Main Text
The production and identification of chamber-specific, induced pluripotent stem cell-derived cardiac myocytes (iPSC-CMs) is a crucial step in the application of this technology in physiology, pharmacology, and regenerative studies. The significance of toxicology tests would be strongly diminished if, for instance, drugs for atrial fibrillation were tested in ventricular myocytes or pathophysiological mechanisms involved in hypertrophic cardiomyopathy were studied in atrial myocytes. One could predict that the transplantation of nodal myocytes in the infarcted ventricle would have a strong arrhythmogenic potential and could be disastrous in the setting of myocardial repair strategies. It is therefore not surprising that our article (1) has stimulated discussion. We are particularly grateful to Giles and Noble (2) for their insightful comments and for giving us the opportunity to clarify further some aspects of our work.
A more detailed characterization of the electrical properties of human iPSC-CMs is desirable particularly when a very sophisticated, chamber-specific phenotype needs to be assigned. We agree with Giles and Noble (2) that, among the many possible parameters, the measurement of the resting membrane potential and IK1 is important. However, an accurate knowledge of these parameters, which are already known to be very different between the iPSC-CMs now used and adult cells, is unlikely to help resolve the existence of distinct populations within the iPSC-CMs that can be categorically ascribed to the atrial or ventricular populations of myocytes. As Giles and Noble (2) remind us, there is already very strong evidence that iPSC-CMs have a limited functional IK1 together with substantial differences in other ion transporter expressions as well as in ultrastructure, in metabolism, etc. This is further proof that iPSC-CMs, in their contemporary status, cannot completely mirror the highly specialized, chamber-specific, human adult myocytes. Moreover, the assertion that reinstating normal levels of IK1 can unveil the existence of subpopulations of myocytes appears to us simplistic (see analysis below).
We came initially to this topic somewhat serendipitously. Having used iPSC-CMs in our work for a number of years, it appeared to us that the numerous assertions in the literature that it is possible to determine the existence of distinct subpopulations of atrial, ventricular, and nodal myocytes from their action potential (AP) morphologies could not, in our hands at least, be supported. Moretti et al. (3) first alluded to isolated iPSC-CMs belonging to a particular cardiac chamber in 2010, based on analysis of AP waveforms. Subsequently, many groups have described individually selected iPSC-CMs using terms such as “ventricular” or “ventricular-like,” based entirely on AP waveform morphology. Notwithstanding that there are no molecular markers specific or selective for adult chamber-specific myocytes, the ascribing of chamber specificity based on AP waveform would presumably require highly specific AP parameters—this, however, this has not been the case.
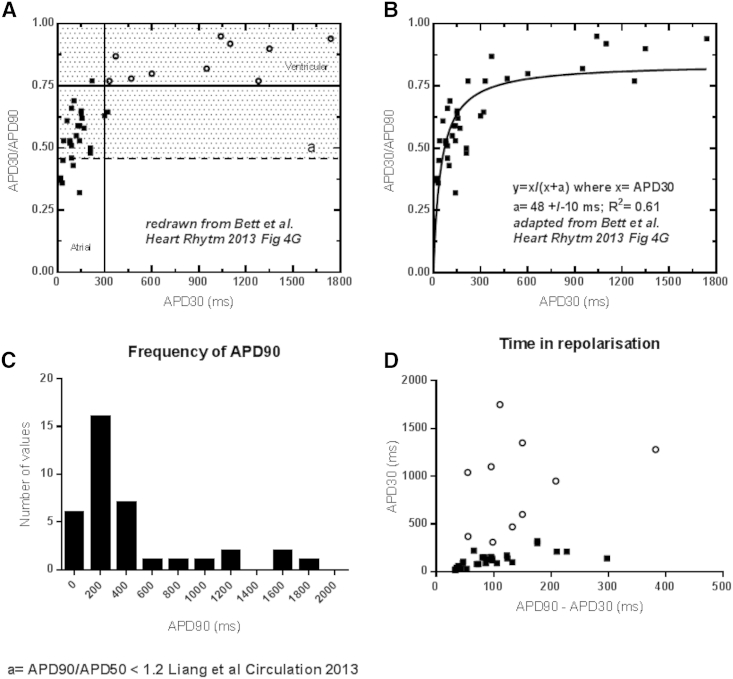
Few authors detail the criteria upon which they judge whether an AP waveform is atrial, ventricular, or nodal. This qualitative approach seemed unsatisfactory to us and raises concerns over the robustness of conclusions derived in this way. By way of example, we have reproduced Fig. 4 D from the work of Bett et al. (4), quoted by Giles and Noble (2), concerning AP morphologies on electronic expression of IK1 in iPSC-CMs. The authors plotted APD30 against APD30/90 and placed arbitrary boundaries to distinguish between atrial and ventricular populations, suggesting that there are two distinct subpopulations. We have redrawn this graph in our Fig. 1 A (1) and changed the symbols for ventricular cells to open circles. We have then drawn the borderline value (Fig. 1 A, dotted line, Du et al. (1)) used in another study to distinguish between atrial and ventricular cells (5). These two analyses lead to a very different categorization, supporting our hypothesis that these atrial, ventricular, and nodal labels are based on the arbitrary clustering of continuous variables. In support of this, in Fig. 1 B (1) we show that there is a continuous, hyperbolic relationship between APD30/90 and APD30 in these cells. As such, one can argue that AP duration is fairly uniformly distributed, even after corrections for IK1. Fig. 1 C (1) shows that APD90, the most commonly used parameter in the literature, also does not cluster in two distinct subpopulations. In addition, the ventricular cells have durations of >1 s that, even for a nonphysiological 0.5-Hz stimulation rate, indicates significant residual repolarization defects, despite IK1 correction. Finally, Fig. 1 D (1) shows that APD90-APD30 (time of repolarization, presumably highly dependent on IK1, independent of APD and expected to be larger in more triangular atrial cell action potentials) does not cluster into two subpopulations and there is no relationship between this distribution and the ventricular label assigned in Fig. 1 A (1). This further suggests that AP morphology cannot be used to determine chamber specificity.
