Abstract
AIM: To investigate whether the expression of platelet-derived growth factor receptor-α-positive (PDGFRα+)-cells is altered in Hirschsprung’s disease (HD).
METHODS: HD tissue specimens (n = 10) were collected at the time of pull-through surgery, while colonic control samples were obtained at the time of colostomy closure in patients with imperforate anus (n = 10). Immunolabelling of PDGFRα+-cells was visualized using confocal microscopy to assess the distribution of these cells, while Western blot analysis was undertaken to quantify PDGFRα protein expression.
RESULTS: Confocal microscopy revealed PDGFRα+-cells within the mucosa, myenteric plexus and smooth muscle in normal controls, with a marked reduction in PDGFRα+-cells in the HD specimens. Western blotting revealed high levels of PDGFRα protein expression in normal controls, while there was a striking decrease in PDGFRα protein expression in the HD colon.
CONCLUSION: These findings suggest that the altered distribution of PDGFRα+-cells in both the aganglionic and ganglionic HD bowel may contribute to the motility dysfunction in HD.
Keywords: Platelet-derived growth factor receptor alpha, Hirschsprung’s disease, Gastrointestinal motility, Aganglionosis, Myenteric plexus
Core tip: Hirschsprung’s disease is a congenital condition characterised by an absence of ganglia in the distal colon. Platelet-derived growth factor receptor-α-positive (PDGFRα+)-cells are a novel cell type recently found to be involved in gastrointestinal neurotransmission and smooth muscle contractility. Our study has revealed a striking decrease in PDGFRα+-cell expression in Hirschsprung’s disease colon compared to normal control colon. These results suggest an exciting new role for PDGFRα+-cells in the pathophysiology of this complex condition.
INTRODUCTION
Gastrointestinal smooth muscle contraction is controlled by co-ordinated interaction of three main cell types: enteric nerve cells, interstitial cells of Cajal (ICCs) and smooth muscle cells (SMCs). In recent years, a fourth cell type has been described as forming part of this complex network, namely platelet-derived growth factor receptor alpha-positive cells (PDGFRα+-cells). These PDGFRα+-cells were, for many years, known as “fibroblast-like cells” or “ICC-like” cells, as they resembled ICCs morphologically, but were c-kit negative[1]. More recently, enhanced green fluorescent protein (eGFP) labelling of these cells, as well as commercial availability of antibodies directed against PDGFRα, has enabled specific and reliable identification of this cell type[2]. PDGFRα+-cells form discrete networks in the region of the myenteric plexus and within the circular and longitudinal muscle layers[3]. PDGFRα+-cells express the small-conductance Ca2+-activated K+ channel (SK3), which is an important mediator of purinergic neurotransmission in gastrointestinal smooth muscle[3,4].
Platelet-derived growth factors (PDGFs) comprise four subtypes (A, B, C, and D), and their receptors, PDGFRs, have two subtypes, PDGFRα and PDGFRβ. It is known that PDGFs exist in epithelial cells, endothelial cells, and fibroblasts of adult mammals. They play an important role in cell proliferation, survival, and migration[5,6]. PDGFs are also present in the extracellular matrix and participate in tissue remodeling. It has been demonstrated that the PDGF/PDGFR signaling pathway plays a crucial role in embryonic development, especially in organogenesis, including alveogenesis, glomerulogenesis, angiogenesis and spermatogenesis[5]. This signaling pathway is also essential for interstitial cell and mesenchymal cell proliferation. Bonner[7] have previously shown that PDGF-A and the PDGFRα are important in gastrointestinal development, as mice lacking PDGF-A or PDGFRα develop abnormal gastrointestinal mucosal lining, which is also associated with a loss of PDGFRα-positive mesenchymal cells underlying the intestinal epithelium.
Hirschsprung’s disease (HSCR) is a congenital gut motility disorder with an incidence of 1 in 5000 live births[8]. It is characterized by an absence of ganglia within the distal colon for varying distances[9]. Most cases present in the newborn period with a failure to pass meconium, abdominal distension and bilious vomiting. A disruption of neural crest cell migration during the early stages of embryonic development is thought to be the main cause of this condition, with neural crest cells failing to complete their cranio-caudal colonization of the gastrointestinal tract. In addition to a lack of ganglia, many studies have documented deficiencies in smooth muscle proteins, extracellular matrix molecules, ion channels and various other important molecules in HSCR colon[10]. Our group has previously reported a reduction of ICCs in HSCR colon[11].
