Abstract
Cu is an essential micronutrient, and its role in an array of critical physiological processes is receiving increasing attention. Among these are wound healing, angiogenesis, protection against reactive oxygen species, neurotransmitter synthesis, modulation of normal cell and tumor growth, and many others. Free Cu is absent inside cells, and a network of proteins has evolved to deliver this essential, but potentially toxic, metal ion to its intracellular target sites following uptake. Although the total body content is low (∼100 mg), dysfunction of proteins involved in Cu homeostasis results in several well-characterized human disease states. The initial step in cellular Cu handling is its transport across the plasma membrane, a subject of study for only about the last 25 years. This review focuses on the initial step in Cu homeostasis, the properties of the major protein, hCTR1, that mediates Cu uptake, and the status of our understanding of this highly specialized transport system. Although a high-resolution structure of the protein is still lacking, an array of biochemical and biophysical studies have provided a picture of how hCTR1 mediates Cu(I) transport and how Cu is delivered to the proteins in the intracellular milieu. Recent studies provide evidence that the transporter also plays a key protective role in the regulation of cellular Cu via regulatory endocytosis, lowering its surface expression, in response to elevated Cu loads.
Main Text
Cu is used as a cofactor in numerous enzymatic mechanisms. Many of these use the dual redox states of Cu(I) and Cu(II) and their relatively facile interconversion, under physiological conditions, in oxidation-reduction cycles. Examples of cellular processes that depend on Cu include, mitochondrial aerobic metabolism as the central metal ion of cytochrome C oxidase; as a cofactor for dopamine β-hydroxylase in the biosynthesis of neurotransmitters and for peptidylglycine α-amidating monooxygenase in neuropeptide biosynthesis; in the important protective role of Cu, Zn-superoxide dismutase (SOD) against reactive oxygen species; and in Cu-dependent lysyl oxidase in cross-linking the collagen in extracellular matrix and connective tissue, during wound healing, metastasis, and growth. Paradoxically, Cu can be a potent inhibitor of many cellular enzymes. Cu ions react promiscuously with a wide array of protein side chains that contain sulfur, nitrogen, and oxygen. In addition, the production of reactive oxygen species by Cu adds another damaging consequence to Cu elevation. How then do cells navigate the critical path between the Scylla of Cu deficiency and the Charybdis of toxicity?
We are familiar with the transport of the major components of physiological media, Na, K, Cl, etc. It is apparent that the concentration of free Cu ions in cells is undetectable and estimated to be <10−18 M (1). Among the consequences of this are that considerations of the energetics of Cu transport contain many uncertainties and that observations of the effects of changes in Cu distribution in cells will be difficult to interpret. Estimates of the dissociation constants of Cu-ligand interactions make it likely that the kinetics of (slow) dissociation steps and protein-protein interactions control many changes in intracellular Cu (see Banci et al. (2) for discussion). The apparent dissociation constants for Cu(I) binding proteins and low molecular weight ligands are, for the intracellular chaperones 2–20 × 10−15 M, and glutathione (GSH, see below) ∼1 × 10−11 M. However, there is also some debate on the best methods to determine the Cu affinities for a range of cellular Cu-binding species (3). These factors also complicate issues in developing reagents for monitoring Cu, already addressed a couple of decades ago, in searching for optical indicators to record changes in the low, but measurable resting cellular Ca concentration (∼100 nM). In the case of Cu, (at <10−18 M) it is particularly challenging to produce indicators that report on the Cu(I) status and do so without inherent disruption of the intracellular Cu distribution. An excellent authoritative review has recently appeared that describes progress that has been made in this area (4). The sizes of the fluxes involved physiologically in Cu homeostasis enter new territory for those interested in transport physiology and biophysics. Comparison of Na and Cu handling in humans illustrates this. Kidneys filter ∼90 L of plasma every 12 h, containing 150 mM Na (or ∼310 g), of which >95% is reabsorbed, largely driven by Na pump fluxes. The turnover rate for the Na pump is ∼120 s−1 or ∼360 Na ions per second. In comparison, the total body Cu content is ∼100 mg and the daily dietary intake is ∼1–2 mg. Thus, cellular fluxes of Cu are likely to be very small. This is accomplished by having a relatively slow transporter (around 5–10 Cu ions per transporter trimer per second (5), see below) and low copy numbers, for example ∼1000 endogenous hCTR1 (human copper transporter 1), trimers per human embryonic kidney (HEK) cell (5). However, size is not everything!
