Abstract
Shifts in the composition of the commensal microbiota are emerging as a hallmark of gastrointestinal inflammation. In particular, outgrowth of γ-proteobacteria has been linked to the etiology of inflammatory bowel disease and the pathologic consequences of infections. Here we show that, following gastrointestinal infection, control of commensal outgrowth is a highly coordinated process involving both the host response and microbial signals. Notably, neutrophil emigration to the lumen results in the generation of organized intra-luminal structures that encapsulate commensals and limit their contact with the epithelium. Formation of these luminal casts depends upon the high-affinity N-formyl peptide receptor, Fpr1. Consequently, after infection, mice deficient in Fpr1 display increased microbial translocation, poor commensal containment and increased mortality. Altogether, our present study describes a novel mechanism by which the host rapidly contains outgrowth of commensal pathobionts during infection. Further, these results reveal Fpr1 as a major mediator of host commensal interaction during dysbiosis.
Introduction
All barrier surfaces are covered by a diverse and abundant microbiota, with the human intestine harboring 1015 microbes, composed of an estimated 4000 individual strains (Eckburg et al., 2005; Ley et al., 2006). A central strategy utilized by the mucosal immune system to maintain its homeostatic relationship with the microbiota is to minimize contact between luminal microorganisms and the epithelial cell surface. This is accomplished by establishment of a structural and immunological barrier, referred to as the mucosal firewall, resulting from the combined action of mucus, IgA, and antimicrobial proteins (Hooper et al., 2012). Under steady state conditions, commensals can promote their own containment by enhancing various aspects of this physical and immunological barrier (Brandl et al., 2008; Vaishnava et al., 2011).
The gastrointestinal (GI) tract represents one of the primary sites of exposure to pathogens. In this highly reactive environment, infections can threaten the homeostatic relationship with the flora. Acute mucosal infections are also characterized by significant shifts in the microbiota, a phenomenon known as dysbiosis. GI infections can also promote expansion of commensals with inflammatory potential, referred to as pathobionts, that can directly exacerbate the pathological process (Egan et al., 2011; Heimesaat et al., 2006; Lupp et al., 2007; Stecher et al., 2007). Paneth cell death and enhanced nitrate levels have both been proposed as mechanisms underlying expansion of proteobacteria during inflammation (Raetz et al., 2012; Winter et al., 2013). In particular, γ-proteobacteria dominance has emerged as a hallmark of acute mucosal infections and enhanced pathology (Egan et al., 2011; Heimesaat et al., 2006; Lupp et al., 2007; Stecher et al., 2007). One of the first examples of this scenario was demonstrated in an oral model of Toxoplasma gondii infection in which γ-proteobacteria-mediated pathology is associated with exuberant sensing of commensals (Benson et al., 2009; Craven et al., 2012; Heimesaat et al., 2006; Raetz et al., 2012). As such, this model provides a powerful tool to examine the relationship between the expansion of γ-proteobacteria and the etiology of inflammatory disease.
The volatile nature and pathogenic potential of commensal populations during an inflammatory response poses a significant challenge for the host, specifically maintaining or restoring microbial diversity and spatial segregation with the microbiota. Indeed, sustained bacterial contact with the epithelium, as well as shifts in microbiota composition or sensing, have been associated with inflammatory bowel disease (IBD), a condition characterized by chronic inflammation of the intestinal tract (Abraham and Cho, 2009). Furthermore, impaired bacterial recognition as well as alterations in the intestinal microbiota have been associated with GI inflammation (Hugot et al., 2001; Man et al., 2011; Mann and Saeed, 2012; Sokol et al., 2009; Willing et al., 2009). Notably, increased levels of proteobacteria, specifically Escherichia coli, Campylobacter concisus and enterohepatic Helicobacter, have all been associated with the pathogenesis of IBD (Darfeuille-Michaud, 2002; Darfeuille-Michaud et al., 2004; Liu et al., 1995; Lodes et al., 2004; Mukhopadhya et al., 2012; Swidsinski et al., 2005). In further support of a link between proteobacteria and intestinal pathology, inability to control outgrowth of this group of bacteria in TLR-5 deficient mice leads to mucosal inflammation (Carvalho et al., 2012). Additionally, chronic mucosal inflammation can induce the expansion of defined strains of E. coli that directly promote invasive carcinoma in genetically susceptible hosts (Arthur et al., 2012). Under inflammatory settings, in order to prevent these pathologic outcomes, the host must restore microbial diversity, contain the outgrowth of commensal pathobionts and limit bacterial contact with the epithelium. How such a complex task is accomplished remains unknown.
To discover immune mechanisms that restrict host-bacteria contact and outgrowth during inflammation, we utilized a model of acute mucosal T. gondii infection. Our results revealed a novel and highly coordinated mechanism of commensal containment during inflammation characterized by the generation of intra-luminal casts. This mechanism, directly triggered by γ-proteobacteria and mediated by the N-formyl peptide chemoattractant receptor Fpr1, allows for the rapid encapsulation of bacterial outgrowth, restores the required segregation between the microbiota and epithelium and limits microbial translocation.
Results
Transient outgrowth of γ-proteobacteria during T. gondii infection
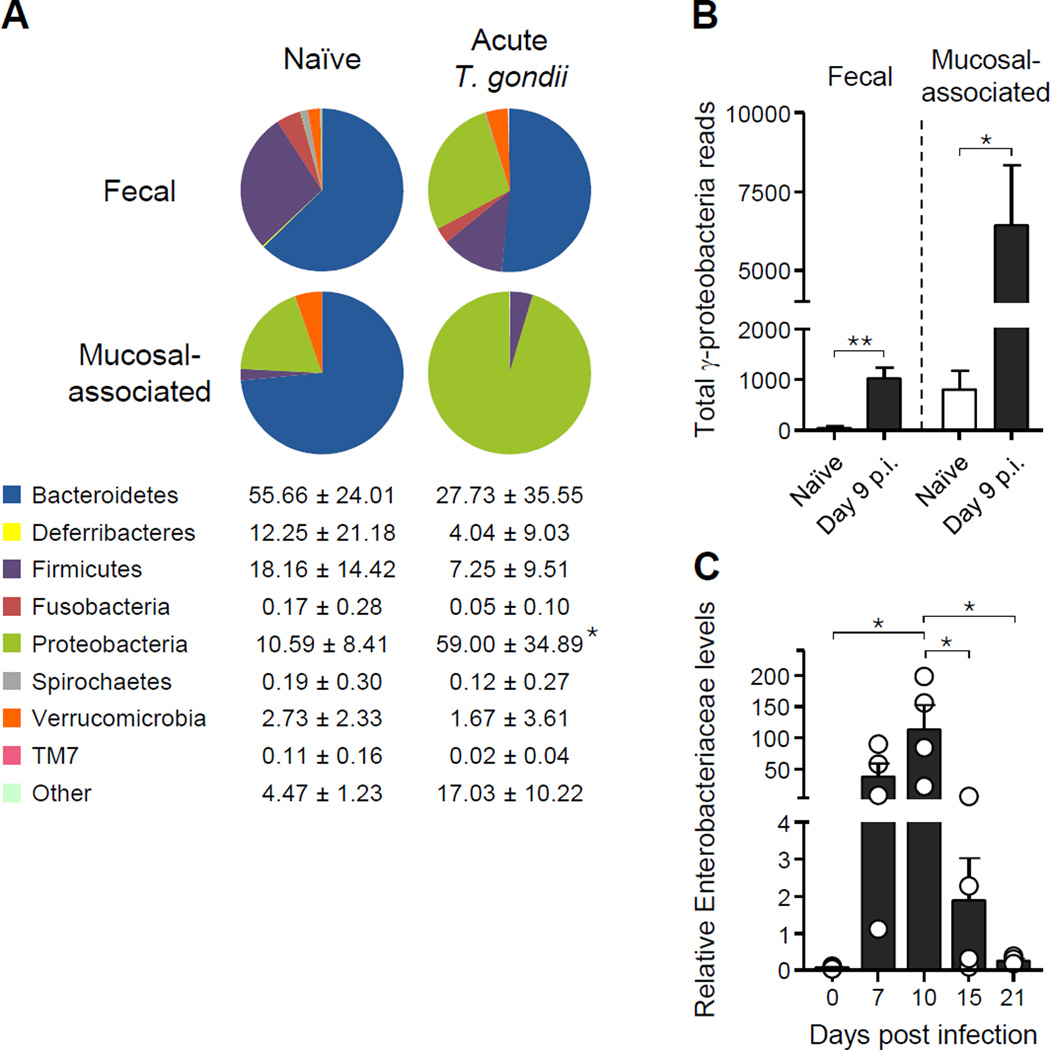
T. gondii infection induces a dramatic shift in the small intestine microbiota with increased bacterial content, reduced species diversity and increase in adherent and invasive E. coli (Heimesaat et al., 2006)(Craven et al., 2012). Expanding upon this observation, metagenomic analysis of the feces and small intestine mucosa-associated bacteria from T. gondii-infected mice revealed a dramatic outgrowth of γ-proteobacteria and more specifically bacteria of the family Enterobacteriaceae (Figures 1A–C and S1A). Further analysis of microbiota composition revealed significant reductions in Firmicutes in particular the class of Clostridia, which have previously been implicated as potent inducers of regulatory T cells (Atarashi et al., 2011) (Figures 1A and S1B). Concomitant with the dysbiosis, T. gondii infection leads to the translocation of aerobic bacteria, in particular the Enterobacteriaceae family members E. coli and Proteus mirabilis, to peripheral organs by day 8 post infection (p.i.) (Hand et al., 2012). Remarkably outgrowth of pathogenic commensals was rapidly contained and restored to the preinfection levels by day 15 p.i. (Figure 1C), suggesting that mechanisms are in place to rapidly restore the host’s homeostatic relationship with the flora.
