Abstract
Background
Regulatory T cells (Treg) are being explored for their tolerance-inducing capabilities. Freezing and banking Treg for future use makes this strategy more clinically applicable. We aimed to devise an improved method of expanding and cryopreserving Treg to maximize yield, purity, and function for use in xenotransplantation.
Methods
Baboon peripheral blood mononuclear cells were isolated from whole blood. CD4+/CD25hi cells were isolated by flow cytometric sorting and expanded for 26 days in culture with interleukin (IL)-2, anti-CD3 antibody, artificial antigen presenting cells transfected with human CD58, CD32, and CD80, and rapamycin with weekly restimulations. Expanded Treg were frozen for 2 months then thawed and cultured for 48 hours in medium plus (1) no additives, (2) IL-2, (3) anti-CD3 antibody, (4) IL-2 + anti-CD3 antibody, and (5) IL-2 + anti-CD3 antibody + L cells. Phenotype and suppression were assessed after expansion, immediately after thawing, and after culturing.
Results
We expanded purified baboon Treg more than 10,000-fold. Expanded Treg exhibited excellent suppression in functional assays. Cryopreservation decreased suppressive function without changing phenotype, but increasing amounts of reactivation after thawing produced significantly better viability and suppressive function with a trend toward greater Treg purity.
Conclusions
We produced numbers of expanded Tregs consistent with clinical use. In contrast to some previous reports, both Treg phenotype and suppressive function were preserved or even enhanced by increasing amounts of restimulation after thawing. Thus, banking of expanded recipient Tregs for in vivo infusion should be possible.
Infusion of regulatory T cells (Treg) is a promising new therapy in the field of transplantation.1 In humans, Treg have been shown to have potential for the prevention of graft-versus-host disease after allogeneic hematopoietic cell transplantation.2 3 4 Treg infusion may also play an important role in preventing rejection or promoting tolerance of transplanted solid organs.5 This is supported by in vitro data demonstrating powerful suppression of alloreactive cells using Treg in mouse,6, 7 primate,8, 9 and human10 models as well as pig-to-primate xenogeneic models.11, 12
Although adoptive transfer of freshly sorted Treg has been shown to have suppressive effects, the number of cells obtained is often limited compared to the dose necessary for clinical effect.13 However, recent protocols have made possible massive expansion of Treg, including human Treg.13, 14 Expansion protocols vary widely, but all techniques begin with sorting a purified Treg population either by magnetic beads or by flow cytometry. These Treg are then expanded in culture conditions including interleukin (IL)-2 and some form of T-cell receptor stimulation, whether anti-CD3 antibody +/− anti-CD28 antibody or artificial antigen-presenting cells (APCs). The use of rapamycin also varies between protocols. All protocols remain experimental, and the only 2 previous reports of baboon (Papio hamadryas) Treg expansion produced approximately 200-fold expansion.11, 12 This is insufficient for a trial of pig-to-primate Treg therapy using baboons as the recipients or for clinical trials.
Although umbilical cord blood was the first Treg source used for expansion in a clinical trial,15 newer protocols have achieved superior results with peripheral blood, producing millions of cells that can be cryopreserved, thawed, and infused with good function.14 This technique could therefore be especially useful for xenotransplantation, in which the ability to prepare for transplantation between a known donor and known recipient at a planned time point makes possible the pre-expansion of donor-specific Treg in large numbers. These can then be cryopreserved and used when sufficient numbers have been generated.
However, the idea of cryopreserving Treg is controversial. Because of decreased viability on thawing, cryopreservation decreases total cell numbers due to decreased viability ranging from 50% to 80%,15 16 17 and it is not clear whether cryopreserved Treg maintain the same phenotype and function as fresh cells. Several studies have demonstrated that cryopreservation changes the phenotype of the frozen cells. Elkord et al18 demonstrated that percentages of CD4+ and CD4+/CD25+ cells decrease with a statistically significant decrease in the number of CD4+/Foxp3+ cells in human peripheral blood mononuclear cells (PBMC) frozen for 3 weeks. Seale et al19 showed that, in blood from human immunodeficiency virus–positive subjects, percentages of CD4+ cells staining positively for CD25 decreased significantly after cryopreservation. Sattui et al20 found that cryopreservation significantly decreased the percentage of CD4+/CD25+/Foxp3+ cells in both healthy and human immunodeficiency virus–infected human subjects. There is also some indication that cryopreservation decreases the suppressive function of Treg, although this function could be restored by a post-thaw period of restimulation or expansion before cryopreservation.21 Other reports suggest that neither Treg phenotype14 17 22 23 24 nor suppressive function14, 23 changed significantly after cryopreservation. Moreover, just as stimulation was shown to restore suppressive function after thawing, a brief (48 hours) restimulation period has been similarly shown to further enhance Foxp3 phenotype after thawing.22
In light of the possible applications of banked Treg for xenotransplantation, which we plan to explore in a preclinical pig-to-baboon model, and given these contradictory findings, we modified the Treg expansion protocol developed by Levings et al5 to optimize baboon Treg expansion. Furthermore, given the reported improvements in phenotype and function of Treg on post-thaw restimulation, we investigated the effect of cryopreservation on expanded baboon Treg by determining Treg phenotype and function immediately after thawing as well as after restimulation under various conditions.
