Abstract
Background
To explore the effectiveness of video-assisted thoracoscopic surgery (VATS) bronchial sleeve resection and reconstruction.
Methods
The clinical data of patients who had received VATS bronchial sleeve lobectomy in our center from January 2008 to February 2015 were retrospectively analyzed.
Results
Totally 118 patients (105 men and 13 women) received the VATS bronchial sleeve lobectomy. The procedures included sleeve resection of right upper lobe (n=59), right middle lobe (n=7), right lower lobe (n=8), left upper lobe (n=34), and left lower lobe (n=10). The lesions were confirmed to be squamous cell carcinoma (n=68), adenocarcinoma (n=16), mucoepidermoid carcinoma (n=8), adenosquamous carcinoma (n=7), large cell carcinoma (n=1), carcinoids (n=5), and others (n=13; including small cell carcinoma, pleomorphic carcinoma, and inflammatory myofibroblastic tumor). Operations lasted 118–223 min [mean ± standard deviations (SD): 124.00±31.75 min]. The length of removed bronchus was 1.50–2.00 cm (mean ± SD: 1.75±0.26 cm). The duration of bronchial anastomosis (from the first puncture to the completion of knotting) was 15–42 min (mean ± SD: 30.20±7.97 min). The number of dissected lymph node stations (at least three mediastinal lymph node stations, including station 7) was 5–9 stations (mean ± SD: 6.50±1.18 min). The number of dissected lymph nodes was 10–46 (mean ± SD: 26.00±10.48). The intraoperative blood loss was 20–400 mL (mean ± SD: 71.00±43.95 mL), and no blood transfusion was performed. All patients were observed in intensive care unit (ICU) for 1 day. Postoperative drainage was performed for 3–8 days (mean ± SD: 5.00±1.49 days). Postoperative hospital stay was 3–8 days (mean ± SD: 5.10±2.07 days).
Conclusions
VATS bronchial sleeve resection and reconstruction is a safe and feasible technique.
Keywords: Video-assisted thoracoscopic surgery (VATS), surgery, lung cancer, sleeve resection, lobectomy
Introduction
Lung cancer is the most fatal cancer worldwide (1). Non-small cell lung cancer (NSCLC) is the most common lung cancer, whereas surgery remains the preferred treatment for the selected NSCLC cases (2). In 2006, the National Comprehensive Cancer Network (NCCN) of the United States listed the complete video-assisted thoracoscopic surgery (VATS) radical treatment as the standard procedure for early lung cancer in its guidelines on NSCLC (3); since then, VATS radical surgery for lung cancer has been widely recognized by the majority of thoracic surgeons worldwide. Many literatures have demonstrated the advantages of VATS techniques, which include small incision, short hospital stay, mild postoperative pain, and small lung damage (3-6). All these advantages are helpful to facilitate post-operative recovery. In recent years, along with improvements in both VATS surgical skills and devices, some doctors have attempted this technique in more challenging procedures such as complete VATS tracheal and bronchial sleeve lobectomy in patients with more complex central-type lung cancer (5,7).
When tumor invades the bronchial openings and main bronchus, simple pulmonary Lobectomy can not thoroughly remove the tumor, whereas total pneumonectomy severely damages the lung function and can not be tolerated by most patients. In these patients, bronchial sleeve lobectomy may be the preferred surgical treatment (8,9). In 1947, Prince Thomas performed the first case of right upper lobe sleeve resection; since then, bronchial sleeve lobectomy has become a standard procedure for lung cancer (10). Compared with total pneumonectomy, bronchial sleeve lobectomy can achieve comparable long-term survival but with small damage to lung function; thus, it can lower the surgical mortality and improve the long-term survival and quality of life even in patients who can tolerate total pneumonectomy, procedures that can maximize the preservation of lung function should be adopted (8,9,11).
Here we will focuse on the surgical skills and considerations of bronchial sleeve lobectomy. Based on the lesion locations, we selected several specific cases to describe different bronchial sleeve lobectomy procedures and meanwhile demonstrate the skills for resection and reconstruction/anastomosis during these procedures.
Patients and methods
Patients
The clinical data of 118 patients (105 men and 13 women) who had undergone VATS bronchial sleeve lobectomy in our center from January 2008 to February 2015 were retrospectively collected and analysed. The procedures included sleeve resection of right upper lobe (n=59), right middle lobe (n=7), right lower lobe (n=8), left upper lobe (n=34), and left lower lobe (n=10) (Table 1).
Table 1. General information of 118 patients receiving the VATS bronchial sleeve lobectomy.
| Variable | Right upper lobe | Right middle lobe | Right lower lobe | Left upper lobe | Left lower lobe |
|---|---|---|---|---|---|
| Number | 59 | 7 | 8 | 34 | 10 |
| Gender | |||||
| Men | 54 | 4 | 6 | 31 | 10 |
| Women | 5 | 3 | 2 | 3 | 0 |
| Stage | |||||
| 1a | 7 | 4 | 0 | 4 | 0 |
| 1b | 8 | 0 | 2 | 9 | 5 |
| 2a | 14 | 0 | 2 | 5 | 0 |
| 2b | 7 | 2 | 0 | 3 | 2 |
| 3a | 18 | 0 | 3 | 8 | 3 |
| 3b | 1 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 |
| Pathologic type | |||||
| Squamous cell carcinoma | 34 | 2 | 6 | 18 | 8 |
| Adenocarcinoma | 11 | 1 | 1 | 3 | 0 |
| Small cell carcinoma | 1 | 0 | 0 | 1 | 0 |
| Adenosquamous carcinoma | 6 | 0 | 0 | 1 | 0 |
| Large cell carcinoma | 1 | 0 | 0 | 0 | 0 |
| Pleomorphic carcinoma | 2 | 1 | 0 | 3 | 0 |
| Carcinoids | 0 | 0 | 0 | 4 | 1 |
| Mucoepidermoid carcinoma | 2 | 3 | 0 | 2 | 1 |
| Others | 2 | 0 | 1 | 2 | 0 |
Pre-operative preparation
Patient preparation was same as in VATS small-incision lobectomy. Among the routine preoperative examinations, computed tomography (CT) or magnetic resonance imaging (MRI) is the main tool for identifying any swollen lymph node in pulmonary hilum, and mediastinum, and or intermediate bronchial trunk and for facilitating staging or decision-making on the use of sleeve resection. Preoperative fiberoptic bronchoscopy is particularly important because it can be applied for: (I) locating: to observe the site, size, and involvement of the tumor or necrosis, so as to decide whether the bronchus condition is feasible for surgery and/or which surgical procedure should be adopted; and (II) characterization: to determine whether the tumor is malignant or benign; if it was a NSCLC, chemotherapy with or without surgery might be considered; if it was a squamous cell carcinoma whose range had extended the openings of lobar bronchus or if imaging indicated the presence of swollen lymph nodes, preoperative neoadjuvant therapy can be considered before the application of surgery. Patients who were scheduled for sleeve lobectomy often had obstructive pneumonia, which needed to be managed with antibiotics before surgery. Fiberoptic bronchoscopy should be performed before surgery to clarify the scope of tumor invasion and to remove purulent sputum (so as to alleviate the obstructive pneumonia).
The required surgical instruments included: a full set of VATS camera equipment; video imaging and recording equipment; endoscope; VATS biopsy cannula; electric knife; endoscopic cutter/stapler; suction device; small thoracotomy devices (e.g., Stoze Retractor System for thoracoscopic surgery), different types of forceps (especially non-invasive vascular blocking forceps), and Prolene sutures (5/0 or 4/0) and absorbable sutures (4/0).
Postoperative management
The postoperative management for patients who had received complete VATS left upper bronchial sleeve lobectomy with pulmonary artery sleeve reconstruction was same as that for routine complete VATS. It mainly included: encourage the patients to cough, carry out respiratory function training, and become ambulatory as soon as possible. Prophylactic use of antibiotics, atomization, and expectorant was applied; bronchofiberoscopy to suction sputum may be performed if required. If a second chest X-ray examination showed that the lungs were well dilated and the daily pleural fluid drainage was <100 mL, the chest tube could be withdrawn.
Statistical analysis
The general information and clinical findings (including surgical duration, intraoperative blood loss, postoperative drainage volume, postoperative drainage days, postoperative hospital stay, number of dissected lymph node stations, and number of dissected lymph nodes) were entered into a database and analysed using the SPSS 19.0 software. The measurement data were expressed as mean ± standard deviations (SD).
