Abstract
AIM: To analyze differences in patients’ clinical course, we compared two regimes of either preemptive therapy or prophylaxis after liver transplantation.
METHODS: This retrospective study was reviewed and approved by the institutional review board of the University of Leipzig. Cytomegalovirus (CMV) prophylaxis with valganciclovir hydrochloride for liver transplant recipients was replaced by a preemptive strategy in October 2009. We retrospectively compared liver transplant recipients 2 years before and after October 2009. During the first period, all patients received valganciclovir daily. During the second period all patients included in the analysis were treated following a preemptive strategy. Outcomes included one year survival and therapeutic intervention due to CMV viremia or infection.
RESULTS: Between 2007 and 2010 n = 226 patients underwent liver transplantation in our center. n = 55 patients were D+/R- high risk recipients and were excluded from further analysis. A further 43 patients had to be excluded since CMV prophylaxis/preemptive strategy was not followed although there was no clinical reason for the deviation. Of the remaining 128 patients whose data were analyzed, 60 received prophylaxis and 68 were treated following a preemptive strategy. The difference in overall mortality was not significant, nor was it significant for one-year mortality where it was 10% (95%CI: 8%-28%, P = 0.31) higher for the preemptive group. No significant differences in blood count abnormalities or the incidence of sepsis and infections were observed other than CMV. In total, 19 patients (14.7%) received ganciclovir due to CMV viremia and/or infections. Patients who were treated according to the preemptive algorithm had a significantly higher rate risk of therapeutic intervention with ganciclovir [n = 16 (23.5%) vs n = 3 (4.9%), P = 0.003)].
CONCLUSION: These data suggest that CMV prophylaxis is superior to a preemptive strategy in patients undergoing liver transplantation.
Keywords: Transplantation, Liver, Cytomegalovirus, Preemptive, Prophylaxis, Valganciclovir, Therapy
Core tip: This retrospective study compares a preemptive therapy to prophylaxis for cytomegalovirus (CMV) infection in 128 patients after liver transplantation (LTx). CMV infections are frequent and increase morbidity and mortality so that preventive strategies are routine procedures. The one-year mortality did not differ significantly between the preemptive (n = 68) and prophylaxis (n = 60) groups, though it was 10% (95%CI: 8%-28%, P = 0.31) higher for the former. Preemptive patients had a significantly higher rate of intervention with ganciclovir (23.5% vs 4.9%, P = 0.003). Our data suggest that CMV prophylaxis is superior to a preemptive strategy after LTx.
INTRODUCTION
Patients who undergo immunosuppressive therapy after solid organ transplantation are at higher risk for opportunistic bacterial, fungal, and viral infections. Infections with cytomegalovirus (CMV) are frequent and have been shown to increase morbidity and mortality in particular shortly after transplantation[1]. Estimates for the incidence of CMV infections after liver transplantation (LTx) range from 22% to 29%[1-3]. Therefore, strategies for preventing CMV infections are a routine procedure after solid organ transplantation[4]. Prophylaxis with antiviral substances leads to a reduction in the incidence and the severity of CMV infections. However, appropriate substances for prophylaxis have major side effects including myelodepression. Therefore, preemptive therapy has been proposed as an alternative regimen[1,5-7]. Preemptive therapy aims at suppressing viral replication after detection of CMV viremia, but prior to the onset of clinical symptoms. Antiviral therapy is then initiated in order to prevent clinically relevant infections[8-13]. However, to date, there is no strong clinical evidence indicating superiority of one regimen in liver transplant recipients over the other. To accumulate more evidence on the clinical course in patients after liver transplantation, we used retrospective data to compare preemptive therapy to prophylaxis during the liver transplantation program of the university hospital of Leipzig.
MATERIALS AND METHODS
The study was approved by the Institutional Review Board of the university of Leipzig (No. 122-12-16042012).
