Abstract
Objective
To provide an overview of the preclinical literature on progesterone for neuroprotection after traumatic brain injury (TBI), and to describe unique features of developmental brain injury that should be considered when evaluating the therapeutic potential for progesterone treatment after pediatric TBI.
Data Sources
National Library of Medicine PubMed literature review.
Data Selection
The mechanisms of neuroprotection by progesterone are reviewed, and the preclinical literature using progesterone treatment in adult animal models of TBI are summarized. Unique features of the developing brain that could either enhance or limit the efficacy of neuroprotection by progesterone are discussed, and the limited preclinical literature using progesterone after acute injury to the developing brain is described. Finally, the current status of clinical trials of progesterone for adult TBI is reviewed.
Data Extraction and Synthesis
Progesterone is a pleotropic agent with beneficial effects on secondary injury cascades that occur after TBI, including cerebral edema, neuroinflammation, oxidative stress, and excitotoxicity. More than 40 studies have used progesterone for treatment after TBI in adult animal models, with results summarized in tabular form. However, very few studies have evaluated progesterone in pediatric animal models of brain injury. To date, two human Phase II trials of progesterone for adult TBI have been published, and two multi-center Phase III trials are underway.
Conclusions
The unique features of the developing brain from that of a mature adult brain make it necessary to independently study progesterone in clinically relevant, immature animal models of TBI. Additional preclinical studies could lead to the development of a novel neuroprotective therapy that could reduce the long-term disability in head-injured children, and could potentially provide benefit in other forms of pediatric brain injury (global ischemia, stroke, statue epilepticus).
Keywords: neurosteroid, developmental brain injury, trauma, children, infant
Introduction
Every year in the United States alone, nearly a half million children sustain TBI, and ∼3,000 children per year die from these injuries (CDC). Despite recent advances in neurointensive care and reduction in the overall mortality rate (1), the long-term morbidity of severe TBI in childhood remains high. Survivors of pediatric TBI suffer from many long-term physical, cognitive, psychological, and emotional impairments (2,3). After TBI, a cascade of secondary insults lead to cell death. The developing brain may be uniquely vulnerable to some of these secondary insults, due to maturational features. The steroid hormone progesterone has been studied extensively in preclinical models of adult TBI. It provides multiple mechanisms of neuroprotection that could be very important after pediatric TBI, such as reducing cerebral edema, as well as anti-inflammatory, antioxidant, anti-apoptotic and antiexcitatory properties. This review will highlight progress to date in using progesterone for neuroprotection after TBI, and will discuss unique features of developmental brain injury that should be considered when evaluating the therapeutic potential for progesterone treatment after pediatric TBI.
Progesterone in Preclinical Studies of Adult TBI
Over the last 25 years, many preclinical studies have demonstrated neuroprotection by progesterone after TBI in adult animal models (4-13). Dr. Stein and colleagues began investigating progesterone after observing that female rats recovered better than male rats after TBI (14). In initial studies, they compared three groups of adult rats (normal male rats, normally cycling female rats in proestrus, and pseudopregnant female rats with high circulating progesterone) (15). They found that normal female rats had less brain edema at 24h after TBI compared to male rats, and that the pseudopregnant females had remarkably little brain edema compared to the other 2 groups. Follow-up studies showed that exogenous treatment of male rats with progesterone reduced cerebral edema, lesion volume and neuronal loss after TBI (15). Similarly, Bramlett and Dietrich compared male rats, normal female rats and ovariectomized female rats showing the smallest lesion volumes in normal females compared to the other 2 groups (16). Dr. Stein's group also showed that the therapeutic window for progesterone could be up to 24h after TBI, when targeting cerebral edema (17), and that there is a u-shaped dose response curve for improving cognitive outcomes, including memory acquisition in the Morris water maze (18). In addition they described potentially detrimental symptoms of abrupt progesterone withdrawal that may warrant tapering (19). Other investigators have confirmed the neuroprotective properties of progesterone using a variety of TBI models in adult animals. We performed a Pubmed search of all original preclinical research studies of progesterone use in adult TBI published between 1992 and 2013. The 46 identified studies are summarized in Supplemental Digital Content - Table 1. In addition, a recent preclinical systematic review summarized the key aspects of progesterone treatment for neuroprotection after TBI and cerebral ischemia in adult animals, looking at effects on lesion volume (20). The main finding was that progesterone was neuroprotective, but limitations in the literature were identified, including insufficient examination of dose-response relationships, therapeutic windows, and evaluation in female or aged adult animals.
Mechanism of Neuroprotection by Progesterone
Progesterone is a pleotropic agent with beneficial effects on various secondary injury cascades that are set into motion after traumatic brain injury (Fig. 1) (21,22). The main therapeutic effect of progesterone and its metabolites is thought to be via decreasing cerebral edema (10,23-25). By modulating p-glycoprotein and aquaporin 4 (AQP4) levels it helps maintain blood brain barrier (BBB) integrity (25). Progesterone upregulates p-glycoprotein levels leading to increases in efflux pump in BBB and decreases in cerebral edema (25,26). AQP4 is from a family of water-selective membrane channels which is mainly expressed in perivascular astrocytic endfeet (27). Guo et al., showed that bilateral contusion injuries of the medial frontal cortex resulted in increased water content in the pericontusional area accompanied by increased expression of AQP4 in the pericontusional area and lateral ventricles (25). In contrast there was a significant decrease in AQP4 expression in the tissue surrounding the third ventricle. Progesterone treatment decreased brain water content and AQP4 expression in the pericontusional areas and in the tissue surrounding the lateral ventricles; while increasing AQP4 expression in the tissue adjacent to the third ventricle. The authors speculated that by increasing AQP4 expression in the osmosensory areas in the hypothalamus surrounding the third ventricle, progesterone might have contributed to enhanced water drainage leading to preservation of osmotic equilibrium in the brain. Corroborating the importance of AQP4 as a therapeutic target for pediatric TBI, a recent study showed decreased edema formation, decreased blood-brain barrier disruption, improved motor and long-term cognitive function with inhibition of AQP4 expression by injection of small-interfering RNA (siRNA) targeting AQP4 after controlled cortical impact injury in the developing brain (28).
Figure 1.
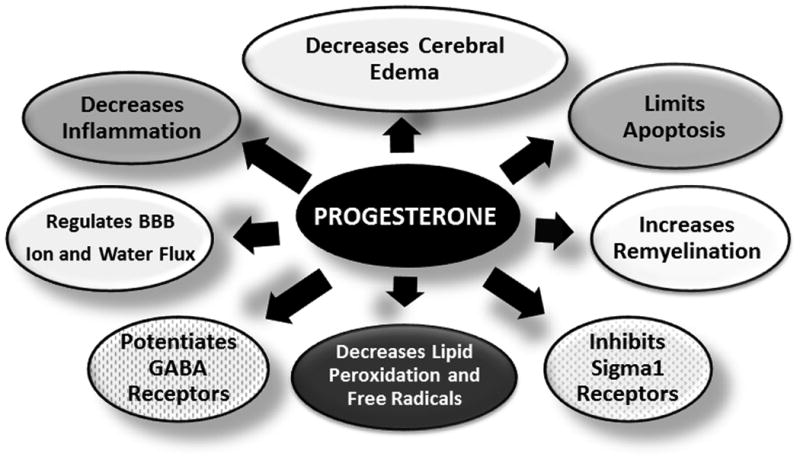
The putative neuroprotective mechanisms of the pleotropic agent progesterone are detailed in this figure. These mechanisms all play a role in secondary injury following TBI.
