Abstract
AIM: To assess the cost-effectiveness of two population-based hepatocellular carcinoma (HCC) screening programs, two-stage biomarker-ultrasound method and mass screening using abdominal ultrasonography (AUS).
METHODS: In this study, we applied a Markov decision model with a societal perspective and a lifetime horizon for the general population-based cohorts in an area with high HCC incidence, such as Taiwan. The accuracy of biomarkers and ultrasonography was estimated from published meta-analyses. The costs of surveillance, diagnosis, and treatment were based on a combination of published literature, Medicare payments, and medical expenditure at the National Taiwan University Hospital. The main outcome measure was cost per life-year gained with a 3% annual discount rate.
RESULTS: The results show that the mass screening using AUS was associated with an incremental cost-effectiveness ratio of USD39825 per life-year gained, whereas two-stage screening was associated with an incremental cost-effectiveness ratio of USD49733 per life-year gained, as compared with no screening. Screening programs with an initial screening age of 50 years old and biennial screening interval were the most cost-effective. These findings were sensitive to the costs of screening tools and the specificity of biomarker screening.
CONCLUSION: Mass screening using AUS is more cost effective than two-stage biomarker-ultrasound screening. The most optimal strategy is an initial screening age at 50 years old with a 2-year inter-screening interval.
Keywords: Two-stage biomarker-ultrasound screening, One-stage abdominal ultrasonography screening, Markov model, Cost-effectiveness, Sensitivity analysis, Age
Core tip: Hepatocellular carcinoma (HCC) mortality could be reduced by early detection. Previous studies have investigated the cost-effectiveness of different surveillance intervals and screening modalities but were restricted to high risk populations. We conducted a cost-effectiveness analysis of mass screening for HCC with abdominal ultrasonography for the general population and compared it to the existing two-stage biomarker-ultrasound screening in an area with high HCC incidence. The findings suggest early detection of HCC with abdominal ultrasonography may be useful for the general population in an area with high HCC incidence not covered by hepatitis B vaccination.
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide[1]. In Taiwan, HCC is the leading cause of cancer death, accounting for more than 7000 deaths annually[2]. Previously, we demonstrated that increasing incidence rather than poor survival accounts for the rapid rise in mortality rate from HCC in Taiwan[3]. Because the major cause of HCC is related to hepatitis B infection in Taiwan[4], a nationwide vaccination program was launched in 1984, resulting in a significant reduction in the incidence of childhood HCC[5]. Nonetheless, adults older than 30 years old are not covered by the nationwide vaccination program, and the incidence of HCC has been increasing in this population. Since the seroprevalence of hepatitis B virus and hepatitis C virus is nearly 20% in our country[6], a large number of individuals are at increased risk of developing HCC. Hence, a population-based screening program is needed for the early detection of HCC to facilitate a favorable survival rate. In many countries, abdominal ultrasonography (AUS)-based imaging technique (with or without α-fetoprotein, AFP) is used to detect early stage HCC[7,8]. Several studies on mass screening for high risk individuals have also demonstrated that screening for HCC using AUS-based tools resulted in improved survival compared with the unscreened control[9,10].
The conventional method for HCC screening is to first identify high-risk individuals by using a constellation of biomarkers. These high-risk individuals are further referred to undergo AUS. In previous studies, population-based two-stage liver cancer screening program for high risk individuals has proven efficacious, with a reduction of mortality up to 41% after adjusting for independent risk factors[10]. On the other hand, AUS, with or without AFP, has been proposed to screen HCC in high HCC endemic countries[11-14]. There are pros and cons for both two-stage biomarker-ultrasound and one-stage AUS. The two-stage method is efficient, but the AUS referral is a barrier for high-risk subjects identified at the first stage. One-stage AUS may dispense with the referral issue but with increased cost and false positive rate. In addition, its relative costs and effectiveness, particularly the long-term outcome, have yet to be estimated.
The choice of either a two-stage method or one-stage AUS screening is of great interest to health policy-makers in high endemic HCC areas. The primary aim of this study is to compare the cost-effectiveness of the two above mentioned strategies. The optimal initial age and inter-screening interval are also investigated.
MATERIALS AND METHODS
Model design and structure
We developed a Markov decision model as the framework to evaluate the economics of two screening strategies for HCC prevention - two-stage biomarker-ultrasound method and mass screening using AUS - compared to no screening for a hypothetical cohort of 40-year-old residents in a high HCC incidence area, such as Taiwan.
Figure S1 shows a schematic representation of our four-state Markov model to represent the natural course of a hypothetical cohort with no screening. Parameters related to disease progress were based on Chen’s model[10]. The population was divided into non-cirrhotic and cirrhotic groups. We utilized a Markov cohort simulation method to follow this hypothetical cohort from 40 to 79 years old or until death, whichever came first. The time cycle for our Markov model was 1 year. The health states were defined to capture the characteristics of HCC with considerations of screening and treatments. We used TreeAge Software for model construction and Winbugs software for parameters synthesis. Parameters, such as sensitivity and specificity of AUS screening and mortality rate from cirrhosis, were estimated based on the literature. The decision tree of the different screening strategies for HCC is also shown (Figure S2).
