Abstract
Spontaneous regression of malignant tumors is rare especially of lung tumor and biological mechanism of such remission has not been addressed. We report the case of a 79-year-old Korean patient with non-small cell lung cancer, squamous cell cancer with a right hilar tumor and multiple lymph nodes, lung to lung metastasis that spontaneously regressed without any therapies. He has sustained partial remission state for one year and eight months after the first histological diagnosis.
Keywords: Squamous cell lung cancer, spontaneous regression, advanced stage
Introduction
Spontaneous regression (SR) of cancer is a rare and is defined as a complete or partial disappearance of all or at least some relevant parameters of soundly diagnosed malignant disease, without any medical treatment (1). The types of cancers that SR were reported are malignant melanoma, renal cell cancer, low grade non-Hodgkin’s lymphoma etc. Total 15 case reports were announced SR of lung cancer from 1954 to 1997 (2). We present a rare and interesting case of a 79-year-old man with advanced squamous cell lung cancer whose tumor has spontaneously regressed with no active treatment.
Case presentation
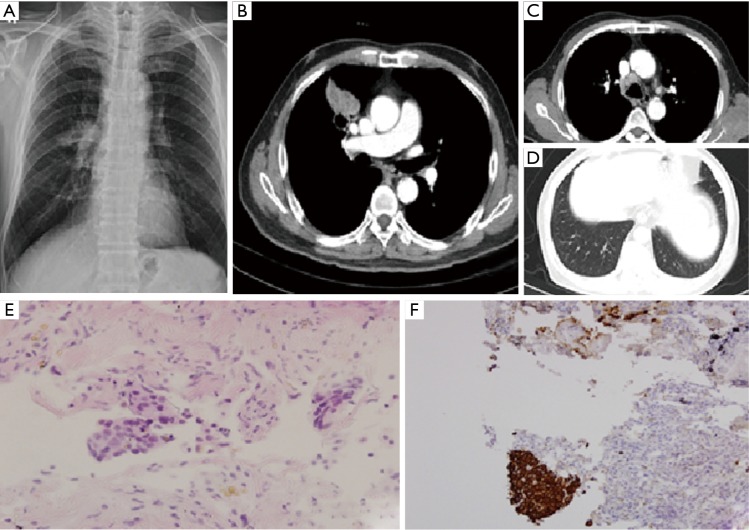
A 79-year-old man was referred to our hospital for right hilar mass on chest X-ray (Figure1A). He had taking medication for high blood pressure and diabetes mellitus. He was taken chest computed tomography (CT), there was a lobulated mass with cavity of right upper lobe anterior segment (Figure 1B). Other findings of lung were metastasis on same lobe, ipsilateral and contralateral lobe and metastatic lymphadenopathy in right highest, paratracheal, and hilar area (Figure 1C,D). Therefore, the patient was admitted for evaluation of lung mass. Endobronchial lesion was not observed on bronchoscopy. Percutaneous needle biopsy was performed in the main mass, and the result was a few atypical cell infiltration, favor squamous cell carcinoma (Figure 1E). The biopsy result was strongly suspected squamous cell carcinoma, but too small in the number of abnormal cells for confirmation. So, additional immunohistochemical study was performed. Thyroid transcription factor-1 (TTF-1) and Napsin A was negative and p63, cytokeratins (CK) 5/6 were positive, respectively (Figure 1F). These results were appropriate for squamous cell carcinoma. PET-CT was taken after histologic confirmation, hypermetabolic lesion was observed in the anterior segment of RUL with transfissural extension to right middle lobe (RML). And cavitary nodules were observed in right lower lobe (RLL) and both upper lobe (BUL), and glucose metabolism of these lesions was increased. Increased glucose metabolism was also observed in the multiple lymph nodes. We recommended patient to undergo chemotherapy, however he refused it and received only conservative therapy.
Figure 1.
(A-D) Chest X-ray and computed tomography (CT) which were taken on December 2012; (E) tissue obtained via CT-guided biopsy showing squamous cell carcinoma in the right upper lobe (hematoxylin-eosin, original magnification ×400); (F) immunohistochemistry revealed specimen expressed CK5/6 (original magnification ×100).