Progesterone has been shown to have anti-inflammatory effects by suppressing generation of pro-inflammatory cytokines and reducing microglial activation (4,23,24,29,30). These anti-inflammatory effects could be especially important after TBI, where marked neuroinflammation contributes to many aspects of secondary brain injury, such as vascular endothelial injury and disruption of the blood brain barrier (31). Progesterone administration after TBI in animal models decreased production of TNF-α, IL1, IL 6, TLR 2, TLR 4 as well as NF-KB binding activity (4,23,24). In addition, primary microglia cultures which were exposed to LPS or pro-inflammatory cytokines(INF-γ and TNF-α) showed an increase in nitric oxide (NO) levels and administration of progesterone decreased levels of NO by inhibiting the production of inducible nitric oxide synthase (iNOS) which catalyzes the synthesis of NO (29,30). Likely related to its anti-inflammatory effects, progesterone has been shown to reduce lipid peroxidation (9). By upregulating expression of superoxide dismutase, progesterone might also have a direct effect in the control of excess superoxide generation after TBI (9,32).
A direct antiapoptotic effect of progesterone has been postulated based on the work showing that progesterone increases anti-apoptotic Bcl-2 protein levels, decreases pro-apoptotic Bax, and Bad protein levels, as well as inhibits TBI-induced release of mitochondrial pro-apoptotic factor cytochrome c, and activation of caspase 3 resulting in improved functional outcome (13,33).
Progesterone also has effects on GABA and NMDA receptors. One of the key mechanisms of secondary injury after TBI is excitotoxicity, which is mediated by the release of excitatory neurotransmitter glutamate into the extracellular space leading to the activation of both ionotropic receptors, labeled according to specific agonists (N-methyl d-aspartate [NMDA], kainate, and [alpha]-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid [AMPA]) and receptors linked to second messenger systems, called metabotropic receptors. Activation of these receptors leads to calcium influx through receptor-gated or voltage-gated channels, or through the release of intracellular calcium stores. Increased intracellular calcium concentration is the trigger for a number of processes that can lead to neuronal death (reviewed in (21)). Thus inhibitors of receptor-gated or voltage-gated rise in intracellular calcium after injury are expected to result in prevention of neuronal death. Progesterone has been shown to attenuate the rise in intracellular calcium by its effects on both the receptor-gated and the voltage-gated channels after focal cerebral ischemia in vivo or prolonged depolarization of striatal neurons in vitro (34,35). Progesterone also has effects on the receptors that respond to gamma-aminobutyric acid (GABA), the chief inhibitory neurotransmitter in the CNS. Studies in oxygen-glucose deprivation model of neuronal ischemia show that progesterone increases GABAergic activity, resulting in decreased neuronal excitability and consequent protection from excitotoxicity (36). It is likely that progesterone increases GABAergic activity indirectly, through metabolites that potentiate GABAA receptors, thus prolonging miniature inhibitory postsynaptic current (chloride), and hyperpolarizing post-synaptic neurons that inhibit further excitation receptor activity (36-38). The studies in ischemia and epilepsy models support a role for progesterone against excitotoxicity after TBI. Direct investigation of the effects of progesterone on GABA and NMDA receptors after TBI is more limited. Studies using the medial frontal cortex injury showed no effect of progesterone on GABAA receptor expression in the medial dorsal thalamic nucleus, an area with significant cell loss in this model (18). They suggest that evaluation of specific subunits of the GABAA receptor may correlate better with functional outcome. Additional studies by this group showed that abrupt progesterone withdrawal, as prompted by intermittent injections, could lead to abrupt decreases in GABAA activity and a more excitotoxic environment (39). Therefore, the approach to progesterone dosing is important when considering NMDA/GABA receptor effects.
A final important aspect of progesterone neuroprotection is through effects on remyelination. The process of remyelination is an important part of long-term recovery following TBI. During remyelination after injury, expression of mRNA for cytochrome P450scc (converts cholesterol to pregnenolone), 3beta-hydroxysteroid dehydrogenase (converts pregnenolone to progesterone) and progesterone receptors are increased (40). Supporting a positive effect of progesterone on remyelination, it has been shown that progesterone treatment increases the number of mature oligodendrocytes and the rate of myelin formation in Schwann cells (41-45), while blocking progesterone biosynthesis results in demyelination (41).
Progesterone metabolism and progesterone receptors in the developing brain
A complete discussion of neurosteroid production, metabolism and receptor action is out of the scope of this review. The reader is referred to several key reviews in the field (46-50). Briefly, progesterone is synthesized from pregnenolone by 3β hydroxysteroid dehydrogenase, an enzyme that has been shown to be present in both neurons and glia in rat brains (50-53). Baulieu et. al. showed that progesterone is a true neurosteroid, by documenting the synthesis of progesterone in the brain (54). Progesterone is metabolized by the enzyme 5alpha reductase to 5α-dihydroprogesterone, and then to the neurosteroid allopregnanolone by the reversible enzyme 3 α hydroxysteroid dehydrogenase (55). Allopregnanolone is felt to be one of the key metabolites responsible for neuroprotection after brain injury. The synthesis, concentration of, and metabolism of progesterone and allopregnanolone change throughout development, and vary by brain region studied. A recent review summarizes these development- and region-specific changes in neurosteroids (50).
In addition to developmental changes in progesterone brain concentration, there are developmental changes in progesterone receptor expression. The laboratory group of Wagner et. al. has produced a large body of work evaluating the role of progesterone and progesterone receptors during normal brain development (49,56). They suggest that the influence of progesterone on the perinatal brain has been overlooked, and that progesterone may play a role in the development of brain and behavior. Furthermore, the progesterone receptor is expressed throughout the forebrain during critical stages of brain maturation in the rodent, and may influence neuronal migration, synaptogenesis, and cell death (57,58). Importantly, Wagner et. al. suggest that this could be true in human brain development, as children of women who were treated with progesterone during pregnancy for prevention of miscarriage had improved intellectual and behavioral performance in childhood (49,59,60).
In summary, the endogenous brain levels of progesterone, as well as regional brain expression of progesterone receptors, are influenced throughout development by species studied, other brain hormones, and specific developmental timepoint being studied. These complex interactions need to be understood and taken into consideration when planning to use progesterone for neuroprotective therapy after pediatric TBI.
Unique features of the developing brain
Many of the pathologic cascades that are activated following TBI are developmentally regulated. Some developmental features could confer improved benefits compared to the adult brain, while other developmental features could limit progesterone's effectiveness after pediatric TBI. For example, progesterone influences neurotransmission by inhibiting NMDA receptors and potentiating GABAA receptors. The balance of excitatory and inhibitory neurotransmission is different in the young brain. There is a heightened sensitivity of the very young brain to excitotoxicity after hypoxic-ischemic injury (61,62), and regional expression of glutamate receptors (NMDA, AMPA, kainate) changes throughout brain development (63,64). Furthermore, the GABAA receptor, which is responsible for inhibitory neurotransmission in the adult brain, can be excitatory in early development (65,66). Taken together, these developmental differences in glutamate receptors could results in increased sensitivity of the young brain to excitotoxicity after injury, making progesterone treatment even more effective than in the adult brain. However, in the very young brain, the excitatory nature of the GABAA receptor could make progesterone treatment result in neurotoxicity.