Cellular copper homeostasis
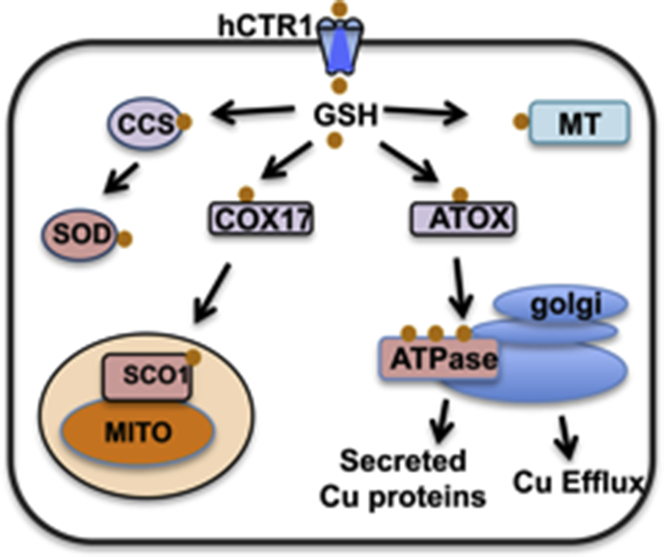
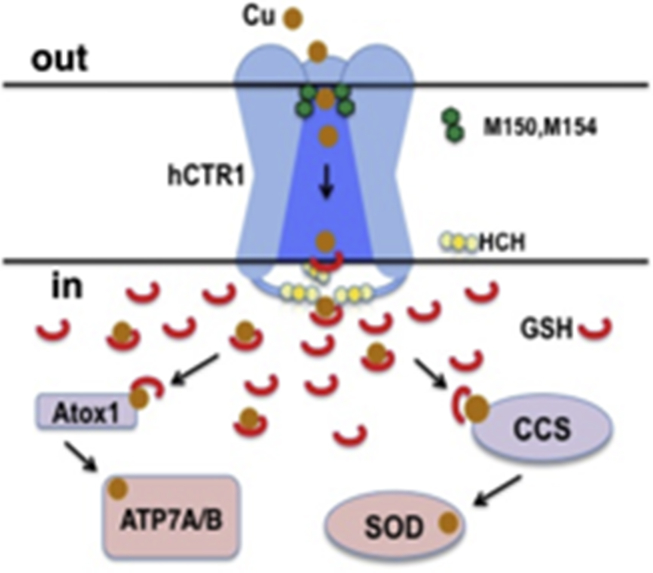
Cu homeostasis is controlled by transporters for the uptake and efflux of Cu ions and the groups of intracellular molecules engaged in handling Cu and delivering it to its specific sites (see Fig. 1). The efflux transporters are Cu-activated P-type ATPases, ATP7A and ATP7B, also known as Menkes disease protein and Wilson disease protein, respectively, for their associated human genetic diseases. These Cu pumps reside in the trans-Golgi membranes where they insert Cu into Cu-dependent enzymes in the secretory pathway. When intracellular Cu is elevated they provide a protective response by trafficking to the plasma membrane in vesicles, into which excess Cu has been pumped, which is then ejected from the cell via exocytosis. When cellular Cu falls they recycle back to the Golgi. The roles of the Cu transporters in Cu homeostasis has been the subject of several reviews (6, 7).
Figure 1.
Copper homeostasis in human cells. Cu enters via hCTR1 and is handed off to copper chaperones, ATOX1, CCS, and COX17, which then deliver Cu to its target proteins. MT, metallothionein may serve as a storage site. The efflux proteins, ATP7A and ATP7B, reside in the trans-Golgi and secretory pathway and relocate via vesicle trafficking and fusion, in conditions of high intracellular Cu, to the plasma membrane.
Because free Cu is not found in cells, intermediaries, the copper chaperones (8), deliver the metal ion to its specific targets. Studies in yeast provided candidates for these functions and mammalian and human orthologs were subsequently identified. There is a specific chaperone that delivers Cu to the ATPases, termed ATOX1 or HAH1, another delivers Cu in the synthesis of cytochrome C oxidase, termed Sco1, and to SOD, called copper chaperone for SOD (CCS). It has been observed that the target enzymes are not always completely dependent on the chaperones for Cu acquisition. Chaperones may obtain the Cu directly from the uptake transporter or from GSH (see below). Additional functions for the chaperones may exist as Cu-dependent transcription factors (9) or as redox regulators to maintain appropriate reductive states of sulfhydryls on proteins involved in Cu homeostasis (10).
The cycling between the Cu(I) and Cu(II) valence states plays an important role in the Cu-dependent enzymes, but it is the Cu(I) form that is transported by the major uptake pathway in mammalian cells. This is based on the homologies between the yeast and human proteins and the evidence in yeast linking the FRE (iron reductase) genes to Cu uptake (8, 11) as well as x-ray absorbance fluorescence spectroscopy studies on recombinant hCTR1 (12). It is also clear from x-ray absorbance fluorescence spectroscopy studies that copper chaperones, such as CCS bind and deliver Cu(I) to their target proteins (13). Ag inhibits Cu uptake (14) and is the only other ion known to be transported by hCTR1.