Prophylaxis with valganciclovir hydrochloride had been the standard treatment for liver transplant recipients in our center irrespective of their CMV serological status. Since patients presented with serious side effects including pancytopenia, prophylaxis was replaced by a preemptive strategy in October 2009. To assess differences in safety and efficacy of the two regimens, we retrospectively compared all liver transplant recipients two years before and after October 2009 during hospital stay after liver transplantation. Data on mortality was collected up to one year after transplantation. All patients treated according to the prophylaxis regimen received 450mg valganciclovir twice daily. If necessary doses were adapted to renal function. For patients treated after October 2009, only high-risk seronegative recipients, who received an organ from a seropositive donor (D+/R-), received prophylaxis and therefore all D+/R- patients in both groups were excluded from analysis. For both regimens, CMV polymerase chain reaction (PCR) was performed twice weekly. When PCR was positive, all patients in both groups were treated with ganciclovir for at least 14 d, whether or not there were clinical symptoms.
The data collected included baseline data, antibody patterns against CMV for donor and recipients (D/R), viremia during the intensive care unit (ICU) stay and occurrence of CMV infections. Furthermore, occurrence of sepsis, thrombocytopenia [platelet count < 50 giga particles (GPT)/L], leukocytopenia (white blood cells < 4 GPT/L) and anemia (hematocrit < 30%) starting 72 h after ICU admission was documented. LabMELD (Model of end stage liver disease) score was calculated for each patient on the day of transplantation. All MELD points were calculated retrospectively using validated laboratory data. Mortality data were collected during initial hospital stay and at day 28, day 90 and 1 year after transplantation. All patients received the same standard immunosuppression with mycophenolate mofetil, steroids (tapered within 8 wk) and tacrolimus (days 1-14 FK506 level 10 ng/mL, days 15-28 8 ng/mL and continuing with 5 ng/mL). In the event of severe infection or side effects, immunosuppressive therapy was chosen by the physicians on an individual basis.
Statistical analysis
Data were collected in an Excel 2010 spreadsheet (Microsoft Corp., Redmond, United States). Statistical analysis was performed using SPSS Statistics 20.0 (SPSS GmbH Software, Munich, Germany) and R version 3.1.0. (R Foundation for Statistical Computing, Vienna, Austria). Survival analyses were performed using a log-rank test with the R package “survival”. Categorical data are expressed as absolute or relative frequencies and the χ2 or Fisher’s exact test was used for inferential statistics depending on the number of expected counts. The confidence interval for differences in proportions makes use of a Wilson confidence interval. Continuous data or categorical ones with fewer than six levels are expressed as mean and standard deviation and a t-test was used for comparing groups. If categorical data has five or fewer levels, then median and interquartile range are presented and differences between groups are compared using the Wilcoxon-Mann-Whitney U test. A P value of less 0.05 was considered to be statistically significant.
RESULTS
Between 2007 and 2010 n = 226 patients underwent liver transplantation in our center. n = 55 patients were D+/R- high risk recipients and were excluded from further analysis. A further 43 patients had to be excluded since CMV prophylaxis/preemptive strategy was not followed although there was no clinical reason for the deviation. These 43 patients do not differ markedly from the remainder with respect to sex, age or labMELD. Of the remaining 128 patients whose data were analyzed, 60 received prophylaxis and 68 were treated following a preemptive strategy.
Mean age of all analyzed patients was 54 ± 10 years, 90 patients were male and 38 were female (Table 1). The mean labMELD score before transplantation was 18.5 ± 9.4. At day 1 after transplantation patients had a mean APACHE II (Acute Physiology And Chronic Health Evaluation II) score of 14.2 ± 6.7 and a SOFA (simplified organ failure assessment) of 9.1 ± 4.0.
Table 1.
