Abstract
Background/Objectives
Older adults do not take medication as prescribed, diminishing the benefits of treatment and increasing costs to individuals and society. A multifaceted prospective memory intervention for improving adherence to antihypertensive medication was tested and assessed if executive function/working memory processes moderated intervention effects.
Design
A two group longitudinal randomized control trial was used.
Setting and Participants and Measurements
The sample consisted of community-based older adults (≥ 65 years of age) without signs of dementia or symptoms of severe depression who were self-managing prescribed medication. Following four weeks of initial adherence monitoring using a medication event monitoring system (MEMS®), individuals with 90% or less adherence were randomly assigned to groups.
Intervention
The prospective memory intervention was designed to provide strategies that switch older adults from relying on executive function/working memory processes (that show effects of cognitive aging) to mostly automatic associative processes (that are relatively spared with normal aging) for remembering to take one’s medications. Strategies included establishing a routine, establishing cues strongly associated with medication taking actions, performing the action immediately upon thinking about it, using a medication organizer, and imagining medication taking to enhance encoding and improve cuing.
Results
There was significant improvement in adherence for the intervention group (57% at baseline to 78% post intervention), but most of these gains were lost after 5 months. The control condition started at 68%, was stable during the intervention, but dropped to 62%. Executive function/working memory moderated the intervention effect, with the intervention producing greater benefit for those with lower executive function/working memory.
Conclusion
The intervention improved adherence, but the benefits were not sustained. Further research is needed to determine how to sustain the substantial initial benefits.
Keywords: medication adherence, intervention, prospective memory, elderly, hypertension
INTRODUCTION
Medication adherence is critically important, yet typical adherence rates for prescribed medications are approximately 50%.1 These rates are particularly low when medications are prescribed for chronic and asymptomatic conditions as is the case with hypertension. People do not adhere to prescribed medications despite extensive interventions to educate, motivate, and support adherence behaviors.1, 2 The purposes of this study were to examine the effects of a four-week multifaceted prospective memory intervention on adherence to a daily prescribed antihypertensive agent among community dwelling older adults (≥ 65 years old) who were self-managing their medications. The immediate and long-term effects (six months post-initiation of the intervention) were examined. Another purpose of this study was to examine executive function/working memory, a set of cognitive abilities that are vulnerable to cognitive aging, as a moderator of the intervention effects on improving medication adherence.
Nonadherence prevents people from fully realizing the benefits of treatment, leading to substantial costs to individuals and society, see for example Dragomir and colleagues.3 Nonadherence to antihypertensive medication is associated with a significantly increased risk of major cardiovascular events and death.4 The need to control hypertension is clear, yet control is often inadequate. Past interventions to improve medication adherence have focused on knowledge, beliefs and attitudes including motivation, for reviews see Viswanathan, et al.2 and Glynn, et al.5 and a few studies have addressed forgetting through reminders.6 Knowledge, beliefs, self-monitoring, decreasing costs and other strategies including ongoing reminders and once a day dosing have been shown to improve adherence, but the effects are often small and not sustained once the intervention is completed.7–9 On the basis of analyzing the cognitive processes involved in taking medications and how these processes are affected by aging, we designed an intervention to support the most vulnerable processes.
An analysis of prospective memory-based tasks suggests that successful medication adherence involves five cognitive components,10 and research has shown that failure with any of these components can undermine adherence.11 These components include: (a) encoding the need to take medications and the conditions for taking them, (b) retaining this information in memory, (c) retrieving the intention to take the medicine at the appropriate time, (d) executing the action of taking the medicine (often in the context of distraction), and (e) monitoring that the medicine was taken (so as not to repeat a dose). The multifaceted prospective memory (remembering to recall an intended activity planned for the future at the right time) intervention tested in this study targeted all five tasks with strategies that have been empirically demonstrated to improve prospective memory performance of older adults. An important and unique feature of the intervention was that it combined these strategies into a comprehensive package that can be easily administered and used; for a complete description of the intervention see Insel and colleagues.12
Given the complexity of the cognitive processes involved in successful medication adherence, it is not surprising that poor adherence is associated with low cognitive functioning in general,13 and particularly with lower executive function/working memory.14, 15 Moreover, robust age-related cognitive declines16 make older adults particularly vulnerable to failures in adherence. An interesting feature of age-related cognitive decline, however, is that normal aging tends to affect certain processes (e.g., executive function and working memory) more than other relatively automatic retrieval process that depend on associative processes rather than more effortful processes.17, 18 Recent research shows that older adults with a history of hypertension show substantial deficits on laboratory prospective memory tasks that rely on the former processes, but not on the latter processes.19 Thus, the major focus of the intervention was to get older adults to rely on the relatively spared cognitive processes when taking medication. Generally, the intervention was aimed at switching dependence from self-initiated processes (processes that show large age-related deficits) that are heavily dependent on working memory and executive resources to environmentally supported and relatively automatic associative processes (processes that are thought to be spared with age).17 Our hypothesis was that individuals in the multifaceted prospective memory intervention group would have greater adherence to antihypertensive medication compared to those in an education control group immediately and over six months. It was also hypothesized that the intervention would be more effective for those with lower executive function/working memory, because these individuals should be less able to effectively rely on these processes to support their medication adherence.