In recent years, several animal studies have reported the expression of the PDGFRα+-cells in the various regions of the gastrointestinal tract. However, there is little information available regarding PDGFRα+-cells distribution in gastrointestinal diseases. We designed this study to test the hypothesis that PDGFRα+-cell distribution is altered in Hirschsprung’s disease.
MATERIALS AND METHODS
Tissue samples
This study was approved by the Ethics Medical Research Committee, Our Lady’s Children’s Hospital, Dublin, Ireland (Ref. GEN/292/12) and tissue samples were obtained with informed parental consent. HD specimens from 10 patients who underwent pull-through surgery were studied. These specimens were divided into aganglionic and ganglionic samples. Patients were aged 6 ± 3 mo old. No additional health issues existed in these patients. Colonic control samples included 10 specimens from patients who underwent colostomy closure following surgical correction of imperforate anus. Control samples were taken from patients who were 11 ± 4 mo old. None of the imperforate anus patients had HD. Tissue specimens were either snap-frozen in liquid nitrogen and stored at -80 °C for protein extraction or embedded in OCT Mounting Compound (VWR International, Leuven, Belgium) for immunofluorescence and stored at -80 °C until use.
Immunofluorescence staining and confocal microscopy
Frozen blocks of HD colon and control samples were sectioned transversely at a thickness of 10 μm, mounted on SuperFrost® Plus slides (VWR International, Leuven, Belgium) and fixed with 10% buffered formalin for 5 min. Sections underwent cell membrane permeabilization with 1% TritonX-100 for 20 min at room temperature. After blocking with 10% normal goat serum (Sigma Aldrich Ltd, Arklow, Ireland) for 30 min to avoid non-specific absorption, sections were incubated with primary antibodies: Primary antibodies including: rabbit anti- PDGFRα (Abcam, Cambridge, United Kingdom), mouse anti-HuC/HuD (Molecular Probes), mouse anti-α-smooth muscle actin, mouse anti-c-kit mouse anti-TLR4, mouse anti-TLR5 (Abcam, Cambridge, United Kingdom), and mouse anti-P2YR1 (Abnova, Taiwan) all used at dilution 1:100, overnight at 4 °C. Sections were then washed in PBS + 0.05% Tween and incubated with corresponding secondary antibodies (goat anti-rabbit Alexa Fluor® 488, dilution 1:200 and goat anti-mouse Alexa Fluor® 647, dilution 1:200, Abcam, Cambridge, United Kingdom) for 1 h at room temperature. After washing, sections were counterstained with DAPI antibody, dilution 1:1000 (Roche Diagnostics GmbH, Mannheim, Germany) for 10 min, washed, mounted and cover-slipped with Fluorescent Mounting Medium (DAKO Ltd, Cambridgeshire, United Kingdom). All sections were independently evaluated by two investigators with a LSM 700 confocal microscope (Carl Zeiss MicroImaging GmbH, Jena, Germany).
Protein extraction and Western blot
Specimens of HD colon and healthy control colon were homogenized in RIPA buffer (Radio Immunoprecipitation Assay, Sigma-Aldrich Ltd., Wicklow, Ireland) containing 1% protease inhibitor cocktail (Sigma-Aldrich Ireland Ltd., Wicklow, Ireland). Protein concentrations were determined using a Bradford assay (Sigma-Aldrich Ireland Ltd., Wicklow, Ireland). A total volume of 20 μL Laemmli sample buffer (Sigma-Aldrich Ireland Ltd., Wicklow, Ireland) containing 10 μg of protein was loaded in the 10% SDS-PAGE gel (NuPAGE Novex Bis-Tris gels, Invitrogen, Carlsbad, United States) for electrophoretic separation. The electrophoresis was performed in MES SDS running buffer (Invitrogen, Carlsbad, United States). Proteins were then transferred to 0.45 μm nitrocellulose membrane (Millipore Corporation, Billerica, United States) by western blotting. Following western blotting, the membranes were blocked in 3% BS-0.05% Tween for 30 min before antibody detection. A primary antibody, rabbit anti-PDGFRα (Abcam, Cambridge, United Kingdom), dilution 1:1000, was used and incubation was performed overnight at 4 oC. Following extensive washing (four times in PBS-0.05% Tween) the membranes were incubated with the appropriate secondary antibody (goat anti-rabbit IgG, HRP-linked Antibody, dilution 1:10000, Abcam, Cambridge, United Kingdom) followed by washing (four times in PBS-0.05% Tween). Detection was performed with the ECL plus chemiluminescence kit (Thermo, Fisher Scientific, Dublin, Ireland). We used GAPDH (mouse anti-GAPDH, dilution 1:1000, Abcam, Cambridge, United Kingdom) as an additional loading control.