Patient demographics n (%)
| All (n = 128) | Prophylaxis (n = 60) | Preemptive therapy (n = 68) | P value | |
| Age (yr) | 54 ± 10 | 52 ± 12 | 56 ± 8.5 | 0.04 |
| Sex | ||||
| Male | 90 (70) | 42 (70) | 48 (71) | 1.00 |
| Female | 38 (30) | 18 (30) | 20 (29) | |
| Weight (kg) | 80 ± 16 | 81 ± 13 | 79 ± 19 | 0.52 |
| SOFA | 9.1 ± 4.0 | 8.8 ± 3.8 | 9.4 ± 4.3 | 0.34 |
| APACHE II | 14.2 ± 6.7 | 13.3 ± 5.5 | 15.0 ± 7.5 | 0.13 |
| Lab. MELD at Transplantation | 18.5 ± 9.4 | 18.8 ± 8.8 | 18.2 ± 9.9 | 0.73 |
| CMV-status D/R | 0.24 | |||
| -/- | 19 (17) | 5 (10) | 14 (22) | |
| -/+ | 33 (29) | 16 (33) | 17 (27) | |
| +/+ | 60 (54) | 28 (57) | 32 (51) | |
| +/- | Excluded |
Data are expressed as mean ± SD or n (%). Complete CMV-status was unavailable for 16 patients, but was known not to be +/-. D/R: Donor/receptor; CMV: Cytomegalovirus; Lab.MELD: Model of end stage liver disease.
Mortality did not differ significantly between the groups (Figure 1). At one year, the mortality was 18/60 (30%, 95%CI: 20%-43%) for the prophylaxis strategy and 27/67 (40%, 95%CI: 29%-52%) for the preemptive strategy, where one censored case accounts for the denominator of 67 instead of 68 patients. However, this difference did not reach significance (P = 0.31).
Figure 1.
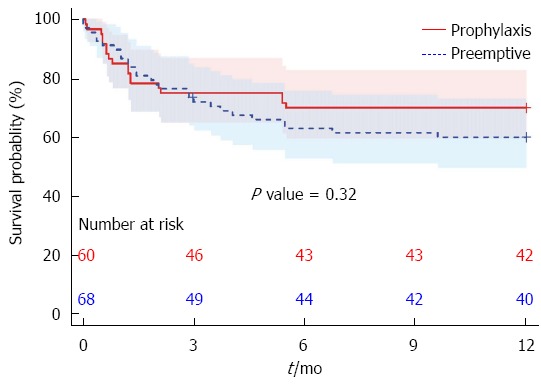
Survival curve. Mortality did not differ significantly between the groups.
There was a nonhomogeneous distribution of donors and recipients serologic CMV patterns in our series, which was not significant. We found 22% D-/R- in the preemptive group compared with 10% in the prophylaxis group (Table 1).
There were no significant differences in blood count abnormalities or the incidence of sepsis and infections other than CMV (Table 2). In total 19 patients (15%) received ganciclovir due to CMV viremia and/or infections. The therapy started 29 ± 20 d after transplantation on average and lasted for a mean of 18 ± 12 d. Patients who were treated according to the preemptive algorithm had a significantly higher rate risk of therapeutic intervention with ganciclovir [n = 16 (24%) vs n = 3 (5%), P = 0.005]. From these 19 patients 16 patients (84.0%) had clinical symptoms allegeable by CMV in context of the viremia [prophylaxis n = 2 (12.5%), preemptive therapy n = 14 (87.5%)].
Table 2.
Infection/blood count abnormalities n (%)
| All (n = 128) | Prophylaxis (n = 60) | Preemptive therapy (n = 68) | P value | |
| Re - LTx | 17 (13) | 10 (17) | 7 (10) | 0.31 |
| Infection | 86 (71) | 40 (69) | 46 (72) | 0.84 |
| Sepsis | 63 (52) | 30 (52) | 33 (52) | 1.00 |
| Thrombocytopenia (PLT < 50 Gpt/L; > 72 h post-LTx | 69 (57) | 32 (55) | 37 (58) | 0.86 |
| Leukocytopenia (WBC < 4 Gpt/L; > 72 h post-LTx) | 38 (31) | 15 (26) | 23 (36) | 0.25 |
| Anaemia (Hct < 30%; > 72 h post-LTx) | 114 (93) | 54 (93) | 60 (94) | 1.00 |
GPT: Giga particles; LTx: Liver transplantation; PLT: Platelet count; WBC: White blood cells; Hct: Haematocrit.
DISCUSSION
We retrospectively compared the effects of a preemptive and a prophylactic strategy to prevent CMV infections during the early phase after liver transplantation. We could demonstrate that a preemptive strategy was associated with significantly more episodes of CMV viremia/infections in patients with mid/low risk without evidence for more side effects of the antiviral substances.