METHOD
The study protocol was approved and monitored by the University Human Subjects Protection Committee. The choice of antihypertensive medication to monitor was made by writing on separate slips of paper the name of each prescribed antihypertensive medication (with the exception of diuretics because sometimes individuals will intentionally withhold a diuretic) and randomly withdrawing a slip from an opaque envelope. All participants were asked to place the monitored medication in a medication container with a MEMS® cap (an electronic monitoring cap). Following 4 weeks of baseline medication monitoring to establish the level of initial adherence for eligibility, defined as ≤ 90% adherence to the inter-dose interval, participants were randomly assigned to either the multifaceted prospective memory intervention group or the educational comparison group (see Figure 1).
Figure 1.
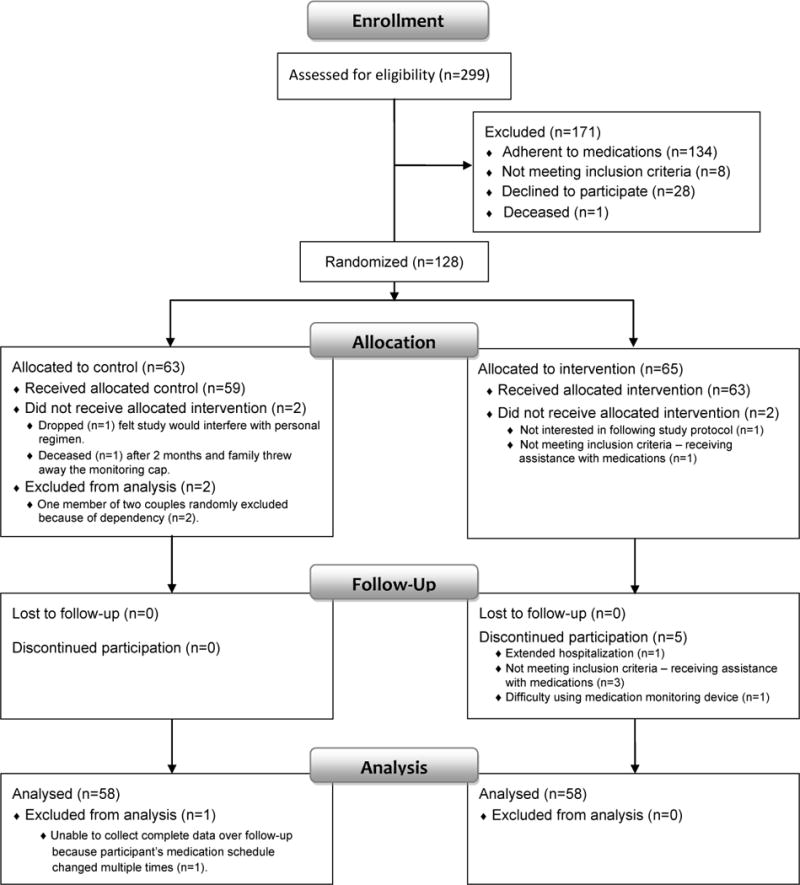
Flow Diagram
Sample
The sample was selected from community dwelling adults (≥ 65 years of age) who were English speaking and self-managing at least one prescribed antihypertensive agent. Individuals residing in nursing homes, indicating symptoms of severe depression a score greater than 11 on the short form of the Geriatric Depression Scale;20 or having signs of dementia based on a MMSE lower than 2421 were excluded from participation.
Individuals, whose adherence was 90% or lower over the initial four weeks of baseline monitoring, were randomly assigned to the intervention or the education comparison group. Random assignment occurred within blocks of two with the first individual randomly assigned to one condition and the second individual assigned to the other condition. For the purpose of calculating the sample size, using an 18% difference (80%–62%) information obtained in a preliminary study22 a standard deviation of 28 from the same preliminary study, a one-tailed alpha of .05, and a power of .90, it was determined that a final sample size of 43 per group, or a total sample size of 86 was needed. Anticipated attrition was 15%, a target sample of 50 was anticipated for each arm.
Participants were recruited at meal sites for seniors, health fairs and community talks. A complete description of the methods used for recruitment and the efforts taken to increase the comfort and security of participants are detailed in McHenry.23 Of the 299 participants who were initially assessed, 134 were adherent to medication (i.e., adherence was over 90% during the four week baseline period) and 8 did not meet the inclusion criteria. Twenty-eight declined to participate following initial data collection or baseline monitoring and one died resulting in 128 individuals randomly assigned to the two conditions. Following randomization two individuals did not complete the allocated control condition and two did not receive the allocated intervention. In total, 58 individuals in each condition continued to the end of the ongoing monitoring phase (see Figure 1).
