O., Streitenberger N., Galvan W.R., Giudici C.J., Gimeno E.J. The effect of anthelmintic resistance on the productivity in feedlot cattle. Vet. Parasitol. 2014;206:240–245. doi: 10.1016/j.vetpar.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Gasbarre L.C. Anthelmintic resistance in cattle nematodes in the US. Vet. Parasitol. 2014;204:3–11. doi: 10.1016/j.vetpar.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Geary T.G., Hosking B.C., Skuce P.J., von Samson-Himmelstijerna G., Maeder S., Holdsworth P., Pomroy W., Vercruysse J. World Association for the Advancement of Veterinary Parasitology (W.L.A.V.P.) Guidelines: anthelmintic combination products targeting nematode infections of ruminants and horses. Vet. Parasitol. 2012;190:306–316. [Google Scholar]
- Geerts S., Gryseels B. Drug resistance in human helminths: current situation and lessons from livestock. Clin. Microbiol. Rev. 2000;13:207–222. doi: 10.1128/cmr.13.2.207-222.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurden T., Christophe Chartier C., Fanke J., Regalbono A.F., Traversa D., Von-Himmelstjerna G.S., Demeler J., Vanimisetti H.B., Bartram D.J., Denwood M.J. Anthelmintic resistance to ivermectin and moxidectin in gastrointestinal nematodes of cattle in Europe. Int. J. Parasitol. Drugs Drug Resist. 2015;5:163–171. doi: 10.1016/j.ijpddr.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Platzer E.G., Bellier A., Aroian R.V. Discovery of a highly synergistic anthelmintic combination that shows mutual hypersusceptibility. PNAS. 2010;107:5955–5960. doi: 10.1073/pnas.0912327107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Instituto FNP . Instituto FNP; São Paulo: 2012. ANUALPEC: Anuário da Pecuária Brasileira; p. 378. [Google Scholar]
- James Catherine E., Hudson Amanda L., Davey Mary W. Drug resistance mechanisms in helminths: is it survival of the fittest? Trends Parasitol. July 2009;25(7):328–335. doi: 10.1016/j.pt.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Lyndal-Murphy M., Swain A.J., Pepper P.M. Methods to determine resistance to anthelmintics when continuing larval development occurs. Vet. Parasitol. 2014;199:191–200. doi: 10.1016/j.vetpar.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Muñiz-Lagunes A., González-Garduño R., López-Arellano M.E., Ramírez-Valverde R., Ruíz-Flores A., García-Muñiz G., Ramírez-Vargas G., Mendoza-de Gives P., Torres-Hernández G. Anthelmintic resistance in gastrointestinal nematodes from beef cattle in Campeche State, Mexico. Trop. Anim. Health Prod. 2015;47:15. doi: 10.1007/s11250-015-0826-3. [DOI] [PubMed] [Google Scholar]
- McKellar Q.A., Jackson F. Veterinary anthelmintics: old and new. Trends Serol. 2004;20:456–461. doi: 10.1016/j.pt.2004.08.002. [DOI] [PubMed] [Google Scholar]
- McKenna P.B. The effect of previous cold storage on the subsequent recovery of infective third stage nematode larvae from sheep faeces. Vet. Parasitol. 1998;80:167–172. doi: 10.1016/s0304-4017(98)00203-9. [DOI] [PubMed] [Google Scholar]
- Mello M.H.A., Depner R.A., Molento M.B., Ferreira J.J. Lateral resistance of macrolactones against cattle nematodes. Arch. Vet. Sci. 2006;11:8–12. [Google Scholar]
- Molento M.B., Verissimo C.J., Amarante A.T., Van Wyk J.A., Chagas A.C.S., Araújo J.V., Borges F.A. Alternatives for the control of gastrointestinal nematoides of small ruminants. Arq. Inst. Biol. São Paulo. 2013;80:253–263. [Google Scholar]
- Neves J.H.D., Carvalho N., Rinaldi L., Cringoli G., Amarante A.F.T. Diagnosis of anthelmintic resistance in cattle in Brazil: a comparison of different methodologies. Vet. Parasitol. 2014;206:216–226. doi: 10.1016/j.vetpar.2014.10.015. [DOI] [PubMed] [Google Scholar]