MATERIALS AND METHODS
Growth Medium
Unless otherwise stated, medium used for this study (“growth medium”) consisted of RPMI (Gibco, Life Technologies) 85%, fetal calf serum (Gibco, Life Technologies) 10%, “nutrient mix” 4%, and 100× GlutaMAX (Gibco, Life Technologies) 1%. Nutrient mix consisted of penicillin-streptomycin 1000 units/mL (Gibco, Life Technologies) 25%, Sodium Pyruvate 100 mM (Gibco, Life Technologies, Grand Island, NY) 25%, Minimum Essential Medium (MEM) Non-Essential Amino Acids (NEAA) (Gibco, Life Technologies) 25%, and 100× GlutaMAX (Gibco, Life Technologies) 25%.
PBMC Isolation
Heparinized baboon blood (20 mL for each experiment) was obtained from the Mannheimer Foundation (Homestead, FL). It was shipped overnight on ice. Blood cells were separated from plasma by centrifugation and were diluted 1:1 with 1× phosphate-buffered saline (PBS, Fisher Scientific, Pittsburgh, PA). Density gradient centrifugation using 60% Percoll (Sigma, St. Louis, MO) was then used to separate PBMC.
Treg Sorting
Peripheral blood mononuclear cells were placed in staining media containing 90% PBS and 10% fetal bovine serum (Gibco, Life Technologies) with penicillin-streptomycin 1000 units/mL (Gibco, Life Technologies) at a concentration of 2.5 × 108 cells in 100 μL and stained for 1 hour with allophycocyanin (APC)-conjugated CD4 (Clone L200) (Beckton, Dickson, and Company, East Rutherford, NJ) and phycoerythrin (PE)-conjugated CD25 (Clone BC96) (BioLegend, San Diego, CA). The cells were then washed with sort medium containing 99.5% PBS, 0.5% fetal bovine serum (FBS), and 1:100 penicillin-streptomycin and sorted on a BD Influx cell sorter (BD Biosciences, San Jose, CA). We selected CD4+ lymphocytes that were in the top 1% of CD25 expression. This cutoff was chosen as it provided high Treg purity while producing enough Treg to be clinically applicable. We chose not to sort our Treg using CD127 for several reasons. First, we achieve good results without it (good expansion numbers, excellent suppression, relatively pure phenotype). Second, we have found in multiple experiments with baboon and cynomolgus monkey cells that CD127 tends to be less useful for differentiating Treg than it is in humans (data not shown). Third, we have found CD127 expression to be even less useful as a marker for identifying Treg after cells have been frozen. Finally, we and others have found that gating on CD127-low cells sometimes permits inclusion of Treg with lower CD25 expression, and these Treg tend to be less stable.25
Treg Expansion
Our protocol used artificial APCs for costimulation, as first reported by Dr. Levings et al.5 In our case, these were specifically modified “L cells,” a mouse fibroblast line virally transfected with the human adhesion molecule CD58 (to bind CD2 on target cells and stabilize the cell-cell interaction), Fc receptor CD32 (to bind the Fc receptor of the CD3 monoclonal antibody in culture to provide T-cell receptor stimulation), and the costimulatory ligand CD80 (obtained from Dr. Levings).26 Human CD58 and CD80 have been shown to cross-react with baboon.27, 28 Our protocol was further modified to include pulsed rapamycin (days 0 and 21) based on the results reported by Singh et al,29 and an additional 5 days in culture with rapamycin was added at the end of the protocol.
Freshly sorted Tregs from 1 of 2 experimental animals used were placed in 48-well flat-bottom tissue culture-treated plates (Falcon, Franklin Lakes, NJ) at a concentration of 1 × 105 or lower. At this and all restimulation time points, we added IL-2 (200 units/mL), purified anti-CD3 antibody (clone SP34, 200 ng/mL on day 0, 100 ng/mL on subsequent restimulation days, BD Pharmingen, San Jose, CA), and modified L cells irradiated at 50 Gy at a concentration of 1 × 105 cell/cm2 of flask area. Rapamycin (Sigma) was added at a concentration of 100 ng/mL on days 0 and 21. Cells were placed in a 37 °C incubator with 5% CO2 and 100% humidity and split as necessary, which was determined by crowded appearance of cells and change in medium color. Interleukin-2 and fresh growth medium were added after splitting. Cells were replaced in 6-well flat-bottom tissue culture-treated plates (Costar, Corning, NY), 75 cm2 flasks (Falcon), and 150 cm2 flasks (Corning, Corning, NY) as they reached confluence during expansion. Restimulations were carried out on days 7, 14, and 21. The cells were harvested on day 26. Throughout this process, all reagents contained antibiotics and were sterilely filtered, and all assays were performed in a level 2 biosafety cabinet. In addition, we performed screening cultures of all samples to detect any contamination.