Recommendations for prevention of CMV infections in solid organ transplantation are heterogeneous and rather difficult to interpret. There are a few prospective randomized studies comparing both regimens after kidney transplantation. Kliem et al[15] demonstrated that prophylaxis reduced the incidence of CMV infections by 65% and the authors suggest that prophylaxis might improve long-term graft survival and recommend limiting preemptive strategies to low-risk patients. In a series of 296 kidney transplant patients, there was a reduction of CMV infections by 28 percentage points in the group who received prophylactic therapy (38.7% vs 11%, P < 0.0001)[16]. On the other hand, Reischig et al[17] and Khoury et al[18] found a similar incidence of CMV infections in both groups (6% vs 9%, P = 0.567) and therefore suggested a comparable efficacy of both strategies. Gerna et al[19] performed a controlled, randomized, open-label study in 21 children after liver transplantation and did not report any CMV disease, irrespective of the procedure. A recent Cochrane analysis concluded that data is not yet sufficient to recommend one strategy over the other prophylaxis or preemptive strategies[14].
For adult liver transplant recipients, data from randomized studies are not available. In clinical practice there is a broad variety of strategies concerning the selection of substances, the timing and the duration of either prophylaxis or preemptive strategies. However, most centers tend to use prophylaxis at least in high-risk patients[16].
All told, there is no strong evidence from randomized studies indicating which of the two strategies is superior after liver transplantation. Accordingly, several authors consider both treatment options to be similarly effective[8,11,12,20-22].
Positive effects of a preemptive strategy can be the reduction of side effects and costs. When compared to no preventive strategy at all, preemptive therapy leads to less graft rejection and may improve CMV specific cell-mediated immunity and lead to a decreased risk of late CMV infections[11,13,23]. On the other hand, the use of prophylactic strategies has been shown to reduce mortality, the incidence of graft loss and opportunistic viral[24], bacterial or fungal infections[25]. Otherwise, prophylaxis increases the risk of drug-resistance and the incidence of late-onset CMV disease[26].
The incidence of CMV infection varies depending upon donor and/or recipient serological status[19,27,28]. Hodson et al[25] suggested in a systematic review of randomized trials, that CMV infections are more frequently in sero-negative patients and current guidelines suggest antiviral prophylaxis at least in D+/R- patients[29,30]. In the presented study, these patients have been excluded from analysis. There is an imbalance of sero-negative patients between the two groups in our study (Table 1) and following the above argument, one might have supposed that the prophylaxis group was at higher risk of infection. It turns out that none of the 19 sero-negative patients in the study had CMV viremia/infection, however, so that this could not have contributed to our findings.
The presented retrospective study of consecutive treatment groups has limitations. Due to the retrospective character and lack of randomization, a variety of factors such as slight changes in therapeutic regimens over-time may have influenced results. On the other hand, we demonstrated that groups did not differ concerning demographic baseline data, severity of liver failure or severity of disease at admission, and therefore suggest that our results strongly support the hypothesis that the higher incidence of CMV viremia was mainly influenced by the introduction of a preemptive strategy. Attributing clinical symptoms such as diarrhea or elevation of liver enzymes in the early course after LTX to CMV remains uncertain as there are a broad variety of possible causes. However, we suggest that our results support the hypothesis that prophylaxis is more effective in preventing CMV viremia and infection even in patients with low or mid risk for CMV infection. In conclusion, we demonstrated a significantly lower rate of CMV viremia/infection with prophylaxis when compared to a preemptive strategy. Our data indicate that prophylaxis might be superior to a preemptive strategy. To confirm this hypothesis, randomized prospective trials in liver transplant recipients are needed.
COMMENTS
Background
Patients after solid organ transplantation who undergo immunosuppressive therapy are at higher risk for infections with cytomegalovirus. They are frequent and have been shown to increase morbidity and mortality, particularly during the early stages after liver transplantation. Therefore, strategies for preventing cytomegalovirus (CMV) infections are a routine procedure after liver transplantation. Prophylaxis with antiviral substances leads to a reduction in the incidence and the severity of CMV infections. Preemptive therapy aims at suppression of viral replication after detection of CMV viremia and prior to the onset of clinical symptoms. The authors compared preemptive therapy to prophylaxis during the liver transplantation program of the university hospital of Leipzig to analyze differences in the clinical course in patients after liver transplantation.