Figure 1. γ-proteobacteria outgrowth is rapidly contained during T. gondii infection.
Mice were orally infected with 15 T. gondii cysts. (A) Fecal and small intestine mucosa-associated bacteria were isolated from naïve and day 9 p.i. mice. Pie charts show the percent of total 16S reads identified with each phylum and are representative examples of 3–5 mice. Columns below indicate the mean percent ± SD of reads from each phylum in mucosa-associated samples from naive (left) and infected (right) mice. (B) Total 16S reads identified as γ-proteobacteria in feces and mucosa-associated compartment from (A). (C) Enterobacteriaceae-specific semi-quantitative PCR from fecal DNA at different timepoints after infection. Open circles represent individual mice and each bar represents the mean ± SEM. All data shown are representative of two independent experiments. *P<0.05, **P<0.01. See also Figure S1.
Intra-luminal cellular casts limit commensal contact with the epithelium and control bacterial translocation
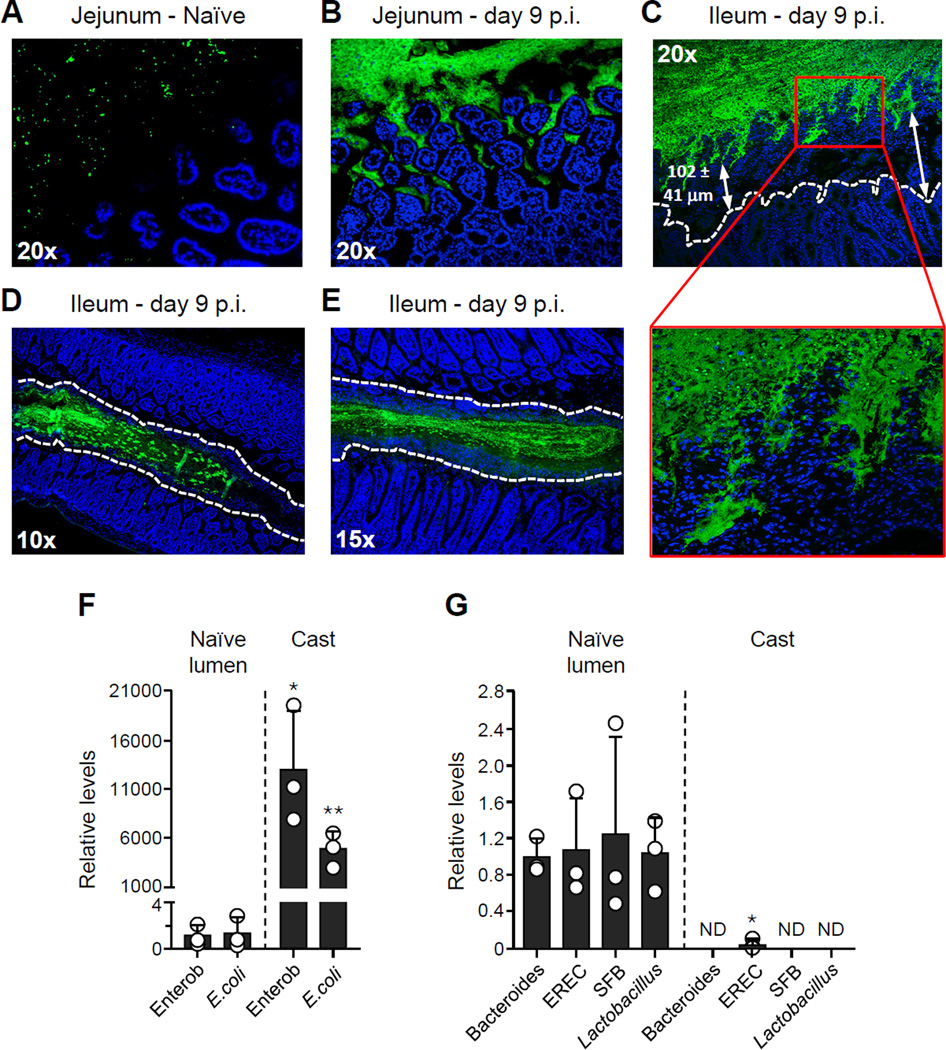
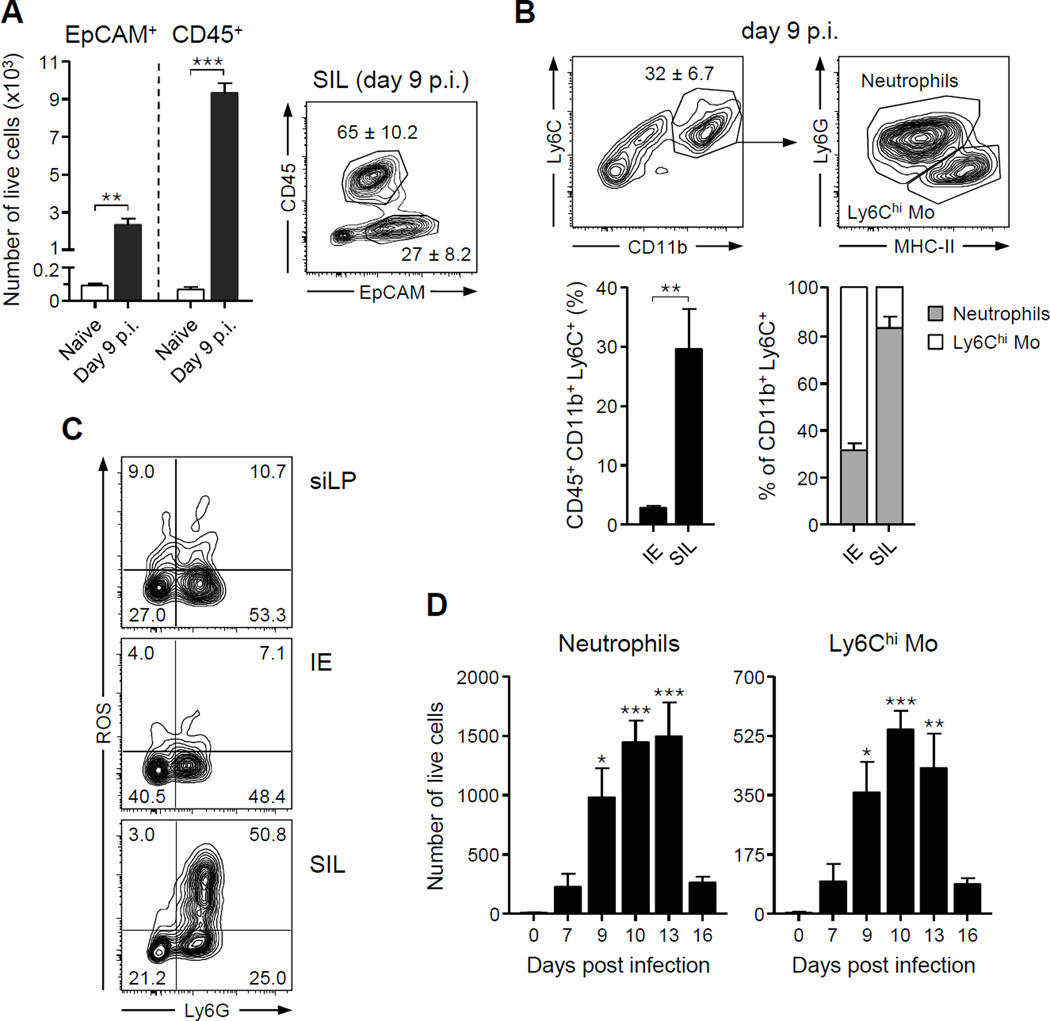
At day 9 after T. gondii infection, fluorescence in-situ hybridization (FISH) with a pan-bacterial 16S probe revealed a close association between bacteria and jejunal epithelium when compared to naïve tissue (Figures 2A–2B). Contrasting this, in the distal portion of the small intestine, the ileum, commensal outgrowth was segregated from the epithelium by an average of greater than 100 µM (Figure 2C). Physical segregation of the microbiota from the ileal epithelium resulted from massive accumulation of host cells in the lumen organized into a defined structure, further referred to as intra-luminal casts, surrounding the bacterial outgrowth (Figures 2C–2E). Compared to the naïve lumen, casts contained increased levels of γ-proteobacteria and more specifically E. coli (Figure 2F–2G). In contrast, other commensals assayed including those from the genus Bacteroides, the Lactobacillus/Lactococcus group and Segmented Filamentous Bacteria (SFB) were not detected (Figures 2F–2G). To assess the cellular composition of these structures, cells were isolated from the small intestine lumen (SIL) and evaluated by flow cytometry (Figure S2). Under steady state conditions, a small number of cells could be recovered from the SIL as part of the normal turnover and delamination of the epithelium (Potten et al., 1992) (Figure 3A). As infection progressed, this compartment became highly enriched in cells of the hematopoietic lineage and in particular neutrophils as well as Ly6Chi inflammatory monocytes, and epithelial cells (Figures 3A–3B). Of note, the small intestine lumen represents a unique immunological niche relative to the epithelial compartment, as the percentage of inflammatory cells and the ratio of neutrophils and inflammatory monocytes were distinct between the two compartments (Figure 3B). Further, SIL neutrophils were highly activated compared to those in both the lamina propria (LP) and the intraepithelial (IE) compartment, with over 50% of the Ly6G+ cells producing reactive oxygen species (ROS) in absence of exogenous stimulation (Figure 3C). Following a similar pattern to γ-proteobacteria expansion and contraction, intra-luminal accumulation of innate cells was restored to basal levels by day 15 p.i. (Figure 3D). Thus, the formation of intra-luminal casts occurs in tandem with the outgrowth of commensal pathobionts during acute infection.