Results
General results
The surgeries were smoothly completed in all 118 patients. The lesions were pathologically confirmed to be squamous cell carcinoma (n=68), adenocarcinoma (n=16), mucoepidermoid carcinoma (n=8), adenosquamous carcinoma (n=7), large cell carcinoma (n=1), carcinoids (n=5), and others (n=13; including small cell carcinoma, pleomorphic carcinoma, and inflammatory myofibroblastic tumor) (Table 1).
Operations lasted 118–223 min (mean ± SD: 124.00±31.75 min). The length of removed bronchus was 1.50–2.00 cm (mean ± SD: 1.75±0.26 cm). The duration of bronchial anastomosis (from the first puncture to the completion of knotting) was 15–42 min (mean ± SD: 30.20±7.97 min). The number of dissected lymph node stations (at least three mediastinal lymph node stations, including station 7) was 5–9 stations (mean ± SD: 6.50±1.18 min). The number of dissected lymph nodes was 10–46 (mean ± SD: 26.00±10.48). The intraoperative blood loss was 20–400 mL (mean ± SD: 71.00±43.95 mL), and no blood transfusion was performed. All patients were observed in intensive care unit (ICU) for 1 day. Postoperative drainage was performed for 3–8 days (mean ± SD: 5.00±1.49 days). Postoperative hospital stay was 3–8 days (mean ± SD: 5.10±2.07 days).
Surgical methods
Anesthesia
After the induction of general anesthesia, the patient was under double-lumen endotracheal intubation. In some cases, intravenous anesthesia under spontaneous breathing was applied.
Body position
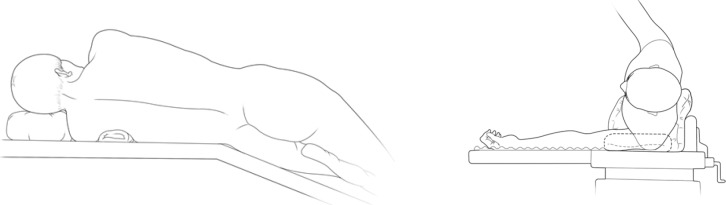
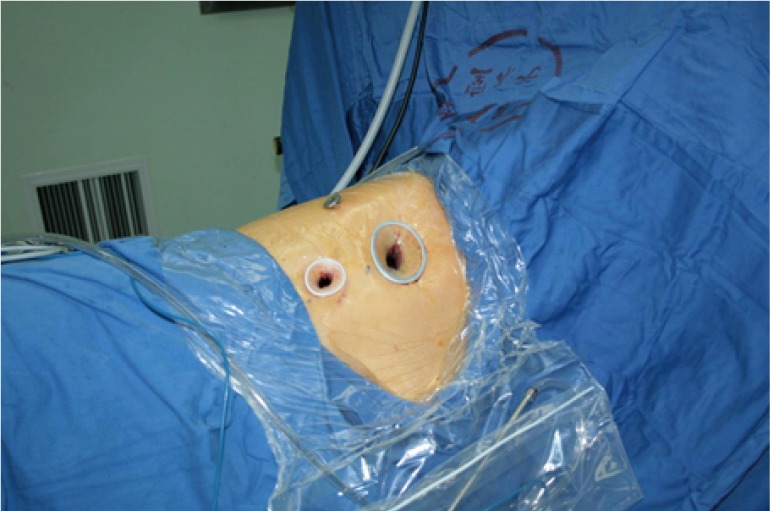
The patients were often placed in a lateral decubitus position on the unaffected side (Figure 1). The waist bridge is elevated to maximize the intercostal spaces and thus facilitate the operation.
Figure 1.
Surgical position.
Design of incisions
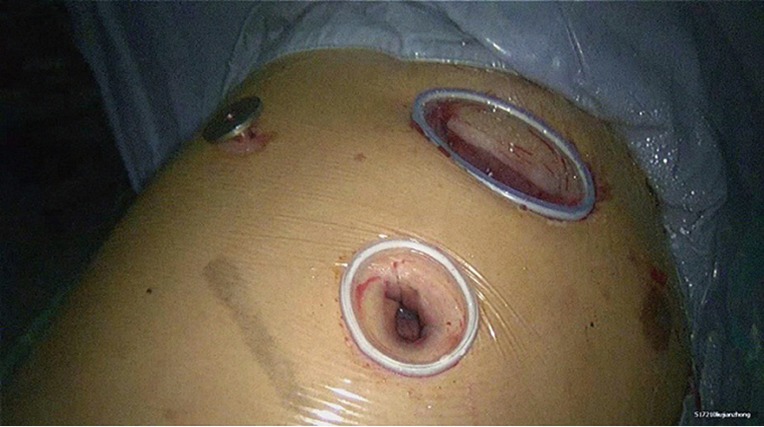
The incisions were designed and selected based on the patients’ individual features and the operator’s habit. A triple-port method was typically used in our center (Figure 2). Observation port: in the 6th or 7th intercostal space at anterior axillary line, about 1cm in length. Main operation port: in the 4th or 5th intercostal space at anterior axillary line, about 3–4 cm in length. Auxiliary operation port: within the same intercostal space with the observation port; in the 6th or 7th intercostal space at posterior axillary line, about 0.5 cm in length.
Figure 2.
Distribution of incisions.
Surgical procedures
Right upper sleeve lobectomy
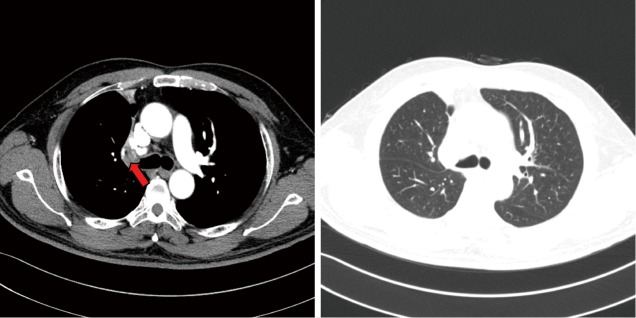
The pre-operative examination results are shown in Figure 3.
Figure 3.
CT scan of right upper lobe mass: contrast-enhanced chest spiral CT shows the presence of a neoplasm at the opening of right upper lobe bronchus (arrow).
The basic process of the resection was as follows:
Explore the thoracic cavity to decide whether the sleeve resection is needed. Dissociate vessels: divide the right hilum to separate each branch of lung arteries and veins in right upper lobe; then, these branches were ligated, or transected using the endoscopic cutter/stapler. During all types of sleeve lobectomy, the pulmonary arteries and veins were handled firstly, followed by the sleeve transection of diseased bronchus and the anastomosis of bronchus. The handling of lung vessels was same as that in routine lobectomy;
Separate the bronchus: separation of bronchus should be performed in normal tissues, approaching the carina if possible. Lobar bronchus and lobar pulmonary arteries were lifted using small plastic tubes preserved. Before the transection of the proximal main bronchus, the tracheal ring proximal to cutting section could be lifted using a suture to prevent the main bronchus to retract into the mediastinum;
Sleeve lobectomy: the intermediate bronchus and right main bronchus were transected separately, followed by the resection of right upper lobe. The stumps of both intermediate bronchus and right main bronchus were confirmed to be negative after intraoperative frozen-section biopsy. Before the bronchus was cut open, bleeding must be carefully stopped to ensure that there was no active bleeding around bronchus, in mediastinum, or on the pulmonary rough surface. Blood in the surgical field must be suctioned to prevent the blood flowed into distal bronchus after the main bronchus is cut open, so as to ensure the anastomosis can be carried out smoothly;
After the sleeve lobectomy was completed, dissociate the lower lung ligament and dissect the lymph nodes before the bronchial reconstruction was performed;
Reconstruction: reconstruction should be adjusted according to factors including bronchial diameter, anastomotic tension, and bronchial wall thickness. Based on his/her experiences and intuition, the operator should make appropriate adjustment and ensure each stitch at the anastomosis has evenly distributed tension, the bronchial lumen is not reversed, and the anastomosis is not air-leaked. A more detailed process of the reconstruction is demonstrated in Figures 4,5,6,7,8,9,10.