Copper entry pathways
The ctr proteins, responsible for the high affinity uptake of Cu, were first identified in the yeast Saccharomyces cerevisiae (15, 16). Subsequently, the human protein hCTR1 (SLC31A1) was identified by rescue of the yeast mutants lacking endogenous Cu transporters (17). The mouse homolog was next identified and sequenced and is 92% identical with hCTR1 (18). However, in work on Cu uptake in cells lacking hCTR1, ∼15–20% of the Cu uptake remained and so other minor transport systems are probably present (14). There are two plausible candidates. These are, DMT1, the divalent metal ion transporter that mediates the uptake of Mn and Zn. Although it has been claimed that DMT1 can also transport Cu(I) or Cu(II) any significant role for DMT1 in Cu transport remains to be established (19). The other candidate(s) for mediating Cu entry emerged from studies on heterologously expressed hCTR1 in mammalian cells (20, 21). In simple salt buffers there was such a high rate of Cu entry, that the effect of overexpression of hCTR1 on the Cu uptake rate was quite small. On the addition of serum, or agents that coordinate Cu, the total uptake was lowered and the major contribution of hCTR1 was evident. In the absence of ligands that coordinate Cu, there was a high rate of 4,4’-diisothiocyano-2,2’-stilbenedisulfonic acid-inhibited Cu entry, independent of the presence or absence of hCTR1 (21). The mechanism is suggested by work in erythrocytes, showing that high rates of Cu uptake were inhibited by 4,4’-diisothiocyano-2,2’-stilbenedisulfonic acid, and occurred via Band III, or AE1, the anion exchanger (22). Metal ions were transported as metalloanion complexes of the [MnCl]n-2- or [MClOH]n-1-types. A detailed discussion of this has appeared (21). In specific locations (duodenal lumen, for example) where pH is low and Cu ligands are protonated or absent, there may be a significant contribution to Cu entry from anion transporters. This may be relevant in the intestine, where entry mechanisms for Cu at the apical surface are still uncertain (7). For most cells, bathed in serum, ∼90% of Cu uptake is mediated by hCTR1. Previous reviews described structure-mechanism relations of hCTR1 (23) and the roles of the CTR family of Cu transporters in health and disease (24). We will now discuss current ideas about the structure, mechanism, and roles of hCTR1 in Cu acquisition and regulation.
hCTR1: sequence and structure
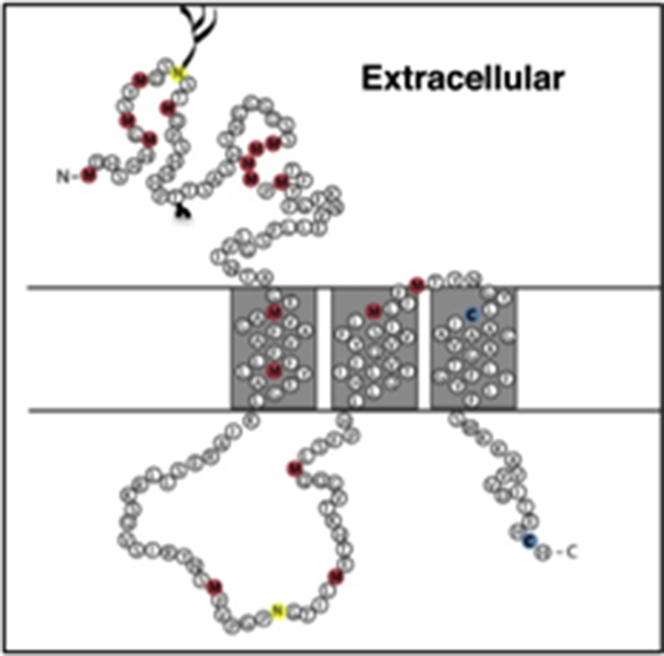
The first sequenced mammalian high affinity Cu transporters were the human (17) and mouse proteins (18) that contain several characteristic features (23). hCTR1 consists of 190 amino acids and several regions with putative metal binding sequences, rich in Met and His. The monomeric polypeptide was proposed to have three transmembrane segments (Fig. 2). Two putative extracellular N-linked glycosylation sites on opposite sides of the first transmembrane segment enabled the membrane topology to be assigned by the demonstration that extensive N-linked glycosylation occurred at Asn-15 (25) (see Fig. 1). hCTR1 also has short O-linked glycosylation at Thr-27 that protects the amino-terminus against cleavage by intracellular proteases (26). The proteolytic product obtained when O-linked glycosylation is prevented is a less efficient transporter than the full-length protein.
Figure 2.
Sequence, topology, and key residues of hCTR1 monomer. The 190 amino acid polypeptide spans the membrane three times. The potential Cu-coordinating residues are shown, Met in red and Cys in blue. The two putative N-linked glycosylation sites are shown in yellow. Extracellular N-glycosylation occurs at Asn-15, O-linked glycosylation occurs at Thr-27.
The small size of the monomeric protein made it likely that the functional unit was an oligomeric form of the protein (14, 15, 25, 27) and this was confirmed in the cryoelectron structure of unglycosylated hCTR1, obtained by overexpression in Pichia pastoris (28). The structure shows a trimer with a central pore, which presumably forms a transmembrane pathway for Cu ions. A full three-dimensional crystallographic structure at 7 Å subsequently revealed a central pore spanning the width of the membrane. The second transmembrane segments of each monomer line the central pore. The pore has a conical structure with an outer diameter of ∼8 Å, widening to ∼22 Å at the cytoplasm (12). The acquisition of structures set the stage for targeted mechanistic studies using mutagenic analysis of hCTR1 that have revealed many interesting features of this unique system.