PBMC and Treg Cryopreservation and Thawing
Before cryopreservation, cells were resuspended in cold freezing medium (10% dimethyl sulfoxide, 90% FBS) at a concentration of 1 to 2 × 106/mL. They were then frozen to −80 °C at 1°/minute using a propanol-based cell freezer (Nalgene, Rochester, NY) and stored for 2 months at −80 °C. At that point, the cells were thawed in a 37 °C water bath and diluted by dripping growth medium. The cells were washed twice with growth medium before use in assays. Viability of thawed cells was assessed by counting in Trypan Blue (Gibco, Life Technologies) diluted 1:1 with PBS.
Post-Thaw Restimulation
The thawed cells were cultured in 5 resting conditions as follows: medium plus (1) no additives, (2) IL-2 (200 units/mL), (3) anti-CD3 (100 ng/mL), (4) IL-2 + anti-CD3, and (5) IL-2 + anti-CD3 + L cells (1 million/well). The 4 × 106 freshly thawed Treg were plated in 3.5 mL of media with the appropriate additives in 1 well of a 6-well plate (Costar) and placed in a 37 °C incubator with 5% CO2 and 100% humidity for 48 hours. This experiment was performed on 3 Treg lines. Analysis of 1 line was performed in triplicate as an internal control to evaluate the experimental variation within a single line, which was minimal (data not shown). The mean percentages of each phenotype from this line were used as comparison with the other 2 lines.
Cell Phenotyping
Phenotypic analysis before and after cryopreservation was performed for a total of 5 times with 3 different Treg lines (1 line in triplicate, as discussed above). Only the reactivation conditions containing IL-2 produced enough viable cells for analysis. Cells were stained for fluorescence-activated cell sorting in medium containing 95% PBS, 5% FBS, and 1 g sodium azide/L (Sigma-Aldrich, St. Louis, MO). The cells were stained with PerCP-Cy5.5-conjugated CD3 (clone SP34-2) (BD Pharmingen), BV510-conjugated CD4 (clone L200) (BD Biosciences), APC-conjugated CD8 (clone BW135/80) (Miltenyi Biotec, San Diego, CA), BV421-conjugated CD25 (clone BC96) (BioLegend), and PE-conjugated Foxp3 (clone 236A/E7) (eBioscience, San Diego, CA). The intracellular Foxp3 staining was facilitated by using a Foxp3 fix/perm buffer set (BioLegend) according to the manufacturer's protocol. Stained cells were analyzed using a FACSCanto II Cell Analyzer (BD Biosciences). Results were analyzed using FlowJo version 9.5.3 (Tree Star, Ashland, OR).
Suppression Assay
Treg were adjusted to a concentration of 2 × 105/mL and plated in a 96-well plate (Costar) in triplicate at 1 × 104 per well in 50 μL. These were then serially diluted with growth medium to a dilution of 1:64. Bulk PBMC from the same animal were then thawed and similarly adjusted to a concentration of 2 × 105 and plated in the same wells at 1 × 104 per well in 50 μL. For stimulation, anti-CD3/anti-CD28 beads (Miltenyi Biotec) were then added in 100 μL/well at a bead:responder concentration of 1:2. Control wells contained Treg alone, PBMC alone, PBMC with beads, and Treg with beads. The cells were placed in a 37 °C incubator with 5% CO2 and 100% humidity for 4 days, at which point 3H-thymidine was added at 1 μCi in 50 μL per well. The plate was incubated for 24 hours longer, harvested using a plate washer (TomTec Life Sciences, Hamden, CT), read on a β counter (PerkinElmer, Waltham, MA), and analyzed using MicroBeta Windows Workstation software (PerkinElmer). Percent proliferation was calculated by dividing the averaged counts per minute of each triplicate by the averaged counts per minute of the positive control wells (no Treg). To ensure the validity of our results (confirmed with multiple linear regression), we used for analysis only the 2 Treg lines that had full data under each condition (precryopreservation, freshly thawed, and the 3 restimulation conditions that included IL-2). However, the results were not substantially different when near-complete data from the other lines were added to the analysis (results not shown). Conditions without IL-2 did not produce enough viable cells to analyze.
Statistics
Statistical analysis and comparisons (analysis of variance with Tukey post hoc test) were performed using GraphPad Prism version 5.04 for Windows (GraphPad Software, San Diego, CA, www.graphpad.com). Multiple linear regression was performed using STATA (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP). Data in bar and line graphs are expressed as mean ± standard deviation.
RESULTS
Expansion
Under our standard expansion protocol of 26 days in culture with IL-2, anti-CD3 antibody, rapamycin, and artificial APCs transfected with human CD58, CD32, and CD80 with weekly restimulations (see Methods), we expanded from a range of 71.5 × 103 to 157 × 103 Treg on day 0 to a range of 184 × 106 to 772 × 106 Treg on day 26. Figure 1 shows the expansion curves for five different expanded baboon Treg lines using our protocol.
FIGURE 1.