A second important consideration is that the baseline and post-injury antioxidant capacity of the immature brain is significantly reduced compared to a mature brain (reviewed in (67,68)). For example, the activity of key antioxidant enzymes, such as CuZnSOD, MnSOD and glutathione peroxidase are 20-40% lower in the young brain compared to the adult brain (68). An in-depth discussion of these developmental vulnerabilities and their influence after TBI in the immature brain is found in a review by Bayir et al. (68). Overall, this would suggest that the antioxidant capacity of progesterone would be especially beneficial in pediatric TBI.
Post-traumatic inflammation is a significant contributor to neuropathology after TBI. Progesterone has anti-inflammatory effects by suppressing microglial activation and generation of pro-inflammatory cytokines. In early development, microglia are predominantly present in the white matter tracts and play a crucial role in remodeling and restructuring (69), moving to the cortex by about 2 years of age in humans (70). Microglial activation after brain injury could damage surrounding oligodendrocytes, worsening the normal myelination process occurring during brain development (71,72). Progesterone's ability to limit inflammation could therefore have age- and brain region-specific neuroprotection.
A fourth key aspect of the immature brain is the dominant role that apoptotic cell death cascades play after injury. With normal programmed cell death that occurring in the postnatal period, pro-apoptotic proteins are expressed at higher levels in the immature brain. This could increase vulnerability to molecular cell death cascades after developmental brain injury (73). Accordingly, progesterone's ability to increase anti-apoptotic Bcl-2 protein levels while decreasing pro-apoptotic protein levels could be beneficial. There are many other aspects of developmental neurobiology that could influence efficacy of progesterone in the young brain, such as protection against mitochondrial dysfunction, and improvement in neurogenesis. In summary, studies evaluating the neuroprotective features of progesterone must take into account the age-specific mechanisms of secondary injury and recovery after pediatric TBI.
Overview of studies utilizing progesterone in the developing brain
There are few studies which evaluated the effects of progesterone in the developing brain. One of the important questions regarding progesterone treatment is whether TBI leads to alterations in the levels of progesterone or its receptor. Although this has not been examined after pediatric TBI, experimental studies in immature seizure and ischemia models report changes in progesterone and its receptor levels early after injury depending on the insult and time after injury. For example, when post-natal day 7 (P7) day old female rats were exposed to hypoxia only (6.5% O2 for 50 min) or hypoxia plus ischemia (HI, ligature of the right carotid artery), progesterone receptor levels decreased at 48 h after hypoxia and markedly increased at 7 days after hypoxia and HI vs control (74). Progesterone and estradiol secretion at 3 and 8 months were unaffected by HI, but levels were not evaluated at earlier times. González-Ramírez et al (75), showed that serum progesterone levels increase 5-6 fold at 30min and 24 h after pentylenetetrazol-induced seizures in P10 male and female rats. These limited studies of progesterone and progesterone receptor levels after brain injury would suggest that the mechanism of injury and timing after injury are important considerations when evaluating progesterone for treatment.
A recent study in adult TBI show that compared with controls, CSF progesterone levels were significantly and persistently elevated during the first two days after TBI, and high CSF progesterone levels were associated with worse GOS at 6 months in bivariate analysis (76). In multivariate analysis high CSF progesterone was indirectly associated with worse outcome through its interactions with cortisol since progesterone is a precursor to cortisol. Thus, it is possible that brain interstitial and CSF levels of progesterone and its physiologically active metabolites (such as allopregnanolone) may be an important determinant of recovery in the injured brain. Progesterone and its metabolites have been associated with anticonvulsant effects (77,78), most likely by acting as powerful positive modulators of GABAA receptors in the brain (79,80). Corroborating this finding, Holmes et al., demonstrated that while progesterone does not have effect on kindling in the adult animal, it markedly inhibits kindling in immature animals and prevents generalization of seizures (81).
As discussed in the previous section, GABA is excitatory for immature neurons, while it is inhibitory for mature neurons. Consistent with this, a recent study reported that exogenous administration of progesterone and allopregnanolone exacerbated brain injury in an age-dependent manner (82). Progesterone (10 mg/kg), allopregnanolone (10 mg/kg), or vehicle was intraperitoneally administered immediately before and then subcutaneously at 6 h and 24 h after hypoxia–ischemia, using the Rice –Vannucci model(83), to P7, P14, and P21 male and female rats. Both progesterone and allopregnanolone exacerbated hemispheric volume loss and histopathological injury score in P7 and P14 rats, but not in P21 rats. Co-administration of the GABAA receptor antagonist, bicuculline, partially mitigated the exacerbating effect of allopregnanolone. The authors speculated that the detrimental effects of progesterone were most likely due to GABAergic neuroexcitatory activity of allopregnanolone. Although the authors identified a potential mechanism for the detrimental effects of progesterone and allopregnanolone in their model, levels of progesterone, allopregnanolone in serum and brain and their corresponding receptors in the brain were not analyzed. It is possible that if the levels of progesterone receptor are decreased after injury as shown previously (74), one might observe off-target effects of the drug more often. Nevertheless this study suggests that caution is required when considering progesterone and its metabolites for neuroprotection in the immature brain.
Two recent studies evaluated the neuroprotective effect of progesterone alone or in combination with magnesium after TBI in the developing brain (84,85). Mixed gender 7 day old Wistar rats were exposed to weight drop injury, and progesterone or magnesium sulfate were administered i.p. immediately after TBI. Combination therapy was found to be superior to progesterone alone for improving long-term (3 week) neuronal survival in the dentate gyrus, while treatment with progesterone alone, magnesium alone, or the two in combination reduced the extent of apoptotic cell death profiles. Furthermore, spatial learning and memory retention at 3 weeks after injury was improved by treatment with progesterone alone, magnesium alone or the two in combination (84). In a separate study the authors reported that progesterone decreased TBI-induced anxiety in the immature rat (85), thought to be related to attenuation of TBI-induced changes in circulating corticosterone and insulin-like growth factor levels by progesterone. Further studies in different models of TBI in the immature brain are needed to determine whether progesterone is beneficial in contusional head injury.
Interestingly, studies performed in a single center in extremely preterm infants report improved neurodevelopmental outcome with exogenous combined estrogen and progesterone administration (86,87). The initial aim of these studies was to evaluate the impact of estradiol and progesterone replacement on postnatal bone mineral accretion (86,88). Male and female infants <1000g were randomized to receive either ESTRA-PRO emulsion or placebo containing same amount of lipids for 4 weeks after birth. The authors followed circulating estrogen and progesterone levels in the patients and aimed to maintain plasma levels equaling intrauterine levels. At the follow-up examination at 5-yr corrected age (87), a significant time-response relationship was found: every day of treatment with estrogen and progesterone was associated with a reduced risk for cerebral palsy, spasticity, and ametropia. Although these results are promising, multicenter trials are necessary to test beneficial effects of estrogen and progesterone on neurodevelopmental outcome in extremely preterm infants.
Clinical trials of progesterone for adult TBI
To date, two human trials of progesterone for TBI in adults have been published (89,90). A Phase II trial was conducted by Wright et al. in 2007 to gather data on drug safety, and to determine the pharmacokinetics of intravenous progesterone in moderate-to-severely injured adult patients (age > 17 years, GCS scores 4-12). One hundred patients were randomly assigned to receive either a 72-hour IV infusion of progesterone (0.71mg/kg over one hour and then 0.5 mg/kg/hour given by infusion for 12 hours per day for 3 days, with a 2 day taper) or an equal volume of placebo. No serious adverse events were attributed to the study drug. Patients treated with progesterone had mortality rates less than one half of controls, and had nearly identical rates of adverse events compared to controls. Moderately brain-injured patients treated with progesterone were less disabled 30 days after injury than similarly injured patients treated with placebo (p=0.02). These findings suggested that intravenous progesterone is safe at the dose selected.
