Abstract
Gating of voltage-dependent cation channels involves three general molecular processes: voltage sensor activation, sensor-pore coupling, and pore opening. KCNQ1 is a voltage-gated potassium (Kv) channel whose distinctive properties have provided novel insights on fundamental principles of voltage-dependent gating. 1) Similar to other Kv channels, KCNQ1 voltage sensor activation undergoes two resolvable steps; but, unique to KCNQ1, the pore opens at both the intermediate and activated state of voltage sensor activation. The voltage sensor-pore coupling differs in the intermediate-open and the activated-open states, resulting in changes of open pore properties during voltage sensor activation. 2) The voltage sensor-pore coupling and pore opening require the membrane lipid PIP2 and intracellular ATP, respectively, as cofactors, thus voltage-dependent gating is dependent on multiple stimuli, including the binding of intracellular signaling molecules. These mechanisms underlie the extraordinary KCNE1 subunit modification of the KCNQ1 channel and have significant physiological implications.
Main Text
Voltage-gated K+, Na+, and Ca2+ ion channels (KV, NaV, and CaV) are responsible for electric activities and Ca2+ signaling in excitable cells such as neurons and cardiac myocytes, as well as in various nonexcitable cells. Since Hodgkin and Huxley discovered voltage-dependent changes of membrane conductance of Na+ and K+ ions in the 1950s (1), functional and structural studies of voltage-gated ion channels have revealed the general principles of voltage-dependent gating. It is known that the voltage sensors and the ionic pore of these channels are distinct structural domains (2, 3) (Fig. 1, A and B). The voltage-dependent movements of voltage sensors regulate the opening of the pore. Thus, voltage-dependent gating of these channels involves three general molecular processes: 1) voltage-dependent movements in the voltage sensor domain (VSD), known as voltage sensor activation; 2) activation gate movements in the pore-gate domain (PGD), known as pore opening; and 3) interactions between the VSD and PGD that propagate the movements from one to the other, known as VSD-PGD coupling. Voltage sensor activation should not be confused with voltage-dependent activation of the channel, which is a term historically used to describe channel opening in response to voltage changes, encompassing all three molecular processes.
Figure 1.
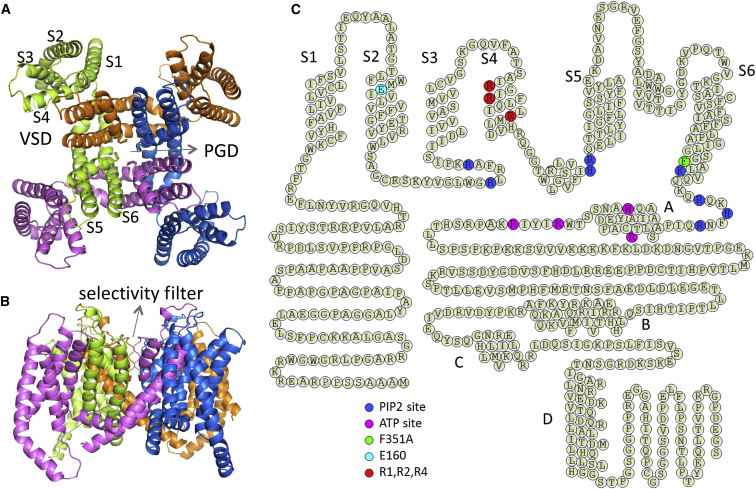
Models of the KCNQ1 channel. (A) Top view of the tetrameric KCNQ1 homology model. Four subunits are shown in different colors. Every subunit consists S1–S6 transmembrane segments as indicated. The S1–S4 form the voltage sensor domain, and the S5-S6 from all four subunits form the pore-gate domain. This open state KCNQ1 structural model is originated from Van Horn et al. (4). (B) Side view of the KCNQ1 channel homology model; the selectivity filter is labeled as indicated. (C) Membrane topology of full length KCNQ1. Each circle represents an amino acid residue with the one-letter symbol. The S1–S6 and cytoplasmic C terminus helices (A–D) are labeled as indicated. The residues important for voltage-dependent gating, including the gating charges in S4 (R1, R2, and R4), E160 in S2, F351 in the cytoplasmic end of S6, and the PIP2 and ATP binding sites are colored as indicated.
The VSD and the PGD, respectively, of voltage-gated ion channels share common structural features. The VSD is comprised of transmembrane helices S1–S4. In this domain, the primary voltage sensitive element is S4, which contains the positively charged residues arginine (R) or lysine (K). The PGD is comprised of S5, S6, and the pore loop between the two helices, which contains the ion selectivity filter (Fig. 1). During voltage sensor activation, S4 moves in the extracellular direction in response to depolarization of the membrane potential (5, 6), while during pore opening, the cytoplasmic lower halves of S6 in the four subunits move apart (7, 8). VSD-PGD coupling is less understood. We know that the interaction between the peptide linking S4 and S5 and the cytoplasmic end of S6 is important for the coupling (9, 10). Other interactions, such as those between S4 and S5 (11, 12, 13) and between S1 and the outer vestibule of the pore (14), may also contribute to the VSD-PGD coupling.
The KCNQ1 channel, also known as KvLQT1 or Kv7.1, was discovered to be a novel Kv channel, and its gene was associated with type 1 long QT syndrome (LQT1) (15), which predisposes inflicted patients to cardiac arrhythmia. Besides having a critical function in the physiology of the heart, KCNQ1 is also involved in the functions of other organs, including the brain, kidney, and gastrointestinal system (16, 17). In different tissues, members of the KCNE auxiliary subunit family (KCNE1–5) associate with KCNQ1 to modify channel properties, creating the various phenotypes important for physiological functions (17). KCNQ1 is the founding member of the KCNQ channel family (KCNQ1–5). KCNQ2–5 form K+ channels that are important for neural functions and share important properties with KCNQ1 (18, 19), but because of the scope of this review, these channels will not be discussed in this article. The mechanisms of voltage-dependent gating are central to the physiological and pathophysiological roles of KCNQ1 channels, highlighted by the fact that KCNE subunits and various disease-associated mutations alter voltage-dependent activation. Structural models of KCNQ1 based on sequence homology and experimental results indicate that KCNQ1 has a canonical Kv channel structure (4, 20, 21, 22, 23): the channel is formed by four KCNQ1 subunits, with the individual VSD from each subunit located at the periphery of the central pore that is formed by the PGD of all four subunits (Fig. 1 A). The mechanism of voltage-dependent gating of KCNQ1 has been investigated mostly within a framework that has been established mainly by studies of the Shaker-related subfamily of Kv channels, and, in general, the mechanism of KCNQ1 gating closely follows the same principles. However, KCNQ1 shows unique properties in voltage-dependent activation. Although these properties could reflect mechanisms of gating specific to KCNQ1 channels, I argue in this mini review that they provide opportunities to reveal novel aspects of the general mechanism of voltage-dependent gating. I first summarize results suggesting that mechanisms common to voltage-gated ion channels also apply to KCNQ1 voltage-dependent gating. Then I focus on the distinctive features of KCNQ1 gating and how these features underlie the modulation of KCNQ1 by its auxiliary subunit, KCNE1. In the end, I discuss how the features of KCNQ1 gating can bring novel insights into the general principles of voltage-dependent gating, especially into the mechanism of VSD-PGD coupling.
General mechanisms of voltage-dependent gating in KCNQ1
Voltage sensor activation
The KCNQ1 S4 contains arginine residues that are conserved in other Kv channels (Fig. 1 C). In Shaker K+ channels, the first four arginines (R1–R4) in the N-terminal half of S4 serve as gating charges for sensing membrane potential (24, 25, 26), but in the KCNQ1 S4 the canonical R3 is replaced by a neutral glutamine (Q234). The C-terminal half of S4 in KCNQ1 also has fewer net positive charges than Shaker. Mutations of S4 residues in KCNQ1 change the voltage dependence of channel activation (27, 28, 29); particularly, neutralization of R231 (R2) with various amino acids (27, 28, 29), and mutation R228E (R1E) (29) eliminate voltage dependence and make the channel constitutively open.
Gating currents resulting from movements of voltage sensors in the membrane were recorded recently from KCNQ1 channels (30). Fluorescence signals from a fluorophore attached to the S3-S4 linker or the N-terminus of S4, measured using voltage clamp fluorometry (VCF), showed kinetics and voltage dependence that were similar to those of gating charge movements measured by gating currents (30, 31), indicating that S4 moves during VSD activation. S4 movements were also demonstrated by the accessibility of cysteine mutations in S4 by extracellular 2-(trimethylammonium) ethyl methanethiosulfonate (MTSET); R228C, G229C, and I230C are accessible only at depolarized voltages where the VSD of KCNQ1 is activated, indicating that S4 moves toward the extracellular side and exposes these residues during voltage-dependent activation (32, 33). Another piece of evidence for S4 movements was provided by the study of interactions of arginine residues in S4 with E160, located at the extracellular end of S2. There, R228 (R1) and R237 (R4) were found to make electrostatic interactions with E160 in the resting state (at hyperpolarized voltages) and the activated state (at depolarized voltages) of the VSD, respectively, indicating an outward movement of S4 during VSD activation (34). These studies also revealed specific characteristics of VSD activation in KCNQ1. First, S4 movements in KCNQ1 are slow, resulting in gating currents with small amplitudes and slow decaying kinetics (time constant >20 ms) (30), which accounts for the slower kinetics of ionic current onset as compared with Shaker K+ channels. Second, the paucity of positive charges in S4 may explain why the channel can be turned to constitutively open by S4 mutations (27). Supporting this idea, the addition of a positive charge by the mutation Q234R (Q3R) recovered voltage dependence of R228E (R1E) (29).
