Abstract
In schistosomiasis, egg-induced hepatic granuloma formation is a cytokine-mediated, predominantly CD4+ Th2 immune response that can give rise to hepatic fibrosis. Hepatic fibrosis is the main cause of increased morbidity and mortality in humans with schistosome infection. Taurine has various physiological functions and hepatoprotective properties as well as anti-inflammatory and immunomodulatory activity. However, little is known about the role of taurine in schistosome egg-induced granuloma formation and fibrosis. We aimed to evaluate the therapeutic potential of taurine as preventative treatment for Schistosoma japonicum infection. Mice infected with S. japonicum cercariae were supplied with taurine drinking water (1% w/v) for 4 weeks starting at 4 weeks post-infection. Taurine supplementation significantly improved the liver pathologic findings, reduced the serum levels of aminotransferases and area of hepatic granuloma, and prevented fibrosis progression. In addition, taurine decreased the expression of the granulomatous and fibrogenic mediators transforming growth factor β1, tumor necrosis factor α, monocyte chemotactic protein 1α and macrophage inflammatory protein 1α as well as the endoplasmic reticulum stress marker glucose-regulated protein 78. Thus, taurine can significantly attenuate S. japonicum egg-induced hepatic granuloma and fibrosis, which may depend in part on the downregulation of some relevant cytokine/chemokines and reducing the endoplasmic reticulum stress response.
Keywords: Schistosomiasis, Schistosoma japonicum, Granuloma, Fibrosis, Taurine
Graphical abstract
Highlights
-
•
Taurine has potential as preventative & therapeutic treatment for schistosomiasis.
-
•
Taurine reduced the development of liver pathology caused by S. japonicum infection.
-
•
Taurine attenuated S. japonicum egg-induced hepatic granuloma and fibrosis.
1. Introduction
Schistosomiasis is one of the most widely occurring neglected tropical diseases, with high incidence in Asia, Africa and Latin America. About 207 million people have been infected worldwide and 780 million people are at risk of infection, and more than 240 million patients require treatment each year (Beckmann et al., 2014, Guimarães et al., 2015). Schistosoma japonicum (S. japonicum) is the major causative agent in Southeast Asia and China. The presence of eggs from S. japonicum in the host liver and intestinal tissue is the major cause of pathologic schistosomiasis. During infection, schistosome eggs are trapped in the host liver and stimulate the granulomatous response. Subsequent significant fibrosis and circulatory impairment can develop in a subset of individuals with extensive or repeated infection and/or lack of treatment (Liu et al., 2013). Hepatic fibrosis is the principal cause of morbidity and mortality in humans with schistosome infection. However, effective medical interventions to control and treat granuloma and fibrosis in schistosomiasis are lacking.
Granulomatous reaction of schistosome is the CD4+ T cell-mediated delayed-type hypersensitivity induced by soluble egg antigen secreted from viable miracidium within eggs trapped in host tissues. The formation of granulomas is a dynamic process that involves the accumulation of inflammatory and immune cells at the site of antigen release, leading to the confinement of the eliciting agent (Chuah et al., 2014). Toward the late stage, fibroblasts are stimulated by egg products and by T-lymphocyte cytokines to proliferate, replacing most of the cellular elements and mediating fibrotic collagenous material deposition around the portal vein tributaries (Olveda et al., 2014). Many factors are involved in regulating the immunopathogenesis of schistosomiasis. T helper 1 cell (Th1) and Th2 cytokines determine the hepatic granuloma size (Liu et al., 2013, Chuah et al., 2014), and Th17 responses have been linked with severe hepatic inflammation in schistosomiasis (Chuah et al., 2014). Chemokines, particularly macrophage inflammatory protein 1α (MIP-1α) and monocyte chemotactic protein 1α (MCP-1α), play major roles in the formation of hepatic granuloma. Mice deficient in MIP-1α show decreased hepatic granuloma size and both fibrosis and eosinophil peroxidase activity (Souza et al., 2005). MCP-1α, can be produced by activated hepatic stellate cells (HSCs) following liver injury, showed increased transcriptional levels during S. japonicum infection correlates with peak fibrosis (Bartley et al., 2006). Transforming growth factor-β1 (TGF-β1) is the most potent fibrogenic cytokine in the liver. TGF-β1 activates and transforms HSCs into myofibroblast-like cells, which express a-smooth muscle actin (a-SMA) and secrete collagens containing hydrohyproline that form extracellular matrix (ECM) fibrosis (Dooley and ten Dijke, 2012, Sun et al., 2015). In addition, pro-inflammatory stimulation, oxidative stress and tissue damage may play important roles in schistosomiasis (Cunha et al., 2012, de Oliveira et al., 2013).
Taurine (2-aminoethane sulfonic acid), a sulfur-containing β-amino acid, is ubiquitously distributed in animal tissues and cells, accounts for approximately 0.1% of total human body weight. It is both synthesized endogenously from cysteine and methionine and ingested directly with certain foodstuffs. According to the European Food Safety Authority, taurine (3–6 g) has been administered daily to a large number of patients (including adults, children and even infants). No adverse health effects have been noted (Schaffer et al., 2014). In recent years taurine has been widely used as a performance-enhancing ingredient in energy drinks (Luckose et al., 2015). In general, oral taurine can be absorbed by gastrointestinal tract, plasma proteins combination with taurine are fewer. Taurine discharges mainly in prototype and kidney can adjust the content of taurine in the body. The normal concentration of taurine in the plasma is very low (e.g.<60 μM in cat) but most tissues contain very high taurine levels (mM range), creating a substantial concentration gradient across the cell membrane (Schaffer et al., 2014). The half-life of turnover of taurine in the mouse was 18.6 days (Huxtable and Lippincott, 1982). Taurine has various physiological functions and protective properties including protection against various types of hepatic damage (Gentile et al., 2011). In addition, taurine possesses anti-inflammatory and immunoregulatory properties (De Luca et al., 2015). Previous studies have demonstrated that exogenous supplementation with taurine can prevent liver injury caused by different harmful substances as well as inhibit ECM deposition on the damaged liver and stop the process of liver fibrosis (Miyazaki et al., 2005, Devi et al., 2009, Devi et al., 2010, Gentile et al., 2011). Mice with hetero- and homozygous knockout of the taurine transporter show chronic liver disease characterized by fibrosis, inflammation, and hepatocyte apoptosis (Warskulat et al., 2006). The hepatoprotective effects of taurine are often accompanied by reduced endoplasmic reticulum (ER) stress, oxidative stress, production of inflammatory and fibrogenic mediators and activation of stellate cells (Erman et al., 2004, Devi et al., 2010, Gentile et al., 2011). However, whether taurine supplementation can affect the pathological processes of hepatic granulomas and fibrosis elicited by S. japonicum infection is not known.