- Pivoto F.L., Machado F.A., Anezi-Junior P.A., Weber A., Cezar A.S., Sangioni L.A., Vogel F.S.F. Improving liveweight gain of lambs infected by multidrug-resistant nematodes using a FECRT-based schedule of treatments. Parasitol. Res. 2014;113:2303–2310. doi: 10.1007/s00436-014-3885-x. [DOI] [PubMed] [Google Scholar]
- Rangel V.B., Leite R.C., Oliveira P.R., Santos E.J., Jr. Resistência de Cooperia spp. e Haemonchus spp. às avermectinas em bovinos de corte. Arq. Bras. Med. Vet. Zootech. 2005;57:186–190. [Google Scholar]
- Sczesny-Moraes E.A., Bianchin I., da Silva K.F., Catto J.B., Honer M.R., Paiva F. Anthelminthic resistance of gastrointestinal nematodes in sheep. Mato Grosso do Sul. Pesq. Vet. Bras. 2010;30:229–236. [Google Scholar]
- Soutello R.G.V., Seno M.C.Z., Amarante A.F.T. Anthelmintic resistance in cattle nematodes in northwestern São Paulo State, Brazil. Vet. Parasitol. 2007;148:360–364. doi: 10.1016/j.vetpar.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Souza A.P., Ramos C.I., Bellato V., Sartor A.A., Schelbauer C.A. Resistência de helmintos gastrintestinais de bovinos a anti-helmínticos no Planalto Catarinense. Cien. Rural. 2008;38:1363–1367. [Google Scholar]
- Taylor M.A., Learmount J., Lunn E., Morgan C., Craig B.H. Multiple resistance to anthelmintics in sheep nematodes and comparison of methods used for their detection. S. Rumin. Res. 2009;86:67–70. [Google Scholar]
- Torgerson P.R., Paul M., Furrer R. Evaluating faecal egg count reduction using a specifically designed package “eggCounts” in R and a user friendly web interface. Int. J. Parasitol. 2014;44:299–303. doi: 10.1016/j.ijpara.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Van Wyk J.A., Mayhew E. Morphological identification of parasitic nematode infective larvae of small ruminants and cattle: a practical lab guide. J. Vet. Res. 2013;80:1–14. doi: 10.4102/ojvr.v80i1.539. [DOI] [PubMed] [Google Scholar]
- Verissimo C.J., Niciura S.C.M., Alberti A.L.L., Rodrigues C.F.C., Barbosa C.M.P., Chiebao D.P., Cardoso D., Silva G.S., Pereira J.R., Margathoi L.D.F., Costa R.L.D., Nardon R.F., Ueno T.E.H., Curci V.C.L.M., Molento M.B. Multidrug resistance in multispecies and sheep flocks from São Paulo state, Brazil. Vet. Parasitol. 2012;187:209–216. doi: 10.1016/j.vetpar.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Waller P.J. FAO, Biological Control of Nematode Parasites of Small Ruminants in Asia. FAO; Rome, Italy: 2002. Global perspectives on nematode parasites control in ruminant livestock: the need to adopt alternatives to chemotherapy, with emphasis on biological control; p. 104. [DOI] [PubMed] [Google Scholar]
- West D., Pomroy W., Kenyon P.R., Morris S.T., Smith S.L., Burnham D.L. Estimating the cost of subclinical parasitism in grazing ewes. S. Rumin. Res. 2009;86:84–86. [Google Scholar]
- Yazwinski T.A., Tucker C.A., Hornsby J.A., Powell J.G., Reynolds J.L., Johnson Z.B., Lindsey W., Silver T.K. Effectiveness evaluation of several cattle anthelmintics via the fecal egg count reduction test. Parasitol. Res. 2009;105:71–76. doi: 10.1007/s00436-009-1364-6. [DOI] [PubMed] [Google Scholar]
- Zanetti Lopes W.D., Felippelli G., Pires Teixeira W.F., Cruz B.C., Maciel W.G., Buzzulini C., Shigaki de Matos L.V., Costa Gomes L.V., Melo Pereira J.C., Fávero F.C., Oliveira G.P., da Costa A.J. Resistance of Haemonchus placei infection, Cooperia punctate and Oesophagostomum radiatum to ivermectin pour-on of 500 mcg kg−1 in cattle herds in Brazil. Cien. Rural. 2014;44:847–853. [Google Scholar]
- Zanetti Lopes W.D., Santos T.R., Sakamoto C.A., de Lima R.C., Valarelli R.L., Paiva P., da Costa A.J. Persistent efficacy of 3.5% doramectin compared to 3.15% ivermectin against gastrointestinal nematodes in experimentally-infected cattle in Brazil. Res. Vet. Sci. 2013;94:290–294. doi: 10.1016/j.rvsc.2012.09.022. [DOI] [PubMed] [Google Scholar]