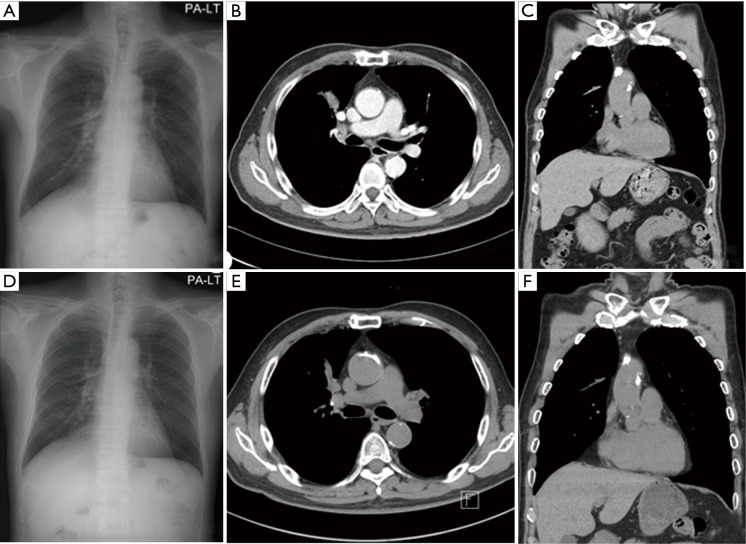
Two months later, the patient complained mild chest discomfort. And we performed chest CT again. There was no difference on the size of main mass and other metastatic nodules and lymph nodes. And four months after diagnosis, the patient didn’t receive chemotherapy still even in some herb medication, we performed chest CT again. Surprisingly, the size of main mass is reduced significantly from 5.4 to 3 cm and those of metastatic nodules were also reduced (Figure 2A-C). The patient took further five times chest CT in two month intervals thereafter to June 2014, main mass was reduced to 1.1 cm finally, and the size of metastatic lung nodules and lymph nodes were no change as compared with those of 2 months after diagnosis to date (Figure 2D-F).
Figure 2.
(A-C) Chest X-ray and computed tomography (CT) which were taken on April 2013; (D-F) chest X-ray and CT on August 2013.
Discussion
SR cancer is a rare phenomenon. Everson and Cole defined SR of cancer as the partial or complete disappearance of a malignant tumor in absence of all treatment, or in the presence of therapy which is considered inadequate to exert a significant influence on neoplastic disease (3). They investigated the cases of cancer has been SR from 1902 to 1990 and found total of 47 cases during that period. Lung cancer was only one of the 47 cases, this was a case that has been regression for five years after underwent thoracotomy and biopsy.
Since then, Kappauf et al. found total 15 cases from 1954 to 1997 targeting lung cancer (2). Among them, seven had squamous cell carcinoma and only three were extensive stage squamous cell cancers which did not received any therapeutic surgical or medical therapy. These cases showed SR duration of two, five, twelve years, respectively. However, the case published in 1954 had three days fever considered inflammatory reactions after performing the exploratory thoracotomy (4). In addition, there are a possibility that because the all cases which were announced in 1988 and 1968 were carried out diagnostic thoracotomy, remission had occurred maybe due to inflammatory response thought to be one of the mechanism of SR of cancer, and it differs from the our case (5,6).
There are several mechanisms responsible for spontaneous cancer regression. The mechanisms which has current discussed are modulated immunological response following systemic infection, differentiation from normal to malignant back to normal, hormonal mechanisms, and psychoneuroimmunological mechanisms (7).
Kumar et al. have developed a new definition of SR in 2010 that modified Everson and Cole criterion (8). This criteria is defined as: (I) the partial or complete disappearance of the tumor in the absence of all systemic or local treatment of the primary or metastatic lesion; (II) patients receiving any systemic therapy were excluded (chemotherapy, radioablative techniques, chemoembolization); (III) primary malignancy was histologically diagnosed or if no biopsy was done to document metastatic spread, the thoracic lesion had to appear metastatic radiographically and in clinical context. If this redesigned definition is applied, primary lung cancer showed SR from 1951 to 2008 is only one case of Sperduto et al. above mentioned (5).
To the best our knowledge, there were eight documented cases which satisfied the modified Everson and Cole criteria to date from 1950. Among them, NSCLC was weven and total of five squamous cell lung cancer cases were documented (Table 1). Moreover, this is a second case of advanced stage IV squamous cell lung cancer since it was announced in 1988.
Table 1. SR of primary non-small cell lung cancer.
| Reference | Histologic type | Stage | Metastasis | Year announced | Author views | Regression period | CR/PR |
|---|---|---|---|---|---|---|---|
| Sperduto et al. (5) | SqCC | IV | Adrenal | 1988 | Psychologic versus depression medication | 2 years | CR |
| Cafferata et al. (9) | Adenocarcinoma | I | None | 2004 | Natural history of disease | 2.8 years | CR |
| Liang et al.(10) | SqCC | IIIA | None | 2004 | Chinese herbal medication | 8 years | CR |
| Horino et al. (11) | SCLC | IV | Pleura | 2006 | PSN | 8 months | CR |
| Pujol et al. (12) | SqCC | I | None | 2007 | Anti-Hu antibody | 4 years | CR |
| Nakamura et al. (13) | Adenocarcinoma | IV | Pleura | 2009 | Anti NY-ESO-1 immunity | 15 months | PR |
| Gladwish et al. (7) | SqCC | IIIB | Double primary breast ca. | 2010 | Herb (essiac tea) | 9 months | PR |
| Choi et al. (14) | SqCC | Endobronchial cancer | None | 2013 | Co infection of pul Tbc | 10 months | CR |
PSN, paraneoplastic sensory neuropathy.