In this paper, we aimed to determine the effect of taurine supplementation on granuloma formation and the fibrosis process in an animal model of S. japonicum infection to assess its potential as preventative and therapeutic treatment for schistosomiasis. Miyazaki et al. (2004) reported that the effective and optimal doses of oral taurine administration for two weeks on a transient exercise performance in rat were between 100 and 500 mg/kg/day. Various reports have described the experimental use of taurine supplemented in drinking water in mice over the concentration range of 0.05%∼5% (Ribeiro et al., 2010, Santora et al., 2013, Santos-Silva et al., 2015). Hence, we used 1% taurine supplementation in our experiment. We found that taurine supplementation could suppress S. japonicum egg-induced liver granuloma and fibrosis in mice.
2. Materials and methods
2.1. Parasite and animals
S. japonicum (Chinese mainland strain)-infected Oncomelania hupensis snails were purchased from the Jiangsu Institute of Parasitic Diseases (Wuxi, Jiangsu, China). Female ICR mice, 6–8 weeks old, were from the Department of Laboratory Animal Science of Peking University Health Science Center (Beijing). All animal care and experimental protocols complied with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication, 8th Edition, 2011) and were approved by the Animal Care Committee of Peking University Health Science Center.
2.2. Infection of mice with S. japonicum
S. japonicum cercariae were shed in a beaker after exposing infected Oncomelania to light for 6 h in 24–28 °C. The mice were infected percutaneously with 30 ± 2 freshly shed cercariae after they had been anesthetized by intraperitoneal injection of ketamine.
2.3. Experimental group and taurine treatment
Mice were randomly divided into three groups for treatment (10 mice per group): 1) control (Con), mice not infected with S. japonicum and fed standard chow; 2) infected (Inf), mice infected with S. japonicum and fed standard chow; 3) infected/taurine (Tau), mice infected with S. japonicum, fed standard chow and 1% taurine (Sigma, MO, USA) in drinking water for 4 weeks starting at 4 weeks post-infection. At 8 weeks post-infection, the body weight of each mouse was weighed and serum was separated from blood taken from the mouse eye socket, then mice were killed by cervical dislocation, and liver tissue and serum samples were collected for analysis.
2.4. Liver and spleen indexes, egg burden
The liver and spleen tissue was weighed. Liver and spleen indexes were calculated as ratios of liver to body weight and spleen to body weight, respectively.
To determine the egg burden, 1 g of each liver was digested with 5% KOH at 37 °C overnight. After centrifugation, released eggs in the liver were then determined by microscopic examination.
2.5. Analysis of liver transaminase activity
Liver injury was assessed by measuring serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) by use of an Olympus AU5800 automatic biochemical analyzer (Olympus, Japan).
2.6. Histology and immunohistochemistry (IHC) of liver sections
Excised livers were instantly fixed in 10% neutral formalin overnight and embedded in paraffin. For histology, according to standard procedures, tissue sections (4 μm) were stained with hematoxylin and eosin (H&E) to examine the area of the granulomas or with Masson trichrome to evaluate the extent of hepatic fibrosis. Granuloma formation and fibrosis were analyzed under a Leica DMI 3000B fluorescence microscope. The granulomatous area as a percentage of total area for each side was measured by computer-assisted morphometric software (Leica Application Suite 4.1). For each specimen, at least three non-contiguous slides were measured and the mean values from 6 to 8 mice for each group were used for statistical analysis.
Tissue underwent immunostaining for anti-α-SMA antibody, a marker of HSC activation, and glucose-regulated protein 78 (GRP78), an ER stress marker (Abcam, Cambridge, UK). Six to 10 microphotographs per mouse liver were recorded under an inverted microscope (Leica DMI 3000B). At least three non-contiguous tissue sections were measured, and the mean values from 6 to 8 mice were used for statistical analysis.
2.7. Quantitative PCR
Total RNA was extracted from liver tissue of each mouse by use of Trizol Reagent (Applygen Technologies, Beijing). In total, 1 μg RNA was reverse-transcribed to cDNA by use of the PrimeScript RT reagent kit (TaKaRa Biotechnology, Dalian, China). Real-time PCR experiments were performed in triplicate with SYBR premix Ex TaqTM Ⅱ (TaKaRa Biotechnology) on an ABI 7500 Fast Real-Time PCR System (Applied Biosystems, Fullerton, CA). The PCR primers were designed by use of the Primer-BLAST tool on the NCBI website; data on primers are in Table 1. Levels of target genes were normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a housekeeping gene (Jakubowski et al., 2005, Tang et al., 2014). Relative mRNA expression was analyzed by use of Applied Biosystems 7500 Software and calculated by the comparative Ct method with the formula 2−△△Ct.
Table 1.