Pore opening
In Kv channels, S6 lines the inner pore. Scanning mutations of the KCNQ1 S6 by substituting alanine or tryptophan for each residue showed that many mutations changed macroscopic current amplitudes and altered the voltage dependence of channel activation (35, 36). Particularly, a cluster of mutations, including P343A, G345A, and I346A, abolished the current but not channel expression in the surface membrane (35, 37), whereas F340W made the channel constitutively open and insensitive to voltage (36). Mutations associated with LQT, F339S, A341V, A341E, and G345E were also found to abolish KCNQ1 currents (38, 39). Interestingly, P343, A344, and G345 are aligned with proline-valine-proline in the Shaker S6 that are important for the motion of S6 during pore opening (7). A glycine residue conserved in the S6 of many K+ channels is thought to also act as a hinge for S6 movements (8). The corresponding residue in the KCNQ1 S6 is A336, and mutations of A336 altered the voltage dependence of channel gating (37). These results are consistent with the idea that mutations in the KCNQ1 S6 around the PAG region may alter the conformation or the motion of the activation gate, resulting in either a constitutively closed or a constitutively open channel. Nonetheless, the structural motifs in KCNQ1 that restrict ionic flow in the closed channel and that move during pore opening are not known.
VSD-PGD coupling
KCNQ1 channels show a constitutive open component that cannot be turned off, even at very negative (∼120 mV) voltages. Many mutations increase the fraction of this constitutive open conductance versus the total conductance, and a large-scale mutational scanning study showed that such an increase was correlated with the shift of the G-V relation of the voltage-dependent component to more negative voltages (40). This relation could be quantitatively explained by a model assuming an allosteric VSD-PGD coupling, according to which the pore can open without voltage sensor activation, but activation of any of the four VSDs increased the probability of pore opening (40). The same allosteric model has been proposed to describe voltage-dependent gating of the BK and hyperpolarization-activated cyclic nucleotide-gated channels (41, 42). In another study, VCF was used to measure voltage sensor movements and the pore opening of channels composed of mutant KCNQ1 subunits with different voltage dependences. The results suggested that the VSD of each subunit moved independently and that pore opening was not tightly correlated with VSD movements of any of the subunits; rather, the results could be well fitted by the same model of allosteric VSD-PGD coupling (43). The allosteric VSD-PGD coupling was also directly shown by locking the pore in an open state that did not prevent the voltage sensor from moving between the resting and activated state (44).
The interaction between the S4-S5 linker and the cytoplasmic end of S6 is important for VSD-PGD coupling in Kv channels (9, 10). In a study of LQT-associated mutations in the KCNQ1 S4-S5 linker, R243C and W248R were found to reduce the opening rate and to shift the voltage dependence of channel opening to more positive voltages, whereas E261K abolished channel function, suggesting the importance of the S4-S5 linker in voltage-dependent gating of KCNQ1 (45). Likewise, a mutation in the cytoplasmic end of S6 associated with LQT, Q357R, also reduced channel function (46). Subsequent studies of scanning mutations of the S4-S5 linker (47) and the cytoplasmic end of S6 (48) found many mutations that had high impact on voltage-dependent gating of KCNQ1. The effects of these mutations from the two different domains share some phenotypical similarities. For instance, one group of mutations, including T247A, L251A, V255W, H258A, and R259A in the S4-S5 linker and S349A, F351A, A352W, and V355W in the cytoplasmic end of S6, reduced the opening rate and shifted the voltage dependence of channel opening to more positive voltages. Another group of mutations, including V254A, L, or E in the S4-S5 linker, and L353A in the cytoplasmic end of S6, led to an increased constitutively open component, and L353E and L353K KCNQ1 even lost voltage dependence entirely, becoming constitutively open. Interestingly, the double mutation V254L/L353A eliminated the constitutively open component, suggesting that the S4-S5 linker and the cytoplasmic end of S6 interacted to close the channel and that the volume of the side chain in residues V254 and L353 was important for the interaction (47). Consistent with this idea, a parallel study showed that isolated peptides corresponding to the S4-S5 linker inhibited the KCNQ1 current, whereas peptides corresponding to the cytoplasmic end of S6 increased the current (49). These results were taken to suggest that the S4-S5 linker in the channel acted as a ligand, binding to the cytoplasmic end of S6 to stabilize the closed state of the channel. Voltage sensor activation prevented this interaction, thereby promoting pore opening. The increased binding of isolated S4-S5 linker peptide to the channel helped stabilize the closed states, whereas the competition for the native S4-S5 linker by the isolated S6 peptide destabilized the closed states (49, 50).
Unique properties of voltage-dependent gating of KCNQ1 channels
Pore opening and VSD-PGD coupling at different stages of voltage sensor activation
In a study of the interaction between S4 arginine residues with E160 in S2 (Fig. 1 C), it was found that the VSD moves in two resolvable steps. Measured in a mutant E160R/R228E/Q234R KCNQ1 coexpressed with the auxiliary subunit KCNE1, VSD movements in the voltage ranges of −100 ∼ −50 mV and −50 ∼ +50 mV showed stepwise increments, suggesting that, during activation, the voltage sensor traversed a stable intermediate state at ∼−50 mV before reaching the activated state at high voltages (34). Also, for the triple mutant C214A/G219C/C331A KCNQ1+KCNE1, stepwise VSD movements were shown using VCF, and the two VSD movements were consistently separated at ∼−50 mV (51). More recently, it was found that in KCNQ1 alone, without KCNE1 association, the VSD also showed stepwise movements during activation. The fluorescence signal measured in VCF experiments showed that the movement at negative voltages (Fmain) shifted to less negative voltages than in KCNQ1+KCNE1, whereas the movement at more positive voltages (Fhigh) was not changed (52).
Stepwise activation of the VSD has been described previously in Shaker K+ channels (53, 54). In these channels, mutations such as the isoleucine-leucine-threonine mutation in S4 (11) and mutations in the gating charge transfer center (55), also known as the hydrophobic plug (56, 57, 58), make the separation in voltage ranges between the two movements more prominent. However, unlike in Shaker, where the pore opens only at the activated state of the VSD, the pore in KCNQ1 opens at both the intermediate and activated states, where the voltage dependence of conductance, the G-V relation, nearly overlaps with that of Fmain (30, 31, 52). Consistently, the double charge reversal mutations, E160R/R231E (E1R/R2E) and E160R/R237E (E1R/R4E), which arrest the VSD at an intermediate and the fully activated state, respectively, make the channel constitutively open (21, 29, 52, 59). Interestingly, it was found that the E1R/R2E and E1R/R4E mutant channels showed different relative Rb+/K+ permeabilities or sensitivities to the inhibitor XE991. Compared with E1R/R4E, E1R/R2E exhibited a larger ratio of inward currents in high Rb+ versus high K+ extracellular solutions; 5 μM XE991 inhibited E1R/R2E currents, but not E1R/R4E (52). The wild-type (WT) KCNQ1 channel showed a larger ratio of inward currents in high Rb+ versus high K+ extracellular solutions (60). The larger Rb+ current was attributed to a reduced flickering block of the pore upon Rb+ binding (60, 61). Consistent with this idea, many mutations in the PGD of KCNQ1 modulated Rb+/K+ permeability, presumably by altering the PGD conformation (62). Thus, the results showing that E1R/R2E and E1R/R4E mutations in the VSD modulate Rb+/K+ permeability suggest that when the VSD is at either the intermediate or activated state, the pore is in different open conformations. The mechanism of XE991 inhibition is not clear. The mutations E1R/R2E and E1R/R4E may alter XE991 inhibition allosterically, which is also consistent with the idea that the conformation of the channel protein differs at the intermediate-open and activated-open states.
The presence of two different open states during KCNQ1 gating was suggested to account for the properties of inactivation (62, 63). Two open states were also shown by a study of Na+ block of the instantaneous KCNQ1 current at the beginning of voltage pulses following a prepulse that activated the channel (64). The currents were blocked more by Na+ when the prepulse duration was longer, suggesting that the open state visited at early times during the prepulse was less sensitive to Na+ block than the open state visited later. Whether these different open states correlate with the intermediate-open and activated-open states remains to be tested.
It was shown that a mutation at the cytoplasmic end of S6, F351A, could abolish the intermediate-open state so that the channel contained predominantly the activated-open state (52). F351 (Fig. 1 C) is highly conserved in many Kv channels and is important for VSD-PGD coupling (48, 65). The mutation selectively suppressed one open state but not the other, suggesting that the VSD-PGD coupling may differ when the VSD is at the intermediate or activated state. This is consistent with results showing that the conformation of the open pore differs when the VSD is at either the intermediate-open or activated-open state, so that different VSD-PGD couplings result in different open pore conformations.
PIP2 is required for VSD-PGD coupling
Phosphatidylinositol 4,5-bisphosphate (PIP2) is an anionic lipid found in the inner leaflet of the plasma membrane. PIP2 is a second messenger for cell signaling and known to directly bind to and regulate a wide variety of ion channels, including KCNQ1 (66). KCNQ1 was found to require PIP2 to function; depletion of PIP2 from the membrane suppresses KCNQ1. The suppression can be prevented or reversed by replenishing PIP2 through cellular metabolisms or exogenous application (44, 67, 68, 69, 70).