hCTR1: mechanism of transport
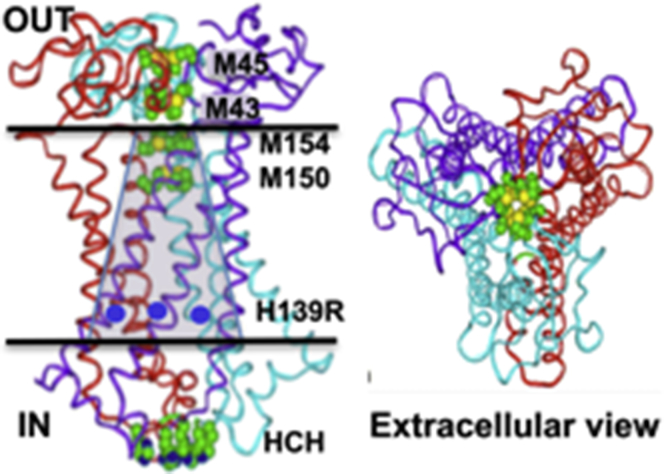
A model for hCTR1-mediated Cu transport emerged from a series of studies on mutations of hCTR1 and their effects on yeast growth in a strain lacking endogenous yeast transporters (18), isotopic Cu64 uptake in baculovirus-infected insect cells (29) or in stably transfected mammalian cells (5). The extracellular amino-terminus, containing several MXXM sequences, is involved in the initial interaction with Cu (or Cu with its serum carrier) and probably delivers the Cu to the pore (30). The sequence of the amino-terminus is not highly conserved across species, although they all contain putative Cu-binding sequences, suggesting similarities in mechanistic significance. Removal or substitution of several of these segments does not greatly affect transport, only the Met residues closest to the membrane (Met-43 and Met-45 in hCTR1) are essential for Cu uptake (30). A computational all-atom model of hCTR1, proposed that the Met-rich sequences in the amino-terminus form stacked rings that guide the Cu ions into the pore (31) (Fig. 3). Two rings of essential Met residues (Met-150 and Met-154), at the entrance to the pore are important in the initial engagement of the metal ions with the pore and form a selectivity filter. Replacement of these Met residues by Leu, which cannot coordinate Cu, shuts off transport (30). Following coordination by the Met triads, Cu ions enter the pore and then emerge at the cytosolic aspect of the pore. The interactions between the Cu ions and the protein during the transport process remain elusive.
Figure 3.
Cartoon of trimeric transporter based on an all-atom model (see Tsigelny et al. (31)). Each monomer of the active trimer are shown in red, cyan, or purple. The putative Cu-coordinating residues, extracellular, Met-43, Met-45, intramembrane, Met-150, Met-154, and intracellular His-188-Cys-189-His-190, in green. His-139 is shown in blue. The conical pore is shaded. Left panel view from side, right panel view from extracellular aspect.
The highly conserved intracellular carboxyl-terminus of 15 amino acid residues is completely conserved among mammalian species and contains a terminal His-Cys-His sequence that, in principle, is able to coordinate Cu ions. A structural study suggested a key role for this terminal sequence (12). It was concluded that Cu binding occurred at this terminal sequence, through the three Cys-189 residues of the trimeric transporter. Furthermore, it was suggested that the higher affinity binding at this site, compared to the Met triads at the pore entry, provides the energetic difference necessary for transport. This proposal had the attractive quality of supplying an essential mechanistic and energetic role for the highly conserved putative terminal Cu binding sequence, His-Cys-His.
More recent observations suggest that this model does not provide an accurate picture of the Cu transport process. We have recently shown that substitution of the terminal His-Cys-His sequence by Ala-Ala-Ala results in a CTR1 molecule with a significantly higher maximal rate of transport (expressed per trimeric transporter, the turnover number is ∼5 Cu ions per second for wild-type compared with ∼17 Cu ions per second for the Ala-substituted mutant) (5). Not only is Cu binding at the intracellular terminal sequence not necessary, but the presence of the His-Cys-His sequence slows, rather than facilitates transport. The ability to bind Cu ions at this terminal site does not enable or drive transport, but actually lowers the transport rate. Not only is the presence of the sequence at the tail important for this modulatory effect, but if its spatial position is altered, by shortening the extra-membrane length, through upstream deletions, it also fails to lower the transport rate (E.B.M. and J.H.K., unpublished data). All mutational changes that elevate the maximal rate of transport also increase the Km for transport of Cu (5). A similar functional effect, an increase in transport rate, has been reported to occur when the C-terminal tail of the yeast ctr1 is deleted (32). However, the cytoplasmic C-terminal tail of the yeast protein consists of >100 residues, whereas hCTR1 has only 15, so that the mechanistic basis of the similar effect is not clear. A related effect, first seen in the insect cell system and subsequently confirmed in mammalian cells emphasizes the importance, of ion-pore interactions. Replacement of His-139, in the second transmembrane segment by Arg, also results in an increased rate of transport (turnover number ∼18 Cu ions per second, per trimer) (5). It is not clear if this modification results in a weakening (via Arg-Cu charge repulsion effects) of Cu interactions with the pore during transit, or if interactions between the three Arg residues (one from each monomer) cause a distortion of the pore. It has been pointed out previously that hCTR1 is unlikely to be a simple pore (28) and earlier evidence suggests that the binding of Cu causes conformational changes in the protein (25).