Gene-specific primers used for quantitative real-time RT-PCR.
| Gene name | Primer sequences (5′→3′) | Annealing temp.(°C) | Length (bp) | Acc.No./ID |
|---|---|---|---|---|
| TNF-α | F: GCCCACGTCGTAGCAAACCACC R: TCGGCTGACGGTGTGGGTGA |
60 | 204 | NM_013693.2 |
| TGF-β1 | F: AGCTGCGCTTGCAGAGATTA R: TTCCGTCTCCTTGGTTCAGC |
60 | 180 | NM_011577.1 |
| MIP-1α | F: CCATATGGAGCTGACACCCC R: GAGCAAAGGCTGCTGGTTTC |
60 | 101 | NM_011337.2 |
| MCP-1α | F: CAGATGCAGTTAACGCCCCA R: AGCTTCTTTGGGACACCTGC |
60 | 116 | NM_011333.3 |
| GRP78 | F: ATCGTGCCTCTCATTGGTGG R: TAGTTGGAGGCCGCTGATTG |
60 | 149 | U16277.1 |
| GAPDH | F: ATGACATCAAGAAGGTGGTGAAG R: TCCTTGGAGGCCATGTAGG |
60 | 238 | NM_008084.2 |
F: forward; R: reverse; TNF-α: tumor necrosis factor-alpha; TGF-β1: transforming growth factor-beta 1; MIP-1α: macrophage inflammatory protein 1 alpha; MCP-1α: monocyte chemotactic protein 1 alpha; GRP78: 78 kDa glucose-regulated protein; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
2.8. Statistical analysis
Results were presented as mean ± SEM. Statistical analysis involved use of GraphPad Prism 5 for Windows (GraphPas Software, San Diego, CA, USA). One-way and two-way ANOVA was used to for analysis. P < 0.05 was considered statistically significant.
3. Results
3.1. Taurine treatment improves the gross appearance and decreases liver and spleen indexes of S. japonicum-infected mice
After infection of S. japonicum, infected mice showed typical manifestations, including diarrhea and athrepsia. The survival rates of infected and infected/taurine-treated groups were 60% and 80%, respectively, at 8 weeks post-infection, and hence, subsequent data collection involved less than the 10 mice initially allocated to each treatment group.
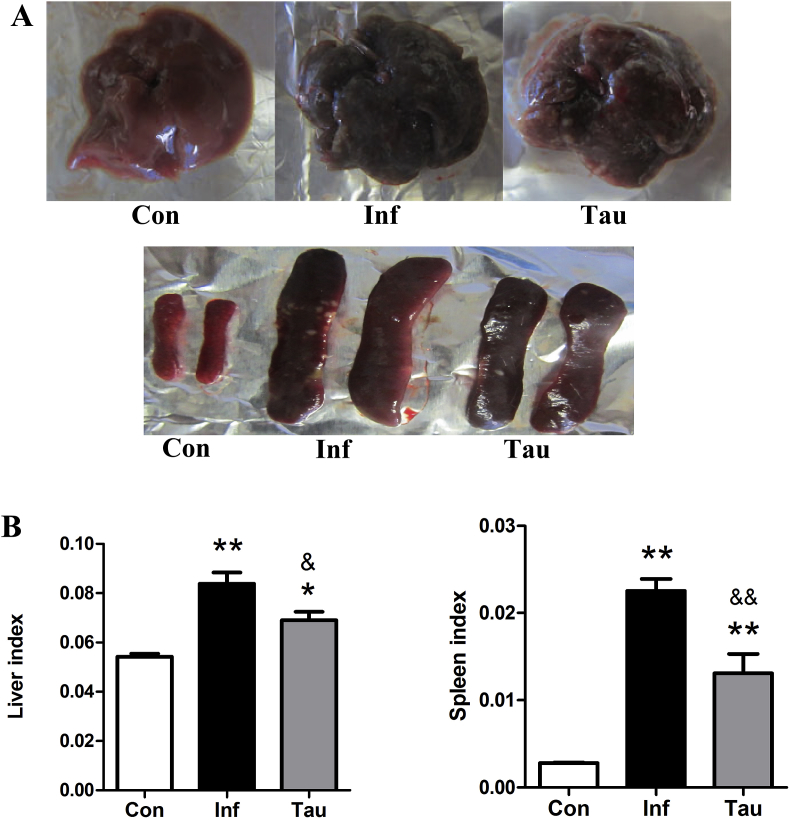
To evaluate the general effect of taurine on the pathologic features of S. japonicum-infected mice, control, infected and infected/taurine-treated mice were weighed and killed, the gross appearance of liver and spleen was observed, and liver and spleen indexes were measured. Normal livers were light red with a smooth surface after blood removal, whereas infected livers were darker red, with many small white spots on the surface, indicating many granuloma nodules. Livers from taurine-treated mice showed an improved appearance over infection alone and fewer white spots (Fig. 1A). The size of spleen increased greatly at 8 weeks post-infection, and taurine treatment attenuated this increase (Fig. 1A). In addition, body weight was lower in infected than control mice but was slightly higher with taurine treatment than with infection alone (data not shown). Liver and spleen indexes were higher for infected than control mice (all p < 0.01) (Fig. 1B). Accordingly, taurine treatment attenuated this increase (p < 0.05 and p < 0.01, respectively). Therefore, taurine treatment improved the gross appearance of livers and decreased liver and spleen indexes of S. japonicum-infected mice.
Fig. 1.
Taurine improves the gross appearance and decreases liver and spleen indexes of Schistosoma japonicum-infected mice. ICR mice were infected percutaneously with 30 ± 2 S. japonicum cercaria. S. japonicum-infected mice were fed 1% taurine in drinking water for 4 weeks starting at 4 weeks post-infection. (A) Gross appearance of the liver and spleen of control, infected and infected/taurine-treated mice. (B) Liver and spleen indexes. Data are mean ± SEM of 6–10 mice/group. Con: control group; Inf: infected group; Tau: infected/taurine-treated group. *p < 0.05, **p < 0.01 vs Con; &: p < 0.05, &&: p < 0.01 vs Inf.