Intervention strategies
The following three different strategies for HCC screening were compared: (1) No intervention. The cohort received no organized screening program for HCC. The patients sought medical intervention only when they had HCC symptoms or signs. This group was not our comparator but provided a reference group to oppose the other two interventions; (2) Two-stage biomarker-ultrasound screening. In the first stage, high risk individuals were identified using fasting blood samples to test for hepatitis B surface antigen (HBsAg), anti-hepatitis C virus (HCV) antibody, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and AFP. In the second stage, AUS was used to screen those with at least one of the following findings: positive HBsAg or anti-HCV antibody, AST ≥ 40 U/L, ALT ≥ 40 U/L, or AFP ≥ 20 ng/mL; and (3) Mass screening using AUS. All residents in this high endemic area received AUS for HCC screening.
For strategies 2 and 3, the inter-screening interval was 1 year.
Clinical surveillance
Only those in the high risk group who had been diagnosed as either HBsAg or anti-HCV positive in the prevalent screen were tested for the three other markers (AST, ALT, and AFP) in the subsequent screen. People who had received AUS and were diagnosed with cirrhosis received three monthly abdominal sonogram surveillance. Under AUS, subjects suspected of malignant nodular lesions were referred to receive further confirmatory diagnosis with liver biopsy and subsequent pathological assessment. The confirmed HCC patients were referred for oncological treatment. Otherwise, those false positive patients returned to a regular screening program.
Input of parameters
Disease progression and mortality rate: Table 1 reports the incidence rates, transitional rates, and mortality rates of HCC used in the model for our cohort. The age-specific incidence rates of HCC were extracted from the annual report of the Cancer Registry of Taiwan[2]. The data sources for parameters used in the natural history estimation are our previous population-based studies and large studies on untreated HCC[10,13,15]. Population-based mortality rates for cirrhosis and non-cirrhosis adjusted for age were obtained from published Taiwan Vital Statistics and meta-analyses[2,16-19].
Table 1.
Base-case estimates and ranges used in sensitivity analysis
| Variables | Base-case | Distribution of probabilistic sensitivity analysis | Ref. | ||
| Natural history and prognosis, per year | |||||
| Prevalence of cirrhosis | 1.77% | Β (1069, 59257) | [23] | ||
| Annual transition rates | |||||
| HCC incidence (1/yr) | NC | Cirrhosis | NC | Cirrhosis | |
| 30-39 yr | 0.00012 | 0.0024 | Gamma (0.06, 510) | Gamma (0.23, 96) | [2,10,23] |
| 40-49 yr | 0.00036 | 0.0070 | Gamma (0.33, 1190) | Gamma (1.2, 220) | |
| 50-59 yr | 0.00100 | 0.0200 | Gamma (3.07, 3614) | Gamma (10, 632) | |
| 60-69 yr | 0.00210 | 0.0410 | Gamma (19, 8928) | Gamma (67, 1640) | |
| 70-79 yr | 0.00430 | 0.0820 | Gamma (79, 18280) | Gamma (269, 3280) | |
| PHCC to CHCC (Non-cirrhosis) | 0.376 (0.157- 0.595) | Gamma (11.3, 30.1) | [10,15] | ||
| PHCC to CHCC (Cirrhosis ) | 0.637 (0.21-1.06) | Gamma (8.7, 13.6) | |||
| CHCC to HCC death | 1.05 (0.93-1.18) | Gamma (1.14, 1.09) | |||
| Age-specific mortality rate of cirrhosis | |||||
| 30-39 yr | 0.0046 | Gamma (0.2, 49.4) | [2,16-19] | ||
| 40-49 yr | 0.0086 | Gamma (0.8, 92.3) | |||
| 50-59 yr | 0.0170 | Gamma (3.1, 182.4) | |||
| 60-69 yr | 0.0380 | Gamma (15.5, 407.7) | |||
| 70-79 yr | 0.0980 | Gamma (103, 1051.5) | |||
| Survival rate of surveillance-detected PHCC (%) | 75 | Gamma (11.2, 15.8) | [15] | ||
| First-stage 5 markers screening characteristics | |||||
| Attendance rate (%) | 60 | Β (23654, 18733) | [9,13,24] | ||
| Sensitivity to Cirrhosis (%) | 80 | Β (62, 15) | [20] | ||
| Sensitivity to HCC | 95 | Β (50, 1) | [13] | ||
| Specificity to HCC | 70 | Β (9493, 4282) | [13] | ||
| Second-stage ultrasonography screening characteristics | |||||
| Compliance rate of ultrasonography (%) | 80 | Β (16394, 3212) | [9,13,24] | ||
| Sensitivity to cirrhosis (%) | 75 | Β (11, 3) | [21] | ||
| Sensitivity to HCC (%) | 83 | Β (48, 10) | [9,12,22] | ||
| Specificity to HCC (%) | 97 | Β (20137, 637) | [9,12,22] | ||
| Direct cost (USD) | |||||
| Biochemical test | |||||
| HBsAg | 4.7 | BNHI | |||
| HCVAb | 7.4 | BNHI | |||
| GOT | 1.5 | BNHI | |||
| GPT | 1.5 | BNHI | |||
| AFP | 5.9 | BNHI | |||
| Ultrasonography | 26 | BNHI | |||
| Confirmation (USD) | |||||
| Triple-phase abdominal CT | 148 | BNHI | |||
| Ultrasonic guidance for biopsy | 38.3 | BNHI | |||
| Liver puncture | 36 | BNHI | |||
| Specimen examinations of pathology | 51.2 | BNHI | |||
| Treatment (USD) | |||||
| Initial cost of HCC treatment | 4892 | Lognormal (8.28, 0.53) | NTUH | ||
| Continuing cost of HCC treatment | 4266 | Lognormal (8.18, 0.46) | NTUH | ||
| Incurable-cancer care (average) | 5691 | Lognormal (8.36, 0.81) | NTUH | ||
| Indirect cost (USD) | |||||
| Screening time (h) | 0.5 | [10,26] | |||
| Person accompanied for screening | 0 | [10,26] | |||
| Time spent for ultrasonography | 4 | [10,26] | |||
| Confirmation time (h) | 8 | NTUH, [26] | |||
| Person accompanied for confirmation | 1 | NTUH, [26] | |||
| Inpatient hospitalization (d) | 15 | NTUH | |||
| Inpatient recovered at home (d) | 15 | [26] | |||
| Person accompanied for inpatient care | 1.69 | [26] | |||
| Outpatient time per visit (h) | 4 | [26] | |||
| Outpatient visit per year | 9.7 | NTUH | |||
| Patient accompanied for outpatient visit | 0.77 | [26] | |||
| Inpatient of terminal care (d) | 30 | NTUH | |||
| Person accompanied for terminal care | 1 | [26] | |||
| Average work per month (h) | 184 | DGBAS | |||
| Production value per hour (USD) | 7.6 | DGBAS | |||
| Discount rate (%) | 3 | ||||
NC: Non-cirrhosis; HCC: Hepatocellular carcinoma; PHCC: Preclinical hepatocellular carcinoma; CHCC: Clinical hepatocellular carcinoma; CT: Computed tomography; GNP: Gross national product; BNHI: Bureau of the National Health Insurance; NTUH: National Taiwan University Hospital; DGBAS: Directorate General of Budget, Accounting and Statistics.