Figure 2. Intra-luminal cellular casts limit commensal contact with epithelium and control bacterial translocation during T. gondii infection.
Mice were orally infected with 15 T. gondii cysts. (A–E) Visualization of relative abundance and localization of bacteria by FISH in the small intestine. Sections were hybridized with a pan-bacterial 16S probe (green) and counterstained with DAPI to visualize nuclei (blue). (A–B) Sections from the jejunum of (A) naïve and (B) day 9 p.i. mice. (C–E) Sections from the ileum of day 9 p.i. mice. The white dashed lines represent the border of the ileal epithelium (C) or the border of the cellular casts that surround the bacteria (D–E). Red box shows area of increased segregation between bacteria and epithelia at higher magnification. (F–G) Bacterial 16S gene analysis of intra-luminal casts by semi-quantitative PCR using primers specific for the following: Enterobacteriaceae (Enterob), Escherichia coli (E.coli), Bacteroides, Eubacterium rectale/Clostridium coccoides (EREC) group, Segmented Filamentous Bacteria (SFB), and Lactobacillus/Lactococcus (Lactobacillus) group. Values are calculated relative to naïve. Open circles represent individual mice and each bar represents the mean ± SEM (*P<0.05, **P<0.01).
Figure 3. Neutrophils and inflammatory monocytes are major components of intra-luminal casts.
Cells from the luminal contents of naïve and T. gondii-infected mice (day 9 p.i.) were isolated and analyzed by flow cytometry. (A) Contour plot (right) shows endothelial and CD45+ hematopoietic cells. Numbers indicate the mean ± SEM percent in each gate. Bar graphs (left) show mean ± SEM of live cells isolated from the lumen of the small intestine. (B) Contour plots show the percent of luminal cells in the CD11b+Ly6C+ innate leukocytes (top left). Number indicates the mean ± SEM percent in the gate. CD11b+Ly6C+ cells were further classified as MHC-II+Ly6G− inflammatory monocytes and MHC-II−Ly6G+ neutrophils (top right). Bar graphs represent mean relative levels ± SEM of CD45+CD11b+Ly6C+ myeloid cells and the ratio of neutrophils and inflammatory monocytes in the Intra-Epithelial (IE) compartment and Small Intestinal Lumen (SIL). (C) ROS production by neutrophils (Ly6G+) and monocytes (Ly6G−) from the small intestine lamina propria (siLP), IE and SIL was analyzed directly ex vivo by flow cytometry. Contour plots are gated on live CD45+Ly6C+CD11b+ cells. Number in each quadrant represents the percent of total. (D) Total number of luminal inflammatory monocytes and neutrophils over the first two weeks of infection. All bar graphs represent the mean ± SEM of three to five mice analyzed. All data shown are representative of two to four independent experiments. *P<.05, **P<.01, ***P<.001. See also Figure S2.
Neutrophils promote intra-luminal containment of commensals during T. gondii infection
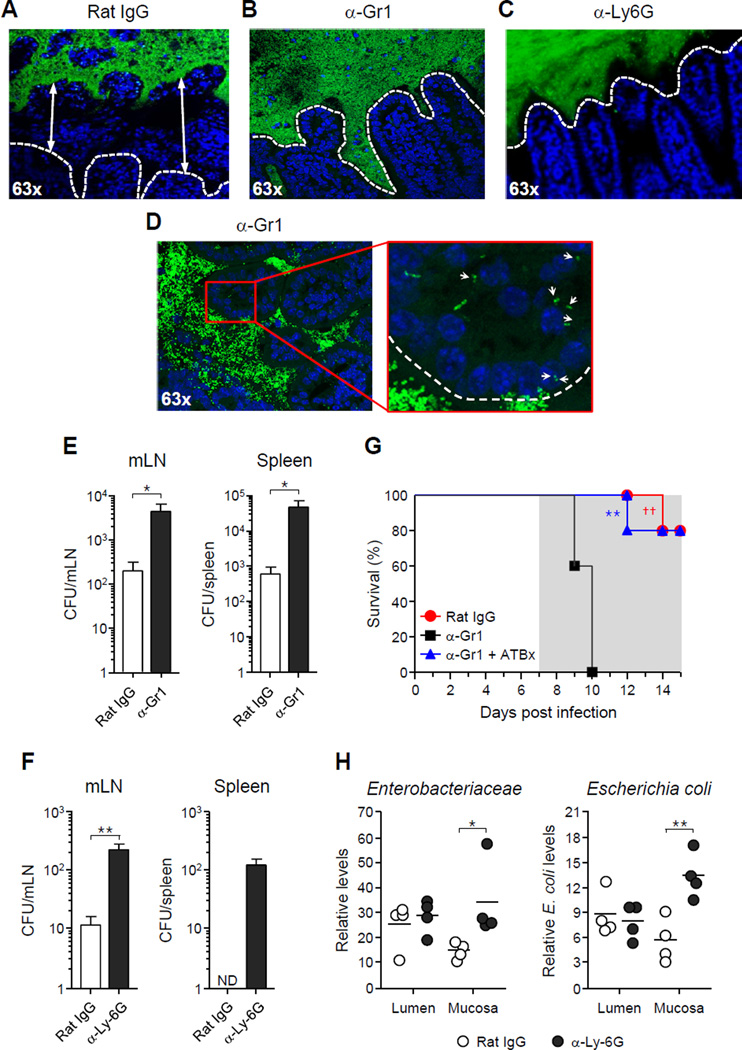
Imaging and flow cytometry analysis supported the hypothesis that (1) intra-luminal containment structures were required to restore the spatial segregation between commensals and the epithelium, and (2) neutrophils and inflammatory monocytes were a major component of the intra-luminal casts (Figures 2E and 3B). To address these points, we depleted T. gondii-infected mice of both neutrophils and inflammatory monocytes using anti-Ly6G/Ly6C antibody (α-Gr1) starting at day 7 p.i. (Figure S3A) (Dunay et al., 2010). Anti-Gr1 treatment abrogated the establishment of the intra-luminal casts (Figures 4A–4B). Treatment of mice with the anti-Ly6G neutrophil specific antibody, 1A8 (Dunay et al., 2010) similarly prevented the formation of the containment structures (Figure 4C). In contrast to isotype control treated mice, the bacterial 16S FISH signal was observed within the intestinal villi of the ileum of mice treated with α-Gr1 antibody (Figure 4D, 63x insert). Consistent with this observation, α-Gr1 as well as α-Ly6G treatment led to increased translocation of bacteria from the GI tract to the spleen and mesenteric lymph node (mLN) (Figures 4E–4F). Neutrophil depletion also resulted in increased levels of mucosa associated γ-proteobacteria (Figure 4H). Moreover, treatment with α-Gr1 antibody was associated with increased mortality despite no increase in parasite burden (Figures 4G and S3B). Mortality in α-Gr1-treated mice could be prevented by injection of broad-spectrum antibiotics, implicating systemic dissemination of commensal bacteria as a major factor contributing to enhanced lethality (Figure 4G). These results demonstrate that neutrophils play a critical role in the control of microbial translocation during T. gondii infection, and that inflammatory cells, in particular neutrophils, are required for the formation of intraluminal casts. Further, our present data suggest that these structures may play an important role in containing the pathogenic outgrowth of commensals intra-luminally and preventing their systemic dissemination.