Intervention
The intervention provided older adults with strategies that helped 1) establish a routine so that medication was taken at the same time in the same circumstances each day and 2) associate the medication taking with a specific environmental cue such as having breakfast rather than a time (say, 8 am). The idea here is that time is not easily noticed but that a salient environmental event like breakfast is and hence more likely to cue relatively automatic retrieval of the intention.24–26 Participants in the intervention condition were also asked to 3) imagine where they will be when they need to take the medication as previous research has shown that forming this kind of implementation intention (consciously linking the action to a triggering cue) further automatizes retrieval.27 The intervention also 4) calls attention to the act of taking the medication (and specifically to elaborating the action) to make the event more memorable and 5) uses an organizer to provide a way to monitor if the medication was taken as intended. These latter two steps were included, because too often, and especially with frequent or habitual prospective memory tasks like taking medication, the act of taking medication becomes routine and is performed without conscious thought.28 The result is that the person may take the medication but then does not remember doing so, leaving open the possibility of repeating a dose. Participants were also encouraged to 6) “do it now” which was intended to get them to perform the action soon as they recalled the intention to take the medication. This was included because previous research has shown that brief delays (as brief as 5 seconds) and interruptions after retrieving an intention (but before being allowed to execute it) greatly interfere with remembering to perform intentions, especially for older adults.29
The education control group was aimed at establishing a relationship with the participants (as was the case in the intervention group) and received the same educational information on hypertension that was given to participants in the intervention group. Time and attention were also equated across the groups by including information on preventing falls and social interaction with the nurses for individuals in the control condition.
Procedure
The intervention and control conditions were delivered by nurses in the home. Participants were visited once each week for four weeks. The initial visit was approximately 90 minutes and the following visits were 45–60 minutes. Participants in both groups received information about hypertension, the deleterious effects of uncontrolled hypertension, and about antihypertensive medications. This education was designed to support the encoding and retention of information needed to take medication successfully. In addition, participants in the intervention condition received the strategy instructions that supported all components of prospective remembering in the first visit and these were reviewed in each of the three other weekly visits. All participants in the intervention group were provided with a medication organizer, a box labeled S, M, T, W, T, F, S where the medication container could be moved from one day to the next. If the medication was twice a day an additional box organizer was provided.
Measures
Medication adherence was measured using the MEMS® system.14, 30 The Medication Event Monitoring System used was a tracking cap that tracks when the cap is opened.31 It was defined by the percent adherence to the inter-dose interval, which is 25% of the interval before and following the targeted prescribed time. If the dose is daily (24 hours), then the inter-dose interval is between 18 hours (24–6 hours) and 30 hours (24+6 hours) since the prior dose. If the dose is twice a day (12 hours), the inter-dose interval is between 9 (12–3) and 15 (12+3) hours from the prior dose. A cut-off point of 90% baseline adherence over the baseline four weeks was used to determine eligibility for the study.
We initially examined whether the intervention improved medication adherence relative to the education control condition. To do so, we computed adherence (percentage adherence to the inter-dose interval) for the baseline period (average over the last two weeks of the four week baseline), nurse-visit period (average over the 3 weeks from the first nurse visit to the fourth), and the additional five month monitoring period (average from the fourth nurse visit to the end of the study). We used the last two weeks over the baseline monitoring period because it has been shown elsewhere that introducing a medication monitoring device may improve adherence, but the effect is diminished after two weeks.32, 33 These data were included in a 2 × 3 mixed ANOVA with the between-subjects variable of condition (education control, intervention) and the within-subjects variable of time (baseline, nurse-visit, monitoring).
A composite measure for executive function/working memory was created using perseveration errors and categories achieved from the Wisconsin Card Sorting Test (WCST)34 and absolute score from Operation Span.35, 36 The WCST assesses abstraction ability and the ability to shift cognitive strategies in response to changing environmental conditions. Successful completion requires planning, organized searching, use of feedback to shift cognitive set, goal-oriented behavior and the ability to modulate impulsive responding.34 Operation span assesses working memory capacity, which is a complex ability that involves holding on to information in the face of distraction and quickly retrieving recently activated information.36 Scores from the WCST on perseveration errors and categories achieved, and absolute scores from operation span were converted to z-scores, then summed and averaged to create the composite measure.
Strategy use for those in the intervention group was determined by “no” = 0, “use sometimes” = 1 and “use always” = 2. There were six strategies with a maximum of two points for each strategy and twelve possible points for the total score.
RESULTS
Demographic information for participants who received the allocated intervention or control is presented in Table 1. Overall the mean age was 76.99 (SD=7.44) and did not differ between groups. Gender was also similarly distributed between groups; however, the overall sample included more women (n=93) than men (n=29). There were no differences in educational preparation (t (1,121) = 1.80, p = .075).
Table 1.