Test characteristics and prognosis of clinical practice: Parameters for test characteristics, such as sensitivity and specificity of biochemical examinations, in the first stage were derived from previous Taiwanese studies[13,20]. The sensitivity and specificity of AUS for both cirrhosis and HCC were extracted from two previous native studies and meta-analyses[9,12,21,22]. The prevalence of cirrhosis was derived from a two-stage screening program, the KCIS program[23]. The treatment outcomes of screening-detected HCC were estimated according to one large hospital-based cohort study in Taiwan[15]. The base-case estimate of 1 year survival was 75% for screening-detected HCC.
Attendance and compliance: A 60% attendance rate was assumed based on our previous experience with KCIS screening programs[24]. In addition, compliance rate and referral rate may vary with screening tools. Because AUS was a non-invasive screening tool, 80% of the referral rate was assumed based on our previous community-based studies[10,13] for individuals screened positive in the first-stage. The compliance rate of AUS was assumed to be 80%, which is comparable to the estimate of previous randomized trials[9,12,24].
Costs: The lifetime costs for HCC encompassed the initial costs (surgery, trans-catheter arterial chemoembolization, and chemotherapy), continuing costs (follow-up and treatment for recurrence), and the eventual cost of terminal care[25]. Costs for screening and confirmation were based on Medicare Payments by the Bureau of National Health Insurance in Taiwan. To acquire the data on costs of treatment, we reviewed the records of HCC patients under treatment in the National Taiwan University Hospital (NTUH). Indirect costs were calculated according to data from our index hospital and previous studies[26,27]. All future costs and life-years were discounted to the present value at an annual rate of 3%. Base-case values and ranges used in the sensitivity analyses are summarized in Table 1.
Model assumptions
Several model assumptions were stated, as follows: (1) HCC incidence varied by age. The age-specific incidence rate of HCC was based on the Cancer Registry of Taiwan. The probability of HCC progression and survival was constant over time; (2) Clinically-detected HCCs often had large tumors and would be treated with palliative measures only, where the prognosis was poor. The survival rate of clinically-detected HCC was not dependent on the presence of liver cirrhosis; (3) False positive cases after referral confirmatory examination would undergo the ultrasonography surveillance at 3-mo intervals for 6 mo and would be resumed to the original screening strategy if negative screening results were found during surveillance; (4) The effect of antiviral therapy on cirrhosis progression and incidence of HCC was not modeled due to the uncertainty of its long-term effects; (5) Liver transplantation for treating HCC was not considered in the model due to the shortage of organ donations and the long waiting time in Taiwan; and (6) Based on the concept of the prevalence pool, the equilibrium state between cirrhosis and non-cirrhosis was stable. Hence, we did not model the transition between non-cirrhotic state and cirrhotic state. We believe this assumption had little influence on the outcome of the HCC estimation.
Cost-effectiveness analysis
We conducted analysis from a societal perspective. The effectiveness of any given screening program was evaluated by the life-year gained after converting mortality reduction as a result of each intervention. Costs are expressed in United States dollars (USD). The direct costs were associated with the screening itself, confirmatory tests, and treatment. The indirect costs were mainly derived from the loss of productivity. A 3% discount rate was used to convert future cost to present value. The results of comparisons between different screening strategies are presented by the incremental cost-effectiveness ratio (ICER). The ceiling ratio of ICER, the maximum amount of willingness to pay (WTP) per life-year saved, was set at the level of USD33000, approximately equivalent to two times per capita the gross national product (GNP) in Taiwan[28].
Sensitivity analyses
We performed one-way and probabilistic sensitivity analyses by varying key model parameters within a specified range in order to compare the main strategy and the reference strategy. Different initial ages and screening intervals were also compared by a series of acceptability curves and ICE scatter plots based on repeated Monte Carlo simulations. The statistical review of this study was performed by a biomedical statistician.