3.2. Taurine treatment attenuates hepatic injury of S. japonicum-infected mice
We further evaluated the effect of taurine on the hepatic injury of S. japonicum-infected mice. The serum ALT and AST levels were higher in infected than control mice (p < 0.05 and p < 0.01, respectively) and were lower with taurine treatment than with infection alone (Fig. 2, all p < 0.05). Hence, taurine treatment may attenuate the hepatic injury of S. japonicum-infected mice.
Fig. 2.
Effect of taurine on the levels of serum transaminases (ALT/AST) of S. japonicum-infected mice. ICR mice were infected percutaneously with 30 ± 2 S. japonicum cercaria. S. japonicum-infected mice were fed 1% taurine in drinking water for 4 weeks starting at 4 weeks post-infection. Serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured by use of a biochemical analyzer. Data are mean ± SEM of 6–10 mice/group. Con: control group; Inf: infected group; Tau: infected/taurine-treated group. *p < 0.05, **p < 0.01 vs Con; &: p < 0.05 vs Inf.
3.3. Egg burden is similar in taurine treated and untreated mice infected with S. japonicum
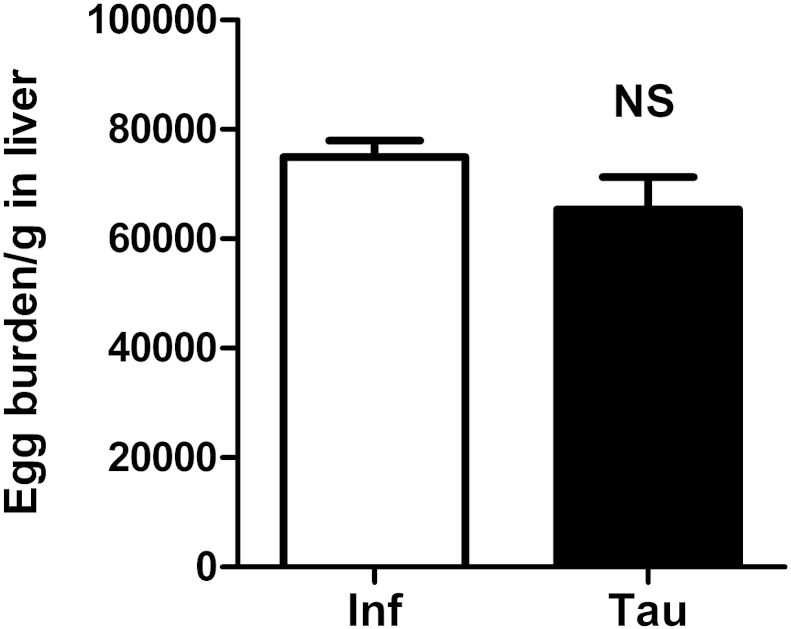
The soluble egg antigen secreted by matured schistosome miracidium within eggs is believed to cause a granulomatous response, so egg load in liver determines the severity of liver pathology. We evaluated the egg burden of liver in taurine treated and untreated mice infected with S. japonicum. Result showed similar liver egg burden between taurine treated and untreated mice (Fig. 3). This result implies that taurine treatment can not affect the deposition of eggs on the liver and the ameliorative effect of taurine on hepatic injury is not caused by difference in schistosome egg.
Fig. 3.
Egg burden is similar in taurine treated and untreated mice infected with S. japonicum. ICR mice were infected percutaneously with 30 ± 2 S. japonicum cercaria. S. japonicum-infected mice were fed 1% taurine in drinking water for 4 weeks starting at 4 weeks post-infection. The number of eggs extracted from the liver was determined by microscopic examination. Data are given as mean ± SEM of 6–8 mice/group. Inf: infected group; Tau: infected/taurine-treated group. NS: no statistical significance.
3.4. Taurine treatment suppresses S. japonicum egg-induced liver granuloma formation
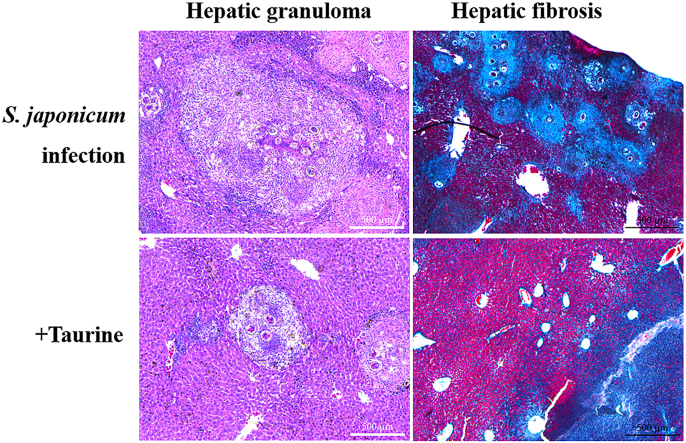
Liver granulomas and fibrosis are the pathological hallmarks of schistosome infection. To investigate the effect of taurine on S. japonicum egg-induced liver granuloma, H&E staining of liver sections was used to analyze the proportion of granulomatous area. Control livers showed normal cellular organization of uninfected hepatic lobules, with the typical actinomorphous distribution of hepatic cords around central veins (Fig. 4A). Infected livers showed markedly altered histological structure. At 8 weeks post-infection, inflammatory granulomatous lesions were seen in the liver tissue around schistosome eggs (Fig. 4B). Taurine treatment markedly reduced the number and diameter of granulomas (Fig. 4C). Compared with infected mice, taurine-treated mice showed significantly decreased proportion of liver granulomatous area (Fig. 4D; p < 0.01).
Fig. 4.