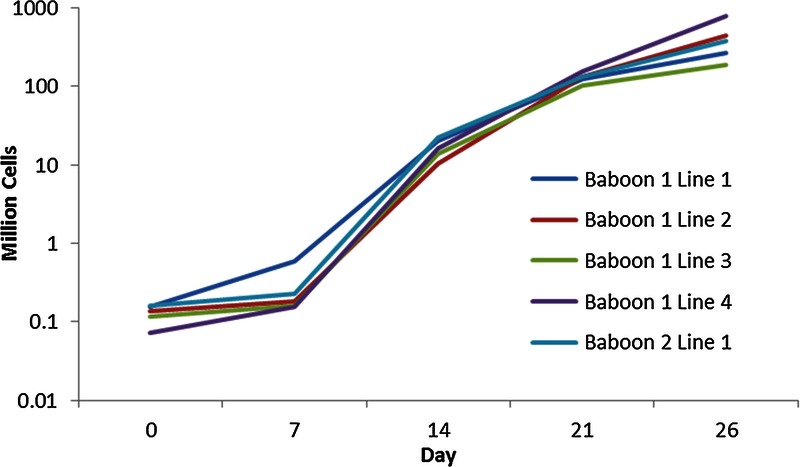
Expanded Treg lines. Treg expansion in five lines between 2 animals using the protocol described in this paper. Expansion levels were as high as 10,000-fold.
To determine the optimal post-thaw restimulation conditions, 4 × 106 thawed cells were plated for 5 different test conditions for 48 hours: medium plus (1) no additives, (2) IL-2, (3) anti-CD3, (4) IL-2 + anti-CD3, and (5) IL-2 + anti-CD3 + L cells (artificial APCs transfected with human CD58, CD32, and CD80, see Methods). Survival was negligible in the 2 conditions lacking IL-2 (conditions 1 and 3). The best survival was in condition 5 (mean 23.25 ± 0.81 × 106 cells) followed by conditions 4 (1.33 ± 0.43 × 106 cells) and 2 (1.23 ± 0.36 × 106 cells) (Figure 2). There was no significant difference in survival between conditions 2 and 4, but the difference in survival between condition 5 and the others was significant (P = 0.009).
FIGURE 2.
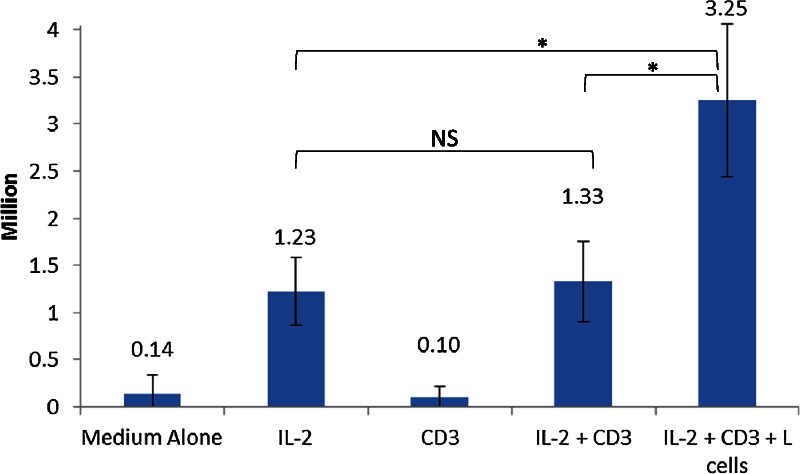
Cell numbers after reactivation. Four million thawed Treg were plated for 48 hours in each of 5 conditions: media plus (1) no additives, (2) IL-2, (3) anti-CD3, (4) IL-2 + anti-CD3, and (5) IL-2 + anti-CD3 + L cells (artificial APCs transfected with human CD58, CD32, and CD80). Average cell numbers and standard deviation for each condition after 48 hours are shown. This experiment was performed 5 times using 3 unique Treg lines. NS: non-significant. *: P < 0.05 by ANOVA. ANOVA, analysis of variance.
Phenotype
There was a trend toward a more pure Treg population after cryopreservation, thawing, and reactivation. CD4 percentage immediately after thawing was 83.6% ± 9.3% (compared to 78.6% ± 10.0% before cryopreservation) and increased to 87.8% ± 10.4% (condition 2), 93.4% ± 4.3% (condition 4), and 90.8% ± 3.2% (condition 5). Almost all CD4+ cells were also CD25+, greater than 98% in all conditions (Figure 3). The remaining CD3+ cells not expressing CD4 were almost entirely CD8+, and the trend toward an increased percentage of CD4+ cells with progressively more restimulation (P = 0.13) corresponded with an even stronger trend toward decreased percentage of CD8+ cells (P = 0.059), producing a more pure Treg phenotype overall. CD8 percentage immediately after thawing was 9.5% ± 7.6% (compared to 16.5% ± 10.1% before cryopreservation) and decreased to 3.08% ± 4.2% (condition 2), 2.16% ± 2.9% (condition 4), and 1.6% ± 2.0% (condition 5). Notably, however, the percentage of CD4+ cells expressing Foxp3 or double-positive for CD25 and Foxp3 did not vary significantly between conditions (Figure 3).
FIGURE 3.