VCF measurements showed that PIP2 is required for VSD-PGD coupling. PIP2 depletion by a voltage-activated lipid phosphatase, CiVSP (71), abolished pore opening but left voltage sensor activation intact (44). This result suggests that either the VSD-PGD coupling or pore opening was disrupted in the absence of PIP2. PIP2 depletion was experimentally shown to actually disrupted VSD-PGD coupling (44). Because of VSD-PGD coupling, constitutive opening of the pore, held by a mutation L353K, shifted the voltage dependence of voltage sensor activation to more negative voltages. After PIP2 depletion, the pore of the mutant KCNQ1 was still constitutively open, but the voltage dependence of voltage sensor activation was no longer altered, indicating that the VSD-PGD coupling was disrupted. Consistent with the role of PIP2 in mediating VSD-PGD coupling, a PIP2 binding site was found at the interface between each VSD and the PGD, which consists of residues in the S2-S3 linker, S4-S5 linker, and the cytoplasmic end of S6 (Fig. 1 C) (44, 72). The location of the PIP2 binding site is consistent with mutational and biochemical results that reveal the importance of some residues in PIP2 binding to the isolated peptides from the KCNQ1 protein (73) and on KCNQ1 function (74) (for a review, see Zaydman and Cui (19)). This PIP2 binding site is a major physiologically important feature of the KCNQ channels that has been preserved during channel evolution (75).
Molecular dynamics simulation and experimental results showed that both protein-protein interactions between the VSD and PGD and protein-PIP2 interactions are involved in VSD-PGD coupling (76). The protein-protein interactions are weakened in KCNQ1 channels by electrostatic interactions such as that between R249 in the S4-S5 linker and K358 in the cytoplasmic end of S6. The negative charges in the PIP2 head group make salt bridges with basic residues in the S2-S3 linker, S4-S5 linker, and the cytoplasmic end of S6; the interaction of PIP2 with each individual residue depends on the open and closed state of the channel to mediate VSD-PGD coupling.
Besides the above site at the interface between the VSD and PGD, mutations of basic amino acids located in other parts of the KCNQ1 protein, such as in the cytoplasmic helix C, including R359W, R555C, R555H, K557E, and R562M (74, 77, 78), also alter the responses of KCNQ1 to PIP2 depletion. Thus, these residues may be also important for PIP2-dependent activation. These residues may form independent PIP2 binding sites to affect channel activation with mechanisms that are not yet known, or may contribute directly or allosterically to the site at the interface between the VSD and PGD (76).
Intracellular ATP is required for pore opening
During patch clamp studies, ATP was required in the intracellular bath solution to maintain the KCNQ1 current in excised inside-out membrane patches (67, 79). ATP was also required for the native KCNQ1 channels (in association with KCNE1) in cardiac myocytes to activate, with an EC50 at ∼1.6 mM. This concentration is close to the cytoplasmic concentration of ATP in physiological conditions, which indicates that ATP-dependent activation of the channel plays a physiological role (79).
It was shown that ATP activates the channel by directly binding to KCNQ1. Mutations of residues W379, R380, K393, and R397 in the cytoplasmic helix A and the A-B linker (Fig. 1 C) abolished not only channel opening but also the binding of an ATP analog (79), suggesting that these residues form the ATP binding site. In the absence of ATP binding, voltage sensors activated normally, although the channel was not open. The voltage dependence of voltage sensor activation was shifted to more negative voltages in the constitutively open L353K KCNQ1, in both the presence and absence of ATP. These results indicate that ATP is not required for voltage sensor activation or VSD-PGD coupling; rather, ATP is required for pore opening (79).
KCNE1 alters VSD-PGD coupling of KCNQ1 to selectively modulate different open states
KCNQ1 is associated with the auxiliary subunit KCNE1, which was discovered before KCNQ1 (80, 81). Together, they form the slowly activating IKs channel in the heart, which is important for action potential repolarization (82, 83). Mutations in either KCNQ1 or KCNE1 are associated with long QT syndrome (LQT), which predisposes patients to cardiac arrhythmias, resulting in syncope and sudden death (15, 84, 85). KCNE1 is a peptide of only 129 amino acids and a single transmembrane helix (80, 86, 87), but its association with KCNQ1 drastically alters every aspect of channel function. The most prominent changes include an increase in the total current amplitude, a shift in the voltage-dependence of activation toward more depolarized potentials, a prolonged activation and deactivation time course (82, 83), the removal of inactivation (63, 88), a decreased Rb+/K+ selectivity (60), altered effects of drugs on channel activity (89, 90, 91, 92, 93), and increased effects of protein kinase A (PKA) phosphorylation on channel function (94, 95). These KCNE1 associated changes in channel gating, ion selectivity, pharmacology, and posttranslational modulation are essential to the proper physiological role of the IKs channels in the heart, and they are fundamental to the understanding and therapeutic treatment of diseases associated with IKs. For instance, in natural fight or flight responses β-adrenergic stimulation increases the rate and contractile force of the heart; at the same time, the β-adrenergic stimulation reduces action potential duration, in part by enhancing IKs currents to facilitate proper diastolic filling at faster rates. The β-adrenergic stimulation enhances IKs currents via PKA dependent phosphorylation of KCNQ1 only in association with KCNE1 (94, 95). The interaction between KCNQ1 and KCNE1 also confers kinetic properties on IKs that make it suitable for participation in rate-dependent adaptation of the cardiac action potential (96, 97). Corroborating with the importance of IKs in rate-dependent adaptation of the cardiac action potential, patients with IKs-associated LQT syndrome often have lethal arrhythmia events during exercise or emotion, when the β-adrenergic pathway was stimulated (98, 99). LQT mutations that abolish the response of IKs to β-adrenergic stimulation pose a much higher risk of death than other mutations in KCNQ1 (100, 101, 102). How can such a small KCNE1 subunit alter the channel function so broadly and radically? Is there an overarching mechanism for all these changes? These questions have been a major driving force in studies of the KCNQ1 and IKs channels.
Structurally, the transmembrane helix of KCNE1 is located in the cleft formed by two adjacent VSDs and the PGD (Fig. 1 A) (4, 22, 23, 87, 103). This location of KCNE1 in the channel complex was identified from disulfide bonds or ion bridges formed between engineered or native cysteine residues in KCNE1 and KCNQ1 proteins at extracellular (23, 104, 105, 106, 107), transmembrane (108), and cytosolic (109) (also see (110, 111)) domains. The location of KCNE1 in the channel suggests that it may interact with the PGD, VSD, or both to modulate channel functions.
Mutations of residues in the transmembrane helix of KCNE1, particularly those of F57, T58, and L59, alter the effects of KCNE1 on voltage-dependent gating (112, 113). These residues were found to have functional interactions with S338, F339, F340, and A341 located in S6 of KCNQ1, because the effects on voltage-dependent gating by the mutations in KCNE1 were altered by the mutations in KCNQ1, and vice versa (36, 103, 114). These functional interactions were cited as evidence for physical interaction between S6 and the transmembrane helix of KCNE1 (87, 103, 115, 116). However, recent studies suggested that interactions between the extracellular domain of KCNE1 with that of the PGD might affect voltage-dependent activation as well (23, 117). A homology model and molecular dynamics simulation of the IKs channel structure based on the crystal structure of the KV1.2/Kv2.1 chimera (118), the NMR structure of KCNE1 (87), and experimental data on the IKs channel, suggested that residues in the transmembrane helix of KCNE1 might not be physically close to those in the KCNQ1 S6. Thus the functional interactions among these sites might derive from an allosteric connection (23). Further studies are needed to resolve whether a physical interaction between the transmembrane helix of KCNE1 and S6 of KCNQ1 is important for KCNE1 to modulate KCNQ1 function.
KCNE1 alters the environment of S4 during activation because KCNE1 association alters the accessibility of extracellular MTS reagents to engineered cysteine residues in the VSD during voltage sensor activation. E160C in S2 is not accessible in KCNQ1 but can be modulated by extracellular MTS reagents in the presence of KCNE1 (34). T224C in S4 is accessible at voltages above −100 mV in the absence of KCNE1 but is accessible only in the presence of KCNE1 at voltages above 0 mV (51). Tryptophan scanning showed that the same mutations of the S4 residues alter voltage-dependent gating differently with or without KCNE1 coexpression, which also suggest interactions altered between S4 and its environment by KCNE1 association (28, 29). Although these differences are prominent for mutations located over the entire S4, the differences at the lower end of S4 were particularly striking. Some of the mutations there caused shifts in the voltage dependence of activation in KCNQ1 alone, but almost a total loss of currents in the presence of KCNE1 (M238, L239, H240, D242, and R243) (29). Recently, it was reported that upon KCNE1 association the interaction between a specific pair of residues, F232 in S4 and F279 in S5, hindered activation of the channel (119). KCNE1 also altered the effects of mutations S140G and V141M in S1, which are associated with atrial fibrillation, on voltage-dependent gating (59, 107, 120, 121). Consistent with these changes, VSD movements measured by VCF shifted to more negative voltages in association with KCNE1 (30, 31, 52). Nevertheless, KCNE1 association does not alter the rate of voltage sensor activation (30, 31, 32, 33, 51, 52) (for a review, see Liin et al. (122)).