Taurine suppresses S. japonicum egg-induced liver granuloma formation. ICR mice were infected percutaneously with 30 ± 2 S. japonicum cercaria. S. japonicum-infected mice were fed 1% taurine in drinking water for 4 weeks starting at 4 weeks post-infection. H&E staining of liver sections in (A) control, (B) S. japonicum-infected and (C) infected/taurine-treated mice (original magnification 50 ×). Arrows indicate granulomatous lesions and arrowheads indicate schistosome eggs. (D) The granulomas area as a percentage of total area was measured by computer-assisted morphometric analysis. Data are mean ± SEM of 6–8 mice/group. Inf: infected group; Tau: infected/taurine-treated group. **p < 0.01 vs Inf.
3.5. Taurine treatment reduces S. japonicum egg-induced liver fibrosis
Liver fibrosis was evaluated by Masson trichrome staining and IHC staining for α-SMA protein. With Masson trichrome staining, the collagen fibers were stained blue, cell nuclei were stained black, and the background was stained red. Control mice showed normal collagen deposition with periportal few collagen. However, the amount of collagen fibers was significantly increased in infected liver, with most around granulomas. Compared with infection alone, taurine treatment decreased collagen deposition (Fig. 5A).
Fig. 5.
Taurine reduces S. japonicum egg-induced liver fibrosis. ICR mice were infected percutaneously with 30 ± 2 S. japonicum cercaria. S. japonicum-infected mice were fed 1% taurine in drinking water for 4 weeks starting at 4 weeks post-infection. Liver tissues were fixed and stained with Masson trichrome or anti-α-smooth muscle actin (SMA) antibody. (A) Masson trichrome staining for collagen content and distribution. Collagen fibers were stained blue, cell nuclei black, and background red (original magnification 50 ×). (B) Immunohistochemistry of expression of α-SMA protein in liver tissues (original magnification 200 ×). The quantitative changes were measured by computer-assisted morphometric analysis. Data are mean ± SEM of 6–8 mice/group. Con: control group; Inf: infected group; Tau: infected/taurine-treated group. *p < 0.05, **p < 0.01 vs Inf. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Activation of HSCs into fibroblasts is the key event in the process of liver fibrosis. Expression of α-SMA is commonly used as a hallmark of the activated HSCs. IHC staining for α-SMA revealed significantly increased amount of α-SMA+ cells in liver of infected mice but reduced with taurine treatment (Fig. 5B), which was consistent with the degree of fibrosis. Taurine treatment may reduce S. japonicum egg-induced liver fibrosis.
3.6. Taurine treatment decreases tumor necrosis factor α (TNF-α), TGF-β1, MCP-1α and MIP-1α mRNA levels
To explore the molecular mechanism underlying the anti-granulomatous and anti-fibrotic effects with taurine supplementation, we analyzed the mRNA levels of several granulomatous and fibrogenic mediators in mice livers. Compared with control mice, infected mice showed increased levels of TNF-α, TGF-β1, MCP-1α and MIP-1α (Fig. 6, all p < 0.01). However, taurine supplementation significantly decreased the levels of these cytokines/chemokines (p < 0.05 for TGF-β1 and MCP-1α, p < 0.01 for TNF-α and MIP-1α). The ameliorative effects of taurine supplementation on S. japonicum egg-induced hepatic granulomas and fibrosis may depend in part on the downregulation of these relevant cytokines/chemokines.
Fig. 6.
Taurine decreases transforming growth factor β1 (TGF-β1), tumor necrosis factor α (TNF-α), monocyte chemotactic protein 1α (MCP-1α) and macrophage inflammatory protein 1α (MIP-1α) mRNA levels. ICR mice were infected percutaneously with 30 ± 2 S. japonicum cercaria. S. japonicum-infected mice were fed 1% taurine in drinking water for 4 weeks starting at 4 weeks post-infection. Real-time quantitative PCR of liver tissue. Data are mean ± SEM of 6–8 mice/group. Con: control group; Inf: infected group; Tau: infected/taurine-treated group. *p < 0.05, **p < 0.01 vs Con; &: p < 0.05, &&: p < 0.01 vs Inf.
3.7. Taurine treatment markedly suppresses GRP78 level
ER stress plays an important role in the development and progression of liver fibrosis. Taurine is a well-known ER stress modulator that prevents abnormal protein aggregation and unfolded protein response. We examined the expression of the ER stress marker GRP78 in mouse liver by IHC staining and real-time PCR. GRP78 expression was greatly increased in livers of infected mice as compared with controls, and taurine supplementation markedly suppressed GRP78 expression (Fig. 7), which suggests that taurine attenuating the hepatic pathologic features of infected mice may involve modulating the ER stress response.
Fig. 7.
Taurine suppresses the expression of endoplasmic reticulum (ER) stress marker glucose-regulated protein 78 (GRP78). ICR mice were infected percutaneously with 30 ± 2 S. japonicum cercaria. S. japonicum-infected mice were fed 1% taurine in drinking water for 4 weeks starting at 4 weeks post-infection. (A) Immunohistochemistry of protein expression of GRP78 in mouse liver tissue (Original magnification: left, 50 ×; right, 200 ×). (B) Quantitative PCR analysis of mRNA level of GRP78 in mouse liver tissue. Data are mean ± SEM of 6–8 mice/group. Con: control group; Inf: infected group; Tau: infected/taurine-treated group. **p < 0.01 vs Con; &: p < 0.05 vs Inf.
4. Discussion
S. japonicum is one of the most common public health problems in China. Schistosome egg-induced hepatic granuloma formation can lead to tissue destruction and fibrosis, which causes much of the morbidity and mortality associated with this disease. Therefore, inhibiting schistosome egg-induced granuloma and subsequent fibrosis can prevent the development of the disease. Here, we found that taurine supplied in drinking water to mice may have significant potential as a preventative and therapeutic treatment to ameliorate hepatic injury and suppress hepatic granuloma formation and fibrosis during S. japonicum infection.