Research frontiers
There is no strong evidence from randomized studies demonstrating which of the strategies is superior after liver transplantation. Accordingly, several authors consider both treatment options to be similarly effective. The authors demonstrated a significantly lower rate of CMV viremia/infection using prophylaxis when compared to a preemptive strategy. The data indicate that prophylaxis might be superior to a preemptive strategy.
Innovations and breakthroughs
Current study found a lower incidence of CMV viremia/infection with prophylaxis compared to a preemptive strategy without significant differences in blood count abnormalities or the incidence of infections and differences in mortality at any time. These data indicate that prophylaxis might be superior to a preemptive strategy.
Applications
The data indicate that prophylaxis might be superior to a preemptive strategy. To confirm this hypothesis randomized prospective trials in liver transplant recipients are needed.
Peer-review
The topic has a great interest given the lack of studies in this field in liver transplant recipients. Nevertheless the higher rate of seronegative patients in the pre-emptive group could explain the higher rate of infections in this group. It would be interesting to include some data regarding duration of treatment in both groups and regarding the specific period of time after transplant in which the CMV infection occurred (early infection vs late infection).
Footnotes
Institutional review board statement: This study was reviewed and approved by the institutional review board of the University of Leipzig, Germany.
Informed consent statement: The institutional review board of the University of Leipzig waived the need for informed consent due to the strongly retrospective and observational nature of the study.
Conflict-of-interest statement: On behalf of all authors, the corresponding author states that there is no conflict of interest.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 20, 2015
First decision: July 19, 2015
Article in press: December 1, 2015
P- Reviewer: Vazquez-Millan MA S- Editor: Yu J L- Editor: A E- Editor: Ma S
References
- 1.Kanj SS, Sharara AI, Clavien PA, Hamilton JD. Cytomegalovirus infection following liver transplantation: review of the literature. Clin Infect Dis. 1996;22:537–549. doi: 10.1093/clinids/22.3.537. [DOI] [PubMed] [Google Scholar]
- 2.Snydman DR. Epidemiology of infections after solid-organ transplantation. Clin Infect Dis. 2001;33 Suppl 1:S5–S8. doi: 10.1086/320897. [DOI] [PubMed] [Google Scholar]
- 3.Limaye AP, Bakthavatsalam R, Kim HW, Randolph SE, Halldorson JB, Healey PJ, Kuhr CS, Levy AE, Perkins JD, Reyes JD, et al. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation. 2006;81:1645–1652. doi: 10.1097/01.tp.0000226071.12562.1a. [DOI] [PubMed] [Google Scholar]
- 4.Park JM, Lake KD, Arenas JD, Fontana RJ. Efficacy and safety of low-dose valganciclovir in the prevention of cytomegalovirus disease in adult liver transplant recipients. Liver Transpl. 2006;12:112–116. doi: 10.1002/lt.20562. [DOI] [PubMed] [Google Scholar]
- 5.Saliba F, Arulnaden JL, Gugenheim J, Serves C, Samuel D, Bismuth A, Mathieu D, Bismuth H. CMV hyperimmune globulin prophylaxis after liver transplantation: a prospective randomized controlled study. Transplant Proc. 1989;21:2260–2262. [PubMed] [Google Scholar]