There are some proposals about the mechanisms of SR of cancer, differentiation, apoptosis, immunological and cytokine mechanisms, hormonal mechanisms, and angiogenesis inhibition might induce SR of cancer (2,7). In this case, there was no suspected reason except he had ate Korean ginseng from 4 months after diagnosis. For anti-cancer effects of ginseng, it has been demonstrated in several papers already (15). However, in this case, the patient had not taken any herbal medication after four months from diagnosis. Therefore, it is hard to say that the cause of SR is ginseng.
Acknowledgements
None.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Cole WH. Efforts to explain spontaneous regression of cancer. J Surg Oncol 1981;17:201-9. 10.1002/jso.2930170302 [DOI] [PubMed] [Google Scholar]
- 2.Kappauf H, Gallmeier WM, Wünsch PH, et al. Complete spontaneous remission in a patient with metastatic non-small-cell lung cancer. Case report, review of the literature, and discussion of possible biological pathways involved. Ann Oncol 1997;8:1031-9. 10.1023/A:1008209618128 [DOI] [PubMed] [Google Scholar]
- 3.Cole WH, Everson TC. Spontaneous regression of cancer: preliminary report. Ann Surg 1956;144:366-83. 10.1097/00000658-195609000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blades B, McCorkle RG, Jr. A case of spontaneous regression of an untreated bronchiogenic carcinoma. J Thorac Surg 1954;27:415-9. [PubMed] [Google Scholar]
- 5.Sperduto P, Vaezy A, Bridgman A, et al. Spontaneous regression of squamous cell lung carcinoma with adrenal metastasis. Chest 1988;94:887-9. 10.1378/chest.94.4.887 [DOI] [PubMed] [Google Scholar]
- 6.Emerson GL, Emerson MS, Sherwood CE, et al. Spontaneous regression of bronchogenic carcinoma. Twelve-year survival. J Thorac Cardiovasc Surg 1968;55:225-30. [PubMed] [Google Scholar]
- 7.Gladwish A, Clarke K, Bezjak A. Spontaneous regression in advanced non-small cell lung cancer. BMJ Case Rep 2010;2010. [DOI] [PMC free article] [PubMed]
- 8.Kumar T, Patel N, Talwar A. Spontaneous regression of thoracic malignancies. Respir Med 2010;104:1543-50. 10.1016/j.rmed.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 9.Cafferata MA, Chiaramondia M, Monetti F, et al. Complete spontaneous remission of non-small-cell lung cancer: a case report. Lung Cancer 2004;45:263-6. 10.1016/j.lungcan.2004.01.026 [DOI] [PubMed] [Google Scholar]
- 10.Liang HL, Xue CC, Li CG. Regression of squamous cell carcinoma of the lung by Chinese herbal medicine: a case with an 8-year follow-up. Lung Cancer 2004;43:355-60. 10.1016/j.lungcan.2003.08.035 [DOI] [PubMed] [Google Scholar]
- 11.Horino T, Takao T, Yamamoto M, et al. Spontaneous remission of small cell lung cancer: a case report and review in the literature. Lung Cancer 2006;53:249-52. 10.1016/j.lungcan.2006.05.011 [DOI] [PubMed] [Google Scholar]
- 12.Pujol JL, Godard AL, Jacot W, et al. Spontaneous complete remission of a non-small cell lung cancer associated with anti-Hu antibody syndrome. J Thorac Oncol 2007;2:168-70. 10.1097/JTO.0b013e31802f1c9d [DOI] [PubMed] [Google Scholar]
- 13.Nakamura Y, Noguchi Y, Satoh E, et al. Spontaneous remission of a non-small cell lung cancer possibly caused by anti-NY-ESO-1 immunity. Lung Cancer 2009;65:119-22. 10.1016/j.lungcan.2008.12.020 [DOI] [PubMed] [Google Scholar]
- 14.Choi SM, Go H, Chung DH, et al. Spontaneous regression of squamous cell lung cancer. Am J Respir Crit Care Med 2013;188:e5-6. 10.1164/rccm.201208-1417IM [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Zhao Y, Rayburn ER, et al. In vitro anti-cancer activity and structure-activity relationships of natural products isolated from fruits of Panax ginseng. Cancer Chemother Pharmacol 2007;59:589-601. 10.1007/s00280-006-0300-z [DOI] [PubMed] [Google Scholar]