Demographics of total sample, control and intervention groups
| Total sample | Control | Intervention | statistic, p | ||
|---|---|---|---|---|---|
| Starting enrollment, n | 122 | 59 | 63 | ||
| Attrition over 6 months, n | 6 | 1 | 5 | ||
| Age | M (SD) | 76.99 (7.44) | 77.20 (7.57) | 76.79 (7.38) | t=0.31, p=.763 |
| Range | 65–94 | 65–94 | 65–91 | ||
| Gender, n | |||||
| Male | 29 | 15 | 14 | χ2=.17, p=.678 | |
| Female | 93 | 44 | 49 | ||
| Education | |||||
| < High school, n | 11 | 2 | 9 | χ2=5.10, p=.164 | |
| High school, n | 17 | 8 | 9 | ||
| Some post-high school, n | 34 | 16 | 18 | ||
| College degree, n | 60 | 33 | 27 | ||
| MMSE | M (SD) | 28.92 (1.43) | 29.08 (1.38) | 28.76 (1.47) | t=1.25, p=.214 |
| Range | 24–30 | 24–30 | 24–30 | ||
| Number of prescribed medications | M (SD) | 8.09 (4.65) | 8.59 (5.37) | 4.59 (3.77) | t=1.16, p=.249 |
| Range | 2–33 | 2–33 | 3–24 | ||
| Number of antihypertensive medications | M (SD) | 2.00 (1.06) | 1.97 (1.08) | 2.03 (1.06) | t=−0.35, p=.729 |
| Range | 1–6 | 1–6 | 1–5 | ||
Intervention Effect on Adherence
A significant condition by time interaction, (Wilks’λ=.887, F(2, 113) = 7.166, p < .01) indicated that the pattern over time was different for the two groups. As can be seen in Figure 2, adherence improved markedly in the intervention group, but this advantage was not sustained over the five month monitoring period. Adherence in the education control group, by contrast, declined slightly over the five months. This observation was supported by the post hoc analyses probing the condition by time interaction. The simple effect of time for the intervention group was significant (Wilks’λ=.715, F(2, 113) = 22.559, p < .01). Pairwise comparisons for the intervention group indicated both a significant increase from baseline (M=57.41, SD=29.84) to the nurse visits (M=77.78, SD=24.42, p<.01) and a significant decrease from the nurse visits through the monitoring phase (M=58.97 SD=32.66, p<.01). The simple effect of time for the control group was not significant (Wilks’λ=.951, F(2, 113) = 2.942, p > .05). Pairwise comparisons for the control group indicated a non-significant change from baseline (M=67.84, SD=28.50) to nurse visits (M=68.35, SD=30.24, p >.05) and a significant decrease from the nurses visit to end of the extended monitoring phase (M=61.12, SD=29.94, p < .05).
Figure 2.
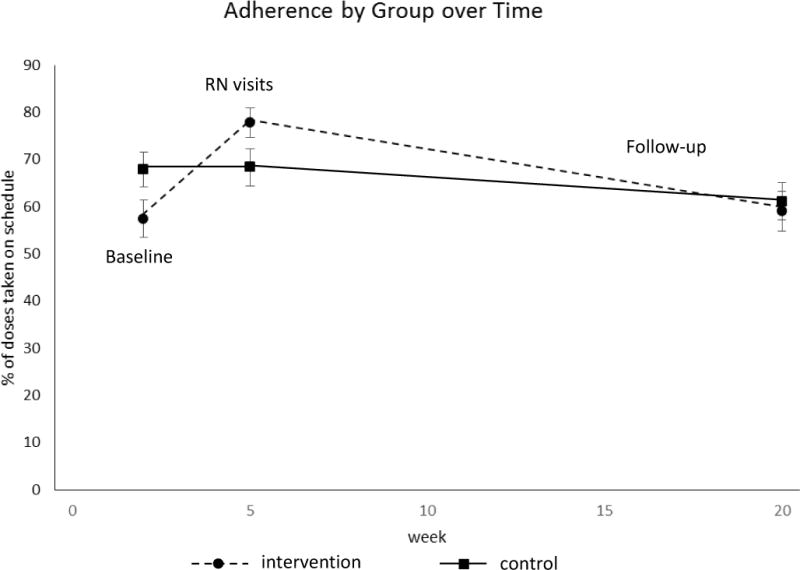
Adherence Over Time for the Intervention and Control Groups
To explore why adherence declined in the intervention group after the nurse visits, we examined change in self-reported strategy use between the last intervention visit and the final visit at the end of the 5 months of additional monitoring. There was a significant decline in strategy use overall from a mean of 10.86 to a mean of 8.84 (t(1,57) = 7.70, p < .001). The following strategies showed a significant decline: cues for remembering (t (1,57)= 3.79, p < .001), “Do it now” (t(1,57) = 2.03, p = .047), “Elaborate the Action” (t(1,57) =4.05, p < .001), and use of “Implementation Intentions” (t (1,57) = 5.20, p < .001). There were no significant differences for the strategies of establishing a routine or using an organizer.
