Abstract
OBJECTIVES
To evaluate the effect of structured physical activity on respiratory outcomes in community-dwelling elders with mobility limitations.
DESIGN
Multicenter, randomized trial of physical activity versus health education, with respiratory variables pre-specified as tertiary outcomes over an intervention period of 24–42 months. Physical activity included walking (goal of 150 minutes/week) and strength, flexibility, and balance training. Health education included workshops on topics relevant to older adults and upper extremity stretching exercises.
SETTING
Lifestyle Interventions and Independence in Elder (LIFE) Study.
PARTICIPANTS
1635 community-dwelling persons, aged 70–89, with Short Physical Performance Battery scores <10.
MEASUREMENTS
Dyspnea severity (defined as moderate-to-severe by a Borg index >2, immediately after a 400-m walk), forced expiratory volume in 1-second (FEV1) (<lower limit of normal (LLN) defined low breathing capacity), and maximal inspiratory pressure (MIP) (<LLN defined respiratory muscle weakness) were assessed at baseline and 6, 18, and 30 months. In addition, hospitalization for exacerbation of obstructive airways disease (EOAD) and pneumonia were ascertained over the 42-month follow-up period.
RESULTS
The randomized groups were similar on baseline demographics, including mean age (79 years) and sex (67% female). Relative to health education, physical activity had no effect on dyspnea severity, FEV1, or MIP, but was associated with a higher likelihood of hospitalization, significantly for EOAD (hazard ratio 2.34 (1.19, 4.61), p=.01) and marginally for pneumonia (hazard ratio 1.54 (0.98, 2.42), p=.06).
CONCLUSION
Among older persons with mobility limitations, physical activity was associated with a higher likelihood of respiratory hospitalization, relative to health education, but this effect was not accompanied by differences in dyspnea severity, FEV1, or MIP — raising the possibility that higher hospital utilization could be attributable to greater participant contact.
Keywords: FEV1, MIP, dyspnea, hospitalization, exercise
INTRODUCTION
Older persons are at risk of having respiratory impairments, given the age-related decline in lung function (e.g. decrease in the forced expiratory volume in 1-second [FEV1]) and in respiratory muscle strength (e.g. decrease in the maximal inspiratory pressure [MIP]), as well as the age-related onset and progression of cardiopulmonary disease (e.g. heart failure, chronic obstructive pulmonary disease, asthma, and interstitial lung disease).1–4 Older persons are also at risk of having mobility limitations, given age-related declines in skeletal muscle mass and function (sarcopenia), and adverse consequences of multimorbidity, including cardiopulmonary and musculoskeletal disease.3–12 Importantly, respiratory impairments and mobility limitations often coexist, and may have bidirectional associations and similar effects on health outcomes, including exertional dyspnea and subsequent disability and death.1–12
There is a strong rationale to promote physical activity.13–15 Based on a comprehensive review of prior work, the U.S. Department of Health and Human Services has concluded that increased physical activity improves cardiovascular, musculoskeletal, and mental health outcomes.14 Whether the benefits of physical activity extend to respiratory outcomes, particularly in older persons with mobility limitations, has not yet been evaluated.
The Lifestyle Interventions and Independence for Elders (LIFE) Study is a randomized controlled trial designed to compare a structured physical activity intervention with a health education intervention in 1,635 elders with mobility limitations, over a planned intervention period of 24 to 42 months.15–17 Although the primary outcome of this study was mobility disability, respiratory variables were included as pre-specified tertiary outcomes, with assessments at baseline and 6, 18, and 30 months, including: 1) modified Borg Index, immediately after a 400 meter walk test — values >2 defined moderate-to-severe dyspnea;18 2) FEV1 — values < lower limit of normal (LLN) defined a low breathing capacity (FEV1 is a strong predictor of the maximal attainable ventilation during exercise);12,19,20 and 3) MIP — values < LLN defined respiratory muscle weakness.21–23 In addition, hospitalizations for exacerbation of obstructive airways disease or pneumonia were ascertained over the 42-month follow-up period. In an earlier cross-sectional analysis, LIFE participants had high rates of moderate-to-severe exertional dyspnea (31.6%), low breathing capacity (17.7%), and respiratory muscle weakness (14.7%) at baseline.10 In the current manuscript, we tested our hypothesis that structured physical activity, compared with a health education intervention, improved several respiratory outcomes over a planned intervention period of 24 to 42 months.
METHODS
Trial design and participants
The LIFE Study is a multicenter, single-blind, parallel randomized trial involving 1,635 sedentary older persons with mobility limitations, conducted at 8 centers across the United States (Appendix A lists the LIFE field centers and investigators).15–17 The study protocol was approved by the institutional review boards at all participating sites. Written informed consent was obtained from all study participants.
Details of the methods were published previously.16 Eligibility criteria included: 1) age 70–89; 2) sedentary status, defined as <20 minutes/week of regular physical activity in the past month and <125 minutes/week of moderate physical activity;24 3) mobility limitations, defined as a Short Physical Performance Battery score <10,7–9 but able to walk 400 meters in ≤15 minutes without sitting, leaning, or the help of another person or walker); and 4) no major cognitive impairment, defined as a Modified Mini-Mental State Examination (3MSE)25 score of no more than 1.5 standard deviations below education- and race-specific norms.