hCTR1: regulation of Cu entry by acute alterations in surface expression
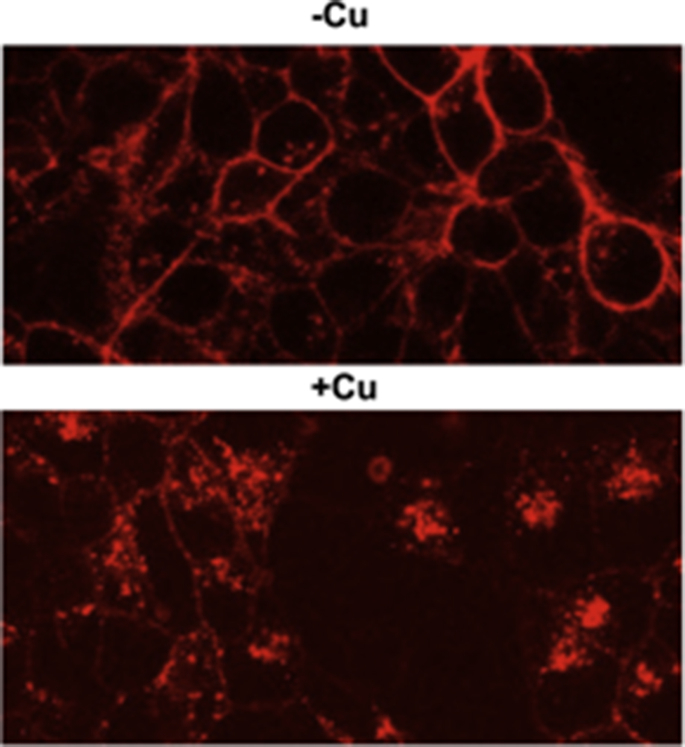
When extracellular Cu is elevated the transporter is internalized (33). This observation offered a plausible mechanism whereby cells might protect themselves against excess Cu entry. Internalization would lead to a regulatory decrease in influx in response to elevated extracellular Cu, in a similar fashion to the increase of Cu efflux (in response to elevated intracellular Cu) by trafficking Cu-activated ATPases to the plasma membrane from intracellular compartments. Recent studies have confirmed that indeed in the face of elevated levels of extracellular Cu, cells protect themselves against the potential adverse effects of Cu overload by rapidly (and reversibly) lowering Cu entry rates (34). This is accomplished by what we have termed regulatory endocytosis. In this mechanism, when medium Cu is raised, endocytosis of hCTR1 occurs and hCTR1 is internalized (Fig. 4). When extracellular Cu is lowered, the transporter recycles back to the plasma membrane. These processes do not involve new protein synthesis and provide an acute regulatory response to increases in the Cu load (34). Although this process is still the subject of investigation several interesting findings have emerged. When extracellular Cu is raised hCTR1 is internalized rapidly (in several minutes). If the exposure to elevated Cu is not prolonged (∼30 min or less), lowering of the extracellular Cu results in the return of hCTR1 to the plasma membrane (over the next 20–45 min) and the recovery of the rate of Cu uptake (34). Recent studies have shown that the internalization is clathrin- and dynamin-dependent (R.J. Clifford, E.B.M., and J.H.K., unpublished data). It is possible that extended exposure to elevated Cu results in some degradation, but acute treatments result in full recovery.
Figure 4.
hCTR1 is internalized in the presence of elevated medium Cu. Live-cell imaging of HEK 293 cells expressing CLIP-labeled hCTR1 (red) in the presence or absence of 50 μM Cu. In the absence of Cu hCTR1 is seen predominantly at the plasma membrane. In the presence of Cu, much of the hCTR1 is internalized. via endocytosis.
A mechanism that might account for this Cu-dependent endocytosis would be that a lower affinity site (lower than the key binding site for transport) binds Cu and causes a conformational change in an internal aspect of hCTR1 that enables the transporter to engage with the cellular protein import machinery. Thus, a critical Cu-bound conformation is key to the internalization process. In the course of analyzing Cu uptake kinetic properties of an array of hCTR1 mutants we have observed that, strikingly, those mutants in the carboxyl-terminus, where the His-Cys-His sequence is removed, that cause an elevated rate of Cu uptake, fail to undergo Cu-dependent endocytosis (5). This implies that Cu binding to this His-Cys-His site triggers interaction with the import machinery. Alternatively, an intermediate in the transport cycle, with extra Cu bound to the transporter interacts with the import machinery, and in the more rapidly transporting forms of hCTR1, the dwell time of the key intermediate is too small to allow the interaction that enables endocytosis. The particular and specific involvement of the carboxyl-terminus is quite provocative, but the observation that His-139, a pore mutant with an elevated maximal transport rate, also fails to undergo endocytosis, suggests that the interaction of hCTR1 with the protein import machinery includes more than just the carboxyl-/terminal sequence or perhaps faster than normal transport cycling, whatever the cause, impedes internalization.
hCTR1: intracellular Cu delivery
The transfer of Cu to intracellular chaperones, via direct interaction with hCTR1, has been proposed as a key step in cellular Cu homeostasis. Recent modeling and docking scenarios, together with some immunoprecipitation data supported this notion (35). However, the idea that the inner face of hCTR1 would interact specifically with several structurally unrelated metallochaperones, each of which would then deliver the Cu specifically to a target protein has always raised some questions about how this might be achieved. In studies performed before the discovery of the Cu chaperones, using biochemical fractionation it was found that the initial intracellular Cu-binding moiety was glutathione (GSH, γ-glutamyl-cysteinyl-glycine), an endogenous Cu-coordinating molecule present in high abundance (often 3–5 mM) in eukaryotic cells (36).