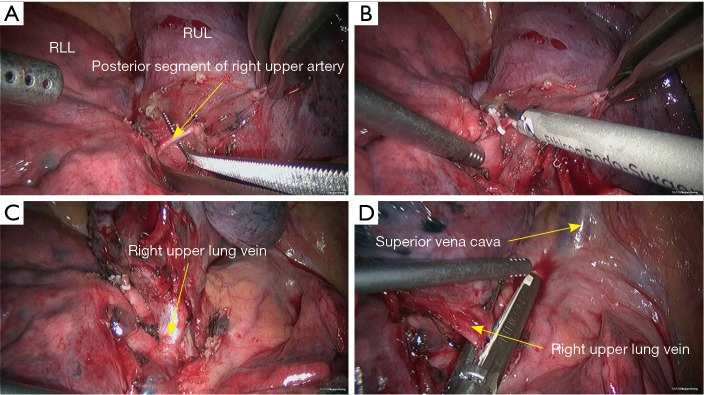
Figure 4.
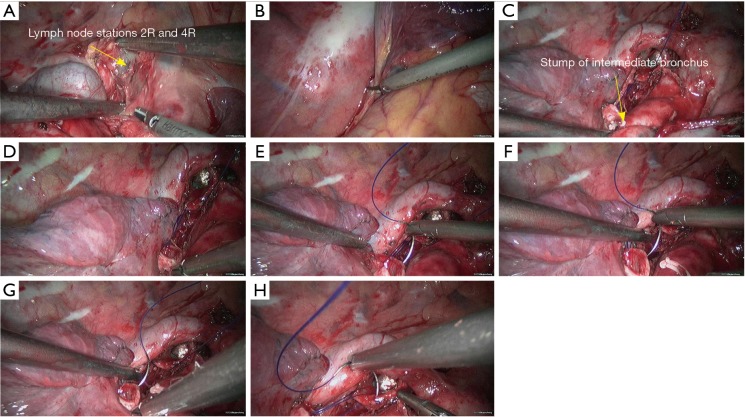
Right upper sleeve lobectomy. (A) Separate the lung fissure to expose the posterior segment of right upper lung artery (recurrent branch); (B) block the posterior segment of right upper lung artery (recurrent branch) using the Hemo Lock and then transect the distal end using HIFU; (C) dissect to separate the right upper lung vein; (D) transect the right upper lung vein using the endoscopic cutter/stapler. RLL, right lower lobe; RUL, right upper lobe; HIFU, high intensity focused ultrasound.
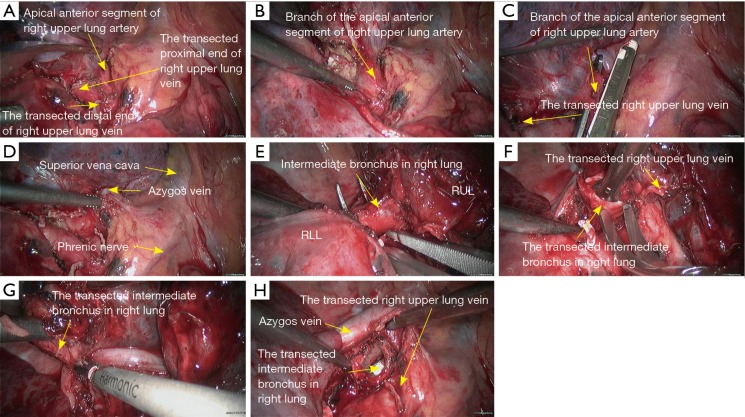
Figure 5.
Right upper sleeve lobectomy. (A) Transect the right upper lung vein to expose the apical anterior segment of right upper lung artery; (B) dissect to expose the apical anterior segment of right upper lung artery; (C) transect the apical anterior segment of right upper lung artery using endoscopic cutter/stapler; (D) the right upper lobe bronchus is then exposed; (E) dissect to separate the intermediate bronchus in right lung; (F) divide its cartilaginous part using a pair of right-angled scissors; (G) divide its membrane part using HIFU; (H) after the sleeve resection of right upper lobe, a tracheal tube is visible via the opened airway. RLL, right lower lobe; RUL, right upper lobe; HIFU, high intensity focused ultrasound.
Figure 6.
Right upper sleeve lobectomy. (A) Dissect the superior mediastinal lymph nodes; (B) release the inferior pulmonary ligament; (C,D) continuous suture of the bronchial membranes using 3-0 Prolene sutures; (E–H) continuous suture of the posterior wall of the bronchus using 3-0 Prolene sutures.
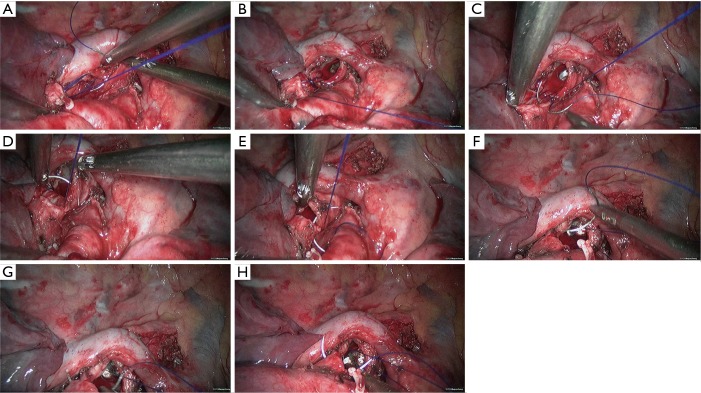
Figure 7.
Right upper sleeve lobectomy. (A–D) Continuous suture of the posterior wall of the bronchus using 3-0 Prolene sutures; (E,F) continuous suture of the lateral wall of the bronchus using 3-0 Prolene sutures; (G,H) continuous suture of the bronchial cartilage at the right side using 3-0 Prolene sutures.
Figure 8.
Right upper sleeve lobectomy. (A,B) Continuous suture of the anterior wall of the bronchus using 3-0 Prolene sutures; (C) anastomotic leak testing is negative.
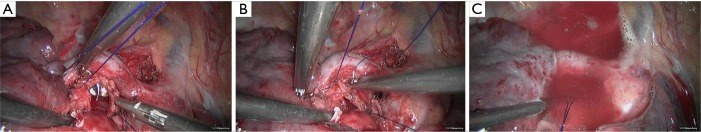
Figure 9.
Design of incision(s): VATS 3-port method was applied: operation port (3.5 cm in length) was located in the 3rd intercostal space in the right axillary line (with incision protector); the observation port (12 mm in length) was located in the 6th intercostal space in the midaxillary line; and the auxiliary port (5 mm in length) was located in the 6th intercostals space in the posterior axillary line (with trocar). VATS, video-assisted thoracoscopic surgery.
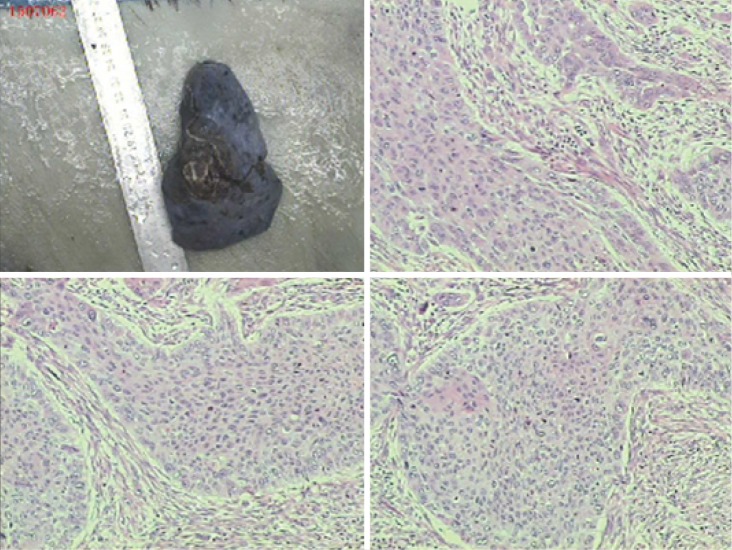
Figure 10.