Moderating Effect of Executive Function/Working Memory
We next examined whether the intervention was more effective for those with lower executive function/working memory by assessing the intervention by executive function/working memory interaction using PROCESS,37 a regression-based program that allows the assessment of quantitative moderators. Using the difference between baseline adherence and adherence during the intervention as the dependent variable and heteroscedasticity-consistent standard errors, a significant interaction was found (t(1,118)=2.24, p <.027). That is, individuals with lower executive function/working memory benefited from the intervention whereas individuals with better executive function/working memory were not as well assisted by the intervention. This interaction is depicted in Figure 3 which shows that the regression lines predicting adherence from executive function for the intervention and control groups have different slopes. Predicted adherence for both groups is the same at the point where these lines intersect. For values of executive function to the left of the point of intersection the intervention group had greater predicted adherence. Using the Johnson-Neyman technique it was found that the intervention condition had significantly higher adherence than the control condition when the executive function/working memory composite was .53 or less. Therefore, participants in this sample who were equal to or lower than a .53 standard deviation above the mean on the executive function/working memory composite were especially likely to benefit from the intervention.
Figure 3.

Experimental and control regression lines depicting the intervention by executive function interaction. The intervention group had significantly greater adherence when executive function Z-score was less than .53.
DISCUSSION
This study tested an intervention directed toward older adults (≥ 65 years) to improve adherence to prescribed antihypertensive medications. Older adults experience cognitive decline in the areas of executive function (inhibiting distractions, focusing and sustaining attention, shifting when needed and updating) and working memory (involved in the temporary storage and processing of information, keeping relevant information activated in the face of distraction).38,36, 39 Executive function/working memory processes can be heavily involved in remembering to take medications as prescribed, such as when someone tries to keep in mind the intention to take medication while performing other activities associated with the demands of life. The intervention significantly improved adherence, presumably because it was designed to decrease the need for effortful self-initiated processes in favor of more automatic associative processes thought to be preserved with normal aging (e.g., associating medication taking with a specific cue like having breakfast so that the occurrence of breakfast triggers retrieval of the intention). Our results are in line with those of past research showing that interventions that focus only on education have few benefits for adherence. Specifically, our education control condition showed no benefit from baseline to the nurse visits and a decline over the following five-months.
In the current study we developed a novel approach that involved providing older adults with strategies for improving medication adherence. The strategies were ones that have been demonstrated in behavioral laboratories to improve prospective remembering in older adults, in part by promoting reliance on processes that tend to be spared with normal aging (e.g., relatively automatic associative retrieval processes) rather than on more vulnerable executive function processes.40 Some intervention studies have examined reminders, or single memory cues (e.g. automated telephone calls, post cards or text messaging) to help individuals adhere with small effects. None have supported all components of successful prospective remembering (encoding, storage, retrieval of the intention to adhere at the right time, execution of the action of taking the medication so as not to forget over a delay, and providing cues to give feedback about whether or not the medication was taken as intended). The intervention markedly improved medication adherence during the nurse visits. The finding that the intervention primarily benefitted participants with lower executive function/working memory capacity suggests that the intervention improved adherence by reducing reliance on these cognitive abilities.
We expected that participants would integrate the trained strategies into their daily life and therefore have no need for continuing contact with the nurses. However, intervention benefits were not sustained over the following five months, perhaps because the strategies learned during the intervention were not fully integrated into the participants’ daily routine. This drop in adherence suggests the need for continuing support for strategy use and converges with other studies demonstrating an improvement as long as the interventionist was in direct contact with the participants, but not after.41 Support for this conclusion comes from a review of studies using reminders covering the years 1968 to 2011.6 All reminder interventions were based on continuous reminders, for example, phone text messages or traditional phone calls rather than the initial intervention described here to set up strategies for remembering, but without ongoing reminders. Ongoing reminders and ongoing coaching of strategy use could be necessary to continue the initial improvement we see in adherence.
Further, a potentially critical feature of hypertension is that it is asymptomatic. This lack of symptoms surely adds to the difficulty of remembering to take medication as there are no physical cues (e.g., aches and pains) that can serve to remind people to take their medications. Research also shows that maintaining behavior change (such as the use of the cognitive strategies implemented in this intervention) is more difficult when people do not receive salient feedback that the new behaviors are producing benefits.42 Thus, the asymptomatic nature of hypertension means that there was no feedback concerning improvements in health that resulted from enhanced medication adherence. At this point, further research is needed to determine how to sustain the use of the highly effective strategies in this cognitive intervention over the long term. This may involve regular self-monitoring of blood pressure so that individuals become more mindful of the health benefits of proper adherence. This approach has been used with success in other investigations.5 It is also possible that technology could be leveraged to help sustain these strategies.43–46
Importantly, the current study demonstrates that executive function/working memory moderates the effect of the intervention on adherence. That is, the intervention was particularly effective for those with lower executive function, i.e. those with executive function/working memory composite z-scores of less than .53. Given that 70% of the population would have z-scores less than .53 we anticipate that our intervention would be significantly effective for 70% of those receiving it. Thus, the intervention has great potential to support medication adherence in those populations that are most at risk for experiencing decline in executive function and working memory. Our findings are consistent with the Institute of Medicine’s recommendations to consider how cognitive aging in older adults affects the performance of everyday activities and demonstrates that normal aging affects some cognitive processes more than others.47 Given that other populations in addition to elderly with compromised executive function also demonstrate a decline in medication adherence,15 our intervention is likely to have general benefits. For example, chemotherapy treatment is associated with cognitive decline48 and lipid disorders or diabetes may accelerate vascular aging and hence decline in cognitive function consistent with what is seen in age associated decline.49–51 Hence, in addition to implications for promoting medication adherence among elderly, the findings in this study may be relevant for other patient populations.