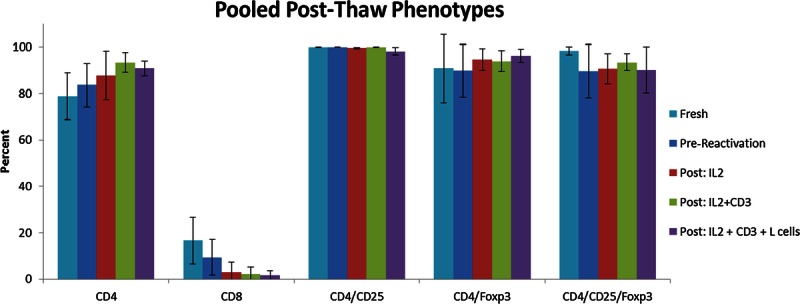
Phenotypes after cryopreservation. Pooled phenotypes as determined by flow cytometry immediately after thawing and after restimulation for 48 hours in the 3 conditions that permitted cell survival. Shown are average percentages and standard deviation for 5 separate experiments using 3 unique Treg lines. Phenotypic differences are not statistically significant (P ≥ 0.05), but there was a trend toward significance for CD4 (P = 0.13) and CD8 (P = 0.059). Gates for CD25 and Foxp3 were established using isotype controls.
Suppressive Function
Cryopreservation significantly decreased the suppressive function of expanded Treg, but function was restored and even somewhat improved by post-thaw reactivation. Overall difference in suppression between conditions was calculated using multiple linear regression controlling for differences in line and titer. Suppression was significantly decreased immediately after thawing compared to other conditions (P < 0.001) and significantly greater than other conditions when thawed cells were maximally restimulated (condition 5, P < 0.001).
This effect was found to be dose-dependent when Treg were plated at various titers with autologous PBMC and anti-CD3/CD28 stimulator beads (Miltenyi Biotec). Higher PBMC proliferation indicates less potent Treg-mediated suppression, and decreased PBMC proliferation indicates more potent Treg-mediated suppression. At greater Treg dilutions (1:32 through 1:4), the percent of PBMC proliferation was significantly greater in wells with freshly thawed Treg compared to wells with Treg plated before cryopreservation or after cryopreservation plus reactivation (P ≤ 0.0001 for all). At 1:32, 1:16, and 1:4 Treg:PBMC titers, PBMC proliferation was higher in wells with freshly thawed Treg than in wells with Treg generated under all other conditions, and at 1:8, it was higher compared to all conditions except those with Treg undergoing the least amount of post-thaw restimulation (condition 2) (P ≤ 0.0001). Thus, suppressive function is clearly diminished by the cryopreservation and thawing process.
However, increasing amounts of reactivation after thawing appeared to restore or even enhance the suppressive function. In fact, at certain dilutions (1:1 through 1:4 as well as 1:64), proliferation was even further suppressed in wells with maximally restimulated Treg (condition 5, P < 0.0007 for all) (Figure 4). At 1:64, 1:4, and 1:1 dilutions, PBMC proliferation was significantly lower in wells with Treg undergoing maximal restimulation after thawing (condition 5) than all others, including fresh Treg before cryopreservation (P < 0.0001), and lower than all except fresh Treg at 1:2 (P = 0.0007). At 1:4, PBMC proliferation was progressively decreased (indicating more potent suppression) with increased reactivation (condition 5 > condition 4 > condition 2 > freshly thawed), with noncryopreserved Treg as suppressive as those in condition 4 (P < 0.0001) (Figure 4).
FIGURE 4.
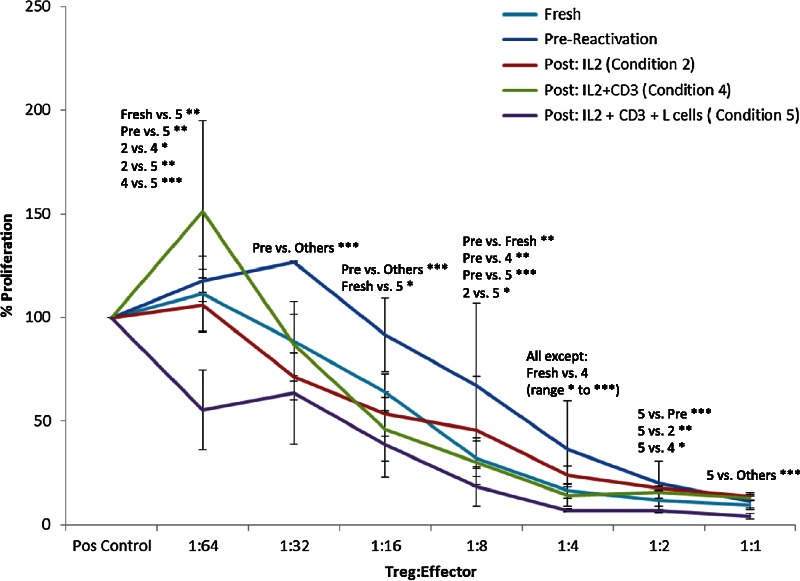
Suppression after cryopreservation. Pooled suppression data from suppression assays carried out using Treg before freezing, immediately after thawing, and after restimulation for 48 hours in the 3 conditions that permitted cell survival. Shown are average percentages and standard deviation for responder cell proliferation stimulated by anti-CD3/CD28 beads at various titrations of Treg from 2 different lines. There is a significant difference in suppression under different conditions at each titer by ANOVA (P = 0.0007 at 1:2, otherwise P ≤ 0.0001). The specific conditions that differ by Tukey post hoc analysis are specified above each titer. For post hoc analysis, *P ≤ 0.05; **P ≤0.01; ***P ≤ 0.001.