The interaction of KCNE1 with the PGD and the consequent changes in VSD movements cannot directly explain the effects of KCNE1 on voltage-dependent gating. KCNE1 does not reduce the rate of VSD activation, although it prolongs the opening and closing time course; KCNE1 shifts the voltage dependence of channel opening to more positive voltages, which is opposite to the direction of KCNE1-induced change in the voltage dependence of voltage sensor activation. Furthermore, little attention was paid to whether these interactions were important for KCNE1 modulation of other properties such as ion selectivity, pharmacology, or modulation. Therefore, the question of how KCNE1 alters such a broad spectrum of channel functions so radically has remained unanswered. However, building on these results and experimental approaches, more recent studies suggested a novel mechanism that may explain the effects of KCNE1 on channel function.
It was found that, although voltage sensor activation showed two resolvable steps in either the absence or presence of KCNE1, pore opening at both the intermediate and activated states of the VSD was observed only in KCNQ1 alone; in the presence of KCNE1 the pore opens only at the activated state (52). Thus, KCNE1 appeared to suppress the intermediate-open state but spare the activated-open state. This mechanism was further examined by the coexpression of KCNE1 with the mutant KCNQ1 channels E1R/R2E and E1R/R4E, which were constitutively open because of the VSD being arrested at an intermediate and active state, respectively. Coexpression with KCNE1 eliminated the currents of E1R/R2E but enhanced the currents of E1R/R4E, although KCNE1 did not alter the expression of the channel proteins in the cell membrane (52). These results suggest that KCNE1 not only suppressed intermediate opening but also enhanced the activated opening.
KCNE1 selectively modulates the two open states by altering the VSD-PGD coupling, because mutation F351A mimicked KCNE1 to suppress the intermediate-open state, producing currents with similar voltage dependence and slow opening/closing kinetics. Similar to KCNE1 association, F351A also altered the Rb+/K+ conductance ratio, response to XE991, and PIP2 sensitivity of the channel (48, 52). F351 is located at the cytoplasmic end of S6, highly conserved in K+ channels and important for VSD-PGD coupling (48, 65). Likewise, mutations in the S4-S5 linker of KCNQ1 that may interact with the cytoplasmic end of S6 for VSD-PGD coupling, including L251A, V255W, H258A, and T247A, also produced KCNE1-like effects on ionic currents, with a right-shifted voltage dependence of activation and reduced opening/closing rates (47). KCNE1 association altered the effects of some mutations in the S4-S5 linker (45), suggesting that KCNE1 affects the conformation and protein interactions in this region.
That KCNE1 alters VSD-PGD coupling is also supported by the findings that KCNE1 association increases the affinity of KCNQ1 to PIP2 (52, 69), which is required to mediate VSD-PGD coupling (44). Applying PIP2 to the intracellular solution rescued currents from run-down in inside-out patch recordings, and it was found that the EC50 for KCNQ1+KCNE1 was more than 100 times lower than for KCNQ1 alone (69). PIP2 depletion inhibited currents from KCNQ1 channels but did not inhibit the current from open KCNQ1+KCNE1 channels; however, PIP2 depletion prevented the closed KCNQ1+KCNE1 channels from reopening (52). These results indicate that PIP2 affinity for the open KCNQ1+KCNE1 channel is so high that the bound PIP2 did not dissociate from the channel until the channel was closed. Thus, association of KCNE1 enhances PIP2 affinity for the activated-open state, which suggests that KCNE1 enhances the VSD-PGD coupling for this state.
A recent study found that the single KCNQ1+KCNE1 channel exhibited long-lived subconductance levels: the channel could open with five different conductance states (123). This result indicates that the active-open state observed via macroscopic current and fluorescence recordings is actually quite complex. The various subconductance levels may be related to a nonconcerted gating of the four KCNQ1 subunits wherein voltage sensor activation in each subunit promotes pore opening to a subconductance level, whereas the pore opens fully only when all four voltage sensors are activated (123). A similar mechanism was also proposed for gating of KCNQ1 channels in the absence of KCNE1 based on time and voltage dependence of voltage sensor activation and channel opening (31). In a study of channels with different numbers of the KCNQ1 subunit that contained a mutation in the voltage sensor, the results suggested that the voltage sensor in each subunit of both the KCNQ1 and KCNQ1+KCNE1 channels independently contributed to channel opening (124). Therefore, although association with KCNE1 alters VSD-PGD coupling, it may not alter the interactions among different subunits.
The above mechanism explains many effects of KCNE1 on channel function. First, a kinetic model depicting the mechanism through which KCNE1 modulates VSD-PGD coupling to suppress the intermediate-open state and enhance the activated-open state could recapitulate the voltage dependence of current onset and of the time course of voltage sensor activation/deactivation (52). Second, the finding that the channels arrested at the intermediate-open (E1R/R2E) and activated-open (E1R/R4E) states show different Rb+/K+ permeation ratios and different responses to XE991 suggests that modulation of the VSD-PGD interactions by KCNE1 can alter ion selectivity and pharmacology. This idea is substantiated by findings that a single mutation, F351A, which affects VSD-PGD interactions directly, can also alter ion selectivity and pharmacology. However, the changes in ion selectivity and drug effects by this mutation were not quantitatively the same as by KCNE1, indicating that the effects of KCNE1 on VSD-PGD interactions are probably more complex than those of the single-point mutation F351A (52). Third, the mechanism also contributes to the increase in current amplitude upon association with KCNE1, because of enhanced VSD-PGD coupling in the activated-open state (52). Besides this mechanism, previous studies also suggested other mechanisms that may contribute to the increase in current amplitude by KCNE1. With an increased PIP2 affinity upon association with KCNE1, the native PIP2 level in the membrane is high enough for most KCNQ1+KCNE1 channels to open. Conversely, for KCNQ1 channels expressed alone, given the low PIP2 affinity, the level of PIP2 in the membrane is not sufficient to saturate the binding sites, thus limiting channel opening (69). Previous studies also suggested that the difference in single-channel conductance between KCNQ1 and KCNQ1+KCNE1 might explain the difference in macroscopic current amplitude between the two channels (125, 126, 127). Using nonstationary noise analysis, these studies found that the single-channel conductance was smaller in KCNQ1 than in KCNQ1+KCNE1. However, more recent studies suggest that single-channel behaviors of KCNQ1 and KCNQ1+KCNE1 channels are complex. These channels may have multiple open states (52, 63, 64) and subconductance open states (123). There is also a fast flickering process in the KCNQ1 pore that is associated with inactivation (60, 62). These data were not available at the time of the studies using noise analysis, a method that assumes only a single open state and that the recording bandwidth is sufficient to capture all relevant timescales. The observed difference in single-channel conductance may reflect the combined effects of all these complications. In sum, the above results suggest that multiple mechanisms, including the ability of KCNE1 to selectively modulate the two open states, may contribute to the KCNE1 effect on current amplitude.
Whether the selective modulation of the two open states by KCNE1 also underlies the elimination of inactivation remains to be determined. KCNQ1 channels exhibit an incomplete inactivation that is masked by the activation process; inactivation only becomes apparent upon repolarization, when inactivated channels first traverse to an open state before closing, resulting in a hook in the tail current (63, 88). Inactivation can be also measured using a triple pulse voltage protocol where a brief hyperpolarizing pulse after depolarization allows the channels to recover from inactivation but not to deactivate (88). Subsequent depolarization does not activate more channels but elicits a larger current because of the recovery of open channels from inactivation. This additional current decays rapidly as the channels inactivate again. One hallmark of KCNE1 association with KCNQ1 is the suppression of inactivation (63, 88). The currents of KCNQ1+KCNE1 do not exhibit a tail hook or a current decay during the triple pulse protocol.
Based on the delay of the onset and the voltage dependence of inactivation, it was suggested that the model accounting for the fast inactivation of KCNQ1 must contain at least two open states (63). The existence of two open states in KCNQ1 was also suggested by the studies of Na+ block (64). In Shaker-like Kv channels, inactivation occurs when open channels spontaneously enter a nonconducting state at voltages that activate the channel. However, the behavior of KCNQ1 that was taken as “fast inactivation” was related to a fast flicker of the open channel that changed the open probability of the pore, but not to an explicit nonconducting inactivation state (62). The flickers were initially suggested by a study of single-channel currents using noise analysis, in which the single-channel current amplitude depended on the frequency bandwidth of recordings (60). The flickers were then related to the fast inactivation because the ratio of Rb+/K+ conductance in KCNQ1 correlated both to the flickers (60) and to the fast inactivation (62). In KCNQ1 channels, a large Rb+/K+ conductance ratio was attributed to the fact that at high Rb+ concentrations the flicker-open state was favored and the flickering rate was slowed (60). On the other hand, some mutations in the pore domain were found to alter both inactivation and Rb+/K+ conductance ratio, and the effects on both were positively correlated (62). The Rb+/K+ conductance ratio also changes upon KCNE1 association (60). These studies suggest that fast inactivation involves two open states with different Rb+/K+ conductance ratios. Consistent with the idea that inactivation may involve conformational changes in the pore domain, it was found that many mutations in the pore domain altered inactivation (62, 128, 129). Whether the two open states that are important for KCNQ1 inactivation are related to the intermediate-open and activated-open states, and whether the association of KCNE1 eliminates inactivation by eliminating one of the open states, are questions yet to be answered. Another remaining question is whether and how the mechanism through which KCNE1 selectively modulates the intermediate-open and activated-open states underlies the changes in channel modulation by PKA phosphorylation.