Another recent Phase II trial demonstrated the potential of progesterone to treat brain-injured adults. This was a single site, prospective, randomized, placebo-controlled trial of progesterone conducted in Hangzhou China by Dr. Guomin Xiao (90). The primary purpose of this trial was to assess the long-term efficacy of progesterone on neurological outcome of patients with severe TBI (GCS ≤ 8). A total of 159 patients were enrolled and randomized to receive either intramuscular progesterone (1mg/kg IM Q12 for 5 days) or placebo. The primary endpoint was the Glasgow Outcome Scale (GOS) score 3 months after brain injury. Secondary efficacy endpoints included the modified Functional Independence Measure (FIM) score and mortality. Three months after treatment the dichotomized GOS showed a favorable outcome for 47% of the patients given progesterone and 31% in the placebo group (p=0.034) and an unfavorable outcome for 53% of the patients given progesterone and 70% of the placebo group (p=0.022), with similar outcomes at six months. The 6 month modified FIM scores suggested good functional outcome in the patients treated with progesterone. There were no adverse events associated with progesterone.
The Neurologic Emergencies Treatment Trials Network (NETT) has commenced a large, multicenter NIH-funded Phase III trial of progesterone for adults with moderate-to-severe TBI (ProTECT III) (91,92). The purpose of this trial is to determine the efficacy of administering intravenous progesterone (initiated within 4 hours of injury, administered for 72 hours, with an additional 24 hour taper) versus placebo for treating adult victims of moderate to severe acute TBI (Glasgow coma scale score 4-12), using the same dosing as the previously discussed Wright study (89). The main outcome measure is the GOS-Extended score at 6 months post injury. The total sample size was planned for 1140 subjects. The NIH announced in January 2014 that enrollment in the trial had been stopped by the independent Data Safety Monitoring Board, when interim review indicated that it was very unlikely that progesterone would demonstrate better outcomes compared to placebo in this trial.
In addition, BHR Pharma LLC is conducting SyNAPSe® (Study of the Neuroprotective Activity of Progesterone in Severe Traumatic Brain Injuries), a global, Phase 3, multi-center trial in severe TBI (93). This study evaluates the effectiveness of its proprietary BHR-100 progesterone product as a neuroprotective agent for treating severe traumatic brain injury (TBI) patients. Approximately 1,200 patients ages 16-65 years with severe (Glasgow Coma Scale scores of 3-8), closed-head TBI are being enrolled in the study at ∼150 centers worldwide. Patients are randomized in a one-to-one allocation to receive progesterone or placebo. The treatment is administered as a five-day/120-hour, continuous intravenous infusion starting within eight hours after injury. The primary study endpoint is the GOS at six months. This study completed enrollment, and we await the final results regarding outcome.
The stopping of the ProTECT III trial certainly has implications for the future study of progesterone treatment for pediatric TBI. It is likely that investigators will need to evaluate the results of the completed SyNAPSe® study, as well as the interim results of the ProTECT III study, before moving forward to clinical trials in pediatric TBI.
Future studies and Conclusions
Traumatic brain injuries differ between children and adults. Although it is believed that the immature brain recovers more fully from TBI, studies indicate that cerebral edema after TBI is three times more likely to occur in children than in adults. This suggests that a novel therapy such as progesterone, which decreases cerebral edema, may have a greater effect on children than adults. Although neurocognitive outcomes are better for children than for adults after TBI in general, neurocognitive recovery and its measurement after TBI are dependent on age and developmental level (94,95). Because of the differences between children and adults, and the lack of effective treatment for TBI, there is a need to complete the preclinical studies necessary to prepare for a potential pediatric clinical trial of progesterone administration. The results of this research could lead to the development of a novel neuroprotective therapy with potential to reduce the profound long-term disability in head-injured chilren. This information could also contribute to the use of progesterone in the treatment of pediatric brain injury from other causes (cardiac arrest, stroke, seizures).
Supplementary Material
Acknowledgments
We would like to thank Ms. Blair Anton, Associate Director of Clinical Information Services with the Johns Hopkins University Welch Library, for her assistance with compilation of published studies for Supplemental Digital Content - Table 1. Author HB received support from NIH (NS061817 and NS076511). Author RS received support from HRSA (1H34MC193530100).
Source of Funding: Author HB received support from NIH grants (NS061817 and NS076511). Author RS received support from HRSA (1H34MC193530100).
Footnotes
Conflicts of Interest: For the remaining authors none were declared.
References
- 1.Tilford JM, Aitken ME, Anand KJ, et al. Hospitalizations for critically ill children with traumatic brain injuries: A longitudinal analysis. Crit Care Med. 2005;33(9):2074–2081. doi: 10.1097/01.ccm.0000171839.65687.f5. [DOI] [PubMed] [Google Scholar]
- 2.Wechsler B, Kim H, Gallagher PR, DiScala C, Stineman MG. Functional status after childhood traumatic brain injury. J Trauma. 2005;58(5):940–9. doi: 10.1097/01.ta.0000162630.78386.98. discussion 950. [DOI] [PubMed] [Google Scholar]
- 3.Yeates KO, Swift E, Taylor HG, et al. Short- and long-term social outcomes following pediatric traumatic brain injury. J Int Neuropsychol Soc. 2004;10(3):412–426. doi: 10.1017/S1355617704103093. [DOI] [PubMed] [Google Scholar]
- 4.Chen G, Shi J, Jin W, et al. Progesterone administration modulates TLRs/NF-kappaB signaling pathway in rat brain after cortical contusion. Ann Clin Lab Sci. 2008;38(1):65–74. [PubMed] [Google Scholar]
- 5.De Nicola AF, Labombarda F, Deniselle MC, et al. Progesterone neuroprotection in traumatic CNS injury and motoneuron degeneration. Front Neuroendocrinol. 2009;30(2):173–187. doi: 10.1016/j.yfrne.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Estrada J, Luquin S, Fernandez AM, Garcia-Segura LM. Dehydroepiandrosterone, pregnenolone and sex steroids down-regulate reactive astroglia in the male rat brain after a penetrating brain injury. Int J Dev Neurosci. 1999;17(2):145–151. doi: 10.1016/s0736-5748(98)00065-3. [DOI] [PubMed] [Google Scholar]