RESULTS
Immunofluorescence staining and confocal microscopy
Confocal microscopy revealed PDGFRα+-cells within the mucosa, myenteric plexus and smooth muscle in colonic controls, with a marked reduction in PDGFRα+-cells in the HD specimens (Figure 1). As other authors have reported, we noticed that PDGFRα+-cells and their projections were located adjacent to and surrounding both enteric neurons and fibres, as well as alongside ICCs in the myenteric plexus and within the smooth muscle layers. In the colonic mucosa, we found PDGFRα+-cells expressed the toll-like receptors 4 and 5, as well as the purinergic receptor, P2Y2R1, as has previously been reported in the murine colonic mucosa[2].
Figure 1.
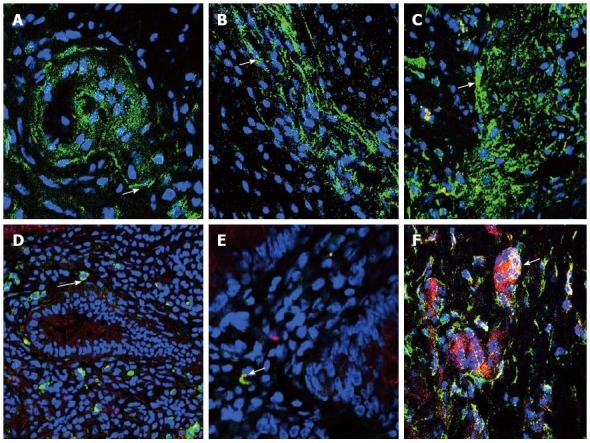
Immunofluorescent staining of platelet-derived growth factor receptor-α-positive-cells. Immunofluorescent staining of platelet-derived growth factor receptor-α-positive (PDGFRα+)-cells (green) in the myenteric plexus of normal control (A), aganglionic (B) and ganglionic (C) colon, arrow shows cell body. Nuclei were stained with DAPI (blue). Mucosal PDGFRα+-cells (green) were seen to co-express TLR4 (red) (D) (arrow), TLR5 (red) (E) (arrow) and P2RY1 (red) (F) (arrow). A-F: Magnification × 40.
Western blot
Western blotting revealed high levels of PDGFRα protein expression in colonic controls, while there was a striking decrease in PDGFRα protein expression in both ganglionic and aganglionic regions of HD (Figure 2). Equal expression was observed in ganglionic vs aganglionic segments.
Figure 2.
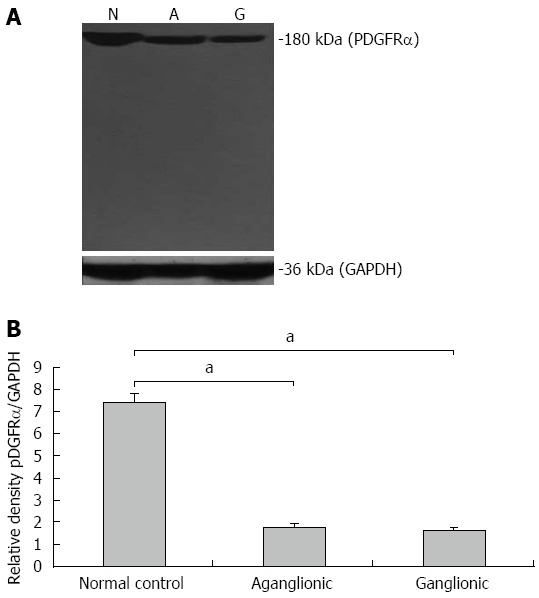
Western blot of platelet-derived growth factor receptor-α protein expression. A: Platelet-derived growth factor receptor-α (PDGFRα) protein expression was high in normal controls and markedly decreased in both ganglionic and aganglionic specimens. The loading control GAPDH was similarly expressed in normal controls, ganglionic and aganglionic specimens; B: Densitometry analysis showed the range of PDGFRα protein expression among the samples studied. aP values are significant vs normal control.