Conclusions
KCNQ1 is a bona fide voltage-gated K+ channel, with conserved Kv channel structure, classical properties, and general mechanisms of voltage-dependent gating that are applicable to all Kv channels. On the other hand, voltage-dependent gating of KCNQ1 channels reveals novel and unique properties. These include the requirement of intracellular signaling molecules, PIP2 and ATP, for VSD-PGD coupling and pore opening, respectively, and pore opening at both the intermediate and activated state of VSD activation. The study of these unique properties of KCNQ1 gating provides an excellent opportunity to reveal novel principles for voltage-dependent gating that may be generally applicable to all voltage-gated channels but hidden in the phenotype of most.
Voltage-dependent gating is subject to modulations by posttranslational modification and the binding of signaling molecules. For instance, phosphorylation of KCNQ1 by protein kinase A affects voltage-dependent gating in the presence of KCNE1 association (94, 95), which underscores the critical physiological role of β-adrenergic regulation of IKs channels in cardiac function. In another example, intracellular Ca2+ binds to BK type K+ channels to increase channel activation (130). These modulations, although altering voltage-dependent gating, are clearly not a required part of the mechanism of voltage-dependent gating; i.e., the channels can be activated by voltage without these modulations. PIP2 and ATP binding, on the other hand, are required for voltage-dependent gating of KCNQ1. In the absence of PIP2 or ATP binding, the channels cannot open (44, 79). It is still not clear if ATP is part of the voltage-dependent gating mechanism or is simply required for setting the PGD conformation ready for opening. However, PIP2 binding is a critical step during voltage-dependent gating; PIP2 acts as a cofactor to mediate VSD-PGD coupling (44, 76). These findings reveal that voltage-dependent gating is a molecular process involving not only the ion channel protein alone, but also involving other molecules. These findings also provide insights into the physiological and pathophysiological roles of the channel. For instance, more than 300 mutations in KCNQ1 are associated with LQT syndrome, of which some were found to reduce PIP2 or ATP binding (44, 74, 77, 78, 79). Thus, the modulation of voltage-dependent gating by cellular signaling is critical to the physiologic functions of these channels, and the dependence of activation on multiple stimuli is one of the reasons for the vulnerability of channel function to so many mutations. ATP dependence of the channel also provides a direct link between the electric activities and energetic metabolism of the heart.
The VSD and PGD in voltage-gated ion channels are distinct structural domains. Functional channels can be formed naturally by a PGD without a VSD (131, 132) or by a PGD artificially isolated from Kv (133, 134) or Nav (135) channels. Likewise, the VSD identified in CiVSP and voltage-sensor only proteins (VSOP) (71, 136, 137) and isolated from Kv channels adopt structures similar to the VSD in voltage-gated ion channels (138, 139, 140). These results suggest that the VSD and PGD may have derived from different ancestors and evolved to merge into voltage-gated ion channels (141). Because these domains are both membrane proteins, it is not surprising that membrane lipid molecules are involved in VSD-PGD coupling. Besides KCNQ channels, Cav channel function also requires PIP2 (142). Although some other Kv channels may not require PIP2 for channel activation (70, 143, 144), the Kv1.2-2.1 crystal structure contains several anionic lipids bound stably at the VSD-PGD interface (118), suggesting that lipid involvement in VSD-PGD coupling may be a principle common to Kv channels. Interestingly, in KCNQ1+KCNE1 channels, the activated-open state has a high affinity for PIP2 (52). Considering that other Kv channels may open only when the VSD is in the activated state, the lipid molecules may bind to these channels tightly and act as a resident cofactor to mediate VSD-PGD coupling.
In their pioneering work on the ionic bases of action potentials, Hodgkin and Huxley derived models for voltage-dependent changes in membrane conductance of K+ and Na+, in which voltage-dependent activation and ion conduction independently contribute to total conductance (1). This notion can still be found in the current understanding of the function of voltage-gated ion channels, which assumes that gating and permeation are independent, so that the VSD conformation changes the probability of pore opening, but does not affect the properties of the open pore. In KCNQ1 channels, both the intermediate and activated states of the voltage sensor yielded robust pore opening, providing a rare opportunity to assess this framework of channel function. Remarkably, the intermediate-open and activated-open states had different permeation and pharmacological properties, and in these two open states the VSD-PGD couplings were different (52). These results revealed that VSD-PGD interactions determine both the open probability and open conformation. VSD-PGD coupling does not just happen at the end of VSD movements; changes in VSD conformation may alter the conformation of PGD during the entire activation transitions via VSD-PGD interactions. Therefore, VSD activation and pore opening are not independent, as assumed in models descending from the Hodgkin and Huxley formulism. Dynamic VSD-PGD interactions may influence VSD movements during activation even for those channels that the pore opens only when the VSD is in the final activated state.
Acknowledgments
I would like to thank Mark Zaydman, Jiajing Xu, Smiruthi Ramasubramanian, Panpan Hou, Ling Zhong, and Yoram Rudy for reading the manuscript and providing helpful comments. Ling Zhong and Panpan Hou made Fig. 1.
This work was supported by NIH Grants R01-HL70393 and R01-NS092570.
Editor: Brian Salzberg
References
- 1.Hodgkin A.L., Huxley A.F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long S.B., Campbell E.B., Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 3.Payandeh J., Scheuer T., Catterall W.A. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Horn W.D., Vanoye C.G., Sanders C.R. Working model for the structural basis for KCNE1 modulation of the KCNQ1 potassium channel. Curr. Opin. Struct. Biol. 2011;21:283–291. doi: 10.1016/j.sbi.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bezanilla F. How membrane proteins sense voltage. Nat. Rev. Mol. Cell Biol. 2008;9:323–332. doi: 10.1038/nrm2376. [DOI] [PubMed] [Google Scholar]
- 6.Tombola F., Pathak M.M., Isacoff E.Y. How does voltage open an ion channel? Annu. Rev. Cell Dev. Biol. 2006;22:23–52. doi: 10.1146/annurev.cellbio.21.020404.145837. [DOI] [PubMed] [Google Scholar]
- 7.Yellen G. The moving parts of voltage-gated ion channels. Q. Rev. Biophys. 1998;31:239–295. doi: 10.1017/s0033583598003448. [DOI] [PubMed] [Google Scholar]
- 8.Jiang Y., Lee A., MacKinnon R. The open pore conformation of potassium channels. Nature. 2002;417:523–526. doi: 10.1038/417523a. [DOI] [PubMed] [Google Scholar]
- 9.Lu Z., Klem A.M., Ramu Y. Ion conduction pore is conserved among potassium channels. Nature. 2001;413:809–813. doi: 10.1038/35101535. [DOI] [PubMed] [Google Scholar]
- 10.Long S.B., Campbell E.B., Mackinnon R. Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science. 2005;309:903–908. doi: 10.1126/science.1116270. [DOI] [PubMed] [Google Scholar]
- 11.Ledwell J.L., Aldrich R.W. Mutations in the S4 region isolate the final voltage-dependent cooperative step in potassium channel activation. J. Gen. Physiol. 1999;113:389–414. doi: 10.1085/jgp.113.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soler-Llavina G.J., Chang T.H., Swartz K.J. Functional interactions at the interface between voltage-sensing and pore domains in the Shaker K(v) channel. Neuron. 2006;52:623–634. doi: 10.1016/j.neuron.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Grabe M., Lai H.C., Jan L.Y. Structure prediction for the down state of a potassium channel voltage sensor. Nature. 2007;445:550–553. doi: 10.1038/nature05494. [DOI] [PubMed] [Google Scholar]
- 14.Lee S.Y., Banerjee A., MacKinnon R. Two separate interfaces between the voltage sensor and pore are required for the function of voltage-dependent K(+) channels. PLoS Biol. 2009;7:e47. doi: 10.1371/journal.pbio.1000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q., Curran M.E., Keating M.T. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat. Genet. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- 16.Goldman A.M., Glasscock E., Noebels J.L. Arrhythmia in heart and brain: KCNQ1 mutations link epilepsy and sudden unexplained death. Sci. Transl. Med. 2009;1:2ra6. doi: 10.1126/scitranslmed.3000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abbott G.W. Biology of the KCNQ1 potassium channel. New J. Sci. 2014;2014:237431. [Google Scholar]