- 7.O'Connor CA, Cernak I, Johnson F, Vink R. Effects of progesterone on neurologic and morphologic outcome following diffuse traumatic brain injury in rats. Exp Neurol. 2007;205(1):145–153. doi: 10.1016/j.expneurol.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 8.Robertson CL, Puskar A, Hoffman GE, Murphy AZ, Saraswati M, Fiskum G. Physiologic progesterone reduces mitochondrial dysfunction and hippocampal cell loss after traumatic brain injury in female rats. Exp Neurol. 2006;197(1):235–243. doi: 10.1016/j.expneurol.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Roof RL, Hoffman SW, Stein DG. Progesterone protects against lipid peroxidation following traumatic brain injury in rats. Mol Chem Neuropathol. 1997;31(1):1–11. doi: 10.1007/BF02815156. [DOI] [PubMed] [Google Scholar]
- 10.Roof RL, Duvdevani R, Heyburn JW, Stein DG. Progesterone rapidly decreases brain edema: Treatment delayed up to 24 hours is still effective. Exp Neurol. 1996;138(2):246–251. doi: 10.1006/exnr.1996.0063. [DOI] [PubMed] [Google Scholar]
- 11.Roof RL, Duvdevani R, Braswell L, Stein DG. Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp Neurol. 1994;129(1):64–69. doi: 10.1006/exnr.1994.1147. [DOI] [PubMed] [Google Scholar]
- 12.Sayeed I, Stein DG. Progesterone as a neuroprotective factor in traumatic and ischemic brain injury. Prog Brain Res. 2009;175:219–237. doi: 10.1016/S0079-6123(09)17515-5. [DOI] [PubMed] [Google Scholar]
- 13.Yao XL, Liu J, Lee E, Ling GS, McCabe JT. Progesterone differentially regulates pro- and anti-apoptotic gene expression in cerebral cortex following traumatic brain injury in rats. J Neurotrauma. 2005;22(6):656–668. doi: 10.1089/neu.2005.22.656. [DOI] [PubMed] [Google Scholar]
- 14.Stein DG. Progesterone exerts neuroprotective effects after brain injury. Brain Res Rev. 2008;57(2):386–397. doi: 10.1016/j.brainresrev.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roof RL, Duvdevani R, Stein DG. Gender influences outcome of brain injury: Progesterone plays a protective role. Brain Res. 1993;607(1-2):333–336. doi: 10.1016/0006-8993(93)91526-x. [DOI] [PubMed] [Google Scholar]
- 16.Bramlett HM, Dietrich WD. Neuropathological protection after traumatic brain injury in intact female rats versus males or ovariectomized females. J Neurotrauma. 2001;18(9):891–900. doi: 10.1089/089771501750451811. [DOI] [PubMed] [Google Scholar]
- 17.Wright DW, Bauer ME, Hoffman SW, Stein DG. Serum progesterone levels correlate with decreased cerebral edema after traumatic brain injury in male rats. J Neurotrauma. 2001;18(9):901–909. doi: 10.1089/089771501750451820. [DOI] [PubMed] [Google Scholar]
- 18.Goss CW, Hoffman SW, Stein DG. Behavioral effects and anatomic correlates after brain injury: A progesterone dose-response study. Pharmacol Biochem Behav. 2003;76(2):231–242. doi: 10.1016/j.pbb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Cutler SM, Pettus EH, Hoffman SW, Stein DG. Tapered progesterone withdrawal enhances behavioral and molecular recovery after traumatic brain injury. Exp Neurol. 2005;195(2):423–429. doi: 10.1016/j.expneurol.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Gibson CL, Gray LJ, Bath PM, Murphy SP. Progesterone for the treatment of experimental brain injury; a systematic review. Brain. 2008;131(Pt 2):318–328. doi: 10.1093/brain/awm183. [DOI] [PubMed] [Google Scholar]
- 21.Kochanek PM, Clark RS, Ruppel RA, et al. Biochemical, cellular, and molecular mechanisms in the evolution of secondary damage after severe traumatic brain injury in infants and children: Lessons learned from the bedside. Pediatr Crit Care Med. 2000;1(1):4–19. doi: 10.1097/00130478-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Stein DG, Wright DW. Progesterone in the clinical treatment of acute traumatic brain injury. Expert Opin Investig Drugs. 2010;19(7):847–857. doi: 10.1517/13543784.2010.489549. [DOI] [PubMed] [Google Scholar]
- 23.Pan DS, Liu WG, Yang XF, Cao F. Inhibitory effect of progesterone on inflammatory factors after experimental traumatic brain injury. Biomed Environ Sci. 2007;20(5):432–438. [PubMed] [Google Scholar]
- 24.Cutler SM, Cekic M, Miller DM, Wali B, VanLandingham JW, Stein DG. Progesterone improves acute recovery after traumatic brain injury in the aged rat. J Neurotrauma. 2007;24(9):1475–1486. doi: 10.1089/neu.2007.0294. [DOI] [PubMed] [Google Scholar]
- 25.Guo Q, Sayeed I, Baronne LM, Hoffman SW, Guennoun R, Stein DG. Progesterone administration modulates AQP4 expression and edema after traumatic brain injury in male rats. Exp Neurol. 2006;198(2):469–478. doi: 10.1016/j.expneurol.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 26.Dazert P, Suofu Y, Grube M, et al. Differential regulation of transport proteins in the periinfarct region following reversible middle cerebral artery occlusion in rats. Neuroscience. 2006;142(4):1071–1079. doi: 10.1016/j.neuroscience.2006.07.056. [DOI] [PubMed] [Google Scholar]
- 27.Nagelhus EA, Ottersen OP. Physiological roles of aquaporin-4 in brain. Physiol Rev. 2013;93(4):1543–1562. doi: 10.1152/physrev.00011.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukuda AM, Adami A, Pop V, et al. Posttraumatic reduction of edema with aquaporin-4 RNA interference improves acute and chronic functional recovery. J Cereb Blood Flow Metab. 2013;33(10):1621–1632. doi: 10.1038/jcbfm.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller L, Hunt JS. Regulation of TNF-alpha production in activated mouse macrophages by progesterone. J Immunol. 1998;160(10):5098–5104. [PubMed] [Google Scholar]
- 30.Drew PD, Chavis JA. Female sex steroids: Effects upon microglial cell activation. J Neuroimmunol. 2000;111(1-2):77–85. doi: 10.1016/s0165-5728(00)00386-6. [DOI] [PubMed] [Google Scholar]
- 31.Ziebell JM, Morganti-Kossmann MC. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics. 2010;7(1):22–30. doi: 10.1016/j.nurt.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moorthy K, Sharma D, Basir SF, Baquer NZ. Administration of estradiol and progesterone modulate the activities of antioxidant enzyme and aminotransferases in naturally menopausal rats. Exp Gerontol. 2005;40(4):295–302. doi: 10.1016/j.exger.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Djebaili M, Guo Q, Pettus EH, Hoffman SW, Stein DG. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J Neurotrauma. 2005;22(1):106–118. doi: 10.1089/neu.2005.22.106. [DOI] [PubMed] [Google Scholar]