The present study has limitations. This intervention focused solely on prospective memory strategies to address nonadherence that develops because of forgetting to take medications as intended. Medication adherence is multifactorial.52 Causal factors for nonadherence have been identified as costs, lack of perceived benefit by the patient, and possible side effects. Certainly each of these factors is a possible causal pathway to nonadherence. However, little attention has been paid to simple forgetting at the intended time as a reason for nonadherence except when addressed by single, albeit ongoing reminders such as phone calls or post-cards to sustain the action and using single reminders even when ongoing, does not always work.53 We believe that single reminders alone can be ineffective because they fail to address all of the component processes necessary for successful prospective remembering.
Little progress in improving medication adherence has been made since Osterberg and Blaschke’s review a decade ago.54 In this review, forgetfulness was cited as the reason for nonadherence in 30% of patients. The current study offers a new and unique approach to assisting older adults and others with asymptomatic diseases to improve adherence to medications so they are less likely to forget to take medications as intended. This multifaceted intervention produced pronounced improvements in medication adherence and especially for vulnerable individuals who are low in executive/working memory function. Further research is needed to determine how to sustain the substantial initial benefits that results from this intervention technique.
Acknowledgments
NIH Funding: This work was funded by National Institute of Nursing Research at the National Institutes of Health, NIH NR010350.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
All authors are affiliated with academic institutions. Two presentations were given at professional meetings, no payment was received.
Author Contributions: Kathie Insel is the P.I. of the study. Gil Einstein and Dan Morrow were Co-Investigators on the study since inception, throughout operationalization, data collection, data analysis. This was the creative team. Dr. Joe Hepworth is the statistician. Kari Koerner was involved in data collection, continuous lab meetings, data analysis, development of the manuscript.
Sponsor’s Role: The sponsor was not involved in design, methods, subject recruitment, data collection, analysis or preparation of the paper. The sponsor is the National Institutes of Health, this application was submitted within a call for applications on self-management.
References
- 1.Haynes RB, Ackloo E, Sahota N, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008:CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Viswanathan M, Golin CE, Jones CD, et al. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: A systematic review. Ann Intern Med. 2012;157:785–795. doi: 10.7326/0003-4819-157-11-201212040-00538. [DOI] [PubMed] [Google Scholar]
- 3.Dragomir A, Cote R, White M, et al. Relationship between adherence level to statins, clinical issues and health-care costs in real-life clinical setting. Value Health. 2010;13:87–94. doi: 10.1111/j.1524-4733.2009.00583.x. [DOI] [PubMed] [Google Scholar]
- 4.Nelson MR, Reid CM, Ryan P, et al. Self-reported adherence with medication and cardiovascular disease outcomes in the Second Australian National Blood Pressure Study (ANBP2) Med J Aust. 2006;185:487–489. doi: 10.5694/j.1326-5377.2006.tb00662.x. [DOI] [PubMed] [Google Scholar]
- 5.Glynn LG, Murphy AW, Smith SM, et al. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev. 2010:CD005182. doi: 10.1002/14651858.CD005182.pub4. [DOI] [PubMed] [Google Scholar]
- 6.Fenerty SD, West C, Davis SA, et al. The effect of reminder systems on patients’ adherence to treatment. Patient Prefer Adherence. 2012;6:127–135. doi: 10.2147/PPA.S26314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosworth HB, Powers BJ, Olsen MK, et al. Home blood pressure management and improved blood pressure control: Results from a randomized controlled trial. Arch Intern Med. 2011;171:1173–1180. doi: 10.1001/archinternmed.2011.276. [DOI] [PubMed] [Google Scholar]
- 8.Bae JP, Dobesh PP, Klepser DG, et al. Adherence and dosing frequency of common medications for cardiovascular patients. Am J Manag Care. 2012;18:139–146. [PubMed] [Google Scholar]
- 9.Choudhry NK, Avorn J, Glynn RJ, et al. Full coverage for preventive medications after myocardial infarction. N Engl J Med. 2011;365:2088–2097. doi: 10.1056/NEJMsa1107913. [DOI] [PubMed] [Google Scholar]