Figure 4. Neutrophils are necessary for luminal cast formation and control of bacterial translocation in T. gondii-infected mice.
T. gondii-infected mice were treated with either rat IgG (control), α-Gr1 or α-Ly6G depleting antibody starting 7 days p.i. Some infected mice were also treated with antibiotics from day 7 p.i. (A–D) Visualization of bacteria localization relative to intestinal villi by FISH (green = pan-bacterial 16S probe; blue = DAPI) in mice treated with antibodies as indicated. White lines indicate the epithelial cell border. Arrows indicate examples of tissue-associated bacteria. (E–F) Systemic translocation of aerobic bacteria in spleen and mLN at day 10 p.i. in mice treated with antibodies as indicated. Each bar represents the mean ± SEM of 4–5 mice analyzed. (G) Survival curve of T. gondii-infected mice treated with either rat IgG, α-Gr1 or α-Gr1 and antibiotics (5 mice per group, ††P <0.01). (H) 16S gene analysis of small intestine luminal and mucosa-associated bacteria using primers specific for Enterobacteriaceae or E. coli. Open circles represent individual mice. All data shown are representative of two independent experiments (*P<0.05, **P<0.01). ND = not detected. See also Figure S3.
N-formyl peptides and γ-proteobacteria promote the formation of intra-luminal casts
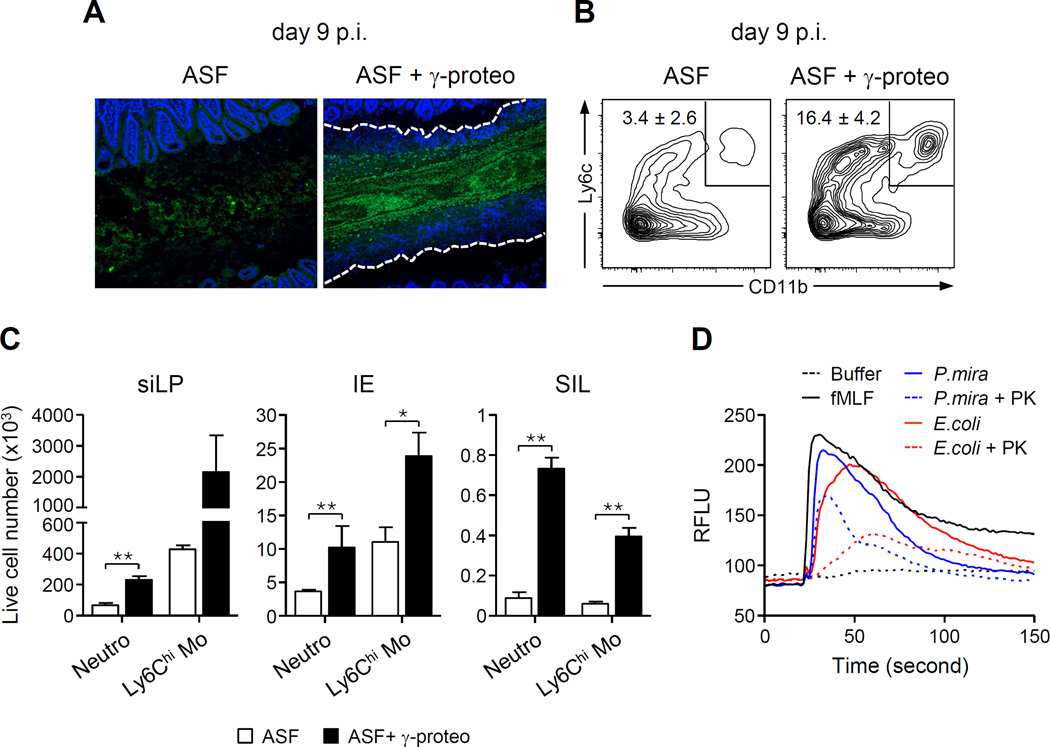
Because of the high pathogenic potential of γ-proteobacteria, we hypothesized that γ-proteobacteria may either directly or indirectly control luminal recruitment of neutrophils and encapsulation of bacterial outgrowth. To address these points, we inoculated mice by oral gavage with two species of γ-proteobacteria isolated from T. gondii-infected mice (E. coli or P. mirabillis) or Lactobacillus paracaseii at day 6 p.i. Increasing levels of γ-proteobacteria but not L. paracaseii led to heightened neutrophil emigration to the lumen, compared to control (Figure S4A). To further establish a link between γ-proteobacteria and intra-luminal casts, we utilized germ free (GF) mice, born in aseptic conditions and reared in isolators. Germ free mice were colonized with a mixture of 8 bacterial species, referred to as Altered Schaedler Flora (ASF), which is non-inflammatory and devoid of γ-proteobacteria (Dewhirst et al., 1999; Geuking et al., 2011). Bacterial outgrowth and recruitment of neutrophils and inflammatory monocytes in the small intestine lumen were minimal in ASF mice following T. gondii infection (Figure 5A). Thus, T. gondii in combination with benign bacteria is not sufficient for the induction of intraluminal casts. Addition of a mixture of γ-proteobacteria (E. coli and P. mirabilis) to mice associated with ASF led to a substantial increase in bacterial load, as well as dramatic increases in cellular transmigration into the small intestine lumen (Figures 5A–5C). Further, in contrast to ASF mice, addition of γ-proteobacteria triggered the formation of intra-luminal casts (Figure 5A). Importantly, these changes were not a consequence of alterations in parasitic load (Figure S4B). Thus, the γ-proteobacteria E. coli and P. mirabilis are sufficient to orchestrate the formation of intra-luminal casts.
Figure 5. γ-Proteobacteria promote luminal recruitment of inflammatory cells.
Germ-free mice were colonized with either ASF bacteria or ASF + γ-proteobacteria and then infected with T. gondii. (A) Representative image of 16S FISH staining of ileal sections. The white dashed lines represent the border of the cellular casts. (B) Contour plots show the percentage of CD11b+Ly6C+ leukocytes isolated from the lumen. Numbers indicate the mean ± SEM percent inside the gate. (C) Number (mean ± SEM) of inflammatory monocytes and neutrophils isolated from the small intestine lamina propria, IE and lumen (SIL) of ASF or ASF + γ-proteobacteria mice. (D) Supernatants isolated from E. coli and P. mirabilis (P.mira) cultures were tested for their ability to induce calcium flux in neutrophils. Histogram indicates the calcium flux over time from purified neutrophils, treated as indicated (fMLF = N-formyl methionine leucyl phenylalanine; PK = proteinase K). See also Figure S4.
Increased emigration of inflammatory cells into the small intestine lumen could result from epithelial or hematopoietic cell derived signals and/or direct signals from the γ-proteobacteria. Indeed, both pathogenic and commensal bacteria can produce or induce numerous factors including chemokines, leukotrienes, and formyl peptides, all of which are able to recruit leukocytes to sites of inflammation (Chin and Parkos, 2007a). In particular, formyl peptides, a major byproduct of bacterial metabolism, activate specific G-protein coupled receptors on phagocytic leukocytes and could potentially drive their emigration to the small intestinal lumen (Schiffmann et al., 1975). Consistent with this, both E. coli and P. mirabilis supernatants were able to potently activate neutrophils, as assessed by the supernatant capacity to induce calcium flux in a manner comparable to the prototypic formyl peptide, N-formyl methionine leucyl phenylalanine (fMLF) (Figure 5D). Also consistent with a potential involvement of formyl peptides, such an effect was greatly diminished by proteinase K treatment (Ojode et al., 2012) (Figure 5D).