- 34.Cai W, Zhu Y, Furuya K, Li Z, Sokabe M, Chen L. Two different molecular mechanisms underlying progesterone neuroprotection against ischemic brain damage. Neuropharmacology. 2008;55(2):127–138. doi: 10.1016/j.neuropharm.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 35.Luoma JI, Stern CM, Mermelstein PG. Progesterone inhibition of neuronal calcium signaling underlies aspects of progesterone-mediated neuroprotection. J Steroid Biochem Mol Biol. 2011 doi: 10.1016/j.jsbmb.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ardeshiri A, Kelley MH, Korner IP, Hurn PD, Herson PS. Mechanism of progesterone neuroprotection of rat cerebellar purkinje cells following oxygen-glucose deprivation. Eur J Neurosci. 2006;24(9):2567–2574. doi: 10.1111/j.1460-9568.2006.05142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhodes ME, Frye CA. Actions at GABA(A) receptors in the hippocampus may mediate some antiseizure effects of progestins. Epilepsy Behav. 2005;6(3):320–327. doi: 10.1016/j.yebeh.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 38.Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Neurosteroid modulation of GABAA receptors. Prog Neurobiol. 2003;71(1):67–80. doi: 10.1016/j.pneurobio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Cutler SM, VanLandingham JW, Murphy AZ, Stein DG. Slow-release and injected progesterone treatments enhance acute recovery after traumatic brain injury. Pharmacol Biochem Behav. 2006;84(3):420–428. doi: 10.1016/j.pbb.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 40.Chan JR, Phillips LJ, 2nd, Glaser M. Glucocorticoids and progestins signal the initiation and enhance the rate of myelin formation. Proc Natl Acad Sci U S A. 1998;95(18):10459–10464. doi: 10.1073/pnas.95.18.10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koenig HL, Schumacher M, Ferzaz B, et al. Progesterone synthesis and myelin formation by schwann cells. Science. 1995;268(5216):1500–1503. doi: 10.1126/science.7770777. [DOI] [PubMed] [Google Scholar]
- 42.Ghoumari AM, Ibanez C, El-Etr M, et al. Progesterone and its metabolites increase myelin basic protein expression in organotypic slice cultures of rat cerebellum. J Neurochem. 2003;86(4):848–859. doi: 10.1046/j.1471-4159.2003.01881.x. [DOI] [PubMed] [Google Scholar]
- 43.Ghoumari AM, Baulieu EE, Schumacher M. Progesterone increases oligodendroglial cell proliferation in rat cerebellar slice cultures. Neuroscience. 2005;135(1):47–58. doi: 10.1016/j.neuroscience.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 44.Ibanez C, Shields SA, El-Etr M, Baulieu EE, Schumacher M, Franklin RJ. Systemic progesterone administration results in a partial reversal of the age-associated decline in CNS remyelination following toxin-induced demyelination in male rats. Neuropathol Appl Neurobiol. 2004;30(1):80–89. doi: 10.1046/j.0305-1846.2003.00515.x. [DOI] [PubMed] [Google Scholar]
- 45.Jung-Testas I, Schumacher M, Robel P, Baulieu EE. The neurosteroid progesterone increases the expression of myelin proteins (MBP and CNPase) in rat oligodendrocytes in primary culture. Cell Mol Neurobiol. 1996;16(3):439–443. doi: 10.1007/BF02088110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herson PS, Koerner IP, Hurn PD. Sex, sex steroids, and brain injury. Semin Reprod Med. 2009;27(3):229–239. doi: 10.1055/s-0029-1216276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoffman GE, Merchenthaler I, Zup SL. Neuroprotection by ovarian hormones in animal models of neurological disease. Endocrine. 2006;29(2):217–231. doi: 10.1385/ENDO:29:2:217. [DOI] [PubMed] [Google Scholar]
- 48.Brinton RD, Thompson RF, Foy MR, et al. Progesterone receptors: Form and function in brain. Front Neuroendocrinol. 2008;29(2):313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagner CK. The many faces of progesterone: A role in adult and developing male brain. Front Neuroendocrinol. 2006;27(3):340–359. doi: 10.1016/j.yfrne.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 50.Mellon SH. Neurosteroid regulation of central nervous system development. Pharmacol Ther. 2007;116(1):107–124. doi: 10.1016/j.pharmthera.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carre JL, Abalain JH, Sarlieve LL, Floch HH. Ontogeny of steroid metabolizing enzymes in rat oligodendrocytes. J Steroid Biochem Mol Biol. 2001;78(1):89–95. doi: 10.1016/s0960-0760(01)00079-6. [DOI] [PubMed] [Google Scholar]
- 52.Guennoun R, Fiddes RJ, Gouezou M, Lombes M, Baulieu EE. A key enzyme in the biosynthesis of neurosteroids, 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4-isomerase (3 beta-HSD), is expressed in rat brain. Brain Res Mol Brain Res. 1995;30(2):287–300. doi: 10.1016/0169-328x(95)00016-l. [DOI] [PubMed] [Google Scholar]
- 53.Kohchi C, Ukena K, Tsutsui K. Age- and region-specific expressions of the messenger RNAs encoding for steroidogenic enzymes p450scc, P450c17 and 3beta-HSD in the postnatal rat brain. Brain Res. 1998;801(1-2):233–238. doi: 10.1016/s0006-8993(98)00585-x. [DOI] [PubMed] [Google Scholar]
- 54.Baulieu EE, Robel P, Schumacher M. Neurosteroids: Beginning of the story. Int Rev Neurobiol. 2001;46:1–32. doi: 10.1016/s0074-7742(01)46057-0. [DOI] [PubMed] [Google Scholar]
- 55.Compagnone NA, Mellon SH. Neurosteroids: Biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21(1):1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- 56.Wagner CK. Progesterone receptors and neural development: A gap between bench and bedside? Endocrinology. 2008;149(6):2743–2749. doi: 10.1210/en.2008-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quadros PS, Pfau JL, Wagner CK. Distribution of progesterone receptor immunoreactivity in the fetal and neonatal rat forebrain. J Comp Neurol. 2007;504(1):42–56. doi: 10.1002/cne.21427. [DOI] [PubMed] [Google Scholar]
- 58.Quadros PS, Wagner CK. Regulation of progesterone receptor expression by estradiol is dependent on age, sex and region in the rat brain. Endocrinology. 2008;149(6):3054–3061. doi: 10.1210/en.2007-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dalton K. Prenatal progesterone and educational attainments. Br J Psychiatry. 1976;129:438–442. doi: 10.1192/bjp.129.5.438. [DOI] [PubMed] [Google Scholar]
- 60.Dalton K. Intelligence and prenatal progesterone: A reappraisal. J R Soc Med. 1979;72(6):397–399. doi: 10.1177/014107687907200602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McDonald JW, Trescher WH, Johnston MV. Susceptibility of brain to AMPA induced excitotoxicity transiently peaks during early postnatal development. Brain Res. 1992;583(1-2):54–70. doi: 10.1016/s0006-8993(10)80009-5. [DOI] [PubMed] [Google Scholar]
- 62.McDonald JW, Silverstein FS, Johnston MV. Neurotoxicity of N-methyl-D-aspartate is markedly enhanced in developing rat central nervous system. Brain Res. 1988;459(1):200–203. doi: 10.1016/0006-8993(88)90306-x. [DOI] [PubMed] [Google Scholar]
- 63.Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABAA, NMDA and AMPA receptors: A developmentally regulated ‘menage a trois’. Trends Neurosci. 1997;20(11):523–529. doi: 10.1016/s0166-2236(97)01147-8. [DOI] [PubMed] [Google Scholar]