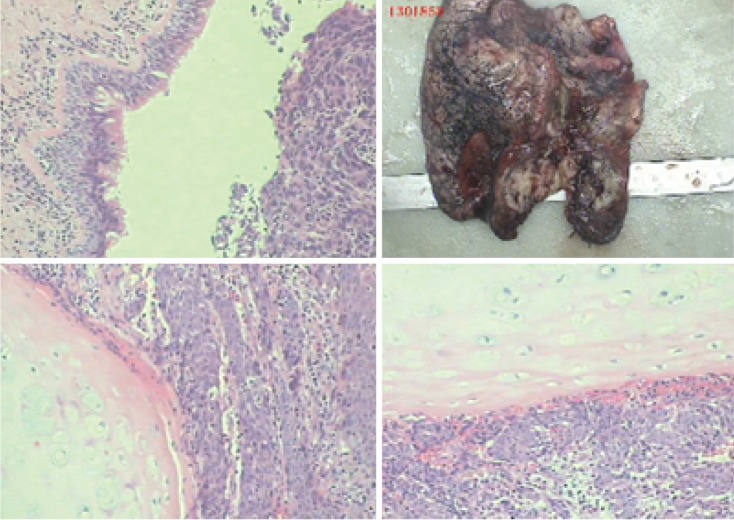
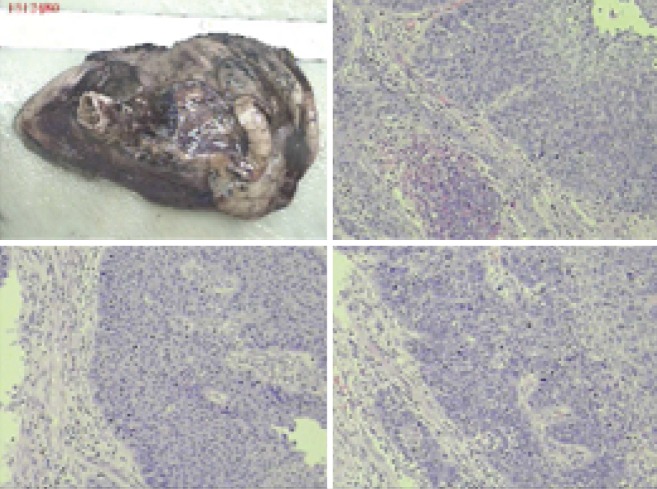
Moderately-differentiated squamous cell carcinoma. Magnification ×100.
Right lower sleeve lobectomy
The pre-operative examination results are shown in Figure 11.
Figure 11.
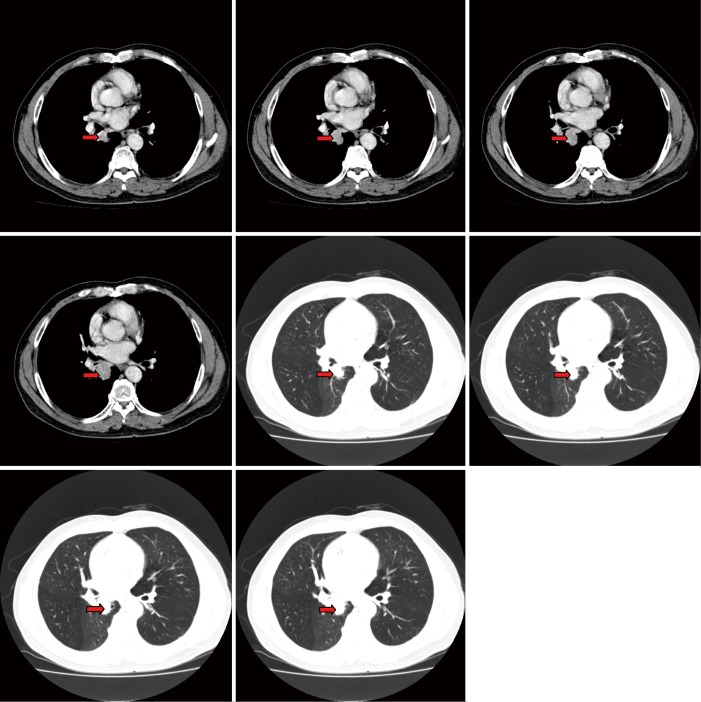
CT scans of right lower lobe mass: contrast-enhanced chest spiral CT show the presence of a neoplasm at the opening of right lower lobe bronchus (arrows).
The basic process of the resection was as follows:
Explore the thoracic cavity to decide whether the sleeve resection is needed. Separate vessels: Dissect the right lung hilum to separate each branch of lung artery and lung vein in right lower lobe, and then each branch was ligated or transected using endoscopic cutter/stapler. During all types of sleeve lobectomy, the pulmonary arteries and veins were handled firstly, followed by the sleeve transection of diseased bronchus and the anastomosis of bronchus. The handling of lung vessels was same as that in routine lobectomy;
Separate the bronchus: separation of bronchus should be performed in normal tissues, during which the lobar bronchus and lobar artery should be preserved;
Sleeve lobectomy: the intermediate bronchus and right main bronchus were transected separately, followed by the resection of right lower lobe. The stumps of both right-intermediate bronchus and right main bronchus were confirmed to be negative after intraoperative frozen-section biopsy. Before the bronchus was cut open, bleeding must be carefully stopped to ensure that there was no active bleeding around bronchus, in mediastinum, or on the pulmonary rough surface. Blood in the surgical field must be suctioned to prevent the blood flowed into distal bronchus after the main bronchus is cut open, so as to ensure the anastomosis can be carried out smoothly;
After the sleeve lobectomy was completed, dissociate the lower lung ligament and dissect the lymph nodes before the bronchial reconstruction was performed;
Reconstruction: reconstruction should be adjusted according to factors including bronchial diameter, anastomotic tension, and bronchial wall thickness. Based on his/her experiences and intuition, the operator should make appropriate adjustment and ensure each stitch at the anastomosis has evenly distributed tension, the bronchial lumen is not reversed, and the anastomosis is not air-leaked. A more detailed process of the reconstruction is demonstrated in Figures 12,13,14,15,16.
Figure 12.
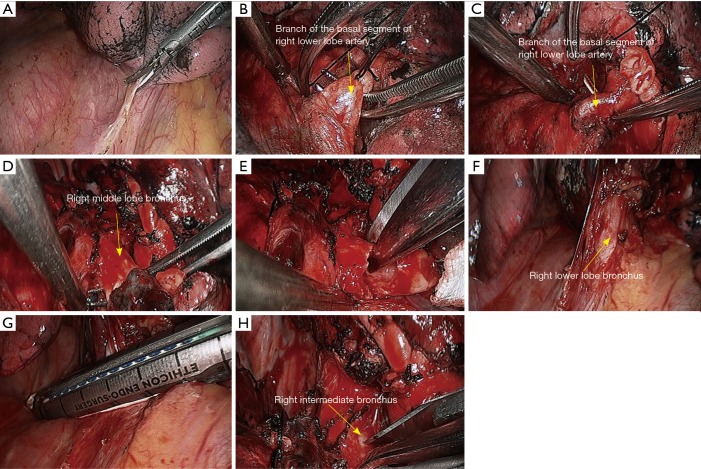
Right lower sleeve lobectomy. (A) Release the inferior pulmonary ligament; (B) dissect to expose the basal segment of right lower lobe artery, which was then transected after having been ligated using a silk suture; (C) dissect to expose the posterior segment of right lower lobe artery, which was then transected after having been ligated using a silk suture; (D) dissect to expose the right middle lobe bronchus; (E) transect the right middle lobe bronchus using a pair of scissors; (F) dissect to expose the right lower lobe vein; (G) transect the right lower lobe vein using endoscopic cutter/stapler; (H) transect the right intermediate bronchus using a scalpel.
Figure 13.
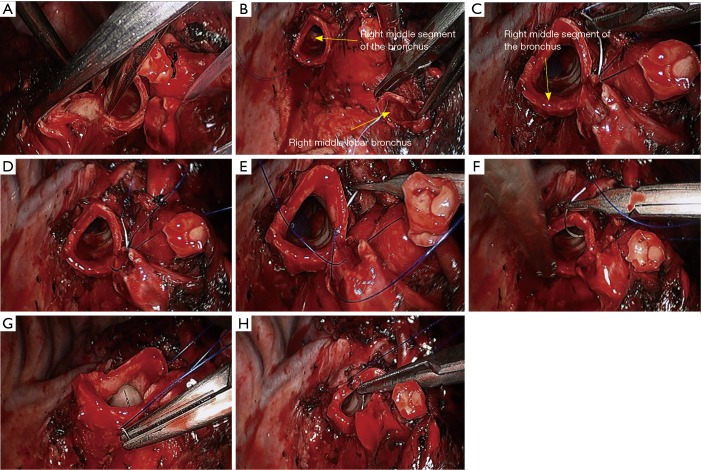
Right lower sleeve lobectomy. (A) Transect the right middle segment of the bronchus using a pair of scissors; (B–H) continuous end-to-end anastomosis of the right middle segment of the bronchus with the residual cartilage of right middle lobar bronchus using 3-0 Prolene sutures.