DISCUSSION
Our protocol for expanding baboon Treg, reported herein, not only produces far greater numbers of Treg than those previously reported for this species (approximately 50-fold more) but also compares favorably with other previously reported protocols for expanding human and nonhuman primate Treg.5 8 10 11 12 16 29 30 31 In contrast to some previous reports, our Treg do not lose their phenotype after cryopreservation, although they do lose suppressive function. However, suppressive function is restored or even enhanced with increasing amounts of restimulation after thawing, coinciding with a trend toward a more pure Treg population. Although increasing amounts of restimulation did not correlate with Foxp3 expression by CD4+ cells, it led to increased CD4 percentage and decreased CD8 percentage, thereby decreasing contamination and representing a trend toward a higher percentage of Treg. Thus, our protocol, at least in this small sample size, produces enough Treg for clinical use and maintains their viability, phenotype, and function after cryopreservation. These achievements are a necessary precursor to using baboon Treg in preclinical xenotransplantation models.
In developing our protocol, we adapted from among those previously published. Almost all involve sorting Treg via flow cytometry for CD4+/CD25hi ± CD127 low. This is followed by culturing in media supplemented by nutrients, antibiotics (penicillin/streptomycin), and 5% or 10% serum. Culture times vary widely from a matter of days31 to more than one month.14 All culture conditions contain IL-2 (usually at 200-300 international units (IU) per mL) and either anti-CD3 beads ± anti-CD28 beads or APCs, whether donor-derived or artificial. More recently, it has been shown that adding rapamycin to the culture conditions enhances the suppressive function of expanded Tregs,29 and there is some evidence that adding IL-15 to culture conditions augments the effect of rapamycin in culture and allows the use of lower doses of IL-2.10 Cells are either split or refed on a specific schedule10 or as needed.8, 29 Among these various options, we chose to use rapamycin and stimulate our Treg with L cells expressing high levels of costimulatory molecules and an Fc receptor. This resulted in excellent Treg expansion with high purity of CD4+/CD25hi/FoxP3+ cells. Of the small number of non-Treg cells produced in our culture, the major population before cryopreservation was CD8+ T cells, and the percentage of these cells decreased markedly after thawing and restimulating.
Relevant to the controversy as to whether cryopreservation causes Treg to lose their characteristic phenotype and function, our results show that Treg numbers decreased, but surviving Treg maintained their phenotype after cryopreservation, although their suppressive function was significantly decreased. However, restimulating in the presence of IL-2 for 48 hours after thawing preserved or even enhanced both phenotypic purity and suppressive function. This finding remains surprising given the many ways that cryopreservation can affect Treg. One main factor is the toxic effect of dimethyl sulfoxide on Treg and indeed all PBMCs,32 but cryopreservation has also been shown to cause oxidative stress from free radicals, cell membrane and structural damage from ice crystals, osmotic injury, and significant changes in cell metabolism and membrane channels.16
In fact, it is more difficult to imagine why cryopreservation does not affect Treg function and phenotype more, especially given previously reported findings. One possibility is suggested by the finding that IL-2 production is increased after cryopreservation compared to fresh cells.33 This could explain both Treg survival as well as our findings that Treg phenotype became more pure after thawing, although it is not clear if IL-2 production would be similarly increased in a nearly-pure culture of Treg. Because Treg are partially defined by their expression of CD25, which is the α chain of the IL-2 receptor,34 increased levels of IL-2 would both promote and favor Treg survival. Even if IL-2 production is not increased in our culture, the exogenously added IL-2 might still favor Treg survival. In the presence of IL-2 but the absence of other survival signals, such as IL-7 and IL-15, we might expect that Treg would have comparatively better survival, especially after a cellular stressor, such as cryopreservation and thawing that makes cells more susceptible to apoptosis.35 This could explain the trend toward increased CD4 and decreased CD8 percentages and why Treg markers also increased with post-thaw stimulation in previously published findings.22
This trend toward increased purity of the Treg population could be responsible for the increased suppression seen after post-thaw restimulation. It is also possible that Treg purity is irrelevant and that post-thaw restimulation simply allows Treg to regain cellular metabolic functions that are lost or impaired during cryopreservation. In fact, the survival signal IL-2 alone was enough to increase suppression, although costimulation augmented this effect. This is also supported by the lack of significant change in the percentage of Foxp3 expression. To the extent that cryopreservation decreases Treg suppressive function, it is worth noting that the effect may be even more pronounced in nonexpanded Treg. Peters et al21 found that, while activation was necessary to restore suppressive function of purified but nonexpanded Treg after cryopreservation, suppressive function of expanded Treg did not decrease.