DISCUSSION
The location of PDGFRα+-cells alongside enteric nerves, ICCs, their close proximity to nerve varicosities and the fact that they are coupled by gap junctions to SMCs, strongly suggest a role for these cells in enteric neurotransmission[12-14]. ICCs are the enteric pacemaker cells, which also function as mediators of neurotransmission, and are important regulators of GI motility. C-kit, which is structurally similar to PDGFRs, is mainly expressed in ICCs of the GI tract. C-kit and PDGFRα are both receptor tyrosine kinases. We postulate that in HD colon, the role of PDGFRα+-cells in purinergic neurotransmission is disrupted leading to bowel dysmotility in both the ganglionic and aganglionic segments.
Many studies have investigated the expression of PDGFRα+-cells in the gastrointestinal tract of various animals in recent years. Iino et al[1] were the first authors to examine PDGFRα+-cells in the murine gastrointestinal tract. They observed these cells in the musculature in all regions of the gastrointestinal tract, closely associated with intramuscular ICCs and enteric nerve fibres. In the myenteric plexus, PDGFRα+-cells were seen to form a cellular network with their ramified processes and encompassed myenteric ganglia. Numerous PDGFRα+-cells were also observed in the sub-serosal plane and showed a multipolar shape[1]. They also analyzed the distribution of PDGFRα+-cells in the ICC-deficient W(v)/W(v) mutant mice and found that the expression pattern of PDGFRα+-cells was the same as that in wild-type mice, suggesting no interdependence between ICCs and PDGFRα+-cells[1]. The authors also reported different morphological features of PDGFRα+-cells dependent on which region of the GI tract they assessed; for example PDGFRα+-cells in the myenteric plexus of the gastric fundus had wider cell bodies than those in other GI regions, while PDGFRα+-cells in the circular muscle layer of the small and large intestines had wider cell bodies and more cytoplasmic extensions than those of the stomach[1].
A later study by Cobine et al[12] examined the distribution of ICCs, PDGFRα+-cells and nitric-oxide-synthase-positive neurons and nerve fibres in the internal anal sphincter of both wild-type mice and KitW/Wv mice, in which ICC numbers are greatly reduced in many regions of the GI tract. They found that PDGFRα+-cells made up 18% of the total tissue volume within the circular muscle layer of the mouse internal anal sphincter, compared to ICC-IM which made up only 5% of tissue volume. They also noted PDGFRα+-cells distributed within the submucosa and along the serosal and myenteric surfaces. They also noted a close morphological arrangement between inhibitory motor neurons, ICC-IM and PDGFRα(+)-IM, which suggested functional interaction between these cell types in the internal anal sphincter[12].
Kurahashi et al[13] were the first group to confirm a functional role for PDGFRα+ cells in gastrointestinal smooth muscle using transgenic mice with constitutive expression of enhanced green fluorescent protein (eGFP) in PDGFRα+-cells, which allowed the authors to isolate and study the function of PDGFRα+-cells. They found that PDGFRα+-cells expressed appropriate receptors and effectors to receive and transduce purinergic neural signals[13]. In 2012, another study by Kurahashi et al[3] used immunohistochemical techniques to study the phenotype and intercellular relationships PDGFRα+-cells widely distributed throughout the tunica muscularis of the human colon. They reported that PDGFRα+-cells form discrete networks in the region of the myenteric plexus and within the circular and longitudinal muscle layers of the human colon. These cells were seen to be distinct from ICCs and were closely associated with varicose processes of neurons expressing the excitatory neurotransmitter substance P or the inhibitory neurotransmitter neuronal nitric oxide synthase. They also found that PDGFRα+-cells express small conductance Ca(2+)-activated K(+) channels (SK3), which are likely to mediate purinergic neural regulation of colonic muscles[3]. Our group has also recently confirmed SK3 channel expression is decreased in HD[4].
