Abstract
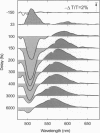
Femtosecond transient absorption measurements of the cis-trans isomerization of the visual pigment rhodopsin clarify the interpretation of the dynamics of the first step in vision. We present femtosecond time-resolved spectra as well as kinetic measurements at specific wavelengths between 490 and 670 nm using 10-fs probe pulses centered at 500 and 620 nm following a 35-fs pump pulse at 500 nm. The expanded spectral window beyond that available (500-570 nm) in our previous study [Schoenlein, R. W., Peteanu, L. A., Mathies, R. A. & Shank, C. V. (1991) Science 254, 412-415] provides the full differential absorption spectrum of the photoproduct as a function of delay time after photolysis. The high time-resolution data presented here contradict an alternative interpretation of the rhodopsin photochemistry offered by Callender and co-workers [Yan, M., Manor, D., Weng, G., Chao, H., Rothberg, L., Jedju, T. M., Alfano, R. R. & Callender, R. H. (1991) Proc. Natl. Acad. Sci. USA 88, 9809-9812]. Our results confirm that the red-shifted (lambda max approximately 570 nm) photo-product of the isomerization reaction is fully formed within 200 fs. Subsequent changes in the differential spectra between 200 fs and 6 ps are attributed to a combination of dynamic ground-state processes such as intramolecular vibrational energy redistribution, vibrational cooling, and conformational relaxation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Busch G. E., Applebury M. L., Lamola A. A., Rentzepis P. M. Formation and decay of prelumirhodopsin at room temperatures. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2802–2806. doi: 10.1073/pnas.69.10.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doukas A. G., Junnarkar M. R., Alfano R. R., Callender R. H., Kakitani T., Honig B. Fluorescence quantum yield of visual pigments: evidence for subpicosecond isomerization rates. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4790–4794. doi: 10.1073/pnas.81.15.4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyring G., Curry B., Broek A., Lugtenburg J., Mathies R. Assignment and interpretation of hydrogen out-of-plane vibrations in the resonance Raman spectra of rhodopsin and bathorhodopsin. Biochemistry. 1982 Jan 19;21(2):384–393. doi: 10.1021/bi00531a028. [DOI] [PubMed] [Google Scholar]
- Eyring G., Curry B., Mathies R., Fransen R., Palings I., Lugtenburg J. Interpretation of the resonance Raman spectrum of bathorhodopsin based on visual pigment analogues. Biochemistry. 1980 May 27;19(11):2410–2418. doi: 10.1021/bi00552a020. [DOI] [PubMed] [Google Scholar]
- Hayward G., Carlsen W., Siegman A., Stryer L. Retinal chromophore of rhodopsin photoisomerizes within picoseconds. Science. 1981 Feb 27;211(4485):942–944. doi: 10.1126/science.7466366. [DOI] [PubMed] [Google Scholar]
- Loppnow G. R., Mathies R. A. Excited-state structure and isomerization dynamics of the retinal chromophore in rhodopsin from resonance Raman intensities. Biophys J. 1988 Jul;54(1):35–43. doi: 10.1016/S0006-3495(88)82928-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palings I., Pardoen J. A., van den Berg E., Winkel C., Lugtenburg J., Mathies R. A. Assignment of fingerprint vibrations in the resonance Raman spectra of rhodopsin, isorhodopsin, and bathorhodopsin: implications for chromophore structure and environment. Biochemistry. 1987 May 5;26(9):2544–2556. doi: 10.1021/bi00383a021. [DOI] [PubMed] [Google Scholar]
- Schoenlein R. W., Peteanu L. A., Mathies R. A., Shank C. V. The first step in vision: femtosecond isomerization of rhodopsin. Science. 1991 Oct 18;254(5030):412–415. doi: 10.1126/science.1925597. [DOI] [PubMed] [Google Scholar]
- Shank C. V. Investigation of ultrafast phenomena in the femtosecond time domain. Science. 1986 Sep 19;233(4770):1276–1280. doi: 10.1126/science.233.4770.1276. [DOI] [PubMed] [Google Scholar]
- Spalink J. D., Reynolds A. H., Rentzepis P. M., Sperling W., Applebury M. L. Bathorhodopsin intermediates from 11-cis-rhodopsin and 9-cis-rhodopsin. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1887–1891. doi: 10.1073/pnas.80.7.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Callender R. H. Primary photochemistry and photoisomerization of retinal at 77 degrees K in cattle and squid rhodopsins. Biophys J. 1981 May;34(2):261–270. doi: 10.1016/S0006-3495(81)84848-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald G. Molecular basis of visual excitation. Science. 1968 Oct 11;162(3850):230–239. doi: 10.1126/science.162.3850.230. [DOI] [PubMed] [Google Scholar]
- YOSHIZAWA T., WALD G. Pre-lumirhodopsin and the bleaching of visual pigments. Nature. 1963 Mar 30;197:1279–1286. doi: 10.1038/1971279a0. [DOI] [PubMed] [Google Scholar]
- Yan M., Manor D., Weng G., Chao H., Rothberg L., Jedju T. M., Alfano R. R., Callender R. H. Ultrafast spectroscopy of the visual pigment rhodopsin. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9809–9812. doi: 10.1073/pnas.88.21.9809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg R., Du-Jeon-Jang, Bitting H. C., El-Sayed M. A. Subpicosecond resonance Raman spectra of the early intermediates in the photocycle of bacteriorhodopsin. Biophys J. 1990 Jul;58(1):135–141. doi: 10.1016/S0006-3495(90)82359-6. [DOI] [PMC free article] [PubMed] [Google Scholar]