- 10.Ellis J. Prospective Memory or the Realization of Delayed Intentions: A Conceptual Framework for Research. In: Brandimonte M, Einstein GO, McDaniel MA, editors. Prospective Memory Theory and Applications. Mahwah, New Jersey: Lawrence Erlbaum Associates; 1996. pp. 1–22. [Google Scholar]
- 11.McDaniel MA, Einstein GO. Prospective memory: An overview and synthesis of an emerging field. Thousand Oaks, CA US: Sage Publications, Inc; 2007. [Google Scholar]
- 12.Insel KC, Einstein GO, Morrow DG, et al. A multifaceted prospective memory intervention to improve medication adherence: Design of a randomized control trial. Contemp Clin Trials. 2013;34:45–52. doi: 10.1016/j.cct.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayes TL, Larimer N, Adami A, et al. Medication adherence in healthy elders: small cognitive changes make a big difference. J Aging Health. 2009;21:567–580. doi: 10.1177/0898264309332836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Insel K, Morrow D, Brewer B, et al. Executive function, working memory, and medication adherence among older adults. J Gerontol B Psychol Sci Soc Sci. 2006;61:102–107. doi: 10.1093/geronb/61.2.p102. [DOI] [PubMed] [Google Scholar]
- 15.Stilley CS, Bender CM, Dunbar-Jacob J, et al. The impact of cognitive function on medication management: Three studies. Health Psychol. 2010;29:50–55. doi: 10.1037/a0016940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park DC, Lautenschlager G, Hedden T, et al. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- 17.McDaniel MA, Einstein GO, Jacoby LL. New considerations in aging and memory: The glass may be half full. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. 3rd. Mahwah, New Jersey: Lawrence Erlabaum Associates; 2008. pp. 251–310. [Google Scholar]
- 18.McDaniel MA, Einstein GO. The neuropsychology of prospective memory in normal aging: A componential approach. Neuropsychologia. 2011;49:2147–2155. doi: 10.1016/j.neuropsychologia.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scullin MK, Gordon BA, Shelton JT, et al. Evidence for a detrimental relationship between hypertension history, prospective memory, and prefrontal cortex white matter in cognitively normal older adults. Cogn Affect Behav Neurosci. 2013;13:405–416. doi: 10.3758/s13415-013-0152-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–173. [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Insel KC, Cole L. Individualizing memory strategies to improve medication adherence. Appl Nurs Res. 2005;18:199–204. doi: 10.1016/j.apnr.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 23.McHenry JC, Insel KC, Einstein GO, et al. Recruitment of older adults: Success may be in the details. Gerontologist. 2015;55:845–853. doi: 10.1093/geront/gns079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mullet HG, Scullin MK, Hess TJ, et al. Prospective memory and aging: evidence for preserved spontaneous retrieval with exact but not related cues. Psychol Aging. 2013;28:910–922. doi: 10.1037/a0034347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Einstein GO, McDaniel MA, Richardson SL, et al. Aging and prospective memory: Examining the influences of self-initiated retrieval processes. J Exp Psychol Learn Mem Cogn. 1995;21:996–1007. doi: 10.1037//0278-7393.21.4.996. [DOI] [PubMed] [Google Scholar]
- 26.Einstein GO, McDaniel MA. Retrieval processes in prospective memory: Theoretical approaches and some new empirical findings. In: Brandimonte Maria, Einstein Gilles O, et al., editors. Prospective memory: Theory and applications. Mahwah, NJ: Lawrence Erlbaum Associates, Publishers; 1996. pp. 115–141. [Google Scholar]
- 27.Gollwitzer PM. Implementation intentions: Strong effects of simple plans. Am Psychol. 1999;54:493–503. [Google Scholar]
- 28.McDaniel MA, Einstein GO, Stout AC, et al. Aging and Maintaining Intentions Over Delays: Do It or Lose It. Psychol Aging. 2003;18:823–835. doi: 10.1037/0882-7974.18.4.823. [DOI] [PubMed] [Google Scholar]
- 29.McDaniel MA, Einstein GO, Graham T, et al. Delaying Execution of Intentions: Overcoming the Costs of Interruptions. Appl Cogn Psychol. 2004;18:533–547. [Google Scholar]
- 30.Aardex C. Advanced Analytic Research on Drug Exposure. Volume 2010. Aardex; 2010. [Google Scholar]
- 31.Aardex C. MEMS 6: Medication Event Monitoring System Volume 2015. 2015 [Google Scholar]
- 32.Morrell RW, Park DC, Kidder DP, et al. Adherence to antihypertensive medications across the life span. Gerontologist. 1997;37:609–619. doi: 10.1093/geront/37.5.609. [DOI] [PubMed] [Google Scholar]
- 33.Park DC, Hertzog C, Leventhal H, et al. Medication adherence in rheumatoid arthritis patients: Older is wiser. J Am Geriatr Soc. 1999;47:172–183. doi: 10.1111/j.1532-5415.1999.tb04575.x. [DOI] [PubMed] [Google Scholar]