Finally, despite repeated rounds of restimulation, our baboon Treg displayed excellent suppressive function. However, there is evidence that Treg that remain in ex vivo culture for too long begin losing their characteristic phenotype and function.36 Similarly, it has been shown that too many restimulations decrease Treg suppressive function despite continued expression of Foxp3.14 We chose to restimulate for 48 hours after thawing to allow Treg sufficient time to recover function after the thawing process. Our entire process involved 4 restimulations, including the post-thaw stimulation. These Treg still maintained robust function at the end of this process, with 50% suppression seen at greater than 1:16 effector-to-Treg ratio.
In summary, we have developed a protocol for massive expansion of baboon Treg to far greater numbers than those previously described. Moreover, our results show that, although these expanded Treg show a significant decrease in their suppressive function after cryopreservation, they maintain their phenotype, and restimulation after thawing maintains or even enhances both. This is likely due to preferential survival of Treg under these conditions. These results have important implications for the future use of baboon recipient Treg in preclinical trials of xenotransplantation and eventual clinical use by facilitating the ability to create and bank expanded donor-specific Treg lines from the recipient before transplantation. The lack of correlation between Foxp3 expression and suppressive function also raises interesting questions for future research.
ACKNOWLEDGMENTS
The authors thank Drs. Bruce Levin and Codruta Chiuzan for their statistical support and Drs. Hao Wei Li and Nichole Danzl for their helpful review of the manuscript.
Footnotes
Published online 6 March 2015.
This work was generously supported by NIH Grant 5T32HL007854-19, the Louis V. Gerstner Foundation, the Columbia Department of Surgery, the John Jones Surgical Society Research Fellowship, the Irving Institute Foundation Grant, and the Kenneth A. Forde Surgical Research Award. These studies were performed in the Columbia Center for Translational Immunology Flow Cytometry Core funded in part through an S10 Shared Instrumentation Grant 1S10RR027050.
The authors declare no conflicts of interest.
J.W. participated in the performance of the research, the data analysis, and the writing of the paper. R.D.S. participated in the data analysis. J.Z. participated in the performance of the research. L.B. and H.S. participated in the research design. A.M. and M.L. participated in the research design. M.S. participated in the research design, the data analysis, and the writing of the paper. A.G. directed the project and participated in the research design, the data analysis, and the writing of the paper.
REFERENCES
- 1. Juvet SC, Whatcott AG, Bushell AR, Wood KJ. Harnessing regulatory T cells for clinical use in transplantation: the end of the beginning. Am J Transplant 2014;14 (4):750. [DOI] [PubMed] [Google Scholar]
- 2. Trzonkowski P, Bieniaszewska M, Juscinska J, et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4 + CD25 + CD127− T regulatory cells. Clin Immunol 2009;133 (1):22. [DOI] [PubMed] [Google Scholar]
- 3. Schliesser U, Streitz M, Sawitzki B. Tregs: application for solid-organ transplantation Curr Opin Organ Transplant. 2012;17 (1):34. [DOI] [PubMed] [Google Scholar]
- 4. Trzonkowski P, Dukat-Mazurek A, Bieniaszewska M, et al. Treatment of graft-versus-host disease with naturally occurring T regulatory cells. BioDrugs 2013;27 (6):605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levings MK, Sangregorio R, Roncarolo MG. Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med 2001;193 (11):1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hanash AM, Levy RB. Donor CD4 + CD25+ T cells promote engraftment and tolerance following MHC-mismatched hematopoietic cell transplantation. Blood 2005;105 (4):1828. [DOI] [PubMed] [Google Scholar]
- 7. Joffre O, Gorsse N, Romagnoli P, Hudrisier D, van Meerwijk JP. Induction of antigen-specific tolerance to bone marrow allografts with CD4 + CD25+ T lymphocytes. Blood 2004;103 (11):4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anderson A, Martens CL, Hendrix R, et al. Expanded nonhuman primate tregs exhibit a unique gene expression signature and potently downregulate alloimmune responses. Am J Transplant 2008;8 (11):2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bashuda H, Kimikawa M, Seino K, et al. Renal allograft rejection is prevented by adoptive transfer of anergic T cells in nonhuman primates. J Clin Invest 2005;115 (7):1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Veerapathran A, Pidala J, Beato F, Yu XZ, Anasetti C. Ex vivo expansion of human Tregs specific for alloantigens presented directly or indirectly. Blood 2011;118 (20):5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Porter CM, Horvath-Arcidiacono JA, Singh AK, Horvath KA, Bloom ET, Mohiuddin MM. Characterization and expansion of baboon CD4 + CD25+Treg cells for potential use in a non-human primate xenotransplantation model. Xenotransplantation 2007;14 (4):298. [DOI] [PubMed] [Google Scholar]