RESULTS
Cost analyses
In total, 158 HCC cases were sampled from the HCC cohort in our index hospital for cost estimation of initial and continuing care. According to the AJCC staging system of HCC, the numbers of patients with stage I to IV tumors were 62 (39%), 48 (30%), 33 (21%), and 15 (10%), respectively. After excluding subjects (n = 42) receiving either palliative treatments or no treatment, the primary modalities for HCC management in our sample were trans-arterial chemoembolization and hepatic resection, which accounted for up to 83% (96/116). The average costs of initial and continuing care were USD4892 (95%CI: USD3359-5936) and USD4266 (95%CI: USD3072-4685) for each individual patient. The average cost of terminal care, based on 93 patients who died within the enrolled year in our index hospital with a mean follow-up period of 4.2 mo, was USD5691 (95%CI: USD4327-7055). The detailed costs and their ranges used for model estimation are listed in Table 1.
Base-case analyses
The ICERs for screening strategies as compared to “No intervention” are listed in Table 2. Both screening strategies yielded more life-year gain and increased total costs compared with no intervention. The ICERs for two-stage screening and AUS screening were USD49733 and USD39825 per life-year gained, respectively. AUS screening was better than the two-stage method.
Table 2.
Simulated results for screening strategies to prevent hepatocellular carcinoma
| Outcome | No intervention | Two-stage screening | Mass screening using ultrasonography |
| Cost per individual screened, USD | 2755 | 3389 | 3359 |
| Life-year gain1 (yr) | 20.4798 | 20.4926 | 20.4950 |
| Comparing with ICER | |||
| No screening as reference | - | 49733 | 39825 |
| Two-stage screening as reference | - | - | Dominant2 |
Screening starting age was 40 years old, screening interval was 1 year;
More effective and less costly than reference strategy. ICER: Incremental cost-effectiveness ratio.
Sensitivity analyses
Results from the one-way sensitivity analyses are summarized to compare the two-stage method and AUS screening (Table S1). The results demonstrated that AUS screening was far more superior to the two-stage method. The superiority of AUS screening was sensitive to the specificity of the biochemical screening and the costs of biochemical screening and AUS. If the cost of biochemical screening was less than USD9.9 or the cost of AUS was greater than USD44.1, the two-stage method was better. Moreover, two-stage screening became more cost-effective if the specificity of biochemical screening was larger than 90%. When such parameters as sensitivity of AUS, cirrhosis prevalence, attendance rate of screening programs, and compliance rate for ultrasonography were varied within a reasonable range, their influence on the superiority of AUS screening was trivial.
In the probabilistic sensitivity test, with a maximum WTP of USD33000, AUS screening had an approximate 15% likelihood of being cost-effective. If the amount of WTP was raised to USD41000 or higher, the probability of AUS screening being cost-effective was over 50% (Figure 1).
Figure 1.
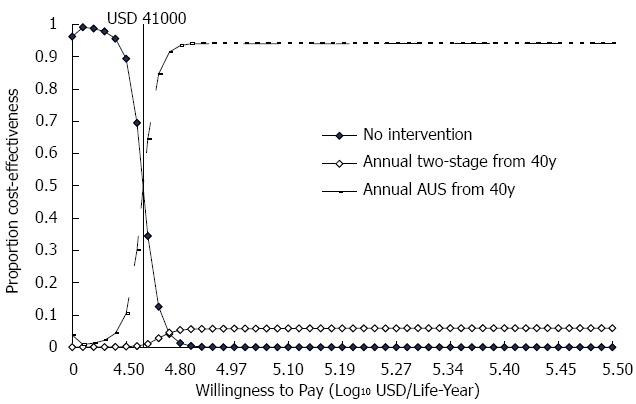
Results of sensitivity analysis: Cost-effectiveness acceptability curves.
Optimal initial screening age
The cost-effectiveness analyses at different initial ages of both screening programs at a given annual screening interval are shown in Figure 2A. The slope of the efficacy frontier showed the optimal ICER among different screening strategies. Other strategies internal to the efficacy frontier were less cost-effective based on the rules of extended dominance. AUS screening was more cost-effective than the two-stage method at any initial age. The most cost-effective strategy by using probabilistic sensitivity analysis was AUS screening with an initiated screening age of 50 years old (Figure S3A).
Figure 2.
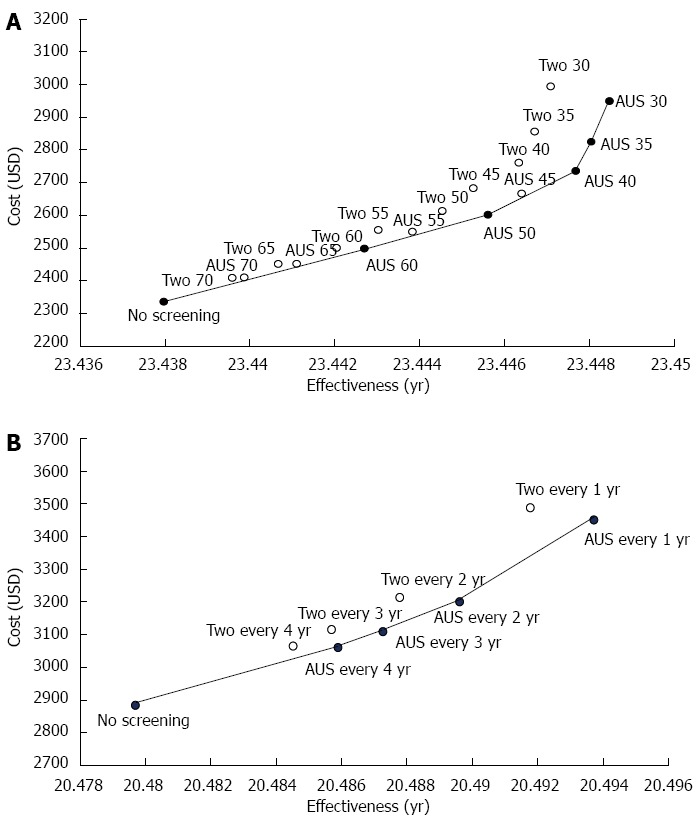
Cost-effectiveness of hepatocellular carcinoma screening with selected initial ages and selected screening intervals. The reference strategies were non-screening programs at the index initial ages. A: Strategies are labeled by the type and initial ages of screening; B: Strategies are labeled by the type and frequency of screening. AUS: Abdominal ultrasonography mass screening; Two: Two-stage biomarker-ultrasound screening.