If handing off Cu from hCTR1 to the chaperones is the final step in the transport process, then large alterations in chaperone levels should influence the rate of uptake. In HEK cells that overexpress hCTR1 (or with endogenous hCTR1), we showed that substantially knocking down or overexpressing ATOX1, CCS, or both, had no effect on the Cu uptake rate (37). However, lowering GSH levels by ∼95% (from 4 mM) by inhibition of GSH synthetase, caused a 50% reduction in the rate of Cu uptake. The uptake rate could be recovered by replenishment of GSH. We suggested that the initial intracellular acceptor at the intracellular face of hCTR1 is GSH. The Cu:GSH complex then delivers Cu to the chaperones. The reversible inhibition of Cu uptake by lowering GSH was demonstrated in a number of other mammalian cells (37). That GSH is the initial Cu acceptor finds support in reported measurements of Cu affinities of the chaperones and GSH, which suggested that GSH was poised by virtue of its relative affinity to act as the initial cellular Cu acceptor and to hand it off to the chaperones (2). A comparison of the relative copy numbers of the chaperones, GSH and hCTR1 in cells shows that GSH is favored over the chaperones by >4 orders of magnitude for interaction with hCTR1 (5). The effects on the rate of Cu uptake, when GSH is lowered or replenished, are seen with hCTR1 mutants lacking the terminal putative metal-binding sequence motif, so that this putative metal-binding site plays no essential role in transfer to GSH. Our favored model currently is that hCTR1 hands off Cu to GSH, a high capacity, low affinity buffer that acts as donor to the chaperones (Fig. 5).
Figure 5.
Glutathione as the initial acceptor. Glutathione, a small tripeptide (orange) picks up the Cu(I) ions as they exit hCTR1 and delivers them to the chaperones, CCS and ATOX1.
A second CTR protein in human cells
In the original yeast complementation studies a second gene member of the human CTR proteins was cloned. Its similarity to hCTR1 was noted and the suggestion made that hCTR2 was also a Cu transporter (17). CTR2 lacks the majority of the amino-terminus but many of the residues thought to be important for transport are conserved, along with the basic membrane topology. It has been suggested that CTR2 is predominantly an intracellular Cu transporter (38, 39), can apparently perform Cu transport when overexpressed (40), and interestingly may play a role in targeting hCTR1 for degradation (41). Its major physiological functions remain unresolved and await more systematic investigation.
Unresolved questions
We pointed out, ∼8 years ago that not only was Cu homeostasis important physiologically and Cu transport interesting biophysically, but most of the important issues were unresolved (42). This remains true and progress has been made. hCTR1 is a very interesting and novel transporter, by virtue of its trimerization to form a pore structure that mediates cellular uptake of a substrate only present at very low levels in the circulation. We pointed out that the identity of the major source of available Cu in the circulation, which donated Cu to hCTR1 was unknown; this is still true. The identity of the initial intracellular acceptor was unknown; now there is evidence that supports the notion of a common acceptor, GSH. The precise role of the extracellular amino-terminus was unknown, other than presumably to interact with the extracellular donor. This uncertainty remains. Meanwhile, the importance of the intracellular carboxyl-terminus has received increasing attention, as it seems to act as a modulator of transport, and also is essential for the protein to undergo regulatory endocytosis. The mechanism of transport has been probed using ideas gleaned from the behavior of other better-characterized transporters and appears to combine elements of channels and transporters. An alternate and provocative possibility remains. hCTR1 may function in a novel way, essentially providing an intramembrane scaffold that enables coordination of Cu at the mouth of a pore allowing access of intracellular GSH to carry out a ligand-exchange reaction, plucking Cu from the Met triad coordinating center and delivering it to the cytosol.
It is clear that the acquisition of Cu by cells is far from being well understood and that its cellular uptake and homeostasis have evolved a complex network of interactions among a fascinating assembly of players. A detailed understanding of these processes will require the application of an array of biophysical approaches.
Editor: Brian Salzberg.