Figure 14.
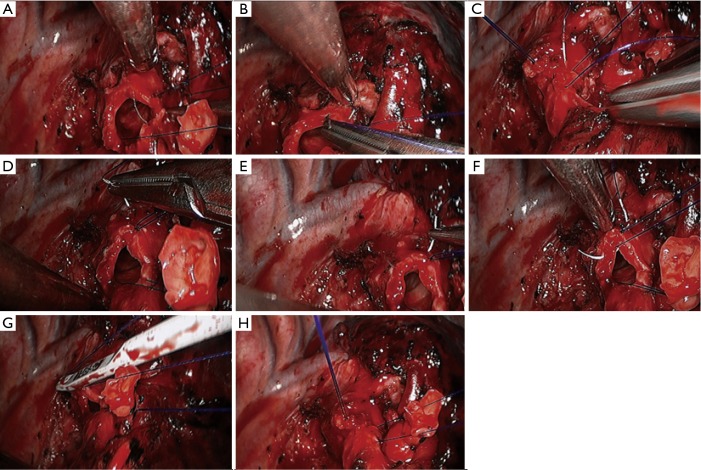
Right lower sleeve lobectomy. (A–C) Continuous end-to-end anastomosis of the right middle segment of the bronchus with the residual cartilage of right middle lobar bronchus using 3-0 Prolene sutures; (D–G) a continuous end-to-end anastomosis of the right middle segment of the bronchus with the residual cartilage of right middle lobar bronchus using 3-0 Prolene sutures; (H) effectiveness after sleeve reconstruction.
Figure 15.
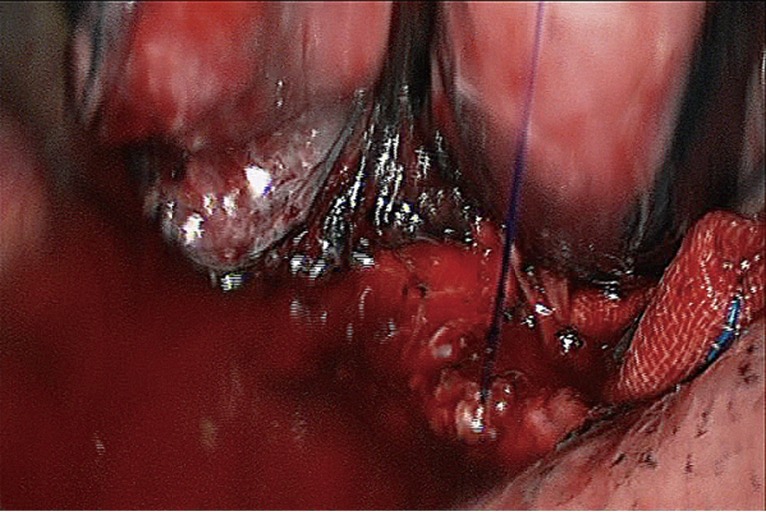
Anastomotic leak testing is negative.
Figure 16.
Poorly-differentiated squamous cell carcinoma. Magnification ×100.
Under general anesthesia with double lumen tube, video-assisted thoracoscopic right upper lobe sleeve lobectomy was performed on May 17th 2015 (Figure 17).
Figure 17.

Under general anesthesia with double lumen tube, video-assisted thoracoscopic right upper lobe sleeve lobectomy (12). Available online: http://www.asvide.com/articles/906
Under general anesthesia with double lumen tube, video-assisted thoracoscopic right lower lobe sleeve lobectomy was performed on February 17th 2013 (Figure 18).
Figure 18.

Under general anesthesia with double lumen tube, video-assisted thoracoscopic right lower lobe sleeve lobectomy (13). Available online: http://www.asvide.com/articles/907
Left upper sleeve lobectomy
The pre-operative examination results are shown in Figure 19.
Figure 19.
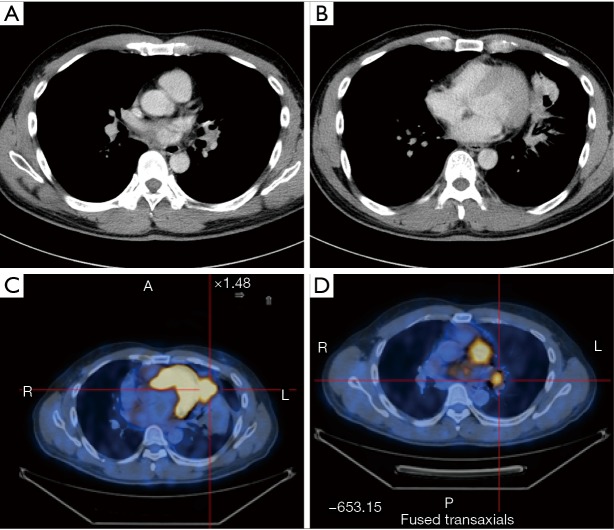
CT scans of left upper lobe mass: contrast-enhanced whole-body PET-CT scans show the presence of a neoplasm at the opening of left upper lobe bronchus.
The basic process of the resection was as follows:
Explore the thoracic cavity to decide whether the sleeve resection is needed. Separate vessels: Dissect the right lung hilum to separate each branch of lung artery and lung vein in left upper lobe, and then each branch was ligated or transected using endoscopic cutter/stapler. During all types of sleeve lobectomy, the pulmonary arteries and veins were handled firstly, followed by the sleeve transection of diseased bronchus and the anastomosis of bronchus. The handling of lung vessels was same as that in routine lobectomy;
Separate the bronchus: separation of bronchus should be performed in normal tissues, approaching the carina if possible. Lobar bronchus and lobar pulmonary arteries were lifted using small plastic tubes preserved. Before the transection of the proximal main bronchus, the tracheal ring proximal to cutting section could be lifted using a suture to prevent the main bronchus to retract into the mediastinum;
Sleeve lobectomy: left lower bronchus and left main bronchus were separately transected to remove the left upper lobe. The stump was confirmed to be negative by intraoperative frozen section examination. Before the bronchus was cut open, bleeding must be carefully stopped to ensure that there was no active bleeding around bronchus, in mediastinum, or on the pulmonary rough surface. Blood in the surgical field must be suctioned to prevent the blood flowed into distal bronchus after the main bronchus is cut open, so as to ensure the anastomosis can be carried out smoothly;
Upon the completion of sleeve lobectomy, the inferior pulmonary ligament was released and the lymph nodes were dissected, followed by the bronchial reconstruction;
Reconstruction: reconstruction should be adjusted according to factors including bronchial diameter, anastomotic tension, and bronchial wall thickness. Based on his/her experiences and intuition, the operator should make appropriate adjustment and ensure each stitch at the anastomosis has evenly distributed tension, the bronchial lumen is not reversed, and the anastomosis is not air-leaked. A more detailed process of the reconstruction is demonstrated in Figures 20,21,22,23,24.
Figure 20.
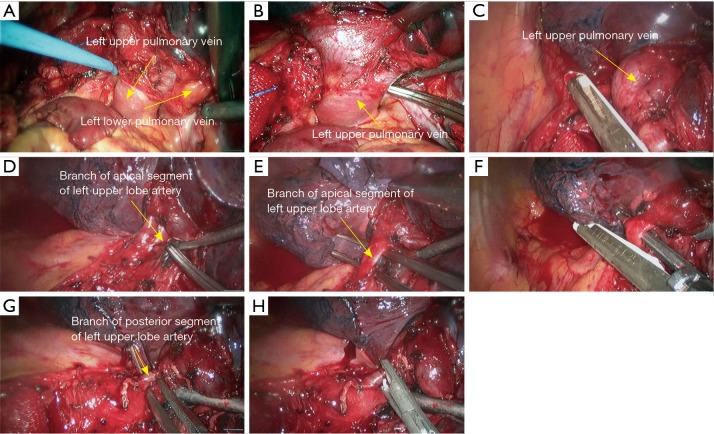
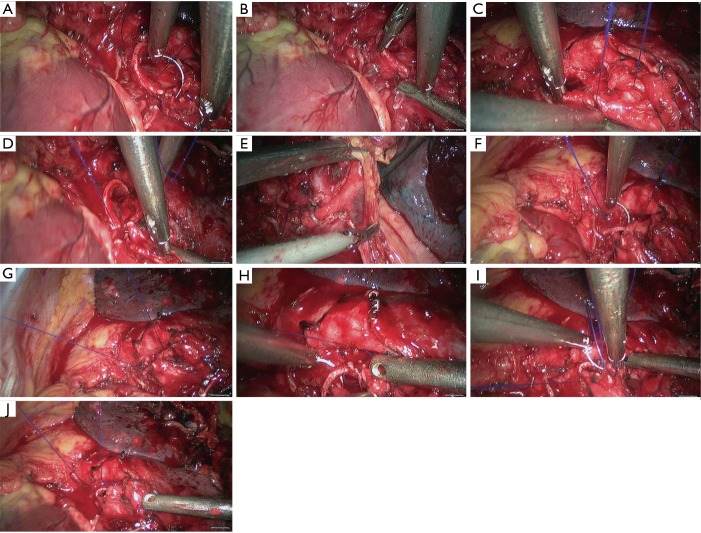
Left upper sleeve lobectomy. (A,B) Pericardiectomy was performed to separate the left upper pulmonary vein; (C) transect the left upper vein using the endoscopic linear cutter stapler; (D,E) dissect to expose the apical segment of left upper lobe artery; (F) transect the apical segment of left upper lobe artery using the endoscopic linear cutter stapler; (G) dissect to expose the posterior segment of left upper lobe artery; (H) block the root of the posterior segment of left upper lobe artery using Hemo Lock.