The formation of schistosome egg-induced hepatic granuloma is a dynamic process triggered by soluble egg antigen secreted from viable miracidium within eggs trapped in liver tissue. Because of the continuous antigenic stimulation from the trapped ova, inflammatory and immune cells are sequentially recruited to the sites of infection, thereby leading to the formation of periovular granulomas (Chuah et al., 2014). The liver gradually hardens and is filled with many granuloma nodules (Fig. 1A). As well, a severe granulomatous response precipitates fibrosis in the liver quickly and eventually causes extensive tissue scarring, which in turn causes severe circulatory impairment of the affected organs. So, granuloma formation is the essential pathological lesion of schistosomiasis, and blocking its formation is critical for the prognosis of disease. In the current study, taurine supplied in drinking water to mice reduced the elevated aminotransferase levels and improved the gross health state of S. japonicum-infected mice (Fig. 1, Fig. 2). Although taurine supplementation could not reduce the egg burden in liver (Fig. 3), it suppressed granuloma formation induced by S. japonicum eggs (Fig. 4). We confirmed that taurine has hepatoprotective effects and can inhibit granuloma formation in schistosome infection.
During the chronic phase of infection, collagen is increasingly deposited in the host liver and fibrosis develops. HSCs are one of the main sources of collagen in the liver and play crucial roles in schistosome-induced fibrogenesis. Quiescent HSCs primarily function to store vitamin A, but in response to liver tissue injury, they are activated and transdifferentiate into myofibroblasts, characterized by the production of ECM components rich in fibrillar collagens (Chuah et al., 2014). Activated HSCs are located at the edges of hepatic granulomas in mice infected with S. japonicum, as shown by IHC staining for the HSC marker a-SMA. Because of increased collagen deposition and portal hypertension, liver function is further damaged, which leads to liver cirrhosis. Fibrosis is reversible, but cirrhosis is irreversible, so fibrosis progressing to cirrhosis must be prevented. Previous studies have demonstrated that taurine can inhibit the deposition of the ECM in the damaged liver and the proliferation of HSC (Chen et al., 2004). Oral taurine administration enhances hepatic taurine accumulation, reduces oxidative stress and prevents the progression of hepatic fibrosis in rats with carbon tetracholoride-induced hepatic damage and inhibits the transformation of HSCs (Miyazaki et al., 2005). In addition, natural taurine can promote HSC apoptosis to inhibit hepatic fibrosis (Deng et al., 2010). Consistent with these results, our studies showed that taurine supplementation could ameliorate S. japonicum-induced hepatic fibrosis (Fig. 5).
The initiation, development, and regression of hepatic granuloma and fibrosis is mediated by the communication of many cytokines and chemokines. Studies in vitro and in experimental models in vivo have demonstrated that TNF-a plays a role in granuloma formation and hepatic fibrosis. In fact, TNF-a seems to play a central role in promoting periportal fibrosis during Schistosoma mansoni infection and was found to be elevated in peripheral blood eosinophils from chronic S. mansoni-infected patients (De Souza Rda et al., 2012). TGF-β1 is the most potent fibrogenic cytokine in the liver; its expression is increased during fibrogenesis and it is the dominant stimulus inducing HSCs to increase ECM synthesis (Dooley and ten Dijke, 2012). Taurine can promote apoptosis in HSCs by inhibiting the expression of TGF-β1, thereby blocking the TGF-β1/Smad pathway, a canonical pathway that can potently regulate hepatic fibrogenesis (Yoshida and Matsuzaki, 2012, Li et al., 2013). In addition, chemokine MCP-1a can trigger migration of monocytes into hepatic granulomas. To that effect, the expression profile of MCP-1a is closely correlated with granuloma diameter and egg burden, so the molecule may play a key role in initiating and maintaining the immune response to tissue egg deposition (Bartley et al., 2006). MIP-1α is a marker of disease severity in schistosome-infected humans, and experimental studies in mice suggest that MIP-1α may be a causative factor in the development of severe schistosomiasis (Souza et al., 2005). We found that taurine supplementation could downregulate these granulomatous and fibrogenic mediators in the liver of S. japonicum-infected mice (Fig. 6), which further confirmed the anti-granulomatous and anti-fibrotic effects of taurine during S. japonicum infection.
ER stress plays an important role in the development and progression of liver fibrosis (Li et al., 2015). The beneficial effects of taurine are often accompanied by reduced ER stress, which suggests a link between the therapeutic properties of taurine and restoration of ER homeostasis. Taurine mitigated palmitate-induced ER stress and cell death in primary hepatocytes and H4IIE liver cells; part of the protective effects of taurine in diet-induced nonalcoholic fatty liver disease were related to the amelioration of ER stress (Gentile et al., 2011). To further determine the underlying mechanisms of taurine improving the hepatic pathologic features of S. japonicum-infected mice, we studied the effect of taurine supplementation on the expression of the ER stress marker GRP78 in the liver of infected mice. Taurine supplementation markedly suppressed GRP78 expression in the liver of infected mice (Fig. 7), which suggested that taurine attenuating the hepatic pathologic features of S. japonicum-infected mice may be related in part to its modulation of the ER stress response. However, the exact mechanism still needs further investigation.
In conclusion, taurine supplementation could reduce hepatic lesions and restrain granuloma formation and resultant fibrosis in S. japonicum-infected mice by downregulating granulomatous and fibrogenic mediators and alleviating ER stress response. Taurine may have significant potential as a preventative and therapeutic treatment in S. japonicum infection.
Acknowledgments
This work was supported by Leading Academic Discipline Project of Beijing Education Bureau (BMU20110254) and the National Natural Science Foundation of China (no. 30901247).
Contributor Information
Yan-Rong Yu, Email: yuyr@bjmu.edu.cn.