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
References
- 1.Rae T.D., Schmidt P.J., O’Halloran T.V. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science. 1999;284:805–808. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- 2.Banci L., Bertini I., Palumaa P. Affinity gradients drive copper to cellular destinations. Nature. 2010;465:645–648. doi: 10.1038/nature09018. [DOI] [PubMed] [Google Scholar]
- 3.Xiao Z., Brose J., Wedd A.G. Unification of the copper(I) binding affinities of the metallo-chaperones Atx1, Atox1, and related proteins: detection probes and affinity standards. J. Biol. Chem. 2011;286:11047–11055. doi: 10.1074/jbc.M110.213074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotruvo J.A., Jr., Aron A.T., Chang C.J. Synthetic fluorescent probes for studying copper in biological systems. Chem. Soc. Rev. 2015;44:4400–4414. doi: 10.1039/c4cs00346b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maryon E.B., Molloy S.A., Kaplan J.H. Rate and regulation of copper transport by human copper transporter 1 (hCTR1) J. Biol. Chem. 2013;288:18035–18046. doi: 10.1074/jbc.M112.442426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan J.H., Lutsenko S. Copper transport in mammalian cells: special care for a metal with special needs. J. Biol. Chem. 2009;284:25461–25465. doi: 10.1074/jbc.R109.031286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta A., Lutsenko S. Human copper transporters: mechanism, role in human diseases and therapeutic potential. Future Med. Chem. 2009;1:1125–1142. doi: 10.4155/fmc.09.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huffman D.L., O’Halloran T.V. Function, structure, and mechanism of intracellular copper trafficking proteins. Annu. Rev. Biochem. 2001;70:677–701. doi: 10.1146/annurev.biochem.70.1.677. [DOI] [PubMed] [Google Scholar]
- 9.Itoh S., Kim H.W., Fukai T. Novel role of antioxidant-1 (Atox1) as a copper-dependent transcription factor involved in cell proliferation. J. Biol. Chem. 2008;283:9157–9167. doi: 10.1074/jbc.M709463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatori Y., Lutsenko S. An expanding range of functions for the copper chaperone/antioxidant protein Atox1. Antioxid. Redox Signal. 2013;19:945–957. doi: 10.1089/ars.2012.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martins L.J., Jensen L.T., Winge D.R. Metalloregulation of FRE1 and FRE2 homologs in Saccharomyces cerevisiae. J. Biol. Chem. 1998;273:23716–23721. doi: 10.1074/jbc.273.37.23716. [DOI] [PubMed] [Google Scholar]
- 12.De Feo C.J., Aller S.G., Unger V.M. Three-dimensional structure of the human copper transporter hCTR1. Proc. Natl. Acad. Sci. USA. 2009;106:4237–4242. doi: 10.1073/pnas.0810286106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eisses J.F., Stasser J.P., Blackburn N.J. Domains I and III of the human copper chaperone for superoxide dismutase interact via a cysteine-bridged Dicopper(I) cluster. Biochemistry. 2000;39:7337–7342. doi: 10.1021/bi000690j. [DOI] [PubMed] [Google Scholar]
- 14.Lee J., Peña M.M., Thiele D.J. Biochemical characterization of the human copper transporter Ctr1. J. Biol. Chem. 2002;277:4380–4387. doi: 10.1074/jbc.M104728200. [DOI] [PubMed] [Google Scholar]
- 15.Dancis A., Yuan D.S., Klausner R.D. Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell. 1994;76:393–402. doi: 10.1016/0092-8674(94)90345-x. [DOI] [PubMed] [Google Scholar]
- 16.Dancis A., Haile D., Klausner R.D. The Saccharomyces cerevisiae copper transport protein (Ctr1p). Biochemical characterization, regulation by copper, and physiologic role in copper uptake. J. Biol. Chem. 1994;269:25660–25667. [PubMed] [Google Scholar]
- 17.Zhou B., Gitschier J. hCTR1: a human gene for copper uptake identified by complementation in yeast. Proc. Natl. Acad. Sci. USA. 1997;94:7481–7486. doi: 10.1073/pnas.94.14.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J., Prohaska J.R., Thiele D.J. Isolation of a murine copper transporter gene, tissue specific expression and functional complementation of a yeast copper transport mutant. Gene. 2000;254:87–96. doi: 10.1016/s0378-1119(00)00287-0. [DOI] [PubMed] [Google Scholar]
- 19.Arredondo M., Mendiburo M.J., Garrick M.D. Mouse divalent metal transporter 1 is a copper transporter in hek293 cells. Biometals. 2014;27:115–123. doi: 10.1007/s10534-013-9691-6. [DOI] [PubMed] [Google Scholar]
- 20.Lee J., Petris M.J., Thiele D.J. Characterization of mouse embryonic cells deficient in the ctr1 high affinity copper transporter. Identification of a Ctr1-independent copper transport system. J. Biol. Chem. 2002;277:40253–40259. doi: 10.1074/jbc.M208002200. [DOI] [PubMed] [Google Scholar]
- 21.Zimnicka A.M., Ivy K., Kaplan J.H. Acquisition of dietary copper: a role for anion transporters in intestinal apical copper uptake. Am. J. Physiol. Cell Physiol. 2011;300:C588–C599. doi: 10.1152/ajpcell.00054.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alda J.O., Garay R. Chloride (or bicarbonate)-dependent copper uptake through the anion exchanger in human red blood cells. Am. J. Physiol. 1990;259:C570–C576. doi: 10.1152/ajpcell.1990.259.4.C570. [DOI] [PubMed] [Google Scholar]