- 6.Strippoli GF, Hodson EM, Jones CJ, Craig JC. Pre-emptive treatment for cytomegalovirus viraemia to prevent cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev. 2006;(1):CD005133. doi: 10.1002/14651858.CD005133.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Müller V, Perrakis A, Meyer J, Förtsch T, Korn K, Croner RS, Yedibela S, Hohenberger W, Schellerer VS. The value of pre-emptive therapy for cytomegalovirus after liver transplantation. Transplant Proc. 2012;44:1357–1361. doi: 10.1016/j.transproceed.2011.11.067. [DOI] [PubMed] [Google Scholar]
- 8.Paya CV, Wilson JA, Espy MJ, Sia IG, DeBernardi MJ, Smith TF, Patel R, Jenkins G, Harmsen WS, Vanness DJ, et al. Preemptive use of oral ganciclovir to prevent cytomegalovirus infection in liver transplant patients: a randomized, placebo-controlled trial. J Infect Dis. 2002;185:854–860. doi: 10.1086/339449. [DOI] [PubMed] [Google Scholar]
- 9.Rubin RH. Preemptive therapy in immunocompromised hosts. N Engl J Med. 1991;324:1057–1059. doi: 10.1056/NEJM199104113241509. [DOI] [PubMed] [Google Scholar]
- 10.Mattes FM, Hainsworth EG, Hassan-Walker AF, Burroughs AK, Sweny P, Griffiths PD, Emery VC. Kinetics of cytomegalovirus load decrease in solid-organ transplant recipients after preemptive therapy with valganciclovir. J Infect Dis. 2005;191:89–92. doi: 10.1086/425905. [DOI] [PubMed] [Google Scholar]
- 11.Singh N, Wannstedt C, Keyes L, Gayowski T, Wagener MM, Cacciarelli TV. Efficacy of valganciclovir administered as preemptive therapy for cytomegalovirus disease in liver transplant recipients: impact on viral load and late-onset cytomegalovirus disease. Transplantation. 2005;79:85–90. doi: 10.1097/01.tp.0000146844.65273.62. [DOI] [PubMed] [Google Scholar]
- 12.Singh N, Yu VL. Preemptive therapy for cytomegalovirus. Liver Transpl. 2006;12:327. doi: 10.1002/lt.20676. [DOI] [PubMed] [Google Scholar]
- 13.Singh N, Wannstedt C, Keyes L, Mayher D, Tickerhoof L, Akoad M, Wagener MM, Cacciarelli TV. Valganciclovir as preemptive therapy for cytomegalovirus in cytomegalovirus-seronegative liver transplant recipients of cytomegalovirus-seropositive donor allografts. Liver Transpl. 2008;14:240–244. doi: 10.1002/lt.21362. [DOI] [PubMed] [Google Scholar]
- 14.Owers DS, Webster AC, Strippoli GF, Kable K, Hodson EM. Pre-emptive treatment for cytomegalovirus viraemia to prevent cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev. 2013;2:CD005133. doi: 10.1002/14651858.CD005133.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kliem V, Fricke L, Wollbrink T, Burg M, Radermacher J, Rohde F. Improvement in long-term renal graft survival due to CMV prophylaxis with oral ganciclovir: results of a randomized clinical trial. Am J Transplant. 2008;8:975–983. doi: 10.1111/j.1600-6143.2007.02133.x. [DOI] [PubMed] [Google Scholar]
- 16.Witzke O, Hauser IA, Bartels M, Wolf G, Wolters H, Nitschke M. Valganciclovir prophylaxis versus preemptive therapy in cytomegalovirus-positive renal allograft recipients: 1-year results of a randomized clinical trial. Transplantation. 2012;93:61–68. doi: 10.1097/TP.0b013e318238dab3. [DOI] [PubMed] [Google Scholar]
- 17.Reischig T, Jindra P, Hes O, Svecová M, Klaboch J, Treska V. Valacyclovir prophylaxis versus preemptive valganciclovir therapy to prevent cytomegalovirus disease after renal transplantation. Am J Transplant. 2008;8:69–77. doi: 10.1111/j.1600-6143.2007.02031.x. [DOI] [PubMed] [Google Scholar]
- 18.Khoury JA, Storch GA, Bohl DL, Schuessler RM, Torrence SM, Lockwood M, Gaudreault-Keener M, Koch MJ, Miller BW, Hardinger KL, et al. Prophylactic versus preemptive oral valganciclovir for the management of cytomegalovirus infection in adult renal transplant recipients. Am J Transplant. 2006;6:2134–2143. doi: 10.1111/j.1600-6143.2006.01413.x. [DOI] [PubMed] [Google Scholar]