Yong-Fen Qi, Email: yongfenqi@163.com.
References
- Bartley P.B., Ramm G.A., Jones M.K., Ruddell R.G., Li Y., McManus D.P. A contributory role for activated hepatic stellate cells in the dynamics of Schistosoma japonicum egg-induced fibrosis. Int. J. Parasitol. 2006;36(9):993–1001. doi: 10.1016/j.ijpara.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Beckmann S., Long T., Scheld C., Geyer R., Caffrey C.R., Grevelding C.G. Serum albumin and α-1 acid glycoprotein impede the killing of Schistosoma mansoni by the tyrosine kinase inhibitor Imatinib. Int. J. Parasitol. Drugs Drug Resist. 2014;4(3):287–295. doi: 10.1016/j.ijpddr.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.X., Zhang X.R., Xie W.F., Li S. Effects of taurine on proliferation and apoptosis of hepatic stellate cells in vitro. Hepatobiliary Pancreat. Dis. Int. 2004;3(1):106–109. [PubMed] [Google Scholar]
- Chuah C., Jones M.K., Burke M.L., McManus D.P., Gobert G.N. Cellular and chemokine-mediated regulation in schistosome-induced hepatic pathology. Trends Parasitol. 2014;30(3):141–150. doi: 10.1016/j.pt.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Cunha G.M., Silva V.M., Bessa K.D., Bitencourt M.A., Macêdo U.B., Freire-Neto F.P., Martins R.R., Assis C.F., Lemos T.M., Almeida M.G., Freire A.C. Levels of oxidative stress markers: correlation with hepatic function and worm burden patients with schistosomiasis. Acta Parasitol. 2012;57(2):160–166. doi: 10.2478/s11686-012-0026-5. [DOI] [PubMed] [Google Scholar]
- De Luca A., Pierno S., Camerino D.C. Taurine: the appeal of a safe amino acid for skeletal muscle disorders. J. Transl. Med. 2015;13:243. doi: 10.1186/s12967-015-0610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira R.B., Senger M.R., Vasques L.M., Gasparotto J., dos Santos J.P., Pasquali M.A., Moreira J.C., Silva F.P., Gelain D.P., Jr. Schistosoma mansoni infection causes oxidative stress and alters receptor for advanced glycation endproduct (RAGE) and tau levels in multiple organs in mice. Int. J. Parasitol. 2013;43(5):371–379. doi: 10.1016/j.ijpara.2012.12.006. [DOI] [PubMed] [Google Scholar]
- De Souza Rda P., Cardoso L.S., Lopes G.T., Almeida M.C., Oliveira R.R., Alcântara L.M., Carvalho E.M., Araujo M.I. Cytokine and chemokine profile in individuals with different degrees of periportal fibrosis due to Schistosoma mansoni infection. J. Parasitol. Res. 2012;2012:394981. doi: 10.1155/2012/394981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X., Liang J., Lin Z.X., Wu F.S., Zhang Y.P., Zhang Z.W. Natural taurine promotes apoptosis of human hepatic stellate cells in proteomics analysis. World J. Gastroenterol. 2010;16(15):1916–1923. doi: 10.3748/wjg.v16.i15.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi S.L., Viswanathan P., Anuradha C.V. Taurine enhances the metabolism and detoxification of ethanol and prevents hepatic fibrosis in rats treated with iron and alcohol. Environ. Toxicol. Pharmacol. 2009;27(1):120–126. doi: 10.1016/j.etap.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Devi S.L., Viswanathan P., Anuradha C.V. Regression of liver fibrosis by taurine in rats fed alcohol: effects on collagen accumulation, selected cytokines and stellate cell activation. Eur. J. Pharmacol. 2010;647(1–3):161–170. doi: 10.1016/j.ejphar.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Dooley S., ten Dijke P. TGF-β in progression of liver disease. Cell Tissue Res. 2012;347(1):245–256. doi: 10.1007/s00441-011-1246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erman F., Balkan J., Cevikbaş U., Koçak-Toker N., Uysal M. Betaine or taurine administration prevents fibrosis and lipid peroxidation induced by rat liver by ethanol plus carbon tetrachloride intoxication. Amino Acids. 2004;27(2):199–205. doi: 10.1007/s00726-004-0105-5. [DOI] [PubMed] [Google Scholar]
- Gentile C.L., Nivala A.M., Gonzales J.C., Pfaffenbach K.T., Wang D., Wei Y., Jiang H., Orlicky D.J., Petersen D.R., Pagliassotti M.J., Maclean K.N. Experimental evidence for therapeutic potential of taurine in the treatment of nonalcoholic fatty liver disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301(6):R1710–R1722. doi: 10.1152/ajpregu.00677.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães M.A., de Oliveira R.N., Véras L.M., Lima D.F., Campelo Y.D., Campos S.A., Kuckelhaus S.A., Pinto P.L., Eaton P., Mafud A.C., Mascarenhas Y.P., Allegretti S.M., de Moraes J., Lolić A., Verbić T., Leite J.R. Anthelmintic activity in vivo of epiisopiloturine against juvenile and adult worms of Schistosoma mansoni. PLoS Negl. Trop. Dis. 2015;9(3):e0003656. doi: 10.1371/journal.pntd.0003656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable R.J., Lippincott S.E. Diet and biosynthesis as sources of taurine in the mouse. J. Nutr. 1982;112(5):1003–1010. doi: 10.1093/jn/112.5.1003. [DOI] [PubMed] [Google Scholar]