Furthermore, another recent study by Peri et al[15] confirmed that SK3 channels are strongly enriched in PDGFRα+-cells and that the expression of these channels in this cell type far exceeded their expression in either ICCs or SMCs. Analysis showed that P2X receptor genes were expressed more highly in PDGFRα+-cells than SMCs. PDGFRα+-cells showed high expression of P2rx1, P2rx3, P2rx4, P2rx5, P2rx6, and particularly P2rx7. In addition, PDGFRα+-cells showed high expression of P2ry1, P2ry2, P2ry12, and P2ry13. Therefore, PDGFRα+-cells might be a target for extracellular ATP acting on ionotropic P2X receptors, as well as P2Y receptors which are activated by adenine, pyridine, and pyrimidine nucleotides[15]. Kurahashi et al[2], also revealed a unique population of PDGFRα+-cells in the mucosal layer of colon. These authors found that sub-epithelial PDGFRα+-cells in the mouse colonic mucosa expressed toll-like receptor genes, purinergic receptor genes, 5-hydroxytryptamine 4 receptor gene, and hedgehog signaling genes[14].
In our current study, we have verified that the mucosal PDGFRα+-cells in the human colon also express TLR4, TLR5 and P2RY1, as has been observed in the mouse colonic mucosa, suggesting a role for these cells in the immune response and in purinergic neurotransmission. The results of our study have revealed a striking decrease in PDGFRα+-cell expression in both ganglionic and aganglionic regions of HD compared to normal control colon. We have previously also shown a marked decrease in the expression of both ICCs and SK3 channels in HD colon compared to controls[11,16]. The reduced volume of PDGFRα+-cell expression indicates a deficiency in inhibitory neurotransmission in HD bowel and may further our understanding of the mechanism by which aganglionic bowel remains in a state of tonic contraction. These results suggest an exciting new role for PDGFRα+-cells in the pathophysiology of this complex condition.
ACKNOWLEDGMENTS
We wish to acknowledge the Department of Histopathology in Our Lady’s Children’s Hospital for their contribution to specimen collection.
COMMENTS
Background
Gastrointestinal smooth muscle contraction has been thought to be controlled by co-ordinated interaction of three main cell types: enteric nerve cells, interstitial cells of Cajal and smooth muscle cells. In recent years, a fourth cell type has been described as forming part of this complex network, namely platelet-derived growth factor receptor-α-positive (PDGFRα+)-cells.
Research frontiers
PDGFRα+-cells have been studied in normal human colon, but as yet have not been analysed in human disease. Hirschsprung’s disease is the most common congenital gut motility disorder. Although the characteristic pathological findings in HSCR are well understood (aganglionosis and nerve cell hypertrophy), the mechanism underlying motility disturbance is not completely understood.
Innovations and breakthroughs
PDGFRα+-cells have been studied in normal human colon but, as yet, their expression in gastrointestinal disease has not been analysed. The authors report the first investigation of PDGFRα+-cell expression in the colon of Hirschsprung’s Disease, and show a deficiency of these cells in this condition.
Applications
In demonstrating reduced expression of PDGFRα+-cells in Hirschsprung’s Disease, this study provides the basis for future functional studies that may seek to determine their exact role in colonic dysmotility in HSCR and potentially in other gut motility disorders.
Terminology
PDGFRα+-cells are a type of interstitial cell involved in neurotransmission in the gastrointestinal tract. Along with interstitial cells of Cajal, enteric neurons and smooth muscle cells, PDGFRα+-cells help maintain peristalsis of the colon.
Peer-review
The authors have explored the expression of PDGFRα+-cells in colon specimens of Hirschsprung’s disease patients. They found, for the first time, a deficiency of PDGFRα+-cells in both the aganglionic and ganglionic colon compared to normal controls. These results suggest a role for PDGFRα+-cells in the pathophysiology of Hirschsprung’s Disease.
Footnotes
Supported by National Children’s Research Centre/Children’s Medical Research Foundation, Ireland.