Inter-screening intervals
The efficacy frontier consisted of a combination of AUS screening with different screening intervals and no screening at a given initial screening age of 40 years (Figure 2B). The cost-effectiveness of both screening strategies with different inter-screening intervals was also evaluated by using 10000 replications from Monte Carlo simulation considering the acceptability curve (Figure S3B). The two-stage screening strategy was less cost-effective than AUS screening at all inter-screening intervals. The most favorable strategy was biennial AUS screening, followed by annual AUS screening.
Cost-effectiveness plane for ultrasonography screening
Because AUS screening has been shown to be superior based on its cost-effectiveness, we further compared different combinations of optimal and suboptimal inter-screening intervals and initial ages for AUS screening. Figure 3A-D illustrates the simulated results of 5000 ICER replicates plotted on a cost-effectiveness plane given the maximum amount of WTP per life-year saved (ceiling ratio) at the level of USD33000. If the ICER lies below the ceiling ratio, the strategy should be implemented. Compared to no screening, the probability of being cost-effective among the different strategies (i.e., annual screening from 40 years, annual screening from 50 years, biennial screening from 40 years, and biennial screening from 50 years) was 15%, 45%, 55%, and 73%, respectively.
Figure 3.
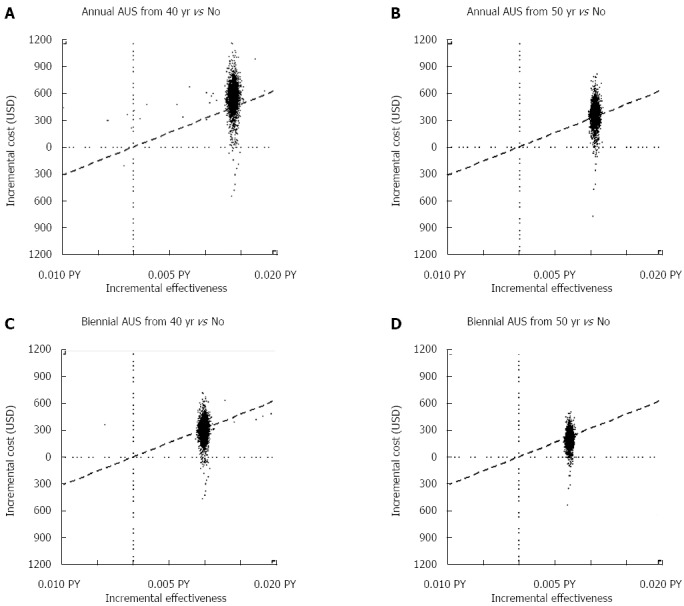
Cost-effectiveness plane for different combinations of optimal, suboptimal initial ages and inter-screening intervals of ultrasonography screening. The slope of the dashed line represents the ceiling ratio. A: Annual screening from 40 years vs no screening; B: Annual screening from 50 years vs no screening; C: Biennial screening from 40 years vs no screening; D: Biennial screening from 50 years vs no screening.
Model validation
The predicted age-specific incidence rate of HCC per 100000 individuals from our model was compared to the empirical data from the Cancer Registry in Taiwan (2007). The empirical figures were as follows: 40-44 years, 23; 45-49 years, 40; 50-54 years, 64; 55-59 years, 99; 60-64 years,146; 65-69 years, 197; 70-74 years, 232; and 75-79 years, 229. The predictive figures were as follows: 40-44 years, 30; 45-49 years, 41; 50-54 years, 81; 55-59 years, 102; 60-64 years,155; 65-69 years, 176; 70-74 years, 237; and 75-79 years, 233. There was no significant statistical difference as tested by χ2 test (χ2 (7) = 8.97, P = 0.26), indicating fair model fitting.
DISCUSSION
Our study is the first to confirm the superiority of AUS screening for HCC (in terms of cost-effectiveness) compared to the conventional two-stage method in a hepatitis endemic area. The results showed that AUS screening was associated with an incremental cost-effectiveness ratio of USD39825 per life-year gained, whereas the two-stage method was associated with an incremental cost-effectiveness ratio of USD49733 per life-year gained, as compared with non-screening. If taking the maximum amount of WTP per life-year saved in our country into account (USD33000), neither AUS screening nor two-stage screening is more cost-effective than non-screening. However, the absolute cost-effectiveness still varies because the cost of management of HCC in different countries may vary, and the decision to implement any screening program also depends on the resources of any given country.