- 18.Brown D.A. Kv7 (KCNQ) potassium channels that are mutated in human diseases. J. Physiol. 2008;586:1781–1783. doi: 10.1113/jphysiol.2008.153007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaydman M.A., Cui J. PIP2 regulation of KCNQ channels: biophysical and molecular mechanisms for lipid modulation of voltage-dependent gating. Front. Physiol. 2014;5:195. doi: 10.3389/fphys.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith J.A., Vanoye C.G., Sanders C.R. Structural models for the KCNQ1 voltage-gated potassium channel. Biochemistry. 2007;46:14141–14152. doi: 10.1021/bi701597s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva J.R., Pan H., Rudy Y. A multiscale model linking ion-channel molecular dynamics and electrostatics to the cardiac action potential. Proc. Natl. Acad. Sci. USA. 2009;106:11102–11106. doi: 10.1073/pnas.0904505106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gofman Y., Shats S., Ben-Tal N. How does KCNE1 regulate the Kv7.1 potassium channel? Model-structure, mutations, and dynamics of the Kv7.1-KCNE1 complex. Structure. 2012;20:1343–1352. doi: 10.1016/j.str.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y., Wang Y., Tseng G.N. Building KCNQ1/KCNE1 channel models and probing their interactions by molecular-dynamics simulations. Biophys. J. 2013;105:2461–2473. doi: 10.1016/j.bpj.2013.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aggarwal S.K., MacKinnon R. Contribution of the S4 segment to gating charge in the Shaker K+ channel. Neuron. 1996;16:1169–1177. doi: 10.1016/s0896-6273(00)80143-9. [DOI] [PubMed] [Google Scholar]
- 25.Seoh S.A., Sigg D., Bezanilla F. Voltage-sensing residues in the S2 and S4 segments of the Shaker K+ channel. Neuron. 1996;16:1159–1167. doi: 10.1016/s0896-6273(00)80142-7. [DOI] [PubMed] [Google Scholar]
- 26.Gandhi C.S., Isacoff E.Y. Molecular models of voltage sensing. J. Gen. Physiol. 2002;120:455–463. doi: 10.1085/jgp.20028678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panaghie G., Abbott G.W. The role of S4 charges in voltage-dependent and voltage-independent KCNQ1 potassium channel complexes. J. Gen. Physiol. 2007;129:121–133. doi: 10.1085/jgp.200609612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shamgar L., Haitin Y., Attali B. KCNE1 constrains the voltage sensor of Kv7.1 K+ channels. PLoS One. 2008;3:e1943. doi: 10.1371/journal.pone.0001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu D., Pan H., Cui J. KCNE1 remodels the voltage sensor of Kv7.1 to modulate channel function. Biophys. J. 2010;99:3599–3608. doi: 10.1016/j.bpj.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruscic K.J., Miceli F., Goldstein S.A. IKs channels open slowly because KCNE1 accessory subunits slow the movement of S4 voltage sensors in KCNQ1 pore-forming subunits. Proc. Natl. Acad. Sci. USA. 2013;110:E559–E566. doi: 10.1073/pnas.1222616110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osteen J.D., Gonzalez C., Kass R.S. KCNE1 alters the voltage sensor movements necessary to open the KCNQ1 channel gate. Proc. Natl. Acad. Sci. USA. 2010;107:22710–22715. doi: 10.1073/pnas.1016300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakajo K., Kubo Y. KCNE1 and KCNE3 stabilize and/or slow voltage sensing S4 segment of KCNQ1 channel. J. Gen. Physiol. 2007;130:269–281. doi: 10.1085/jgp.200709805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rocheleau J.M., Kobertz W.R. KCNE peptides differently affect voltage sensor equilibrium and equilibration rates in KCNQ1 K+ channels. J. Gen. Physiol. 2008;131:59–68. doi: 10.1085/jgp.200709816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu D., Delaloye K., Cui J. State-dependent electrostatic interactions of S4 arginines with E1 in S2 during Kv7.1 activation. J. Gen. Physiol. 2010;135:595–606. doi: 10.1085/jgp.201010408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seebohm G., Westenskow P., Sanguinetti M.C. Mutation of colocalized residues of the pore helix and transmembrane segments S5 and S6 disrupt deactivation and modify inactivation of KCNQ1 K+ channels. J. Physiol. 2005;563:359–368. doi: 10.1113/jphysiol.2004.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panaghie G., Tai K.K., Abbott G.W. Interaction of KCNE subunits with the KCNQ1 K+ channel pore. J. Physiol. 2006;570:455–467. doi: 10.1113/jphysiol.2005.100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seebohm G., Strutz-Seebohm N., Lang F. Differential roles of S6 domain hinges in the gating of KCNQ potassium channels. Biophys. J. 2006;90:2235–2244. doi: 10.1529/biophysj.105.067165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Z., Tristani-Firouzi M., Sanguinetti M.C. Functional effects of mutations in KvLQT1 that cause long QT syndrome. J. Cardiovasc. Electrophysiol. 1999;10:817–826. doi: 10.1111/j.1540-8167.1999.tb00262.x. [DOI] [PubMed] [Google Scholar]
- 39.Hoosien M., Ahearn M.E., Bishopric N.H. Dysfunctional potassium channel subunit interaction as a novel mechanism of long QT syndrome. Heart Rhythm. 2013;10:728–737. doi: 10.1016/j.hrthm.2012.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma L.J., Ohmert I., Vardanyan V. Allosteric features of KCNQ1 gating revealed by alanine scanning mutagenesis. Biophys. J. 2011;100:885–894. doi: 10.1016/j.bpj.2010.12.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horrigan F.T., Cui J., Aldrich R.W. Allosteric voltage gating of potassium channels I. Mslo ionic currents in the absence of Ca(2+) J. Gen. Physiol. 1999;114:277–304. doi: 10.1085/jgp.114.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Altomare C., Bucchi A., DiFrancesco D. Integrated allosteric model of voltage gating of HCN channels. J. Gen. Physiol. 2001;117:519–532. doi: 10.1085/jgp.117.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osteen J.D., Barro-Soria R., Larsson H.P. Allosteric gating mechanism underlies the flexible gating of KCNQ1 potassium channels. Proc. Natl. Acad. Sci. USA. 2012;109:7103–7108. doi: 10.1073/pnas.1201582109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaydman M.A., Silva J.R., Cui J. Kv7.1 ion channels require a lipid to couple voltage sensing to pore opening. Proc. Natl. Acad. Sci. USA. 2013;110:13180–13185. doi: 10.1073/pnas.1305167110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Franqueza L., Lin M., Sanguinetti M.C. Long QT syndrome-associated mutations in the S4-S5 linker of KvLQT1 potassium channels modify gating and interaction with minK subunits. J. Biol. Chem. 1999;274:21063–21070. doi: 10.1074/jbc.274.30.21063. [DOI] [PubMed] [Google Scholar]
- 46.Boulet I.R., Raes A.L., Snyders D.J. Functional effects of a KCNQ1 mutation associated with the long QT syndrome. Cardiovasc. Res. 2006;70:466–474. doi: 10.1016/j.cardiores.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Labro A.J., Boulet I.R., Snyders D.J. The S4-S5 linker of KCNQ1 channels forms a structural scaffold with the S6 segment controlling gate closure. J. Biol. Chem. 2011;286:717–725. doi: 10.1074/jbc.M110.146977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boulet I.R., Labro A.J., Snyders D.J. Role of the S6 C-terminus in KCNQ1 channel gating. J. Physiol. 2007;585:325–337. doi: 10.1113/jphysiol.2007.145813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choveau F.S., Rodriguez N., Loussouarn G. KCNQ1 channels voltage dependence through a voltage-dependent binding of the S4-S5 linker to the pore domain. J. Biol. Chem. 2011;286:707–716. doi: 10.1074/jbc.M110.146324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Choveau F.S., Abderemane-Ali F., Loussouarn G. Opposite effects of the S4-S5 linker and PIP(2) on voltage-gated channel function: KCNQ1/KCNE1 and other channels. Front. Pharmacol. 2012;3:125. doi: 10.3389/fphar.2012.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barro-Soria R., Rebolledo S., Larsson H.P. KCNE1 divides the voltage sensor movement in KCNQ1/KCNE1 channels into two steps. Nat. Commun. 2014;5:3750. doi: 10.1038/ncomms4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaydman M.A., Kasimova M.A., Cui J. Domain-domain interactions determine the gating, permeation, pharmacology, and subunit modulation of the IKs ion channel. eLife. 2014;3:e03606. doi: 10.7554/eLife.03606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zagotta W.N., Hoshi T., Aldrich R.W. Shaker potassium channel gating. III: evaluation of kinetic models for activation. J. Gen. Physiol. 1994;103:321–362. doi: 10.1085/jgp.103.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bezanilla F., Perozo E., Stefani E. Gating of Shaker K+ channels: II. The components of gating currents and a model of channel activation. Biophys. J. 1994;66:1011–1021. doi: 10.1016/S0006-3495(94)80882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tao X., Lee A., MacKinnon R. A gating charge transfer center in voltage sensors. Science. 2010;328:67–73. doi: 10.1126/science.1185954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lacroix J.J., Bezanilla F. Control of a final gating charge transition by a hydrophobic residue in the S2 segment of a K+ channel voltage sensor. Proc. Natl. Acad. Sci. USA. 2011;108:6444–6449. doi: 10.1073/pnas.1103397108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lacroix J.J., Hyde H.C., Bezanilla F. Moving gating charges through the gating pore in a Kv channel voltage sensor. Proc. Natl. Acad. Sci. USA. 2014;111:E1950–E1959. doi: 10.1073/pnas.1406161111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lacroix J.J., Pless S.A., Bezanilla F. Intermediate state trapping of a voltage sensor. J. Gen. Physiol. 2012;140:635–652. doi: 10.1085/jgp.201210827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Restier L., Cheng L., Sanguinetti M.C. Mechanisms by which atrial fibrillation-associated mutations in the S1 domain of KCNQ1 slow deactivation of IKs channels. J. Physiol. 