- 12. Singh AK, Seavey CN, Horvath KA, Mohiuddin MM. Ex-vivo expanded baboon CD4+ CD25 Hi Treg cells suppress baboon anti-pig T and B cell immune response. Xenotransplantation 2012;19 (2):102. [DOI] [PubMed] [Google Scholar]
- 13. Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity 2009;30 (5):656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hippen KL, Merkel SC, Schirm DK, et al. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med 2011;3 (83):83ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics Blood. 2011;117 (3):1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Golab K, Leveson-Gower D, Wang XJ, et al. Challenges in cryopreservation of regulatory T cells (Tregs) for clinical therapeutic applications. Int Immunopharmacol 2013;16 (3):371. [DOI] [PubMed] [Google Scholar]
- 17. Van Hemelen D, Oude Elberink JN, Heimweg J, van Oosterhout AJ, Nawijn MC. Cryopreservation does not alter the frequency of regulatory T cells in peripheral blood mononuclear cells. J Immunol Methods 2010;353 (1–2):138. [DOI] [PubMed] [Google Scholar]
- 18. Elkord E. Frequency of human T regulatory cells in peripheral blood is significantly reduced by cryopreservation. J Immunol Methods 2009;347 (1–2):87. [DOI] [PubMed] [Google Scholar]
- 19. Seale AC, de Jong BC, Zaidi I, et al. Effects of cryopreservation on CD4+ CD25+ T cells of HIV-1 infected individuals. J Clin Lab Anal 2008;22 (3):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sattui S, de la Flor C, Sanchez C, et al. Cryopreservation modulates the detection of regulatory T cell markers. Cytometry B Clin Cytom 2012;82 (1):54. [DOI] [PubMed] [Google Scholar]
- 21. Peters JH, Preijers FW, Woestenenk R, Hilbrands LB, Koenen HJ, Joosten I. Clinical grade Treg: GMP isolation, improvement of purity by CD127 Depletion, Treg expansion, and Treg cryopreservation. PLoS One. 2008;3 (9):e3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kivling A, Nilsson L, Faresjo M. How and when to pick up the best signals from markers associated with T-regulatory cells? J Immunol Methods 2009;345 (1–2):29. [DOI] [PubMed] [Google Scholar]
- 23. Mavin E, Dickinson A, Wang XN. Do cryopreserved regulatory T cells retain their suppressive potency? Transplantation 2013;95 (11):e68. [DOI] [PubMed] [Google Scholar]
- 24. Venet F, Malcus C, Ferry T, Poitevin F, Monneret G. Percentage of regulatory T cells CD4 + CD25 + CD127− in HIV-infected patients is not reduced after cryopreservation. J Immunol Methods 2010;357 (1–2):55. [DOI] [PubMed] [Google Scholar]
- 25. Komatsu N, Okamoto K, Sawa S, et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med 2014;20 (1):62. [DOI] [PubMed] [Google Scholar]
- 26. de Waal Malefyt R, Verma S, Bejarano MT, Ranes-Goldberg M, Hill M, Spits H. CD2/LFA-3 or LFA-1/ICAM-1 but not CD28/B7 interactions can augment cytotoxicity by virus-specific CD8+ cytotoxic T lymphocytes. Eur J Immunol 1993;23 (2):418. [DOI] [PubMed] [Google Scholar]
- 27. Chisholm PL, Williams CA, Jones WE, et al. The effects of an immunomodulatory LFA3-IgG1 fusion protein on nonhuman primates. Ther Immunol 1994;1 (4):205. [PubMed] [Google Scholar]
- 28. Lazetic S, Leong SR, Chang JC, Ong R, Dawes G, Punnonen J. Chimeric co-stimulatory molecules that selectively act through CD28 or CTLA-4 on human T cells. J Biol Chem. 2002;277 (41):38660. [DOI] [PubMed] [Google Scholar]
- 29. Singh K, Kozyr N, Stempora L, et al. Regulatory T cells exhibit decreased proliferation but enhanced suppression after pulsing with sirolimus. Am J Transplant 2012;12 (6):1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gregori S, Bacchetta R, Passerini L, Levings MK, Roncarolo MG. Isolation, expansion, and characterization of human natural and adaptive regulatory T cells. Methods Mol Biol 2007;380:83. [DOI] [PubMed] [Google Scholar]
- 31. Putnam AL, Safinia N, Medvec A, et al. Clinical grade manufacturing of human alloantigen-reactive regulatory T cells for use in transplantation. Am J Transplant 2013;13 (11):3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kloverpris H, Fomsgaard A, Handley A, Ackland J, Sullivan M, Goulder P. Dimethyl sulfoxide (DMSO) exposure to human peripheral blood mononuclear cells (PBMCs) abolish T cell responses only in high concentrations and following coincubation for more than two hours. J Immunol Methods 2010;356 (1–2):70. [DOI] [PubMed] [Google Scholar]
- 33. Venkataraman M. Cryopreservation-induced enhancement of interleukin-2 production in human peripheral blood mononuclear cells. Cryobiology 1992;29 (2):165. [DOI] [PubMed] [Google Scholar]
- 34. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995;155 (3):1151 [PubMed] [Google Scholar]
- 35. Dai Z, Zhang S, Xie Q, et al. Natural CD8 + CD122+ T cells are more potent in suppression of allograft rejection than CD4 + CD25+ regulatory T cells. Am J Transplant 2014;14 (1):39 [DOI] [PubMed] [Google Scholar]
- 36. Marek N, Bieniaszewska M, Krzystyniak A, et al. The time is crucial for ex vivo expansion of T regulatory cells for therapy. Cell Transplant 2011;20 (11–12):1747. [DOI] [PubMed] [Google Scholar]