Both the deterministic and probabilistic modeling approaches revealed that AUS screening is more cost-effective than two-stage screening. Several factors accounted for such results. First, the difference of cost between AUS (USD27.6) and first stage biochemical tests (USD22.2) is relatively low in Taiwan. Two-stage screening became a better strategy if the costs of AUS were more expensive than USD44.1 or if the cost of the biochemical test was lowered to USD9.9. Second, the specificity of the five biochemical makers for HCC screening is low. Two-stage screening is superior only when the specificity of biochemical screening is greater than 90%. A low platelet value has recently been reported as a surrogate for cirrhosis[29]. It is necessary to further assess the optimal utility ratio based on the comparisons between different combinations of biochemical markers. However, at present, there is still no biomarker with relative good sensitivity and specificity for HCC surveillance[7,30,31].
Costs for initial, continuing, and terminal phases of care reported in previous cost-effectiveness studies of cancer care are summarized[25]. The average costs of initial care, continuing care, and terminal care are USD4892, USD4266, and USD5691, respectively. Initial care and terminal care costs are higher than continuing care costs for the treatment of HCC. These results are compatible with a previous study[27]. It should also be noted that our costs are based on those at a medical center, NTUH. As the leading hospital in Taiwan with a 118-year history, this hospital serves patients and accepts referrals evenly distributed from every part of Taiwan. Therefore, the patients of NTUH could represent all HCC patients in Taiwan without substantial bias, with perhaps a slight skew to severe cases. Costs estimated at community hospitals may be somewhat lower. Besides, it is difficult to distinguish asymptomatic and symptomatic cases by retrospective chart reviewing. We assume the same costs for initial treatment for clinically-detected and screen-detected cases. A sensitivity test with a wide range for cost of initial treatment between clinically- and screen-detected tumors did not change the superiority between screening strategies.
As far as the validity of the simulated model is concerned, there is evidence supporting our results. Firstly, the estimated parameters of natural history and variables were largely generated from two previous studies based on the same community cohort in Taiwan[10,13]. The heterogeneity among different studies could be overcome. Secondly, the predicted age-specific incidence rate of HCC was close to the observed one (χ2 (7) = 8.97, P = 0.26). Thirdly, by taking different time horizons into consideration, AUS screening was still more cost-effective than two-stage screening. This means that changes in time horizons had little effect on our results.
Our study was different from several previous decision analyses studies regarding HCC screening[32-37], probably due to the following reasons. Firstly, our study included a four-state ‘micro-simulation’ model and community-based screening in Taiwan, a viral hepatitis endemic area. Previous studies were restricted to high risk populations, such as cirrhotic patients or patients waiting for liver transplantation. Secondly, the rates of incidence of HCC increased with age. We have used age-specific HCC incidence rates based on the Cancer Registry of Taiwan. The study by Arguedas et al[36] evaluated the incremental cost-effectiveness of no screening versus AUS and AFP every year in patients with cirrhosis. The results of base-case analyses were USD22500 per life year saved. Another study by Shih et al[37] from Taiwan that compared no screening and two-stage screening in patients with either hepatitis B or hepatitis C, reported an ICER of USD15600 per life year saved. Their ICERs were lower than our estimate. The difference was more likely attributed to the fact that we included indirect costs and our screening subjects were the general population rather than high risk individuals.
Taken together, our data support AUS screening for the general population in high HCC endemic areas. However, the success of this screening largely depends on a sufficiently well-trained staff to perform AUS. In some countries without this staff, it may be feasible to develop a risk-scoring system with subsequent referral for AUS[38]. On the other hand, in high-risk populations, such as those with advanced cirrhosis, where the detection rate for HCC by AUS is low, use of AUS in combination with biomarkers would be valuable.
There are some limitations in our model. Recently, the incidence of HCC was shown to be reduced with antiviral therapies[39]. However, we cannot model antiviral treatment effects on the natural history of HCC because the data on reversibility of advanced liver disease are not well established. On the other hand, liver transplantation is the optimal treatment for HCC because it simultaneously removes the tumor and underlying cirrhosis, thus reducing the risk of HCC recurrence[40]. This therapeutic option was excluded due to the shortage of organ resources in Taiwan and because decision making for liver transplantation was not based only on medical concerns.
In conclusion, AUS mass screening is more cost-effective than the two-stage method. Its relative cost-effectiveness may vary depending on the cost of the screening tools and the specificity of the biochemical test. We found that screening programs with initial screening at 50 years of age and subsequent biennial screening intervals were the optimal strategy.
ACKNOWLEDGMENTS
We appreciate Ms Sou-Shang Yang from National Taiwan University Hospital, Taipei, Taiwan for her kind help in data collection of HCC management cost.
COMMENTS
Background
Two-stage biomarker-ultrasound method and mass screening using abdominal ultrasonography (AUS) have been proposed for the early detection of hepatocellular carcinoma (HCC). The cost-effectiveness of these two HCC screening strategies remains unclear, particularly regarding aspects such as the optimal initial age and inter-screening interval.
Research frontiers
This study contributes significantly to understanding the cost-effectiveness of mass screening the general population for HCC with AUS compared with the existing two-stage biomarker-ultrasound screening strategy in an area with high HCC incidence.
Innovations and breakthroughs
Mass screening using ultrasonography is more cost-effective than two-stage biomarker-ultrasound screening. The costs of screening tools and the specificity of biomarker screening play an important role in the relative cost-effectiveness of screening strategies.
Applications
Early detection of HCC with abdominal ultrasonography may be suggested for the general population in areas with a high incidence of HCC that had not been covered by hepatitis B vaccination. Optimal age to begin screening and inter-screening interval of HCC could be determined in clinical decision making for early diagnosis of HCC
Peer-review
It is a well written manuscript assessing the cost-effectiveness of two kinds of HCC screening programs.