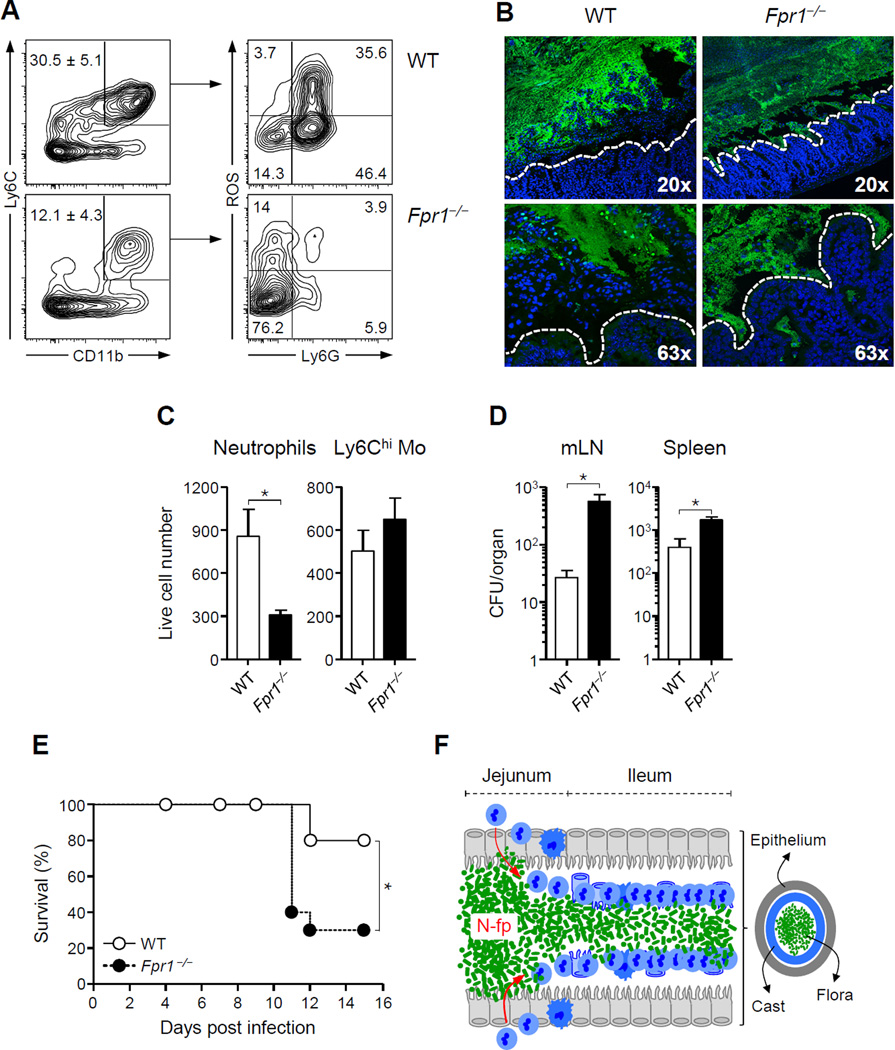
During T. gondii infection, the high-affinity N-formyl peptide receptor Fpr1 was the formyl peptide receptor most expressed on neutrophils isolated from the lamina propria (Figure S5A). To address a potential role for γ-proteobacteria-derived formyl peptides in the formation of intra-luminal casts, mice lacking expression of Fpr1 (Hartt et al., 1999; Le et al., 2002) were orally infected with T. gondii. Recruitment of neutrophils was greatly diminished, and luminal casts failed to form in Fpr1−/− mice at day 9 p.i. (Figures 6A–6C). Loss of containment led to enhanced contact of commensals with epithelial cells in Fpr1−/− mice compared to wild-type (WT) mice (Figure 6B). Concomitantly, significantly increased translocation of commensals to mLN and spleen and enhanced mortality was observed in Fpr1−/− mice compared to WT mice (Figures 6D–6E). Increased lethality was not associated with higher parasite burden in Fpr1−/− mice, or with changes in effector T cell responses (Figure S5B–S5C). At day 11 post-infection, in the surviving Fpr1−/− mice, some neutrophils were recruited to the lumen although total numbers remained significantly reduced compared to WT mice (Figure S5D). Thus, although alternative mechanisms may be able to compensate for Fpr1 deficiency, early and optimal neutrophil recruitment and containment of bacteria in the ileum is highly dependent on this pathway. These results support the idea that γ-proteobacteria sensing, via an Fpr1-dependent mechanism, controls the establishment of intra-luminal casts that in turn can control pathology by promoting bacterial clearance and limiting contact between the epithelium and the commensal outgrowth.
Figure 6. Formyl peptide receptor 1 (FPR1) is required for the establishment of intra-luminal cast during T. gondii infection.
Fpr1−/− and wild-type (WT) control mice were infected orally with 15 T. gondii cysts. (A) Representative contour plots showing the percent (mean ± SEM) of CD11b+Ly6C+ cells (left) and ROS production by Ly6G+ neutrophils (right) isolated from the lumen of WT and Fpr1−/− mice at day 9 p.i. (B) Representative image of 16S FISH staining of sections from infected WT and Fpr1−/− ileum. The white dashed line indicates the border of the epithelium. (C) Total numbers (mean ± SEM) of neutrophils and inflammatory monocytes in the SIL of WT and Fpr1−/− mice at day 9 p.i. (D) Systemic translocation of aerobic bacteria in spleen and mLN of WT mice or Fpr1−/− mice at day 10 p.i. All bar graphs represent the mean ± SEM of two to five mice analyzed. (E) Survival curve of T. gondii-infected wild-type and Fpr1−/− mice (10 mice per group). All data shown in (A–E) are representative of 2–3 independent experiments (*P<0.05). (F) Model of intra-luminal cast formation during γ-proteobacteria outgrowth. Upon infection, outgrowth of bacteria in the jejunum leads to enhanced microbial contact with the epithelium and increased density of N-formyl peptides. This triggers a cascade of responses eventually leading to innate cell recruitment into the lumen in an Fpr1-dependent manner, concomitantly inducing delamination of the epithelium. These events lead to the formation of temporary containment structures that physically contain the bacteria from the ileal epithelium, and allow for elimination of the bacteria via peristalsis as a solid structure. See also Figures S5.
Discussion
Outgrowth of commensals with inflammatory potential represents a formidable challenge for the immune system. Notably, γ-proteobacteria dominance and emergence of strains with enhanced invasive properties has been linked to severe conditions ranging from IBD to carcinogenesis (Arthur et al., 2012; Darfeuille-Michaud et al., 2004; Gulati et al., 2012; Liu et al., 1995; Lodes et al., 2004; Mukhopadhya et al., 2012; Swidsinski et al., 2005). How the host controls outgrowth of commensal pathobionts during infection and prevents their deleterious consequences remains poorly understood. Our present work reveals a previously unappreciated host response limiting the pathogenic consequences of microbiotal dysbiosis associated with acute mucosal infection.
The central feature of the mechanism we describe is a novel multicellular cast in the ileal lumen of T. gondii infected mice that is composed of a layer of inflammatory cells including neutrophils, along with epithelial cells that “wall-off” potentially harmful commensals. These casts are distinct from classic white cell casts present in the collecting system of the kidney and excreted in the urine of patients with acute pyelonephritis (Bagshaw et al., 2006; Ivanyi et al., 1988). On the other hand, like nephritic casts, commensal casts in the ileum represent a tissue-specific response to acute infection. Further, intraluminal casts occur in response to commensal outgrowth and are therefore distinct from pseudomembranes that develop in response to bacterial toxins and in the context of infections such as Clostridium difficile. We propose that during inflammation, outgrowth of bacteria in the jejunum resulted in enhanced microbial contact with epithelial cells triggering a cascade of responses eventually leading to neutrophil transmigration. Neutrophil migration across the epithelium then leads to enhanced delamination of epithelial cells. These cumulative effects could ultimately orchestrate the formation of containment structures that are then physically eliminated by peristalsis (Figure 6F). The formation of luminal casts can be hypothesized to have several outcomes: (1) maintain segregation between the epithelium and inflammatory bacteria in order to prevent sustained inflammation in the ileum and large intestine; (2) reduce translocation of invasive microbes and (3) physically remove bacterial outgrowth in a solid structure.
Despite the beneficial role of commensals for host physiology, a fraction of this complex community of microbes has the potential to trigger and contribute to pathology. These pathogenic bacteria, or pathobionts, can rapidly expand and dominate over other commensal species as a consequence of inflammation. Although we show that cast formation is associated with the control of commensal γ-proteobacteria outgrowth during acute infection with T. gondii, these structures are likely involved in the control of all bacteria with enhanced inflammatory properties including “bona fide” pathogens.