2008;586:4179–4191. doi: 10.1113/jphysiol.2008.157511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pusch M., Bertorello L., Conti F. Gating and flickery block differentially affected by rubidium in homomeric KCNQ1 and heteromeric KCNQ1/KCNE1 potassium channels. Biophys. J. 2000;78:211–226. doi: 10.1016/S0006-3495(00)76586-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu Y., Wang Y., Tseng G.N. Probing binding sites and mechanisms of action of an I(Ks) activator by computations and experiments. Biophys. J. 2015;108:62–75. doi: 10.1016/j.bpj.2014.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seebohm G., Sanguinetti M.C., Pusch M. Tight coupling of rubidium conductance and inactivation in human KCNQ1 potassium channels. J. Physiol. 2003;552:369–378. doi: 10.1113/jphysiol.2003.046490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pusch M., Magrassi R., Conti F. Activation and inactivation of homomeric KvLQT1 potassium channels. Biophys. J. 1998;75:785–792. doi: 10.1016/S0006-3495(98)77568-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pusch M., Ferrera L., Friedrich T. Two open states and rate-limiting gating steps revealed by intracellular Na+ block of human KCNQ1 and KCNQ1/KCNE1 K+ channels. J. Physiol. 2001;533:135–143. doi: 10.1111/j.1469-7793.2001.0135b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haddad G.A., Blunck R. Mode shift of the voltage sensors in Shaker K+ channels is caused by energetic coupling to the pore domain. J. Gen. Physiol. 2011;137:455–472. doi: 10.1085/jgp.201010573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suh B.C., Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr. Opin. Neurobiol. 2005;15:370–378. doi: 10.1016/j.conb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 67.Loussouarn G., Park K.H., Escande D. Phosphatidylinositol-4,5-bisphosphate, PIP2, controls KCNQ1/KCNE1 voltage-gated potassium channels: a functional homology between voltage-gated and inward rectifier K+ channels. EMBO J. 2003;22:5412–5421. doi: 10.1093/emboj/cdg526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang H., Craciun L.C., Logothetis D.E. PIP(2) activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron. 2003;37:963–975. doi: 10.1016/s0896-6273(03)00125-9. [DOI] [PubMed] [Google Scholar]
- 69.Li Y., Zaydman M.A., Cui J. KCNE1 enhances phosphatidylinositol 4,5-bisphosphate (PIP2) sensitivity of IKs to modulate channel activity. Proc. Natl. Acad. Sci. USA. 2011;108:9095–9100. doi: 10.1073/pnas.1100872108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kruse M., Hammond G.R., Hille B. Regulation of voltage-gated potassium channels by PI(4,5)P2. J. Gen. Physiol. 2012;140:189–205. doi: 10.1085/jgp.201210806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murata Y., Iwasaki H., Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- 72.Eckey K., Wrobel E., Seebohm G. Novel Kv7.1-phosphatidylinositol 4,5-bisphosphate interaction sites uncovered by charge neutralization scanning. J. Biol. Chem. 2014;289:22749–22758. doi: 10.1074/jbc.M114.589796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thomas A.M., Harmer S.C., Tinker A. Characterization of a binding site for anionic phospholipids on KCNQ1. J. Biol. Chem. 2011;286:2088–2100. doi: 10.1074/jbc.M110.153551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park K.H., Piron J., Loussouarn G. Impaired KCNQ1-KCNE1 and phosphatidylinositol-4,5-bisphosphate interaction underlies the long QT syndrome. Circ. Res. 2005;96:730–739. doi: 10.1161/01.RES.0000161451.04649.a8. [DOI] [PubMed] [Google Scholar]
- 75.Li X., Liu H., Jegla T. Major diversification of voltage-gated K+ channels occurred in ancestral parahoxozoans. Proc. Natl. Acad. Sci. USA. 2015;112:E1010–E1019. doi: 10.1073/pnas.1422941112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kasimova M.A., Zaydman M.A., Tarek M. PIP2-dependent coupling is prominent in Kv7.1 due to weakened interactions between S4–S5 and S6. Sci. Rep. 2015;5:7474. doi: 10.1038/srep07474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dvir M., Strulovich R., Attali B. Long QT mutations at the interface between KCNQ1 helix C and KCNE1 disrupt I(KS) regulation by PKA and PIP2. J. Cell Sci. 2014;127:3943–3955. doi: 10.1242/jcs.147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Coyan F.C., Abderemane-Ali F., Loussouarn G. A long QT mutation substitutes cholesterol for phosphatidylinositol-4,5-bisphosphate in KCNQ1 channel regulation. PLoS One. 2014;9:e93255. doi: 10.1371/journal.pone.0093255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Y., Gao J., Cui J. Intracellular ATP binding is required to activate the slowly activating K+ channel I(Ks) Proc. Natl. Acad. Sci. USA. 2013;110:18922–18927. doi: 10.1073/pnas.1315649110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takumi T., Ohkubo H., Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science. 1988;242:1042–1045. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- 81.Kaczmarek L.K., Blumenthal E.M. Properties and regulation of the minK potassium channel protein. Physiol. Rev. 1997;77:627–641. doi: 10.1152/physrev.1997.77.3.627. [DOI] [PubMed] [Google Scholar]
- 82.Sanguinetti M.C., Curran M.E., Keating M.T. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 83.Barhanin J., Lesage F., Romey G. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- 84.Splawski I., Tristani-Firouzi M., Keating M.T. Mutations in the hminK gene cause long QT syndrome and suppress IKs function. Nat. Genet. 1997;17:338–340. doi: 10.1038/ng1197-338. [DOI] [PubMed] [Google Scholar]
- 85.Keating M.T., Sanguinetti M.C. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 2001;104:569–580. doi: 10.1016/s0092-8674(01)00243-4. [DOI] [PubMed] [Google Scholar]
- 86.Murai T., Kakizuka A., Nakanishi S. Molecular cloning and sequence analysis of human genomic DNA encoding a novel membrane protein which exhibits a slowly activating potassium channel activity. Biochem. Biophys. Res. Commun. 1989;161:176–181. doi: 10.1016/0006-291x(89)91577-5. [DOI] [PubMed] [Google Scholar]
- 87.Kang C., Tian C., Sanders C.R. Structure of KCNE1 and implications for how it modulates the KCNQ1 potassium channel. Biochemistry. 2008;47:7999–8006. doi: 10.1021/bi800875q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tristani-Firouzi M., Sanguinetti M.C. Voltage-dependent inactivation of the human K+ channel KvLQT1 is eliminated by association with minimal K+ channel (minK) subunits. J. Physiol. 1998;510:37–45. doi: 10.1111/j.1469-7793.1998.037bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Busch A.E., Busch G.L., Stühmer W. The role of the IsK protein in the specific pharmacological properties of the IKs channel complex. Br. J. Pharmacol. 1997;122:187–189. doi: 10.1038/sj.bjp.0701434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sesti F., Tai K.K., Goldstein S.A. MinK endows the I(Ks) potassium channel pore with sensitivity to internal tetraethylammonium. Biophys. J. 2000;79:1369–1378. doi: 10.1016/S0006-3495(00)76389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lerche C., Seebohm G., Busch A.E. Molecular impact of MinK on the enantiospecific block of I(Ks) by chromanols. Br. J. Pharmacol. 2000;131:1503–1506. doi: 10.1038/sj.bjp.0703734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang H.S., Brown B.S., Cohen I.S. Molecular basis for differential sensitivity of KCNQ and I(Ks) channels to the cognitive enhancer XE991. Mol. Pharmacol. 2000;57:1218–1223. [PubMed] [Google Scholar]
- 93.Yu H., Lin Z., Li M. Dynamic subunit stoichiometry confers a progressive continuum of pharmacological sensitivity by KCNQ potassium channels. Proc. Natl. Acad. Sci. USA. 2013;110:8732–8737. doi: 10.1073/pnas.1300684110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Marx S.O., Kurokawa J., Kass R.S. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science. 2002;295:496–499. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- 95.Kurokawa J., Chen L., Kass R.S. Requirement of subunit expression for cAMP-mediated regulation of a heart potassium channel. Proc. Natl. Acad. Sci. USA. 2003;100:2122–2127. doi: 10.1073/pnas.0434935100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Silva J., Rudy Y. Subunit interaction determines IKs participation in cardiac repolarization and repolarization reserve. Circulation. 2005;112:1384–1391. doi: 10.1161/CIRCULATIONAHA.105.543306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nekouzadeh A., Rudy Y. Continuum molecular simulation of large conformational changes during ion-channel gating. PLoS One. 2011;6:e20186. doi: 10.1371/journal.pone.0020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ackerman M.J., Tester D.J., Porter C.J. Swimming, a gene-specific arrhythmogenic trigger for inherited long QT syndrome. Mayo Clin. Proc. 1999;74:1088–1094. doi: 10.4065/74.11.1088. [DOI] [PubMed] [Google Scholar]
- 99.Schwartz P.J., Priori S.G., Bloise R. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 100.Brink P.A., Crotti L., Schwartz P.J. Phenotypic variability and unusual clinical severity of congenital long-QT syndrome in a founder population. Circulation. 2005;112:2602–2610. doi: 10.1161/CIRCULATIONAHA.105.572453. [DOI] [PubMed] [Google Scholar]
- 101.Crotti L., Spazzolini C., Crimi G. The common long-QT syndrome mutation KCNQ1/A341V causes unusually severe clinical manifestations in patients with different ethnic backgrounds: toward a mutation-specific risk stratification. Circulation. 2007;116:2366–2375. doi: 10.1161/CIRCULATIONAHA.107.726950. [DOI] [PubMed] [Google Scholar]