Footnotes
Supported by Kaohsiung Municipal Min-Seng Hospital (KMSH 9702).
Conflict-of-interest statement: The authors had no conflict of interest to declare.
Data sharing statement: No further data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 3, 2015
First decision: September 9, 2015
Article in press: December 21, 2015
P- Reviewer: Tomizawa M S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Zhang DN
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Cancer statistics. Taiwan Cancer Registry. Assessed 2010-05-20. Available from: http://crs.cph.ntu.edu.tw/main.php.
- 3.Jan CF, Chen CJ, Chen HH. Causes of increased mortality from hepatocellular carcinoma in high incidence country: Taiwan experience. J Gastroenterol Hepatol. 2005;20:521–526. doi: 10.1111/j.1440-1746.2005.03602.x. [DOI] [PubMed] [Google Scholar]
- 4.Beasley RP, Hwang LY, Lin CC, Chien CS. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22 707 men in Taiwan. Lancet. 1981;2:1129–1133. doi: 10.1016/s0140-6736(81)90585-7. [DOI] [PubMed] [Google Scholar]
- 5.Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855–1859. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 6.Chen CH, Yang PM, Huang GT, Lee HS, Sung JL, Sheu JC. Estimation of seroprevalence of hepatitis B virus and hepatitis C virus in Taiwan from a large-scale survey of free hepatitis screening participants. J Formos Med Assoc. 2007;106:148–155. doi: 10.1016/S0929-6646(09)60231-X. [DOI] [PubMed] [Google Scholar]
- 7.Daniele B, Bencivenga A, Megna AS, Tinessa V. Alpha-fetoprotein and ultrasonography screening for hepatocellular carcinoma. Gastroenterology. 2004;127:S108–S112. doi: 10.1053/j.gastro.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 8.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–422. doi: 10.1007/s00432-004-0552-0. [DOI] [PubMed] [Google Scholar]
- 9.Wun YT, Dickinson JA. Alpha-fetoprotein and/or liver ultrasonography for liver cancer screening in patients with chronic hepatitis B. Cochrane Database Syst Rev. 2003;(2):CD002799. doi: 10.1002/14651858.CD002799. [DOI] [PubMed] [Google Scholar]
- 10.Chen TH, Chen CJ, Yen MF, Lu SN, Sun CA, Huang GT, Yang PM, Lee HS, Duffy SW. Ultrasound screening and risk factors for death from hepatocellular carcinoma in a high risk group in Taiwan. Int J Cancer. 2002;98:257–261. doi: 10.1002/ijc.10122. [DOI] [PubMed] [Google Scholar]
- 11.Mima S, Sekiya C, Kanagawa H, Kohyama H, Gotoh K, Mizuo H, Ijiri M, Tanabe T, Maeda N, Okuda K. Mass screening for hepatocellular carcinoma: experience in Hokkaido, Japan. J Gastroenterol Hepatol. 1994;9:361–365. doi: 10.1111/j.1440-1746.1994.tb01256.x. [DOI] [PubMed] [Google Scholar]
- 12.Sherman M, Peltekian KM, Lee C. Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: incidence and prevalence of hepatocellular carcinoma in a North American urban population. Hepatology. 1995;22:432–438. [PubMed] [Google Scholar]
- 13.Chen CJ, Lu SN, You SL, Wu MH, Wang LY, Lee LT, Huang GT, Yang PM, Lee HS. [Community-based hepatocellular carcinoma screening in seven townships in Taiwan] J Formos Med Assoc. 1995;94 Suppl 2:S94–S102. [PubMed] [Google Scholar]
- 14.Zhang B, Yang B. Combined alpha fetoprotein testing and ultrasonography as a screening test for primary liver cancer. J Med Screen. 1999;6:108–110. doi: 10.1136/jms.6.2.108. [DOI] [PubMed] [Google Scholar]
- 15.Yu EW, Chie WC, Chen TH. Does screening or surveillance for primary hepatocellular carcinoma with ultrasonography improve the prognosis of patients? Cancer J. 2004;10:317–325. doi: 10.1097/00130404-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61:1942–1956. doi: 10.1002/1097-0142(19880515)61:10<1942::aid-cncr2820611003>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 17.Liaw YF, Lin DY, Chen TJ, Chu CM. Natural course after the development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Liver. 1989;9:235–241. doi: 10.1111/j.1600-0676.1989.tb00405.x. [DOI] [PubMed] [Google Scholar]
- 18.Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, Nevens F, Solinas A, Mura D, Brouwer JT, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463–472. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 19.Serfaty L, Aumaître H, Chazouillères O, Bonnand AM, Rosmorduc O, Poupon RE, Poupon R. Determinants of outcome of compensated hepatitis C virus-related cirrhosis. Hepatology. 1998;27:1435–1440. doi: 10.1002/hep.510270535. [DOI] [PubMed] [Google Scholar]
- 20.Kuo MJ, Yeh HZ, Chen GH, Poon SK, Yang SS, Lien HC, Chang CS. Improvement of tissue-adhesive obliteration of bleeding gastric varices using adjuvant hypertonic glucose injection: a prospective randomized trial. Endoscopy. 2007;39:487–491. doi: 10.1055/s-2007-966267. [DOI] [PubMed] [Google Scholar]