Our data support the idea that N-formyl peptides are key contributors to the commensal containment process. N-formyl peptides can be produced in nature by the degradation of either bacterial or host cell mitochondrial proteins, all of which are formylated on the N-terminal methionine. Thus, N-formyl peptides represent a major byproduct of bacterial and mitochondrial metabolism (Chadwick et al., 1988; Schiffmann et al., 1975). As such these peptides are not only ubiquitous in the context of inflammation or infection, but are highly diverse structurally and functionally (Ye et al., 2009). Recognition of N-formyl peptides by mammalian cells occurs via a family of seven transmembrane domain G-protein coupled receptors, with Fpr1 representing the high-affinity receptor for fMLF, the prototypic N-formyl peptide isolated from E. coli (Marasco et al., 1984; Schiffmann et al., 1975; Ye et al., 2009). These peptides, considered as danger-associated molecular pattern molecules (DAMPs), are capable of alerting the immune system in the context of enhanced cell death or exposure to pathogenic bacteria. For instance, Fpr1-deficient mice display increased susceptibility to Listeria monocytogenes infection (Gao et al., 1999; Liu et al., 2012). How formyl peptide receptors control interaction of commensals with their host was previously unknown. Under steady state conditions the major leukocyte chemotactic factors in the mouse intestinal lumen appear to be Fpr1 independent (Ojode et al., 2012), suggesting that in the absence of inflammation, formyl peptides may not represent a dominant signal in this dialogue. On the other hand, our data propose that, during inflammation, a major function of these receptors may be associated with their capacity to promote commensal containment.
Containment of γ-proteobacteria during inflammation was mediated by the capacity of neutrophils to migrate to the lumen in an Fpr1-dependent manner. Based on the multiple potential targets and sources of these peptides, we could propose several non-exclusive scenarios for the requirement of γ-proteobacteria in this phenomenon. Based on the unique capacity of E. coli or P. mirabilis to expand during inflammation, the sheer density of these bacteria may reach an optimal threshold of formyl peptide concentration required for neutrophil recruitment to the lumen. Further, given the inflammatory potential of γ-proteobacteria, formyl peptides could be also released by injured epithelial cells and necrotic inflammatory cells. Such effects may additionally be linked to our observation that during infection, γ-proteobacteria thrives in the mucosa-associated fraction of the jejunum. In addition to direct neutrophil activation by formyl peptide, part of the effect observed could be indirect, as formyl peptides can also activate epithelial cells and potentially promote epithelial cell secretion of various chemoattractants. Finally, we cannot exclude the possibility that E. coli and P. mirabilis isolates from the T. gondii-infected host produce highly potent formyl peptides compared to more benign commensal species. Indeed, pathogens such as L. monocytogenes and Staphylococcus aureus produce formyl peptides with enhanced neutrophil activity compared to the prototypic E. coli derived formyl peptide, N-formyl-Met-Leu-Phe (Southgate et al., 2008). In addition to their potent chemotactic activity, N-formyl peptides may directly promote pathobiont killing in the lumen, as high concentrations of N-formyl peptides lead to the generation of superoxide (Ye et al., 2009). Consistent with this observation, neutrophils present in the lumen during T. gondii infection are highly activated and produce large amounts of ROS. Intriguingly, neutrophil recruitment to the lamina propria of the large intestine is Fpr1-independent in a model of acute mucosal injury mediated by DSS (Farooq and Stadnyk, 2012). However, in this study, the capacity of neutrophils to transmigrate to the lumen was not assessed. In contrast, and consistent with our present findings, Fpr1-deficient mice treated with DSS displayed increased inflammation, supporting the notion of poor commensal containment in the absence of this receptor (Farooq and Stadnyk, 2012). Although deciphering the exact mechanism of action and target of these peptides in the GI tract remains complex, our present work is, to the best of our knowledge, the first to reveal a role for commensal-derived and/or triggered formyl peptides in the recruitment of neutrophils to the GI tract and in the control of dysbiosis.
Neutrophils not only play a major role in microbial clearance but are also critical mediators of collateral damage to tissue (Chin and Parkos, 2007b; Grisham and Granger, 1988). Of note, crypt abscesses, a classical feature of Ulcerative Colitis result from transmigration of large numbers of neutrophils across tight junctions and accumulation within epithelial crypts (Xavier and Podolsky, 2007). Further, neutrophils contribute to pathology occurring during acute infections (Dunay et al., 2010) (Grainger et al., 2013). Of high relevance to our present findings, Fpr1 polymorphisms in humans have been linked to severe diseases occurring at sites colonized by commensals, including severe periodontitis, stomach cancer, and recurrence of colon cancer (Huang et al., 2010; Otani et al., 2011; Perez et al., 1991; Seifert and Wenzel-Seifert, 2001). Further N-formyl peptide receptor expression has been reported to be increased in Crohn’s disease (CD) patients (Anton et al., 1989). Together with these clinical associations, our present findings support the idea that when uncontrolled, the response to commensal derived formyl peptides may play an important role in promoting tissue damage and inflammation.
Inflammatory bowel disease represents a major health burden (Loftus, 2004) and while many studies have demonstrated a capacity for the microbiota to initiate and exacerbate IBD, few studies have addressed how the host responds to re-establish control over dysbiosis during acute flares (Saleh and Elson, 2011). Our present model of acute mucosal infection reproduces some of the key features of Crohn’s disease. Namely, T. gondii infection is characterized by massive mucosal inflammation associated with commensal shifts, reminiscent of the ones observed in CD patients (Darfeuille-Michaud et al., 2004; Liu et al., 1995; Lodes et al., 2004; Mukhopadhya et al., 2012; Swidsinski et al., 2005). Based on our present findings, we propose that the Fpr1-dependent containment strategy has developed as a means to control bystander outgrowth of bacteria during infection and that failure to develop or control this response may lead to severe disorders such as inflammatory bowel diseases.
Experimental Procedures
Mice and parasite
C57BL/6 (WT) and Fpr1−/− (backcrossed 10 generations onto the C57BL/6NTac background) were obtained from Taconic Farms. Germ-free (GF) C57BL/6 mice were bred at Taconic Farms and maintained in isolators at the NIAID gnotobiotic facility. All mice were bred and maintained under pathogen-free conditions at an American Association for the Accreditation of Laboratory Animal Care-accredited animal facility at the NIAID animal facility and housed in accordance with the procedures outlined in the Guide for the Care and Use Committee. Gender- and age-matched mice between 8–12 weeks of age were used for all experiments. In experiments using knockout mice, wild-type and knockout mice were co-housed for two weeks prior infection. ME-49 clone C1 (expressing RFP) was used for infection as described previously (Oldenhove et al., 2009).
Colonization of germ-free mice
Altered Schaedler Flora (ASF)-colonized germ-free C57BL/6 mice were generated by inoculating GF mice with ASF bacteria according to standard protocol. Briefly, GF mice were first gavaged with bacteria ASF 360 (Lactobacillus acidophilus), 361 (Lactobacillus murinus) and 519 (Bacteroides distasonis), and then two or three days later with bacteria ASF 356 (Clostridium sp.), 457 (Mucispirillum schaedleri), 492 (Eubacterium plexicaudatum), 500 (Firmicutes sp.), 502 (Clostridium sp.). In some experiments, GF mice were also colonized with Escherichia coli and Proteus mirabilis isolated from the intestinal flora of T. gondii-infected mice. Colonization was confirmed by performing PCR using DNA isolated from fecal samples, and primers specific for the variable region of 16S rDNA of each individual bacterium. Mice were infected with T. gondii two weeks after colonization.
Cell depletion and antibiotic treatment
For depletion of neutrophils and/or inflammatory monocytes, mice were injected intra-peritoneally (i.p.) with anti-Gr1 antibody (clone RB6-8C5, 250µg), anti-Ly6G antibody (clone 1A8, 500µg) or rat IgG isotype control every 24 hours starting on day 7 p.i. and until the end of the experiment. In some experiments, T. gondii-infected mice were injected i.p. with ampicillin (200 µg), vancomycin (100 µg) neomycin sulfate (200 µg), and metronidazole (200 µg) (Sigma-Aldrich) starting 7 days after the infection.
Cell isolation
Cells from spleen, lymph nodes, intraepithelial compartment (IE) and the siLP were isolated as previously described (Oldenhove et al., 2009). Cells from the lumen were isolated by flushing 15 ml of RPMI 3% FBS through the small intestine. The flushed material was filtered through a 70 µM cell strainer, re-suspended in Nycodenz gradient (Accurate Chemical) and overlaid with RPMI. Cells were then spun at 1500g and the interphase was collected.