- 64.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12(3):529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 65.Nunez JL, McCarthy MM. Evidence for an extended duration of GABA-mediated excitation in the developing male versus female hippocampus. Dev Neurobiol. 2007;67(14):1879–1890. doi: 10.1002/dneu.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perrot-Sinal TS, Auger AP, McCarthy MM. Excitatory actions of GABA in developing brain are mediated by l-type Ca2+ channels and dependent on age, sex, and brain region. Neuroscience. 2003;116(4):995–1003. doi: 10.1016/s0306-4522(02)00794-7. [DOI] [PubMed] [Google Scholar]
- 67.Fan P, Yamauchi T, Noble LJ, Ferriero DM. Age-dependent differences in glutathione peroxidase activity after traumatic brain injury. J Neurotrauma. 2003;20(5):437–445. doi: 10.1089/089771503765355513. [DOI] [PubMed] [Google Scholar]
- 68.Bayir H, Kochanek PM, Kagan VE. Oxidative stress in immature brain after traumatic brain injury. Dev Neurosci. 2006;28(4-5):420–431. doi: 10.1159/000094168. [DOI] [PubMed] [Google Scholar]
- 69.Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: Implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol. 2012;71(4):444–457. doi: 10.1002/ana.22620. [DOI] [PubMed] [Google Scholar]
- 70.Billiards SS, Haynes RL, Folkerth RD, et al. Development of microglia in the cerebral white matter of the human fetus and infant. J Comp Neurol. 2006;497(2):199–208. doi: 10.1002/cne.20991. [DOI] [PubMed] [Google Scholar]
- 71.Li J, Baud O, Vartanian T, Volpe JJ, Rosenberg PA. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc Natl Acad Sci U S A. 2005;102(28):9936–9941. doi: 10.1073/pnas.0502552102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ferrazzano P, Chanana V, Uluc K, et al. Age-dependent microglial activation in immature brains after hypoxia- ischemia. CNS Neurol Disord Drug Targets. 2013;12(3):338–349. doi: 10.2174/1871527311312030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pohl D, Bittigau P, Ishimaru MJ, et al. N-methyl-D-aspartate antagonists and apoptotic cell death triggered by head trauma in developing rat brain. Proc Natl Acad Sci U S A. 1999;96(5):2508–2513. doi: 10.1073/pnas.96.5.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ezquer ME, Valdez SR, Seltzer AM, Jahn GA. Advancement of reproductive senescence and changes in the early expression of estrogen, progesterone and micro-opioid receptors induced by neonatal hypoxia in the female rat. Brain Res. 2008;1214:73–83. doi: 10.1016/j.brainres.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 75.Gonzalez-Ramirez M, Razo-Juarez LI, Sauer-Ramirez JL, Gonzalez-Trujano ME, Salgado-Ceballos H, Orozco-Suarez S. Anticonvulsive effect of vitamin C on pentylenetetrazol-induced seizures in immature rats. Pharmacol Biochem Behav. 2010;97(2):267–272. doi: 10.1016/j.pbb.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 76.Santarsieri M, Niyonkuru C, McCullough EH, et al. Cerebrospinal fluid cortisol and progesterone profiles and outcomes prognostication after severe traumatic brain injury. J Neurotrauma. 2014;31(8):699–712. doi: 10.1089/neu.2013.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beyenburg S, Stoffel-Wagner B, Bauer J, et al. Neuroactive steroids and seizure susceptibility. Epilepsy Res. 2001;44(2-3):141–153. doi: 10.1016/s0920-1211(01)00194-2. [DOI] [PubMed] [Google Scholar]
- 78.Grosso S, Luisi S, Mostardini R, et al. Inter-ictal and post-ictal circulating levels of allopregnanolone, an anticonvulsant metabolite of progesterone, in epileptic children. Epilepsy Res. 2003;54(1):29–34. doi: 10.1016/s0920-1211(03)00042-1. [DOI] [PubMed] [Google Scholar]
- 79.Majewska MD. Neurosteroids: Endogenous bimodal modulators of the GABAA receptor. mechanism of action and physiological significance. Prog Neurobiol. 1992;38(4):379–395. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- 80.Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003;100(24):14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Holmes GL, Weber DA. The effect of progesterone on kindling: A developmental study. Brain Res. 1984;318(1):45–53. doi: 10.1016/0165-3806(84)90061-0. [DOI] [PubMed] [Google Scholar]
- 82.Tsuji M, Taguchi A, Ohshima M, Kasahara Y, Ikeda T. Progesterone and allopregnanolone exacerbate hypoxic-ischemic brain injury in immature rats. Exp Neurol. 2012;233(1):214–220. doi: 10.1016/j.expneurol.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 83.Rice JE, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9(2):131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- 84.Uysal N, Baykara B, Kiray M, et al. Combined treatment with progesterone and magnesium sulfate positively affects traumatic brain injury in immature rats. Turk Neurosurg. 2013;23(2):129–137. doi: 10.5137/1019-5149.JTN.5582-11.1. [DOI] [PubMed] [Google Scholar]
- 85.Baykara B, Aksu I, Buyuk E, et al. Progesterone treatment decreases traumatic brain injury induced anxiety and is correlated with increased serum IGF-1 levels; prefrontal cortex, amygdala, hippocampus neuron density; and reduced serum corticosterone levels in immature rats. Biotech Histochem. 2013;88(5):250–257. doi: 10.3109/10520295.2013.769630. [DOI] [PubMed] [Google Scholar]
- 86.Trotter A, Bokelmann B, Sorgo W, et al. Follow-up examination at the age of 15 months of extremely preterm infants after postnatal estradiol and progesterone replacement. J Clin Endocrinol Metab. 2001;86(2):601–603. doi: 10.1210/jcem.86.2.7176. [DOI] [PubMed] [Google Scholar]
- 87.Trotter A, Steinmacher J, Kron M, Pohlandt F. Neurodevelopmental follow-up at five years corrected age of extremely low birth weight infants after postnatal replacement of 17beta-estradiol and progesterone. J Clin Endocrinol Metab. 2012;97(3):1041–1047. doi: 10.1210/jc.2011-2612. [DOI] [PubMed] [Google Scholar]
- 88.Trotter A, Maier L, Grill HJ, Kohn T, Heckmann M, Pohlandt F. Effects of postnatal estradiol and progesterone replacement in extremely preterm infants. J Clin Endocrinol Metab. 1999;84(12):4531–4535. doi: 10.1210/jcem.84.12.6180. [DOI] [PubMed] [Google Scholar]
- 89.Wright DW, Kellermann AL, Hertzberg VS, et al. ProTECT: A randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med. 2007;49(4):391–402. doi: 10.1016/j.annemergmed.2006.07.932. [DOI] [PubMed] [Google Scholar]
- 90.Xiao G, Wei J, Yan W, Wang W, Lu Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: A randomized controlled trial. Crit Care. 2008;12(2):R61. doi: 10.1186/cc6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.NIH. Neurological emergencies treatment trials (NETT) network [internet] [Accessed April 10, 2010]; http://www.nett.umich.edu/nett/welcome.
- 92.Wright D. Progesterone for traumatic brain injury: Experimental clinical treatment; NLM identifier: NCT00822900. [Accessed April 13, 2009]; http://clinicaltrials.gov/show/ NCT00822900.