- Jakubowski A., Ambrose C., Parr M., Lincecum J.M., Wang M.Z., Zheng T.S., Browning B., Michaelson J.S., Baetscher M., Wang B., Bissell D.M., Burkly L.C. TWEAK induces liver progenitor cell proliferation. J. Clin. Investig. 2005;115(9):2330–2340. doi: 10.1172/JCI23486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Wang Y., Wang H., Huang C., Huang Y., Li J. Endoplasmic reticulum stress is the crossroads of autophagy, inflammation, and apoptosis signaling pathways and participates in liver fibrosis. Inflamm. Res. 2015;64(1):1–7. doi: 10.1007/s00011-014-0772-y. [DOI] [PubMed] [Google Scholar]
- Li Y., Luo Y., Zhang X., Lin X., He M., Liao M. Combined taurine, epigallocatechin gallate and genistein therapy reduces HSC-T6 cell proliferation and modulates the expression of fibrogenic factors. Int. J. Mol. Sci. 2013;14:20543–20554. doi: 10.3390/ijms141020543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Chen P., Büchele B., Dong S., Huang D., Ren C., Zhang Y., Hou X., Simmet T., Shen J. A boswellic acid-containing extract attenuates hepatic granuloma in C57BL/6 mice infected with Schistosoma japonicum. Parasitol. Res. 2013;112(3):1105–1111. doi: 10.1007/s00436-012-3237-7. [DOI] [PubMed] [Google Scholar]
- Luckose F., Pandey M.C., Radhakrishna K. Effects of amino acid derivativeson physical, mental, and physiological activities. Crit. Rev. Food Sci. Nutr. 2015;55(13):1793–1807. doi: 10.1080/10408398.2012.708368. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Matsuzaki Y., Ikegami T., Miyakawa S., Doy M., Tanaka N., Bouscarel B. Optimal and effective oral dose of taurine to prolong exercise performance in rat. Amino Acids. 2004;27(3–4):291–298. doi: 10.1007/s00726-004-0133-1. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Karube M., Matsuzaki Y., Ikegami T., Doy M., Tanaka N., Bouscarel B. Taurine inhibits oxidative damage and prevents fibrosis in carbon tetrachloride-induced hepatic fibrosis. J. Hepatol. 2005;43(1):117–125. doi: 10.1016/j.jhep.2005.01.033. [DOI] [PubMed] [Google Scholar]
- Olveda D.U., Olveda R.M., McManus D.P., Cai P., Chau T.N., Lam A.K., Li Y., Harn D.A., Vinluan M.L., Ross A.G. The chronic enteropathogenic disease schistosomiasis. Int. J. Infect. Dis. 2014;28:193–203. doi: 10.1016/j.ijid.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Ribeiro R.A., Vanzela E.C., Oliveira C.A., Bonfleur M.L., Boschero A.C., Carneiro E.M. Taurine supplementation: involvement of cholinergic/phospholipase C and protein kinase A pathways in potentiation of insulin secretion and Ca2+ handling in mouse pancreatic islets. Br. J. Nutr. 2010;104:1148–1155. doi: 10.1017/S0007114510001820. [DOI] [PubMed] [Google Scholar]
- Santora A., Neuwirth L.S., L'Amoreaux W.J., Idrissi A.E. The effects of chronic taurine supplementation on motor learning. Adv. Exp. Med. Biol. 2013;775:177–185. doi: 10.1007/978-1-4614-6130-2_15. [DOI] [PubMed] [Google Scholar]
- Santos-Silva J.C., Ribeiro R.A., Vettorazzi J.F., Irles E., Rickli S., Borck P.C., Porciuncula P.M., Quesada I., Nadal A., Boschero A.C., Carneiro E.M. Taurine supplementation ameliorates glucose homeostasis, prevents insulin and glucagon hypersecretion, and controls β, α, and δ-cell masses in genetic obese mice. Amino Acids. 2015;47:1533–1548. doi: 10.1007/s00726-015-1988-z. [DOI] [PubMed] [Google Scholar]
- Schaffer S.W., Shimada K., Jong C.J., Ito T., Azuma J., Takahashi K. Effect of taurine and potential interactions with caffeine on cardiovascular function. Amino Acids. 2014;46(5):1147–1157. doi: 10.1007/s00726-014-1708-0. [DOI] [PubMed] [Google Scholar]
- Souza A.L., Roffê E., Pinho V., Souza D.G., Silva A.F., Russo R.C., Guabiraba R., Pereira C.A., Carvalho F.M., Barsante M.M., Correa-Oliveira R., Fraga L.A., Negrão-Correa D., Teixeira M.M. Potential role of the chemokine macrophage inflammatory protein 1alpha in human and experimental schistosomiasis. Infect. Immun. 2005;73(4):2515–2523. doi: 10.1128/IAI.73.4.2515-2523.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Zhang L., Wang J., Chen J., Zhu D., Shen P., He X., Pan J., Peng W., Duan Y. Schistosoma japonicum protein SjP40 inhibits TGF-β1-induced activation of hepatic stellate cells. Parasitol. Res. 2015;114:4251–4257. doi: 10.1007/s00436-015-4663-0. [DOI] [PubMed] [Google Scholar]
- Tang J., Huang H., Ji X., Zhu X., Li Y., She M., Yan S., Fung M., Li Z. Involvement of IL-13 and tissue transglutaminase in liver granuloma and fibrosis after Schistosoma japonicum infection. Mediat. Inflamm. 2014;2014:753483. doi: 10.1155/2014/753483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warskulat U., Borsch E., Reinehr R., Heller-Stilb B., Monnighoff I., Buchczyk D., Donner M., Flogel U., Kappert G., Soboll S., Beer S., Pfeffer K., Marschall H.U., Gabrielsen M., Amiry-Moghaddam M., Ottersen O.P., Dienes H.P., Haussinger D. Chronic liver disease is triggered by taurine transporter knockout in the mouse. FASEB J. 2006;20:574–576. doi: 10.1096/fj.05-5016fje. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Matsuzaki K. Differential regulation of TGF-β/Smad signaling in hepatic stellate cells between acute and chronic liver injuries. Front. Physiol. 2012;3:53. doi: 10.3389/fphys.2012.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]