Antibodies and flow cytometry
All antibodies used for flow cytometry were purchased from eBioscience, except for Ly6G (BD Biosciences). The following antibodies were used: CD45 (30-F11), CD11b (M1/70), Ly6C (HK1.4), Ly6G (1A8), IFN-γ (XMG1.2) IL-17A (eBio17B7), IL-1-α (ALF-161), IL-1β (NJTEN3). Dead cells were discriminated in all experiments using DAPI or LIVE/DEAD Fixable Blue Dead Cell Stain Kit (Life Technologies) and all stains were carried out in media containing anti-CD16/32 blocking antibody (clone 93, eBioscience). Reactive oxygen species production was measured using Dihydrorhodamine (Life Technologies). All flow cytometry data were acquired on an LSRII FACS analyzer (BD Bioscience) and analyzed on FlowJo software (Tree Star).
Immunofluorescence/Histology/Electron Microscopy
For Fluorescence In Situ Hybridization (FISH), the small intestines were prepared by fixation in 60% Methanol, 30% Chloroform, 10 % Acetic acid, washed in 70% ethanol and embedded in paraffin. Longitudinal sections (5–25 µm) were hybridized to a bacterial 16S rRNA gene probe: [AminoC6+Alexa488]-GCTGCCTCCCGTAGGAGT-[AmC7-Q+Alexa488] (Eurofins MWG Operon) as previously published (Ismail et al., 2011) and counterstained with DAPI. Sections were visualized on a Leica DM IRBE fluorescent microscope.
Bacterial translocation
Mice were sacrificed and tissues isolated in a laminar flow hood under sterile conditions. Tissues were mechanically disassociated, diluted and plated directly onto tryptic soy agar plates (Sigma-Aldrich). Twenty-four hours later bacterial colonies were counted.
Neutrophil calcium release assay
Isolated bone marrow cells were resuspended in PBS and layered on top of a Histopaque 1077/1119 gradient (3 mL Histopaque 1119 overlayed with 3 mL Histopaque 1077) and centrifuged at room temperature for 30 min at 700g without break. The resulting neutrophil layer (lower layer) was removed, washed, and plated for the calcium release assay. Intracellular calcium measurements were performed using a FlexStation 3 microplate reader and the FLIPR Calcium 3 Assay Explorer Kit (Molecular Devices) as previously described (Ojode et al., 2012). Changes in intracellular calcium concentration were recorded as relative fluorescence units (RFLU).
Analysis of bacterial 16S genes
Fecal pellets were excised from the colon and immediately frozen on dry ice. Macroscopically visible casts were isolated from the luminal contents after flushing the small intestine with sterile PBS. Mucosa-associated fractions were isolated by removing luminal contents and vigorously washing the apical surface of the small intestine. Genomic DNA was isolated using the QIAmp DNA stool kit (Qiagen). 454 sequencing of 16S rRNA genes was performed as previously described (Naik et al., 2012). For specific 16S ribosomal measurements, bacterial genomic DNA was analyzed by SYBR Green real-time PCR (Bio-Rad) using 16S rRNA gene primers specific for different bacteria (see Table S1). The amount of specific 16S ribosomal DNA was calculated by ΔCT method as compared to the total 16S ribosomal DNA in each associated DNA sample.
Statistical Analysis
Groups were compared with Prism software (GraphPad) using either the two-tailed unpaired Student’s t-test, one way ANOVA, or Log-rank (Mantel-Cox) Test for the survival analysis. For all experiments P<0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. We thank Dr. Lora Hooper and Shipra Vaishnava (UT southwestern) for sharing protocols, the NIAID gnotobiotic facility staff and in particular Cesar Acevedo and Dana Tragesercesler, Dr. Kevin Holmes and the NIAID sorting facility, Kim Beacht for technical assistance, Dr. Lily Koo and the NIAID Biological Imaging Facility. We thank Clayton Deming for thoughtful discussion and Amiko Uchida for critical reading of the manuscript.
References
- Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton PA, Targan SR, Shanahan F. Increased neutrophil receptors for and response to the proinflammatory bacterial peptide formyl-methionyl-leucyl-phenylalanine in Crohn's disease. Gastroenterology. 1989;97:20–28. doi: 10.1016/0016-5085(89)91410-8. [DOI] [PubMed] [Google Scholar]
- Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshaw SM, Langenberg C, Bellomo R. Urinary biochemistry and microscopy in septic acute renal failure: a systematic review. Am J Kidney Dis. 2006;48:695–705. doi: 10.1053/j.ajkd.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Benson A, Pifer R, Behrendt CL, Hooper LV, Yarovinsky F. Gut commensal bacteria direct a protective immune response against Toxoplasma gondii. Cell host & microbe. 2009;6:187–196. doi: 10.1016/j.chom.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl K, Plitas G, Mihu CN, Ubeda C, Jia T, Fleisher M, Schnabl B, DeMatteo RP, Pamer EG. Vancomycin-resistant enterococci exploit antibiotic-induced innate immune deficits. Nature. 2008;455:804–807. doi: 10.1038/nature07250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho FA, Koren O, Goodrich JK, Johansson ME, Nalbantoglu I, Aitken JD, Su Y, Chassaing B, Walters WA, Gonzalez A, et al. Transient Inability to Manage Proteobacteria Promotes Chronic Gut Inflammation in TLR5-Deficient Mice. Cell Host Microbe. 2012;12:139–152. doi: 10.1016/j.chom.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick VS, Mellor DM, Myers DB, Selden AC, Keshavarzian A, Broom MF, Hobson CH. Production of peptides inducing chemotaxis and lysosomal enzyme release in human neutrophils by intestinal bacteria in vitro and in vivo. Scandinavian journal of gastroenterology. 1988;23:121–128. doi: 10.3109/00365528809093861. [DOI] [PubMed] [Google Scholar]
- Chin AC, Parkos CA. Pathobiology of neutrophil transepithelial migration: implications in mediating epithelial injury. Annu Rev Pathol. 2007a;2:111–143. doi: 10.1146/annurev.pathol.2.010506.091944. [DOI] [PubMed] [Google Scholar]
- Chin AC, Parkos CA. Pathobiology of neutrophil transepithelial migration: implications in mediating epithelial injury. Annu Rev Pathol. 2007b;2:111–143. doi: 10.1146/annurev.pathol.2.010506.091944. [DOI] [PubMed] [Google Scholar]
- Craven M, Egan CE, Dowd SE, McDonough SP, Dogan B, Denkers EY, Bowman D, Scherl EJ, Simpson KW. Inflammation drives dysbiosis and bacterial invasion in murine models of ileal Crohn's disease. PLoS ONE. 2012;7:e41594. doi: 10.1371/journal.pone.0041594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darfeuille-Michaud A. Adherent-invasive Escherichia coli: a putative new E. coli pathotype associated with Crohn's disease. Int J Med Microbiol. 2002;292:185–193. doi: 10.1078/1438-4221-00201. [DOI] [PubMed] [Google Scholar]
- Darfeuille-Michaud A, Boudeau J, Bulois P, Neut C, Glasser AL, Barnich N, Bringer MA, Swidsinski A, Beaugerie L, Colombel JF. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology. 2004;127:412–421. doi: 10.1053/j.gastro.2004.04.061. [DOI] [PubMed] [Google Scholar]
- Dewhirst FE, Chien CC, Paster BJ, Ericson RL, Orcutt RP, Schauer DB, Fox JG. Phylogeny of the defined murine microbiota: altered Schaedler flora. Appl Environ Microbiol. 1999;65:3287–3292. doi: 10.1128/aem.65.8.3287-3292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunay IR, Fuchs A, Sibley LD. Inflammatory monocytes but not neutrophils are necessary to control infection with Toxoplasma gondii in mice. Infect Immun. 2010;78:1564–1570. doi: 10.1128/IAI.00472-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan CE, Cohen SB, Denkers EY. Insights into inflammatory bowel disease using Toxoplasma gondii as an infectious trigger. Immunol Cell Biol. 2011 doi: 10.1038/icb.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq SM, Stadnyk AW. Neutrophil infiltration of the colon is independent of the FPR1 yet FPR1 deficient mice show differential susceptibilities to acute versus chronic induced colitis. Digestive diseases and sciences. 2012;57:1802–1812. doi: 10.1007/s10620-012-2082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao JL, Lee EJ, Murphy PM. Impaired antibacterial host defense in mice lacking the N-formylpeptide receptor. J Exp Med. 1999;189:657–662. doi: 10.1084/jem.189.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, McCoy KD, Macpherson AJ. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34:794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Grainger JR, Wohlfert EA, Fuss IJ, Bouladoux N, Askenase MH, Legrand F, Koo LY, Brenchley JM, Fraser ID, Belkaid Y. Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection. Nat Med. 2013;19:713–721. doi: 10.1038/nm.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisham MB, Granger DN. Neutrophil-mediated mucosal injury. Role of reactive oxygen metabolites. Dig Dis Sci. 1988;33:6S–15S. doi: 10.1007/BF01538126. [DOI] [PubMed] [Google Scholar]