Figure 21.
Left upper sleeve lobectomy. (A) Block the root of the posterior segment of left upper lobe artery using Hemo Lock; (B) transect the distal end of the posterior segment of left upper lobe artery using HIFU; (C) dissect to expose the posterior segment of left upper pulmonary trunk; (D) dissect to expose the left lower pulmonary artery; (E) block the proximal end of the left upper pulmonary artery using a vascular blocking forceps; (F) block the distal end of the left upper pulmonary artery using a vascular blocking forceps; (G,H) transect the lingular segment of left upper lobe artery using the endoscopic linear cutter stapler. HIFU, high intensity focused ultrasound.
Figure 22.
Left upper sleeve lobectomy. (A) Transect the lingular segment of left upper lobe artery using the endoscopic linear cutter/stapler; (B–E) continuous suture of the bronchus using 3-0 Prolene sutures; (F–H) continuous suture of the posterior segment and basal segment of left lower lobe bronchus using 3-0 Prolene sutures.
Figure 23.
Left upper sleeve lobectomy. (A–D) Continuous suture of the posterior segment and basal segment of left lower lobe bronchus using 3-0 Prolene sutures; (E) release the left inferior pulmonary ligament; (F–J) continuous suture using 3-0 Prolene sutures; sleeve reconstruction of left upper lobe bronchus.
Figure 24.
Left upper sleeve lobectomy. (A) Air-leak test; (B) moderately-and poorly-differentiated squamous cell carcinoma. Magnification ×100.
Under general anesthesia with double lumen tube, video-assisted thoracoscopic left upper lobe sleeve lobectomy was performed on March 18th 2015 (Figure 25).
Figure 25.

Under general anesthesia with double lumen tube, video-assisted thoracoscopic left upper lobe sleeve lobectomy (14). Available online: http://www.asvide.com/articles/908
Sleeve resection of left lower lobe
The pre-operative examination results are shown in Figure 26.
Figure 26.
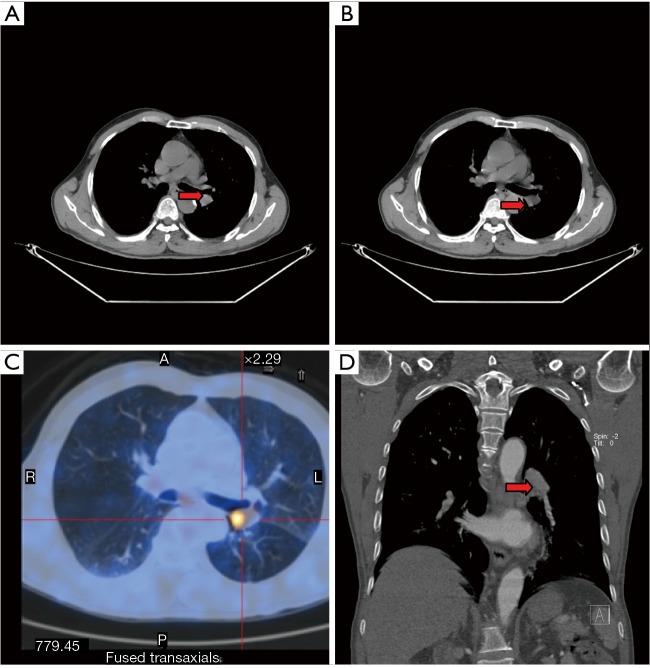
CT scan of left lower lobe mass: contrast-enhanced whole-body PET-CT scan shows the presence of a neoplasm at the opening of left lower lobe bronchus (arrow).
The basic process of the resection was as follows:
Explore the thoracic cavity to decide whether the sleeve resection is needed. Dissociate vessels: divide the left hilum to separate each branch of lung arteries and veins in left lower lobe; then, these branches were ligated, or transected using the endoscopic cutter/stapler. During all types of sleeve lobectomy, the pulmonary arteries and veins were handled firstly, followed by the sleeve transection of diseased bronchus and the anastomosis of bronchus. The handling of lung vessels was same as that in routine lobectomy;
Separate the bronchus: separation of bronchus should be performed in normal tissues;
Sleeve lobectomy: the left upper lobe bronchus and left main bronchus were separately transected to remove the left lower lobe. The stumps of left upper lobe bronchus and left main bronchus were confirmed to be negative by intraoperative frozen section examination. Before the bronchus was cut open, bleeding must be carefully stopped to ensure that there was no active bleeding around bronchus, in mediastinum, or on the pulmonary rough surface. Blood in the surgical field must be suctioned to prevent the blood flowed into distal bronchus after the main bronchus is cut open, so as to ensure the anastomosis can be carried out smoothly;
Upon the completion of sleeve lobectomy, the inferior pulmonary ligament was released and the lymph nodes were dissected, followed by the bronchial reconstruction;
Reconstruction: reconstruction should be adjusted according to factors including bronchial diameter, anastomotic tension, and bronchial wall thickness. Based on his/her experiences and intuition, the operator should make appropriate adjustment and ensure each stitch at the anastomosis has evenly distributed tension, the bronchial lumen is not reversed, and the anastomosis is not air-leaked. A more detailed process of the reconstruction is demonstrated in Figures 27,28,29,30,31.
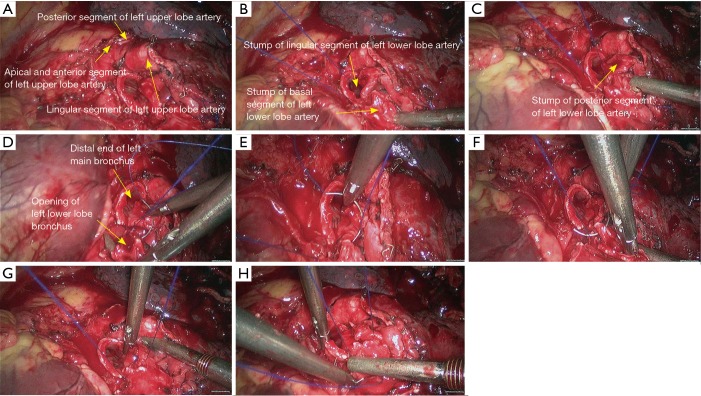
Figure 27.