- 21.Lin DY, Sheen IS, Chiu CT, Lin SM, Kuo YC, Liaw YF. Ultrasonographic changes of early liver cirrhosis in chronic hepatitis B: a longitudinal study. J Clin Ultrasound. 1993;21:303–308. doi: 10.1002/jcu.1870210502. [DOI] [PubMed] [Google Scholar]
- 22.Yang B, Zhang B, Tang Z. [Randomized controlled prospective study of secondary prevention for primary liver cancer] Zhonghua Yi Xue Zazhi. 1999;79:887–889. [PubMed] [Google Scholar]
- 23.Wu GH, Boucher BJ, Chiu YH, Liao CS, Chen TH. Impact of chewing betel-nut (Areca catechu) on liver cirrhosis and hepatocellular carcinoma: a population-based study from an area with a high prevalence of hepatitis B and C infections. Public Health Nutr. 2009;12:129–135. doi: 10.1017/S1368980008002073. [DOI] [PubMed] [Google Scholar]
- 24.Chen TH, Chiu YH, Luh DL, Yen MF, Wu HM, Chen LS, Tung TH, Huang CC, Chan CC, Shiu MN, et al. Community-based multiple screening model: design, implementation, and analysis of 42,387 participants. Cancer. 2004;100:1734–1743. doi: 10.1002/cncr.20171. [DOI] [PubMed] [Google Scholar]
- 25.Taplin SH, Barlow W, Urban N, Mandelson MT, Timlin DJ, Ichikawa L, Nefcy P. Stage, age, comorbidity, and direct costs of colon, prostate, and breast cancer care. J Natl Cancer Inst. 1995;87:417–426. doi: 10.1093/jnci/87.6.417. [DOI] [PubMed] [Google Scholar]
- 26.Wu CL, Yang MC. Morbidity Costs and Associated Factors of Patients with Hepatocellular Carcinoma from a Medical Center. Chin J Pub Health. 1998:148–157. [Google Scholar]
- 27.Yang SS, Tang TS, Hong WH. Medical Cost Analysis on Patients with Hepatocellular Carcinoma. Taipei: Medical Center Taipei Medical University; 2003. p. 67. [Google Scholar]
- 28.Eichler HG, Kong SX, Gerth WC, Mavros P, Jönsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health. 2004;7:518–528. doi: 10.1111/j.1524-4733.2004.75003.x. [DOI] [PubMed] [Google Scholar]
- 29.Lu SN, Wang JH, Liu SL, Hung CH, Chen CH, Tung HD, Chen TM, Huang WS, Lee CM, Chen CC, et al. Thrombocytopenia as a surrogate for cirrhosis and a marker for the identification of patients at high-risk for hepatocellular carcinoma. Cancer. 2006;107:2212–2222. doi: 10.1002/cncr.22242. [DOI] [PubMed] [Google Scholar]
- 30.Rockey DC, Bissell DM. Noninvasive measures of liver fibrosis. Hepatology. 2006;43:S113–S120. doi: 10.1002/hep.21046. [DOI] [PubMed] [Google Scholar]
- 31.Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069–1075. doi: 10.1016/S0140-6736(00)04258-6. [DOI] [PubMed] [Google Scholar]
- 32.Bolondi L, Sofia S, Siringo S, Gaiani S, Casali A, Zironi G, Piscaglia F, Gramantieri L, Zanetti M, Sherman M. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut. 2001;48:251–259. doi: 10.1136/gut.48.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarasin FP, Giostra E, Hadengue A. Cost-effectiveness of screening for detection of small hepatocellular carcinoma in western patients with Child-Pugh class A cirrhosis. Am J Med. 1996;101:422–434. doi: 10.1016/S0002-9343(96)00197-0. [DOI] [PubMed] [Google Scholar]
- 34.Saab S, Ly D, Nieto J, Kanwal F, Lu D, Raman S, Amado R, Nuesse B, Durazo F, Han S, et al. Hepatocellular carcinoma screening in patients waiting for liver transplantation: a decision analytic model. Liver Transpl. 2003;9:672–681. doi: 10.1053/jlts.2003.50120. [DOI] [PubMed] [Google Scholar]
- 35.Lin OS, Keeffe EB, Sanders GD, Owens DK. Cost-effectiveness of screening for hepatocellular carcinoma in patients with cirrhosis due to chronic hepatitis C. Aliment Pharmacol Ther. 2004;19:1159–1172. doi: 10.1111/j.1365-2036.2004.01963.x. [DOI] [PubMed] [Google Scholar]
- 36.Arguedas MR, Chen VK, Eloubeidi MA, Fallon MB. Screening for hepatocellular carcinoma in patients with hepatitis C cirrhosis: a cost-utility analysis. Am J Gastroenterol. 2003;98:679–690. doi: 10.1111/j.1572-0241.2003.07327.x. [DOI] [PubMed] [Google Scholar]
- 37.Shih ST, Crowley S, Sheu JC. Cost-effectiveness analysis of a two-stage screening intervention for hepatocellular carcinoma in Taiwan. J Formos Med Assoc. 2010;109:39–55. doi: 10.1016/s0929-6646(10)60020-4. [DOI] [PubMed] [Google Scholar]
- 38.Yeh YP, Hu TH, Cho PY, Chen HH, Yen AM, Chen SL, Chiu SY, Fann JC, Su WW, Fang YJ, et al. Evaluation of abdominal ultrasonography mass screening for hepatocellular carcinoma in Taiwan. Hepatology. 2014;59:1840–1849. doi: 10.1002/hep.26703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 40.El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]