- 23.Pope C.R., Flores A.G., Unger V.M. Structure and function of copper uptake transporters. Curr. Top. Membr. 2012;69:97–112. doi: 10.1016/B978-0-12-394390-3.00004-5. [DOI] [PubMed] [Google Scholar]
- 24.Kim H., Wu X., Lee J. SLC31 (CTR) family of copper transporters in health and disease. Mol. Aspects Med. 2013;34:561–570. doi: 10.1016/j.mam.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisses J.F., Kaplan J.H. Molecular characterization of hCTR1, the human copper uptake protein. J. Biol. Chem. 2002;277:29162–29171. doi: 10.1074/jbc.M203652200. [DOI] [PubMed] [Google Scholar]
- 26.Maryon E.B., Molloy S.A., Kaplan J.H. O-linked glycosylation at threonine 27 protects the copper transporter hCTR1 from proteolytic cleavage in mammalian cells. J. Biol. Chem. 2007;282:20376–20387. doi: 10.1074/jbc.M701806200. [DOI] [PubMed] [Google Scholar]
- 27.Zhou H., Thiele D.J. Identification of a novel high affinity copper transport complex in the fission yeast Schizosaccharomyces pombe. J. Biol. Chem. 2001;276:20529–20535. doi: 10.1074/jbc.M102004200. [DOI] [PubMed] [Google Scholar]
- 28.Aller S.G., Unger V.M. Projection structure of the human copper transporter CTR1 at 6-A resolution reveals a compact trimer with a novel channel-like architecture. Proc. Natl. Acad. Sci. USA. 2006;103:3627–3632. doi: 10.1073/pnas.0509929103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisses J.F., Kaplan J.H. The mechanism of copper uptake mediated by human CTR1: a mutational analysis. J. Biol. Chem. 2005;280:37159–37168. doi: 10.1074/jbc.M508822200. [DOI] [PubMed] [Google Scholar]
- 30.Puig S., Lee J., Thiele D.J. Biochemical and genetic analyses of yeast and human high affinity copper transporters suggest a conserved mechanism for copper uptake. J. Biol. Chem. 2002;277:26021–26030. doi: 10.1074/jbc.M202547200. [DOI] [PubMed] [Google Scholar]
- 31.Tsigelny I.F., Sharikov Y., Howell S.B. An all-atom model of the structure of human copper transporter 1. Cell Biochem. Biophys. 2012;63:223–234. doi: 10.1007/s12013-012-9358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu X., Sinani D., Lee J. Copper transport activity of yeast Ctr1 is down-regulated via its C terminus in response to excess copper. J. Biol. Chem. 2009;284:4112–4122. doi: 10.1074/jbc.M807909200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petris M.J., Smith K., Thiele D.J. Copper-stimulated endocytosis and degradation of the human copper transporter, hCtr1. J. Biol. Chem. 2003;278:9639–9646. doi: 10.1074/jbc.M209455200. [DOI] [PubMed] [Google Scholar]
- 34.Molloy S.A., Kaplan J.H. Copper-dependent recycling of hCTR1, the human high affinity copper transporter. J. Biol. Chem. 2009;284:29704–29713. doi: 10.1074/jbc.M109.000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pope C.R., De Feo C.J., Unger V.M. Cellular distribution of copper to superoxide dismutase involves scaffolding by membranes. Proc. Natl. Acad. Sci. USA. 2013;110:20491–20496. doi: 10.1073/pnas.1309820110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freedman J.H., Ciriolo M.R., Peisach J. The role of glutathione in copper metabolism and toxicity. J. Biol. Chem. 1989;264:5598–5605. [PubMed] [Google Scholar]
- 37.Maryon E.B., Molloy S.A., Kaplan J.H. Cellular glutathione plays a key role in copper uptake mediated by human copper transporter 1. Am. J. Physiol. Cell Physiol. 2013;304:C768–C779. doi: 10.1152/ajpcell.00417.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rees E.M., Lee J., Thiele D.J. Mobilization of intracellular copper stores by the ctr2 vacuolar copper transporter. J. Biol. Chem. 2004;279:54221–54229. doi: 10.1074/jbc.M411669200. [DOI] [PubMed] [Google Scholar]
- 39.van den Berghe P.V., Folmer D.E., Klomp L.W. Human copper transporter 2 is localized in late endosomes and lysosomes and facilitates cellular copper uptake. Biochem. J. 2007;407:49–59. doi: 10.1042/BJ20070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bertinato J., Swist E., L’abbé M.R. Ctr2 is partially localized to the plasma membrane and stimulates copper uptake in COS-7 cells. Biochem. J. 2008;409:731–740. doi: 10.1042/BJ20071025. [DOI] [PubMed] [Google Scholar]
- 41.Öhrvik H., Nose Y., Thiele D.J. Ctr2 regulates biogenesis of a cleaved form of mammalian Ctr1 metal transporter lacking the copper- and cisplatin-binding ecto-domain. Proc. Natl. Acad. Sci. USA. 2013;110:E4279–E4288. doi: 10.1073/pnas.1311749110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maryon E.B., Molloy S.A., Kaplan J.H. Copper entry into human cells: progress and unanswered questions. Biometals. 2007;20:355–364. doi: 10.1007/s10534-006-9066-3. [DOI] [PubMed] [Google Scholar]