Institutional review board statement: The study was reviewed and approved by the Ethics (Medical Research) Committee of Our Lady’s Children’s Hospital Crumlin, Dublin, Ireland, Ref. GEN/292/12.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Data sharing statement: Dataset is available from the corresponding author at prem.puri@ncrc.ie.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 8, 2015
First decision: November 5, 2015
Article in press: December 21, 2015
P- Reviewer: Lee HC, Zimmer A S- Editor: Yu J L- Editor: A E- Editor: Wang CH
References
- 1.Iino S, Horiguchi K, Horiguchi S, Nojyo Y. c-Kit-negative fibroblast-like cells express platelet-derived growth factor receptor alpha in the murine gastrointestinal musculature. Histochem Cell Biol. 2009;131:691–702. doi: 10.1007/s00418-009-0580-6. [DOI] [PubMed] [Google Scholar]
- 2.Kurahashi M, Nakano Y, Peri LE, Townsend JB, Ward SM, Sanders KM. A novel population of subepithelial platelet-derived growth factor receptor α-positive cells in the mouse and human colon. Am J Physiol Gastrointest Liver Physiol. 2013;304:G823–G834. doi: 10.1152/ajpgi.00001.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurahashi M, Nakano Y, Hennig GW, Ward SM, Sanders KM. Platelet-derived growth factor receptor α-positive cells in the tunica muscularis of human colon. J Cell Mol Med. 2012;16:1397–1404. doi: 10.1111/j.1582-4934.2011.01510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coyle D, O’Donnell AM, Puri P. Altered distribution of small-conductance calcium-activated potassium channel SK3 in Hirschsprung’s disease. J Pediatr Surg. 2015;50:1659–1664. doi: 10.1016/j.jpedsurg.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Karlsson L, Lindahl P, Heath JK, Betsholtz C. Abnormal gastrointestinal development in PDGF-A and PDGFR-(alpha) deficient mice implicates a novel mesenchymal structure with putative instructive properties in villus morphogenesis. Development. 2000;127:3457–3466. doi: 10.1242/dev.127.16.3457. [DOI] [PubMed] [Google Scholar]
- 6.Chan F, Liu Y, Sun H, Li X, Shang H, Fan D, An J, Zhou D. Distribution and possible role of PDGF-AA and PDGFR-alpha in the gastrointestinal tract of adult guinea pigs. Virchows Arch. 2010;457:381–388. doi: 10.1007/s00428-010-0946-0. [DOI] [PubMed] [Google Scholar]
- 7.Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004;15:255–273. doi: 10.1016/j.cytogfr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Spouge D, Baird PA. Hirschsprung disease in a large birth cohort. Teratology. 1985;32:171–177. doi: 10.1002/tera.1420320204. [DOI] [PubMed] [Google Scholar]
- 9.Butler Tjaden NE, Trainor PA. The developmental etiology and pathogenesis of Hirschsprung disease. Transl Res. 2013;162:1–15. doi: 10.1016/j.trsl.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wetherill C, Sutcliffe J. Hirschsprung disease and anorectal malformation. Early Hum Dev. 2014;90:927–932. doi: 10.1016/j.earlhumdev.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Rolle U, Piotrowska AP, Nemeth L, Puri P. Altered distribution of interstitial cells of Cajal in Hirschsprung disease. Arch Pathol Lab Med. 2002;126:928–933. doi: 10.5858/2002-126-0928-ADOICO. [DOI] [PubMed] [Google Scholar]
- 12.Cobine CA, Hennig GW, Kurahashi M, Sanders KM, Ward SM, Keef KD. Relationship between interstitial cells of Cajal, fibroblast-like cells and inhibitory motor nerves in the internal anal sphincter. Cell Tissue Res. 2011;344:17–30. doi: 10.1007/s00441-011-1138-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurahashi M, Zheng H, Dwyer L, Ward SM, Koh SD, Sanders KM. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J Physiol. 2011;589:697–710. doi: 10.1113/jphysiol.2010.201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker SA, Hennig GW, Salter AK, Kurahashi M, Ward SM, Sanders KM. Distribution and Ca(2+) signalling of fibroblast-like (PDGFR(+)) cells in the murine gastric fundus. J Physiol. 2013;591:6193–6208. doi: 10.1113/jphysiol.2013.264747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peri LE, Sanders KM, Mutafova-Yambolieva VN. Differential expression of genes related to purinergic signaling in smooth muscle cells, PDGFRα-positive cells, and interstitial cells of Cajal in the murine colon. Neurogastroenterol Motil. 2013;25:e609–e620. doi: 10.1111/nmo.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piotrowska AP, Rolle U, Chertin B, De Caluwé D, Bianchi A, Puri P. Alterations in smooth muscle contractile and cytoskeleton proteins and interstitial cells of Cajal in megacystis microcolon intestinal hypoperistalsis syndrome. J Pediatr Surg. 2003;38:749–755. doi: 10.1016/jpsu.2003.50159. [DOI] [PubMed] [Google Scholar]