- 102.Barsheshet A., Goldenberg I., Lopes C.M. Mutations in cytoplasmic loops of the KCNQ1 channel and the risk of life-threatening events: implications for mutation-specific response to β-blocker therapy in type 1 long-QT syndrome. Circulation. 2012;125:1988–1996. doi: 10.1161/CIRCULATIONAHA.111.048041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Strutz-Seebohm N., Pusch M., Seebohm G. Structural basis of slow activation gating in the cardiac I Ks channel complex. Cell. Physiol. Biochem. 2011;27:443–452. doi: 10.1159/000329965. [DOI] [PubMed] [Google Scholar]
- 104.Xu X., Jiang M., Tseng G.N. KCNQ1 and KCNE1 in the IKs channel complex make state-dependent contacts in their extracellular domains. J. Gen. Physiol. 2008;131:589–603. doi: 10.1085/jgp.200809976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chung D.Y., Chan P.J., Kass R.S. Location of KCNE1 relative to KCNQ1 in the I(KS) potassium channel by disulfide cross-linking of substituted cysteines. Proc. Natl. Acad. Sci. USA. 2009;106:743–748. doi: 10.1073/pnas.0811897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang Y.H., Jiang M., Tseng G.N. Gating-related molecular motions in the extracellular domain of the IKs channel: implications for IKs channelopathy. J. Membr. Biol. 2011;239:137–156. doi: 10.1007/s00232-010-9333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chan P.J., Osteen J.D., Kass R.S. Characterization of KCNQ1 atrial fibrillation mutations reveals distinct dependence on KCNE1. J. Gen. Physiol. 2012;139:135–144. doi: 10.1085/jgp.201110672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tapper A.R., George A.L., Jr. Location and orientation of minK within the I(Ks) potassium channel complex. J. Biol. Chem. 2001;276:38249–38254. doi: 10.1074/jbc.M103956200. [DOI] [PubMed] [Google Scholar]
- 109.Lvov A., Gage S.D., Kobertz W.R. Identification of a protein-protein interaction between KCNE1 and the activation gate machinery of KCNQ1. J. Gen. Physiol. 2010;135:607–618. doi: 10.1085/jgp.200910386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tapper A.R., George A.L., Jr. MinK subdomains that mediate modulation of and association with KvLQT1. J. Gen. Physiol. 2000;116:379–390. doi: 10.1085/jgp.116.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Haitin Y., Wiener R., Attali B. Intracellular domains interactions and gated motions of I(KS) potassium channel subunits. EMBO J. 2009;28:1994–2005. doi: 10.1038/emboj.2009.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Melman Y.F., Domènech A., McDonald T.V. Structural determinants of KvLQT1 control by the KCNE family of proteins. J. Biol. Chem. 2001;276:6439–6444. doi: 10.1074/jbc.M010713200. [DOI] [PubMed] [Google Scholar]
- 113.Melman Y.F., Krumerman A., McDonald T.V. A single transmembrane site in the KCNE-encoded proteins controls the specificity of KvLQT1 channel gating. J. Biol. Chem. 2002;277:25187–25194. doi: 10.1074/jbc.M200564200. [DOI] [PubMed] [Google Scholar]
- 114.Mikuni I., Torres C.G., Kwok W.M. Partial restoration of the long QT syndrome associated KCNQ1 A341V mutant by the KCNE1 β-subunit. Biochim. Biophys. Acta. 2011;1810:1285–1293. doi: 10.1016/j.bbagen.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Melman Y.F., Um S.Y., McDonald T.V. KCNE1 binds to the KCNQ1 pore to regulate potassium channel activity. Neuron. 2004;42:927–937. doi: 10.1016/j.neuron.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 116.Li P., Liu H., Ding J. Differential modulations of KCNQ1 by auxiliary proteins KCNE1 and KCNE2. Sci. Rep. 2014;4:4973. doi: 10.1038/srep04973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nakajo K., Nishino A., Kubo Y. KCNQ1 subdomains involved in KCNE modulation revealed by an invertebrate KCNQ1 orthologue. J. Gen. Physiol. 2011;138:521–535. doi: 10.1085/jgp.201110677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Long S.B., Tao X., MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 119.Nakajo K., Kubo Y. Steric hindrance between S4 and S5 of the KCNQ1/KCNE1 channel hampers pore opening. Nat. Commun. 2014;5:4100. doi: 10.1038/ncomms5100. [DOI] [PubMed] [Google Scholar]
- 120.Chen Y.H., Xu S.J., Huang W. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299:251–254. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 121.Hong K., Piper D.R., Brugada R. De novo KCNQ1 mutation responsible for atrial fibrillation and short QT syndrome in utero. Cardiovasc. Res. 2005;68:433–440. doi: 10.1016/j.cardiores.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 122.Liin S.I., Barro-Soria R., Larsson H.P. The KCNQ1 channel—remarkable flexibility in gating allows for functional versatility. J. Physiol. 2015;593:2605–2615. doi: 10.1113/jphysiol.2014.287607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Werry D., Eldstrom J., Fedida D. Single-channel basis for the slow activation of the repolarizing cardiac potassium current, I(Ks) Proc. Natl. Acad. Sci. USA. 2013;110:E996–E1005. doi: 10.1073/pnas.1214875110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Meisel E., Dvir M., Attali B. KCNQ1 channels do not undergo concerted but sequential gating transitions in both the absence and the presence of KCNE1 protein. J. Biol. Chem. 2012;287:34212–34224. doi: 10.1074/jbc.M112.364901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pusch M. Increase of the single-channel conductance of KvLQT1 potassium channels induced by the association with minK. Pflugers Arch. 1998;437:172–174. doi: 10.1007/s004240050765. [DOI] [PubMed] [Google Scholar]
- 126.Sesti F., Goldstein S.A. Single-channel characteristics of wild-type IKs channels and channels formed with two minK mutants that cause long QT syndrome. J. Gen. Physiol. 1998;112:651–663. doi: 10.1085/jgp.112.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang Y., Sigworth F.J. Single-channel properties of IKs potassium channels. J. Gen. Physiol. 1998;112:665–678. doi: 10.1085/jgp.112.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Seebohm G., Scherer C.R., Lerche C. Identification of specific pore residues mediating KCNQ1 inactivation. A novel mechanism for long QT syndrome. J. Biol. Chem. 2001;276:13600–13605. doi: 10.1074/jbc.M008373200. [DOI] [PubMed] [Google Scholar]
- 129.Gibor G., Yakubovich D., Attali B. An inactivation gate in the selectivity filter of KCNQ1 potassium channels. Biophys. J. 2007;93:4159–4172. doi: 10.1529/biophysj.107.107987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang H., Zhang G., Cui J. BK channels: multiple sensors, one activation gate. Front. Physiol. 2015;6:29. doi: 10.3389/fphys.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Doyle D.A., Morais Cabral J., MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 132.Kuo A., Gulbis J.M., Doyle D.A. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 2003;300:1922–1926. doi: 10.1126/science.1085028. [DOI] [PubMed] [Google Scholar]
- 133.Santos J.S., Grigoriev S.M., Montal M. Molecular template for a voltage sensor in a novel K+ channel. III. Functional reconstitution of a sensorless pore module from a prokaryotic Kv channel. J. Gen. Physiol. 2008;132:651–666. doi: 10.1085/jgp.200810077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Santos J.S., Asmar-Rovira G.A., Montal M. Crystal structure of a voltage-gated K+ channel pore module in a closed state in lipid membranes. J. Biol. Chem. 2012;287:43063–43070. doi: 10.1074/jbc.M112.415091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shaya D., Kreir M., Minor D.L., Jr. Voltage-gated sodium channel (NaV) protein dissection creates a set of functional pore-only proteins. Proc. Natl. Acad. Sci. USA. 2011;108:12313–12318. doi: 10.1073/pnas.1106811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sasaki M., Takagi M., Okamura Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science. 2006;312:589–592. doi: 10.1126/science.1122352. [DOI] [PubMed] [Google Scholar]
- 137.Ramsey I.S., Moran M.M., Clapham D.E. A voltage-gated proton-selective channel lacking the pore domain. Nature. 2006;440:1213–1216. doi: 10.1038/nature04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chakrapani S., Cuello L.G., Perozo E. Structural dynamics of an isolated voltage-sensor domain in a lipid bilayer. Structure. 2008;16:398–409. doi: 10.1016/j.str.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Takeshita K., Sakata S., Nakagawa A. X-ray crystal structure of voltage-gated proton channel. Nat. Struct. Mol. Biol. 2014;21:352–357. doi: 10.1038/nsmb.2783. [DOI] [PubMed] [Google Scholar]
- 140.Li Q., Wanderling S., Perozo E. Structural mechanism of voltage-dependent gating in an isolated voltage-sensing domain. Nat. Struct. Mol. Biol. 2014;21:244–252. doi: 10.1038/nsmb.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jegla T.J., Zmasek C.M., Nayak S.K. Evolution of the human ion channel set. Comb. Chem. High Throughput Screen. 2009;12:2–23. doi: 10.2174/138620709787047957. [DOI] [PubMed] [Google Scholar]
- 142.Wu L., Bauer C.S., Yang J. Dual regulation of voltage-gated calcium channels by PtdIns(4,5)P2. Nature. 2002;419:947–952. doi: 10.1038/nature01118. [DOI] [PubMed] [Google Scholar]
- 143.Rodriguez-Menchaca A.A., Adney S.K., Logothetis D.E. PIP2 controls voltage-sensor movement and pore opening of Kv channels through the S4-S5 linker. Proc. Natl. Acad. Sci. USA. 2012;109:E2399–E2408. doi: 10.1073/pnas.1207901109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Abderemane-Ali F., Es-Salah-Lamoureux Z., Loussouarn G. Dual effect of phosphatidylinositol (4,5)-bisphosphate PIP(2) on Shaker K(+) [corrected] channels. J. Biol. Chem. 2012;287:36158–36167. doi: 10.1074/jbc.M112.382085. [DOI] [PMC free article] [PubMed] [Google Scholar]