- Gulati AS, Shanahan MT, Arthur JC, Grossniklaus E, von Furstenberg RJ, Kreuk L, Henning SJ, Jobin C, Sartor RB. Mouse background strain profoundly influences Paneth cell function and intestinal microbial composition. PLoS ONE. 2012;7:e32403. doi: 10.1371/journal.pone.0032403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartt JK, Barish G, Murphy PM, Gao JL. N-formylpeptides induce two distinct concentration optima for mouse neutrophil chemotaxis by differential interaction with two N-formylpeptide receptor (FPR) subtypes. Molecular characterization of FPR2, a second mouse neutrophil FPR. J Exp Med. 1999;190:741–747. doi: 10.1084/jem.190.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimesaat MM, Bereswill S, Fischer A, Fuchs D, Struck D, Niebergall J, Jahn HK, Dunay IR, Moter A, Gescher DM, et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. Journal of immunology. 2006;177:8785–8795. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Chen K, Chen J, Gong W, Dunlop NM, Howard OM, Gao Y, Bian XW, Wang JM. The G-protein-coupled formylpeptide receptor FPR confers a more invasive phenotype on human glioblastoma cells. British journal of cancer. 2010;102:1052–1060. doi: 10.1038/sj.bjc.6605591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- Ismail AS, Severson KM, Vaishnava S, Behrendt CL, Yu X, Benjamin JL, Ruhn KA, Hou B, DeFranco AL, Yarovinsky F, et al. Gammadelta intraepithelial lymphocytes are essential mediators of host-microbial homeostasis at the intestinal mucosal surface. Proc Natl Acad Sci U S A. 2011;108:8743–8748. doi: 10.1073/pnas.1019574108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanyi B, Rumpelt HJ, Thoenes W. Acute human pyelonephritis: leukocytic infiltration of tubules and localization of bacteria. Virchows Arch A Pathol Anat Histopathol. 1988;414:29–37. doi: 10.1007/BF00749735. [DOI] [PubMed] [Google Scholar]
- Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends Immunol. 2002;23:541–548. doi: 10.1016/s1471-4906(02)02316-5. [DOI] [PubMed] [Google Scholar]
- Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Liu M, Chen K, Yoshimura T, Liu Y, Gong W, Wang A, Gao JL, Murphy PM, Wang JM. Formylpeptide receptors are critical for rapid neutrophil mobilization in host defense against Listeria monocytogenes. Scientific reports. 2012;2:786. doi: 10.1038/srep00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, van Kruiningen HJ, West AB, Cartun RW, Cortot A, Colombel JF. Immunocytochemical evidence of Listeria, Escherichia coli, and Streptococcus antigens in Crohn's disease. Gastroenterology. 1995;108:1396–1404. doi: 10.1016/0016-5085(95)90687-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, Fort M, Hershberg RM. Bacterial flagellin is a dominant antigen in Crohn disease. The Journal of clinical investigation. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell host & microbe. 2007;2:204. doi: 10.1016/j.chom.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Man SM, Kaakoush NO, Mitchell HM. The role of bacteria and pattern-recognition receptors in Crohn's disease. Nat Rev Gastroenterol Hepatol. 2011;8:152–168. doi: 10.1038/nrgastro.2011.3. [DOI] [PubMed] [Google Scholar]
- Mann EA, Saeed SA. Gastrointestinal infection as a trigger for inflammatory bowel disease. Curr Opin Gastroenterol. 2012;28:24–29. doi: 10.1097/MOG.0b013e32834c453e. [DOI] [PubMed] [Google Scholar]
- Marasco WA, Phan SH, Krutzsch H, Showell HJ, Feltner DE, Nairn R, Becker EL, Ward PA. Purification and identification of formyl-methionyl-leucyl-phenylalanine as the major peptide neutrophil chemotactic factor produced by Escherichia coli. J Biol Chem. 1984;259:5430–5439. [PubMed] [Google Scholar]
- Mukhopadhya I, Hansen R, El-Omar EM, Hold GL. IBD-what role do Proteobacteria play? Nat Rev Gastroenterol Hepatol. 2012;9:219–230. doi: 10.1038/nrgastro.2012.14. [DOI] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, et al. Compartmentalized control of skin immunity by resident commensals. Science. 2012;337:1115–1119. doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojode T, Schneider EH, Tiffany HL, Yung S, Gao JL, Murphy PM. The Major Leukocyte Chemotactic and Activating Factors in the Mouse Gut Lumen Are Not N-Formylpeptide Receptor 1 Agonists. Journal of innate immunity. 2012 doi: 10.1159/000339572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenhove G, Bouladoux N, Wohlfert EA, Hall JA, Chou D, Dos Santos L, O'Brien S, Blank R, Lamb E, Natarajan S, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani T, Ikeda S, Lwin H, Arai T, Muramatsu M, Sawabe M. Polymorphisms of the formylpeptide receptor gene (FPR1) and susceptibility to stomach cancer in 1531 consecutive autopsy cases. Biochem Biophys Res Commun. 2011;405:356–361. doi: 10.1016/j.bbrc.2010.12.136. [DOI] [PubMed] [Google Scholar]
- Perez HD, Kelly E, Elfman F, Armitage G, Winkler J. Defective polymorphonuclear leukocyte formyl peptide receptor(s) in juvenile periodontitis. J Clin Invest. 1991;87:971–976. doi: 10.1172/JCI115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten CS, Kellett M, Roberts SA, Rew DA, Wilson GD. Measurement of in vivo proliferation in human colorectal mucosa using bromodeoxyuridine. Gut. 1992;33:71–78. doi: 10.1136/gut.33.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz M, Hwang SH, Wilhelm CL, Kirkland D, Benson A, Sturge CR, Mirpuri J, Vaishnava S, Hou B, Defranco AL, et al. Parasite-induced T(H)1 cells and intestinal dysbiosis cooperate in IFN-gamma-dependent elimination of Paneth cells. Nat Immunol. 2012 doi: 10.1038/ni.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh M, Elson CO. Experimental inflammatory bowel disease: insights into the host-microbiota dialog. Immunity. 2011;34:293–302. doi: 10.1016/j.immuni.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann E, Corcoran BA, Wahl SM. N-formylmethionyl peptides as chemoattractants for leucocytes. Proc Natl Acad Sci U S A. 1975;72:1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert R, Wenzel-Seifert K. Defective Gi protein coupling in two formyl peptide receptor mutants associated with localized juvenile periodontitis. J Biol Chem. 2001;276:42043–42049. doi: 10.1074/jbc.M106621200. [DOI] [PubMed] [Google Scholar]
- Sokol H, Seksik P, Furet JP, Firmesse O, Nion-Larmurier I, Beaugerie L, Cosnes J, Corthier G, Marteau P, Dore J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis. 2009;15:1183–1189. doi: 10.1002/ibd.20903. [DOI] [PubMed] [Google Scholar]
- Southgate EL, He RL, Gao JL, Murphy PM, Nanamori M, Ye RD. Identification of formyl peptides from Listeria monocytogenes and Staphylococcus aureus as potent chemoattractants for mouse neutrophils. J Immunol. 2008;181:1429–1437. doi: 10.4049/jimmunol.181.2.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, Chaffron S, Macpherson AJ, Buer J, Parkhill J, et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007;5:2177–2189. doi: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol. 2005;43:3380–3389. doi: 10.1128/JCM.43.7.3380-3389.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing B, Halfvarson J, Dicksved J, Rosenquist M, Jarnerot G, Engstrand L, Tysk C, Jansson JK. Twin studies reveal specific imbalances in the mucosa-associated microbiota of patients with ileal Crohn's disease. Inflamm Bowel Dis. 2009;15:653–660. doi: 10.1002/ibd.20783. [DOI] [PubMed] [Google Scholar]
- Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM, Laughlin RC, Gomez G, Wu J, Lawhon SD, et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science. 2013;339:708–711. doi: 10.1126/science.1232467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- Ye RD, Boulay F, Wang JM, Dahlgren C, Gerard C, Parmentier M, Serhan CN, Murphy PM. International Union of Basic and Clinical Pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacological reviews. 2009;61:119–161. doi: 10.1124/pr.109.001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.