- 93.BHR Pharma LLC. SyNAPSe: The global phase 3 trial of progesterone in severe traumatic brain injury. http://www.synapse-trial.com/
- 94.Yeates KO, Taylor HG, Wade SL, Drotar D, Stancin T, Minich N. A prospective study of short- and long-term neuropsychological outcomes after traumatic brain injury in children. Neuropsychology. 2002;16(4):514–523. doi: 10.1037//0894-4105.16.4.514. [DOI] [PubMed] [Google Scholar]
- 95.Marquez de la Plata CD, Hart T, Hammond FM, et al. Impact of age on long-term recovery from traumatic brain injury. Arch Phys Med Rehabil. 2008;89(5):896–903. doi: 10.1016/j.apmr.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roof RL, Duvdevani R, Stein DG. Progesterone treatment attenuates brain edema following contusion injury in male and female rats. Restor Neurol Neurosci. 1992;4(6):425–427. doi: 10.3233/RNN-1992-4608. [DOI] [PubMed] [Google Scholar]
- 97.Galani R, Hoffman SW, Stein DG. Effects of the duration of progesterone treatment on the resolution of cerebral edema induced by cortical contusions in rats. Restor Neurol Neurosci. 2001;18(4):161–166. [PubMed] [Google Scholar]
- 98.Shear DA, Galani R, Hoffman SW, Stein DG. Progesterone protects against necrotic damage and behavioral abnormalities caused by traumatic brain injury. Exp Neurol. 2002;178(1):59–67. doi: 10.1006/exnr.2002.8020. [DOI] [PubMed] [Google Scholar]
- 99.Djebaili M, Hoffman SW, Stein DG. Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat pre-frontal cortex. Neuroscience. 2004;123(2):349–359. doi: 10.1016/j.neuroscience.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 100.Grossman KJ, Goss CW, Stein DG. Effects of progesterone on the inflammatory response to brain injury in the rat. Brain Res. 2004;1008(1):29–39. doi: 10.1016/j.brainres.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 101.He J, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, enhances behavioral recovery and decreases neuronal loss after traumatic brain injury. Restor Neurol Neurosci. 2004;22(1):19–31. [PubMed] [Google Scholar]
- 102.He J, Evans CO, Hoffman SW, Oyesiku NM, Stein DG. Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury. Exp Neurol. 2004;189(2):404–412. doi: 10.1016/j.expneurol.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 103.Jones NC, Constantin D, Prior MJ, Morris PG, Marsden CA, Murphy S. The neuroprotective effect of progesterone after traumatic brain injury in male mice is independent of both the inflammatory response and growth factor expression. Eur J Neurosci. 2005;21(6):1547–1554. doi: 10.1111/j.1460-9568.2005.03995.x. [DOI] [PubMed] [Google Scholar]
- 104.O'Connor CA, Cernak I, Vink R. Both estrogen and progesterone attenuate edema formation following diffuse traumatic brain injury in rats. Brain Res. 2005;1062(1-2):171–174. doi: 10.1016/j.brainres.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 105.Pettus EH, Wright DW, Stein DG, Hoffman SW. Progesterone treatment inhibits the inflammatory agents that accompany traumatic brain injury. Brain Res. 2005;1049(1):112–119. doi: 10.1016/j.brainres.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 106.VanLandingham JW, Cekic M, Cutler S, Hoffman SW, Stein DG. Neurosteroids reduce inflammation after TBI through CD55 induction. Neurosci Lett. 2007;425(2):94–98. doi: 10.1016/j.neulet.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen G, Shi JX, Qi M, Wang HX, Hang CH. Effects of progesterone on intestinal inflammatory response, mucosa structure alterations, and apoptosis following traumatic brain injury in male rats. J Surg Res. 2008;147(1):92–98. doi: 10.1016/j.jss.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 108.Gilmer LK, Roberts KN, Scheff SW. Efficacy of progesterone following a moderate unilateral cortical contusion injury. J Neurotrauma. 2008;25(6):593–602. doi: 10.1089/neu.2007.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.VanLandingham JW, Cekic M, Cutler SM, et al. Progesterone and its metabolite allopregnanolone differentially regulate hemostatic proteins after traumatic brain injury. J Cereb Blood Flow Metab. 2008;28(11):1786–1794. doi: 10.1038/jcbfm.2008.73. [DOI] [PubMed] [Google Scholar]
- 110.Wright DW, Hoffman SW, Virmani S, Stein DG. Effects of medroxyprogesterone acetate on cerebral oedema and spatial learning performance after traumatic brain injury in rats. Brain Inj. 2008;22(2):107–113. doi: 10.1080/02699050701867399. [DOI] [PubMed] [Google Scholar]
- 111.Kasturi BS, Stein DG. Progesterone decreases cortical and sub-cortical edema in young and aged ovariectomized rats with brain injury. Restor Neurol Neurosci. 2009;27(4):265–275. doi: 10.3233/RNN-2009-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sayeed I, Parvez S, Wali B, Siemen D, Stein DG. Direct inhibition of the mitochondrial permeability transition pore: A possible mechanism for better neuroprotective effects of allopregnanolone over progesterone. Brain Res. 2009;1263:165–173. doi: 10.1016/j.brainres.2009.01.045. [DOI] [PubMed] [Google Scholar]
- 113.Shahrokhi N, Khaksari M, Soltani Z, Mahmoodi M, Nakhaee N. Effect of sex steroid hormones on brain edema, intracranial pressure, and neurologic outcomes after traumatic brain injury. Can J Physiol Pharmacol. 2010;88(4):414–421. doi: 10.1139/y09-126. [DOI] [PubMed] [Google Scholar]
- 114.Anderson GD, Farin FM, Bammler TK, et al. The effect of progesterone dose on gene expression after traumatic brain injury. J Neurotrauma. 2011;28(9):1827–1843. doi: 10.1089/neu.2011.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Barha CK, Ishrat T, Epp JR, Galea LA, Stein DG. Progesterone treatment normalizes the levels of cell proliferation and cell death in the dentate gyrus of the hippocampus after traumatic brain injury. Exp Neurol. 2011;231(1):72–81. doi: 10.1016/j.expneurol.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cekic M, Cutler SM, VanLandingham JW, Stein DG. Vitamin D deficiency reduces the benefits of progesterone treatment after brain injury in aged rats. Neurobiol Aging. 2011;32(5):864–874. doi: 10.1016/j.neurobiolaging.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Grossman KJ, Goss CW, Stein DG. Sickness behaviors following medial frontal cortical contusions in male rats. Behav Brain Res. 2011;217(1):202–208. doi: 10.1016/j.bbr.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hua F, Wang J, Ishrat T, et al. Genomic profile of toll-like receptor pathways in traumatically brain-injured mice: Effect of exogenous progesterone. J Neuroinflammation. 2011;8:42. doi: 10.1186/1742-2094-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Khaksari M, Soltani Z, Shahrokhi N, Moshtaghi G, Asadikaram G. The role of estrogen and progesterone, administered alone and in combination, in modulating cytokine concentration following traumatic brain injury. Can J Physiol Pharmacol. 2011;89(1):31–40. doi: 10.1139/y10-103. [DOI] [PubMed] [Google Scholar]
- 120.Wali B, Sayeed I, Stein DG. Improved behavioral outcomes after progesterone administration in aged male rats with traumatic brain injury. Restor Neurol Neurosci. 2011;29(1):61–71. doi: 10.3233/RNN-2011-0579. [DOI] [PubMed] [Google Scholar]
- 121.Cekic M, Johnson SJ, Bhatt VH, Stein DG. Progesterone treatment alters neurotrophin/proneurotrophin balance and receptor expression in rats with traumatic brain injury. Restor Neurol Neurosci. 2012;30(2):115–126. doi: 10.3233/RNN-2011-0628. [DOI] [PubMed] [Google Scholar]
- 122.Hua F, Reiss JI, Tang H, et al. Progesterone and low-dose vitamin D hormone treatment enhances sparing of memory following traumatic brain injury. Horm Behav. 2012;61(4):642–651. doi: 10.1016/j.yhbeh.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li Z, Wang B, Kan Z, et al. Progesterone increases circulating endothelial progenitor cells and induces neural regeneration after traumatic brain injury in aged rats. J Neurotrauma. 2012;29(2):343–353. doi: 10.1089/neu.2011.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shahrokhi N, Haddad MK, Joukar S, Shabani M, Keshavarzi Z, Shahozehi B. Neuroprotective antioxidant effect of sex steroid hormones in traumatic brain injury. Pak J Pharm Sci. 2012;25(1):219–225. [PubMed] [Google Scholar]
- 125.Sarkaki AR, Khaksari Haddad M, Soltani Z, Shahrokhi N, Mahmoodi M. Time- and dose-dependent neuroprotective effects of sex steroid hormones on inflammatory cytokines after a traumatic brain injury. J Neurotrauma. 2013;30(1):47–54. doi: 10.1089/neu.2010.1686. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