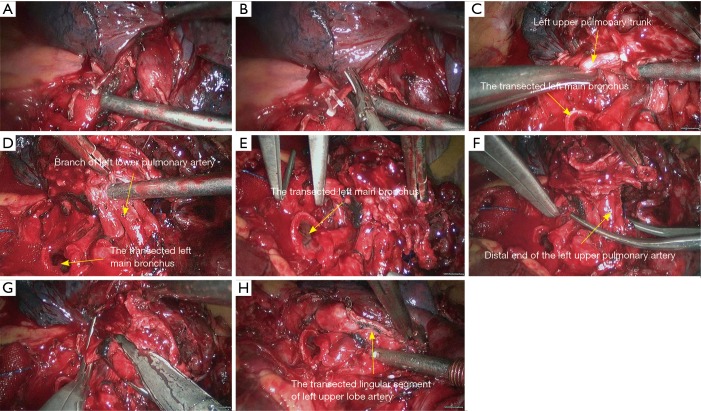
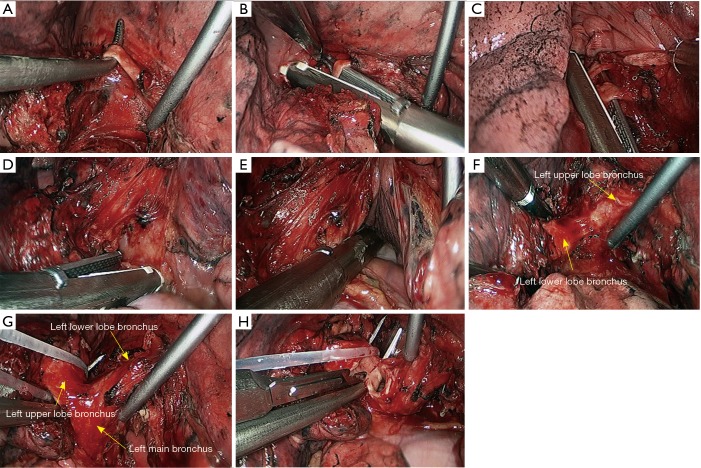
Left lower sleeve lobectomy. (A) Dissect to expose the basal segment of left lower pulmonary artery; (B,C) transect the basal segment of left lower lobe artery using the endoscopic linear cutter stapler; (D,E) transect the left lower pulmonary vein using the endoscopic linear cutter/stapler; (F) thoroughly expose the left lower lobe bronchus; (G) thoroughly expose the left lower lobe bronchus and left upper lobe bronchus; (H) transect the left upper lobe bronchus using a scalpel.
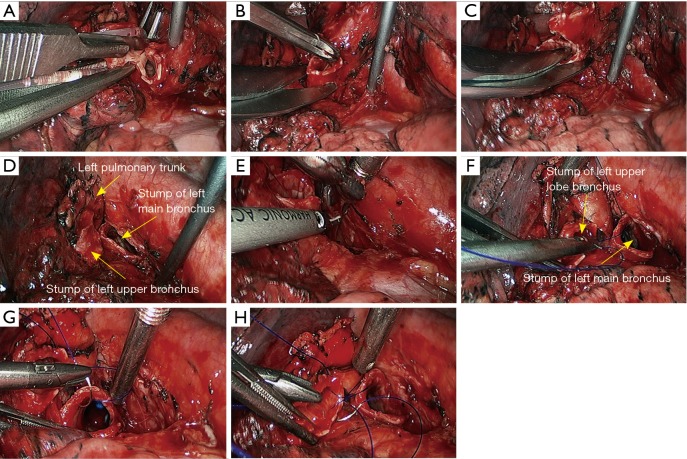
Figure 28.
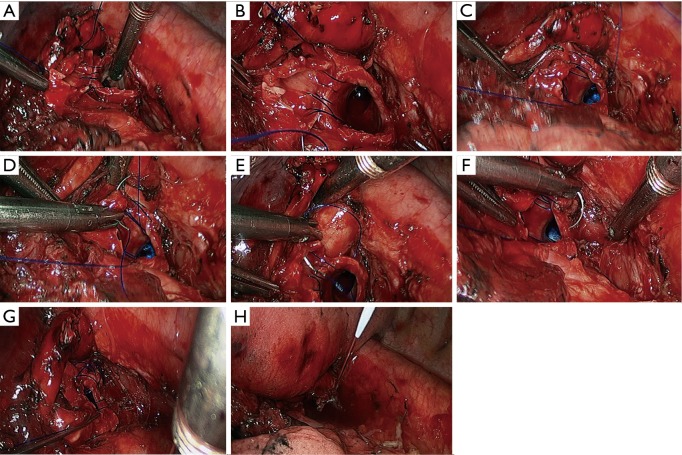
Left lower sleeve lobectomy. (A) Transect the left upper lobe bronchus using a scalpel; (B,C) transect the left main bronchus using a pair of tissue scissors; (D) the exposed stumps of left main bronchus and left upper lobe bronchus; (E) dissect the lymph node station 7; (F–H) continuous suture of the bronchus using 3-0 Prolene sutures.
Figure 29.
Left lower sleeve lobectomy. (A–H) Continuous suture of the bronchus using 3-0 Prolene sutures.
Figure 30.
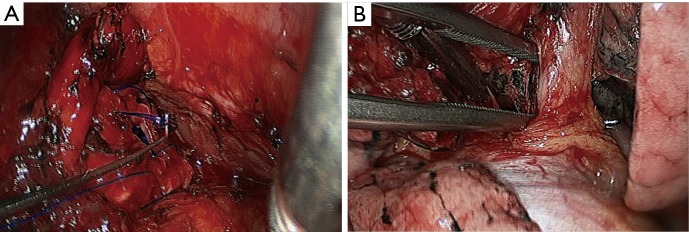
Left lower sleeve lobectomy. (A) Continuous suture of the bronchus using 3-0 Prolene sutures; (B) thoroughly release the left upper pulmonary vein.
Figure 31.
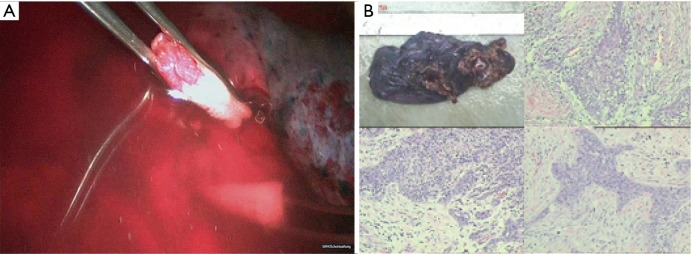
Moderately differentiated squamous cell carcinoma. Magnification ×100.
Under general anesthesia with double lumen tube, video-assisted thoracoscopic left lower lobe sleeve lobectomy was performed on September 3rd 2013 (Figure 32).
Figure 32.

Under general anesthesia with double lumen tube, video-assisted thoracoscopic left lower lobe sleeve lobectomy (15). Available online: http://www.asvide.com/articles/909
Post-operative complications and follow-up
In one patient who had received sequential radiotherapy and chemotherapy before surgery, bronchial anastomotic leakage occurred on the 12th postoperative day, and the patient died of massive hemoptysis on the 15th postoperative day. Another patient who had received six cycles of chemotherapy before surgery suffered from acute pneumonia after surgery, and he was discharged on the 20th postoperative day. No postoperative complication was reported in the remaining 116 patients.
Discussion
Bronchial sleeve anastomosis: VATS sleeve lobectomy has been widely carried out in China along with the advances in various minimally invasive surgical instruments such as cutter/stapler and electric hook and with the accumulation of experiences in this procedure. For bronchial sleeve lobectomy that requires complex anastomosis and reconstruction, there are still technical bottlenecks for the standardization of the operational progress and the anastomosis techniques, making this procedure one of the most challenging operation in the field of minimally invasive thoracic surgery.
The main challenges during complete VATS bronchial sleeve anastomosis include: (I) the operation port is narrow and small, making the complete VATS anastomosis from multiple angles particularly difficult; (II) the VATS anastomosis sites can be varied, whereas the site of the operation port is relatively fixed due to the restriction of the narrow intercostal spaces; (III) the three-dimensional operation of anastomosis is shown at two-dimensional angles in the monitor. The angle of puncture needs to be repeatedly attempted, and the angle of needle-holding need to be frequently adjusted outside the operation port (using forward or backward needle pitch), which remarkably slows down the operation. However, these difficulties can be overcome after rigorous training. Other useful countermeasures may include: (I) Use a good suture. Prolene suture can be selected because it has the following advantages: it has high tensile strength within tissues and is soft and smooth, and therefore is less invasive to the tissues; it has good histocompatibility and thus facilitates the healing of the anastomosis; it will not leave foreign objects, avoiding any complications such as cough due to the anastomotic thread irritation; (II) appropriate anastomosis.
The main bronchial anastomotic methods include sleeve-type and end-to-end anastomoses (16-18). The sleeve-type anastomosis requires the continuous suture of bronchial membranes, followed by the horizontal mattress suture of the cartilaginous part. This method can reduce the ischemia at the airway anastomosis and avoid anastomotic leakage; however, the bronchial sleeve may easily lead to bronchial anastomotic stenosis. In contrast, the end-to-end bronchial anastomosis enables the continuous suture of bronchial membranes, followed by the simple interrupted suture of bronchial cartilage. This method has high technical requirements, and the operators must have a solid knowledge of the technical details. Initially, we also applied the interrupted suture for VATS anastomosis. However, the interrupted suture is quite time-consuming; meanwhile, many sutures may be retracted from the incision and thus affect the operation, resulting in the tangling of sutures. Then, we adopted the simple continuous suture method. In our center, twin-needle stitch and bi-directional continuous full-thickness suture were applied in most patients. Literature has also demonstrated that the simple continuous suture for the anastomosis of bronchus is safe and reliable (19,20). We also had applied this method for the repair of large airway wound repair and achieved good effectiveness. We have found that this anastomosis method is fast and seldom cause the tangling of sutures; therefore, it is a feasible technique in VATS procedures.