Figure 1.
Categorization of a continuous variable leads to the incorrect use of action potential morphology to determine the chamber specificity of iPSC-CM. This figure shows a reanalysis of data from Bett et al. (4). In (A), we have redrawn the original graph where Bett et al. (4) plotted APD30 against APD30/90 and placed arbitrary boundaries to distinguish between atrial and ventricular populations. We have changed the symbols for ventricular cells to open circles. We have then drawn the borderline value (dotted line) used in another study to distinguish between atrial and ventricular cells (5). These two analyses lead to a very different categorization. (B) Shows that there is a continuous, hyperbolic relationship between APD30/90 and APD30 in these cells. (C) Shows the distribution of APD90 in these experiments with no evidence that this parameter clusters in two distinct subpopulations. In (D), APD90-APD30, a more direct index of the time of fast repolarization, was plotted against APD30. Once again, this parameter does not cluster into two subpopulations and there is no relationship between this distribution and the ventricular label assigned in (A). This demonstrates that AP morphology cannot be used to determine chamber specificity in this experiment.
We entirely agree with Giles and Noble (2) that microelectrode recording of electrophysiological parameters is the most accurate way to study AP morphology and patch-clamping is the only way to directly measure ion currents. However, this method has several limitations in this setting. Firstly, it is a very low throughput technique and is strongly subjected to selection bias (i.e., data can only be acquired from cells that are successfully impaled). Secondly, iPSC-CMs are morphologically very heterogeneous, extremely flat, and difficult to impale compared with adult myocytes. These problems may be addressed with the new high-throughput automatic patch-clamping techniques, although a component of selection bias may be present even with these. Thirdly, and more worryingly, patch-clamping can only be performed in isolated cells, often soon after enzymatic digestion; while we agree again with Giles and Noble (2) that cell density strongly affects ion transporter expression and regulation (and we indirectly show it here), we cannot support their view that single cell analysis should be preferred to multicellular analysis. If these cells should be used for understanding disease and testing drugs, with the myocardium being a syncytium, this view is hardly tenable. For this reason patch-clamp data obtained from isolated iPSC-CMs should be interpreted with caution.
We were not the first to consider this weakness of work to date, with Gorospe et al. (6) turning to computational methods to perform more quantitative comparisons of optically recorded AP waveforms with typical chamberlike morphologies, albeit in embryonic stem cell-derived cardiomyocytes. Tellingly, while this approach was able to distinguish a number of discrete AP phenotypes, this varied from just one to four or more distinct sets of morphologies depending on how the data was interpreted.
Our approach was to look for evidence of discrete populations of chamber-specific iPSC-CMs based on AP morphology, without any prior assumption of their existence. Further, having noted the apparent difference in our own work using confluent monolayers of iPSC-CMs and single cell work in the literature, we decided to compare datasets between confluent and single cell preparations.
Efforts were made to address limitations of previous studies. Utilizing an optical mapping system allowed us to analyze hundreds of cells, because sample sizes of cells from previous studies that used patch-clamping techniques provide inadequate statistical power. This also reduced operator bias of individually selecting cells. Further, we were able to avoid the electrophysiological variability and heterogeneity, which is a natural consequence of individual cells that have been enzymatically digested and then isolated or suspended. The ability to analyze cells in a two-dimensional monolayer allowed us to introduce some complexity to the model that cannot exist in patch-clamping experiments.