- 34.Heaton RK, Chelune GJ, Talley JL, et al. Wisconsin Card Sorting Test (WCST) Manual Revised and Expanded. Odessa, FL: Psychological ASsessment Resources; 1993. [Google Scholar]
- 35.Conway ARA, Kane MJ, Bunting MF, et al. Working memory span tasks: A methodological review and user’s guide. Psychonom Bull Rev. 2005;12:769–786. doi: 10.3758/bf03196772. [DOI] [PubMed] [Google Scholar]
- 36.Engle RW. Working memory capacity as executive attention. Curr Direct Psychol Sci. 2002;11:19–23. [Google Scholar]
- 37.Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis. New York: Guilford Press; 2013. [Google Scholar]
- 38.Miyake A, Friedman NP. The nature and organization of individual differences in executive functions: Four general conclusions. Curr Dir Psychol Sci. 2012;21:8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Otsuka Y, Osaka N, Morishita M, et al. Decreased activation of anterior cingulate cortex in the working memory of the elderly. Neuroreport. 2006;17:1479–1482. doi: 10.1097/01.wnr.0000236852.63092.9f. [DOI] [PubMed] [Google Scholar]
- 40.McDaniel MA, Einstein GO, Rendell PG. The puzzle of inconsistent age-related declines in prospective memory: A multiprocess explanation. In: Kliegel M, McDaniel MA, Einstein GO, editors. Prospective memory: Cognitive, neuroscience, developmental, and applied perspectives. New York, NY: Taylor & Francis Group/Lawrence Erlbaum Associates; 2008. pp. 141–160. [Google Scholar]
- 41.Murray MD, Young J, Hoke S, et al. Pharmacist intervention to improve medication adherence in heart failure: A randomized trial. Ann Intern Med. 2007;146:714–725. doi: 10.7326/0003-4819-146-10-200705150-00005. [DOI] [PubMed] [Google Scholar]
- 42.Rothman AJ, Sheeran P, Wood W. Reflective and automatic processes in the initiation and maintenance of dietary change. Ann Behav Med. 2009;38(Suppl 1):S4–17. doi: 10.1007/s12160-009-9118-3. [DOI] [PubMed] [Google Scholar]
- 43.Mohammed S, Siddiqi O, Ali O, et al. User engagement with and attitudes towards an interactive SMS reminder system for patients with tuberculosis. J Telemed Telecare. 2012;18:404–408. doi: 10.1258/jtt.2012.120311. [DOI] [PubMed] [Google Scholar]
- 44.Cassimatis M, Kavanagh DJ. Effects of type 2 diabetes behavioural telehealth interventions on glycaemic control and adherence: a systematic review. J Telemed Telecare. 2012;18:447–450. doi: 10.1258/jtt.2012.gth105. [DOI] [PubMed] [Google Scholar]
- 45.Bosworth HB. How can innovative uses of technology be harnessed to improve medication adherence? Expert Rev Pharmacoecon Outcomes Res. 2012;12:133–135. doi: 10.1586/erp.12.6. [DOI] [PubMed] [Google Scholar]
- 46.Williams A. Issue Brief: Medication Adherence and Health IT. Office of the National Coordinator for Health Information Technology: Department of Health and Human Services; 2014. [Google Scholar]
- 47.Blazer D, Yaffe K, Liverman CE. Cognitive Aging: Progress in Understanding and Opportunities for Action. Washington DC: National Academy of Sciences; 2015. [PubMed] [Google Scholar]
- 48.Hess LM, Insel KC. Chemotherapy-related change in cognitive function: A conceptual model. Oncol Nurs Forum. 2007;34:981–994. doi: 10.1188/07.ONF.981-994. [DOI] [PubMed] [Google Scholar]
- 49.Dufouil C, de Kersaint-Gilly A, Besancon V, et al. Longitudinal study of blood pressure and white matter hyperintensities: The EVA MRI Cohort. Neurology. 2001;56:921–926. doi: 10.1212/wnl.56.7.921. [DOI] [PubMed] [Google Scholar]
- 50.Balasubramanyam M, Adaikalakoteswari A, Monickaraj SF, et al. Telomere shortening & metabolic/vascular diseases. Indian J Medl Res. 2007;125:441–450. [PubMed] [Google Scholar]
- 51.Tzourio C, Dufouil C, Ducimetiere P, et al. Cognitive decline in individuals with high blood pressure: A longitudinal study in the elderly. EVA Study Group. Epidemiology of Vascular Aging. Neurology. 1999;53:1948–1952. doi: 10.1212/wnl.53.9.1948. [DOI] [PubMed] [Google Scholar]
- 52.Rosenbaum L, Shrank WH. Taking our medicine–improving adherence in the accountability era. N Engl J Med. 2013;369:694–695. doi: 10.1056/NEJMp1307084. [DOI] [PubMed] [Google Scholar]
- 53.Marquez Contreras E, de la Figuera von Wichmann M, Gil Guillen V, et al. Effectiveness of an intervention to provide information to patients with hypertension as short text messages and reminders sent to their mobile phone (HTA-Alert) Atencion primaria/Sociedad Espanola de Medicina de Familia y Comunitaria. 2004;34:399–405. doi: 10.1016/S0212-6567(04)78922-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Osterberg L, Blacschke T. Adherence to Medications. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]