During the anastomosis, sputum in distal end must be thoroughly suctioned, so as to prevent the development of early atelectasis after the reconstruction. Meanwhile, the two ends of the bronchus are parallel transected, so as to prevent the forming of anastomosis angle that may influence sputum discharge. Excessive separation of bronchus should be avoided to ensure the sufficient blood supply at the bronchial resection margin. Generally, the stripping extent of both bronchial ends should be no more than 0.5 cm away from the resection margin. During the anastomosis, suture marks should be made firstly on the left and right walls of the distal and proximal bronchial ends. Bronchial distortion, rotation, or angulation should be avoided during the suture. No matter which anastomosis method is applied, the anastomosis can be embedded in adjacent tissues such as Azygos vein, pericardial tissue, and parietal pleura, so as to reduce the incidence of anastomotic leakage.
Many useful skills during anastomosis have been described (21,22). According to our experiences, incision protector is very useful during the surgical operation and therefore should be listed as a required device. For an experienced VATS team, changing the operation port is feasible and the time consumed is acceptable during the anastomotic operation. Furthermore, when we are increasingly familiar with the VATS sleeve lobectomy, especially when the anastomotic methods have been optimized, the anastomotic operation has become less difficult.
The most severe complications following bronchial sleeve lobectomy include bronchopleural fistula and stenosis at the anastomosis. Intraoperative aseptic practices must be strictly followed. During the bronchial anastomosis, absorbable sutures should be used to keep the anastomosis tension-free. Effective and reliable embedding of the sutures outside the anastomosis is a feasible way to prevent the above-mentioned complications. In our series, one patients whose wound were closed with silk sutures experienced postoperative irritative cough, which was improved after the thread was removed via a fiberoptic bronchoscope. After the surgery, early pulmonary atelectasis should be prevented to keep the patency of the respiratory tract. Also, the patients should be encouraged and assisted to cough and cough up phlegm. Endobronchial ultrasound-assisted aerosol inhalation can be applied, along with the administration of a certain dosage of steroids, to prevent edema and scar formation at the anastomosis. In patients with weak cough or sputum production, bronchofibroscopic sputum suctioning should be performed as early as possible. In our series, one patient developed the symptoms of early pulmonary atelectasis, which were resolved after bronchofibroscopic sputum suctioning.
VATS sleeve lobectomy is a really challenging surgery. However, similar as VATS pulmonary lobectomy plus lymph node dissection for lung cancer, although VATS sleeve lobectomy were extremely difficult for most operators at beginning, it has been widely applied after intensive training and accumulation of experiences.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Siegel RL, Jemal A. Lung Cancer Statistics. Adv Exp Med Biol 2016;893:1-19. 10.1007/978-3-319-24223-1_1 [DOI] [PubMed] [Google Scholar]
- 3.Swanson SJ, Herndon JE, 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. 10.1200/JCO.2007.12.6649 [DOI] [PubMed] [Google Scholar]
- 4.Hennon M, Sahai RK, Yendamuri S, et al. Safety of thoracoscopic lobectomy in locally advanced lung cancer. Ann Surg Oncol 2011;18:3732-6. 10.1245/s10434-011-1834-9 [DOI] [PubMed] [Google Scholar]
- 5.He J, Shao W, Cao C, et al. Long-term outcome of hybrid surgical approach of video-assisted minithoracotomy sleeve lobectomy for non-small-cell lung cancer. Surg Endosc 2011;25:2509-15. 10.1007/s00464-011-1576-6 [DOI] [PubMed] [Google Scholar]
- 6.He J, Shao W, Cao C, et al. Long-term outcome and cost-effectiveness of complete versus assisted video-assisted thoracic surgery for non-small cell lung cancer. J Surg Oncol 2011;104:162-8. 10.1002/jso.21908 [DOI] [PubMed] [Google Scholar]
- 7.Xu X, Chen H, Yin W, et al. Thoracoscopic half carina resection and bronchial sleeve resection for central lung cancer. Surg Innov 2014;21:481-6. 10.1177/1553350613509728 [DOI] [PubMed] [Google Scholar]
- 8.Li S, Chai H, Huang J, et al. Hybrid video-assisted thoracic surgery with segmental-main bronchial sleeve resection for non-small cell lung cancer. Surg Innov 2014;21:180-6. 10.1177/1553350613492026 [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto K, Miyamoto Y, Ohsumi A, et al. Sleeve lung resection for lung cancer: analysis according to the type of procedure. J Thorac Cardiovasc Surg 2008;136:1349-56. 10.1016/j.jtcvs.2008.05.018 [DOI] [PubMed] [Google Scholar]
- 10.Thomas CP. Conservative resection of the bronchial tree. J R Coll Surg Edinb 1956;1:169-86. [PubMed] [Google Scholar]
- 11.Rea F, Marulli G, Schiavon M, et al. A quarter of a century experience with sleeve lobectomy for non-small cell lung cancer. Eur J Cardiothorac Surg 2008;34:488-92; discussion 492. 10.1016/j.ejcts.2008.05.027 [DOI] [PubMed] [Google Scholar]
- 12.He J, Huang J, Li S, et al. Under general anesthesia with double lumen tube, video-assisted thoracoscopic right upper lobe sleeve lobectomy. Asvide 2016;3:151. Available online: http://www.asvide.com/articles/906
- 13.Chen H, Huang J, He J, et al. Under general anesthesia with double lumen tube, video-assisted thoracoscopic right lower lobe sleeve lobectomy. Asvide 2016;3:152. Available online: http://www.asvide.com/articles/907
- 14.He J, Yin W, Huang J, et al. Under general anesthesia with double lumen tube, video-assisted thoracoscopic left upper lobe sleeve lobectomy. Asvide 2016;3:153. Available online: http://www.asvide.com/articles/908
- 15.He J, Huang J, Li S, et al. Under general anesthesia with double lumen tube, video-assisted thoracoscopic left lower lobe sleeve lobectomy. Asvide 2016;3:154. Available online: http://www.asvide.com/articles/909
- 16.Nakanishi K. Video-assisted thoracic surgery lobectomy with bronchoplasty for lung cancer: initial experience and techniques. Ann Thorac Surg 2007;84:191-5. 10.1016/j.athoracsur.2007.03.008 [DOI] [PubMed] [Google Scholar]
- 17.Mahtabifard A, Fuller CB, McKenna RJ, Jr. Video-assisted thoracic surgery sleeve lobectomy: a case series. Ann Thorac Surg 2008;85:S729-32. 10.1016/j.athoracsur.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 18.Schmid T, Augustin F, Kainz G, et al. Hybrid video-assisted thoracic surgery-robotic minimally invasive right upper lobe sleeve lobectomy. Ann Thorac Surg 2011;91:1961-5. 10.1016/j.athoracsur.2010.08.079 [DOI] [PubMed] [Google Scholar]
- 19.Aigner C, Jaksch P, Seebacher G, et al. Single running suture--the new standard technique for bronchial anastomoses in lung transplantation. Eur J Cardiothorac Surg 2003;23:488-93. 10.1016/S1010-7940(03)00018-6 [DOI] [PubMed] [Google Scholar]
- 20.Huang J, Li J, Qiu Y, et al. Thoracoscopic double sleeve lobectomy in 13 patients: a series report from multi-centers. J Thorac Dis 2015;7:834-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe A, Koyanagi T, Nakashima S, et al. How to clamp the main pulmonary artery during video-assisted thoracoscopic surgery lobectomy. Eur J Cardiothorac Surg 2007;31:129-31. 10.1016/j.ejcts.2006.10.017 [DOI] [PubMed] [Google Scholar]
- 22.Kamiyoshihara M, Nagashima T, Igai H, et al. Video-assisted thoracic lobectomy with bronchoplasty for lung cancer, with special reference to methodology. Interact Cardiovasc Thorac Surg 2011;12:534-8. 10.1510/icvts.2010.258228 [DOI] [PubMed] [Google Scholar]