- 19.Gerna G, Lilleri D, Callegaro A, Goglio A, Cortese S, Stroppa P, Torre G. Prophylaxis followed by preemptive therapy versus preemptive therapy for prevention of human cytomegalovirus disease in pediatric patients undergoing liver transplantation. Transplantation. 2008;86:163–166. doi: 10.1097/TP.0b013e31817889e4. [DOI] [PubMed] [Google Scholar]
- 20.Gane E, Saliba F, Valdecasas GJ, O’Grady J, Pescovitz MD, Lyman S, Robinson CA. Randomised trial of efficacy and safety of oral ganciclovir in the prevention of cytomegalovirus disease in liver-transplant recipients. The Oral Ganciclovir International Transplantation Study Group [corrected] Lancet. 1997;350:1729–1733. doi: 10.1016/s0140-6736(97)05535-9. [DOI] [PubMed] [Google Scholar]
- 21.Paya C, Humar A, Dominguez E, Washburn K, Blumberg E, Alexander B, Freeman R, Heaton N, Pescovitz MD. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2004;4:611–620. doi: 10.1111/j.1600-6143.2004.00382.x. [DOI] [PubMed] [Google Scholar]
- 22.Singh N, Paterson DL, Gayowski T, Wagener MM, Marino IR. Cytomegalovirus antigenemia directed pre-emptive prophylaxis with oral versus I.V. ganciclovir for the prevention of cytomegalovirus disease in liver transplant recipients: a randomized, controlled trial. Transplantation. 2000;70:717–722. doi: 10.1097/00007890-200009150-00002. [DOI] [PubMed] [Google Scholar]
- 23.Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Snydman DR, Allen U, Humar A. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795. doi: 10.1097/TP.0b013e3181cee42f. [DOI] [PubMed] [Google Scholar]
- 24.Razonable RR, Brown RA, Humar A, Covington E, Alecock E, Paya CV. Herpesvirus infections in solid organ transplant patients at high risk of primary cytomegalovirus disease. J Infect Dis. 2005;192:1331–1339. doi: 10.1086/466529. [DOI] [PubMed] [Google Scholar]
- 25.Hodson EM, Jones CA, Webster AC, Strippoli GF, Barclay PG, Kable K, Vimalachandra D, Craig JC. Antiviral medications to prevent cytomegalovirus disease and early death in recipients of solid-organ transplants: a systematic review of randomised controlled trials. Lancet. 2005;365:2105–2115. doi: 10.1016/S0140-6736(05)66553-1. [DOI] [PubMed] [Google Scholar]
- 26.Singh N. Late-onset cytomegalovirus disease as a significant complication in solid organ transplant recipients receiving antiviral prophylaxis: a call to heed the mounting evidence. Clin Infect Dis. 2005;40:704–708. doi: 10.1086/427506. [DOI] [PubMed] [Google Scholar]
- 27.Razonable RR, van Cruijsen H, Brown RA, Wilson JA, Harmsen WS, Wiesner RH, Smith TF, Paya CV. Dynamics of cytomegalovirus replication during preemptive therapy with oral ganciclovir. J Infect Dis. 2003;187:1801–1808. doi: 10.1086/375194. [DOI] [PubMed] [Google Scholar]
- 28.Singh N, Wannstedt C, Keyes L, Wagener MM, Cacciarelli TV. Who among cytomegalovirus-seropositive liver transplant recipients is at risk for cytomegalovirus infection? Liver Transpl. 2005;11:700–704. doi: 10.1002/lt.20417. [DOI] [PubMed] [Google Scholar]
- 29.Singh N Cytomegalovirus. Am J Transplant. 2004;4 Suppl 10:51–58. doi: 10.1111/j.1600-6135.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- 30.Preiksaitis JK, Brennan DC, Fishman J, Allen U. Canadian society of transplantation consensus workshop on cytomegalovirus management in solid organ transplantation final report. Am J Transplant. 2005;5:218–227. doi: 10.1111/j.1600-6143.2004.00692.x. [DOI] [PubMed] [Google Scholar]