While in their article Giles and Noble (2) assert that this is the case, at no point did we come to the conclusion that long or short, spike and dome, and triangular AP waveforms cannot be identified from these preparations. Indeed, we highlight that the vast spread of APDs seen in singly seeded cells may account for the observations of many other chamberlike waveforms in patch-clamp studies; any very long or short APs may exist in the extremes of the bell-shaped curve. What we do conclude is that given the observed plasticity of AP morphologies upon variation of culture density, attempts to assign chamber types on this basis are predicated on a false syllogism: ventricular cardiomyocytes have a long AP, therefore cardiomyocytes with long APs are ventricular. In the study of terminally differentiated adult cardiomyocytes, one would not consider such logic appropriate. While it is possible that chamber-specific cells may be reliably identified by some other measure, we would strongly argue that AP morphology is not a suitable means to achieve that end.
This opens the important and unanswered question: are adult features of atrial and ventricular cardiac myocytes embryologically determined (and as such capable of showing electrophysiological differences in iPSC-CMs), or does distinct phenotype depend on the exposure to the mechanical and chemical environment of the different cardiac chambers? More evidence supports the latter hypothesis (7), and it is therefore difficult to see how chamber specificity is even expected in iPSC-CMs. iPSC-CMs, being derived from fibroblasts in vitro, have not been exposed to any specific cardiac chamber environment. Their development/differentiation is carried out using a minimalistic in vitro approach that cannot resemble the complex cardiac developmental program occurring in the embryo. Our results show that AP morphology does not supply evidence of chamber specificity and as such, we feel that there is, as of this writing, insufficient evidence to support the idea that iPSC-CMs spontaneously form distinct, chamber-specific subtypes. Manipulating the environment with chemical and mechanical stimuli, such as with retinoic acid (8), anisotropism (9), or increased mechanical load, may lead to the selection of iPSC-CMs enriched with certain ion transporters that better simulate a particular chamber-specific phenotype. It remains that validation of these strategies cannot rely entirely on AP morphology, and more specific markers are required.
Moving forward, we are confident that the development of more sophisticated techniques for optical recording of membrane voltage, such as those involving genetically encoded or more quantitative and less toxic ratiometric dyes, will make possible to achieve the deeper degree of electrophysiological analysis in large multicellular preparations that we, with Giles and Noble (2), feel is necessary to understand the physiology of iPSC-CMs. This will maximize the potential of these intriguing and promising cell preparations for advancements in drug testing and cardiac tissue regeneration.
Editor: Godfrey Smith.
References
- 1.Du D.T.M., Hellen N., Terracciano C.M.N. Action potential morphology of human induced pluripotent stem cell-derived cardiomyocytes does not predict cardiac chamber specificity and is dependent on cell density. Biophys. J. 2015;108:1–4. doi: 10.1016/j.bpj.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giles W.R., Noble D. Comment to the editor. Biophys. J. 2016;110:280–282. [Google Scholar]
- 3.Moretti A., Bellin M., Laugwitz K.L. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N. Engl. J. Med. 2010;363:1397–1409. doi: 10.1056/NEJMoa0908679. [DOI] [PubMed] [Google Scholar]
- 4.Bett G.C.L., Kaplan A.D., Rasmusson R.L. Electronic “expression” of the inward rectifier in cardiocytes derived from human-induced pluripotent stem cells. Heart Rhythm. 2013;10:1903–1910. doi: 10.1016/j.hrthm.2013.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang P., Lan F., Wu J.C. Drug screening using a library of human induced pluripotent stem cell-derived cardiomyocytes reveals disease-specific patterns of cardiotoxicity. Circulation. 2013;127:1677–1691. doi: 10.1161/CIRCULATIONAHA.113.001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorospe G., Zhu R., Vidal R. Automated grouping of action potentials of human embryonic stem cell-derived cardiomyocytes. IEEE Trans. Biomed. Eng. 2014;61:2389–2395. doi: 10.1109/TBME.2014.2311387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mikawa T., Fischman D.A. The polyclonal origin of myocyte lineages. Annu. Rev. Physiol. 1996;58:509–521. doi: 10.1146/annurev.ph.58.030196.002453. [DOI] [PubMed] [Google Scholar]
- 8.Devalla H.D., Schwach V., Passier R. Atrial-like cardiomyocytes from human pluripotent stem cells are a robust preclinical model for assessing atrial-selective pharmacology. EMBO Mol. Med. 2015;7:394–410. doi: 10.15252/emmm.201404757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rao C., Prodromakis T., Terracciano C.M. The effect of microgrooved culture substrates on calcium cycling of cardiac myocytes derived from human induced pluripotent stem cells. Biomaterials. 2013;34:2399–2411. doi: 10.1016/j.biomaterials.2012.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]