Interventions
The physical activity intervention involved primarily walking, with a goal of 150 minutes/week, as well as strength, flexibility, and balance training.15,16 The intervention included attendance at two center-based sessions per week and home-based activity 3–4 times per week for the duration of the study. The physical activity sessions progressed towards a goal of 30 minutes of walking five-days a week at a moderate intensity (based on the Borg perceived exertion scale), 10 minutes of primarily lower extremity strength training (ankle weights), 10 minutes of balance training, and large muscle group flexibility exercises. Participants began with lighter intensity exercise and gradually increased intensity over the first 2–3 weeks of the intervention. Of the 818 participants who were randomized to physical activity, 118 discontinued the intervention after a median of 15.8 months. The physical activity group attended 63% of scheduled sessions after excluding medical leave (median [interquartile range] of 71% [50%–83%]) over a median of 28.5 months.
The health education intervention focused on weekly workshops during the first 26 weeks, and monthly sessions thereafter.15,16 Workshops included topics such as how to negotiate the health care system, how to travel safely, preventive services and screenings at different ages, and where to go for reliable health information and nutrition. Each workshop also included a 5–10 minute instructor-led program of gentle upper extremity stretching or flexibility exercises. Of the 817 participants who were randomized to health education, 160 discontinued the intervention after a median of 32.5 months. The health education group attended 73% of scheduled sessions (median [interquartile range] of 82% [63%–90%]) over a median of 32.5 months.
Adherence to the intervention was based on attendance, CHAMPS questionnaire, and accelerometry data, as previously reported in the main trial of the LIFE Study.15 In both groups, discontinuation of the intervention was operationalized as no attendance at any intervention session during the 6 months prior to the last planned follow-up visit date — deaths and intervention withdrawals are included in these numbers.15 For a detailed flow of participants through the intervention period, please refer to Figure 1 in reference 15.
Demographics and Clinical Characteristics
The baseline characteristics included age, sex, race/ethnicity (non-Hispanic white versus other), body mass index (BMI, in kg/m2), smoking status, chronic conditions, health status, respiratory medications, and oxygen therapy.10,16 The chronic conditions were ascertained by self-report. To assess health status, participants were asked, “Would you say your health is excellent, very good, good, fair, or poor?” Decreased health was defined based on participant’s self-reported rating of “fair” or “poor.” Respiratory medications included bronchodilators and corticosteroids, specifically in the prior 3 days. Oxygen therapy included intermittent use, at night or with exercise (regular use of oxygen therapy was an exclusion criterion in the LIFE Study).
Mobility Impairment and Physical Inactivity
Baseline mobility was evaluated by the Short Physical Performance Battery (SPPB) and 400 meter walk test (400MWT).15,16 The SPPB is a composite measure that consists of time to walk 4 meters at usual pace, time to complete five chair stands, and three increasingly difficult standing balance maneuvers.7–9 An SPPB score <8 identified moderate-to-severe mobility impairment.7–9 The 400MWT was completed at the participant’s usual walking pace over a 40-meter course, with a slow gait speed defined as <0.8 m/s.26
Baseline physical inactivity was established by accelerometry (ActiGraph GT3X and ActiLife software [version 5]; Pensacola, FL), over a planned 7-day period.10,15,16 After dressing each morning, participants placed the accelerometer on their right hip (waistline belt), thereafter removing the monitor just prior to going to bed at night. Sedentary time was defined as the percent of accelerometry wear time with activity <100 counts/minute (approximated sitting time),27 averaged across at least 5 days of monitoring, including 10-hours on each day (this amount of wear time correlates well with 3 weeks of wear time).28 Accelerometry was measured at baseline and 6, 12, and 24 months.
Dyspnea
Dyspnea was evaluated at baseline and 6, 18, and 30 months, using the modified Borg index immediately after the 400MWT. The modified Borg dyspnea index is a 10-level severity scale, with moderate-to-severe dyspnea based on a threshold of >2.18
Forced Expiratory Volume in 1-Second (FEV1)
Spirometric data were collected by centrally-trained, certified research staff at baseline and 6, 18, and 30 months, using the EasyOne™ PLUS spirometer (NDD Medical Technologies; Andover, MA) and protocols from the American Thoracic Society (ATS).19 Participants performed at least three trials of a forceful exhalation maneuver that started from maximal inspiration and concluded with a 6-second end-of-test criterion.19 Spirometric performance was reviewed by the independent LIFE Study quality control spirometry committee, evaluating each set of spirometry tracings and providing monthly feedback to the certified research staff. Grades were assigned to each FEV1, where “C” or better ratings were used in the analysis (achieved at least two ATS acceptable trials). The FEV1 was selected as a primary respiratory outcome, for at least three reasons: 1) it is more likely to be successfully completed than the forced vital capacity (FVC) in older persons; 2) it is a strong predictor of the maximal attainable ventilation during exercise; and 3) it is associated with important health outcomes, including respiratory symptoms, physical disability, hospitalization, and death.1–3,10,12,19,20,29–32
For comparisons between measured and predicted FEV1 values, we used reference equations from the Global Lung Function Initiative (GLI).33 The use of GLI equations rigorously account for age-related changes in lung function.33 Using the GLI equations, Z-scores for FEV1 were calculated for each participant, with a Z-score of −1.64 defining the lower limit of normal (LLN) as the 5th percentile of distribution.33,34 Participants were classified as having low breathing capacity if FEV1 <LLN.10,12
Maximal Inspiratory Pressure (MIP)
MIP readings (cm H2O) were obtained at baseline and 6, 18, and 30 months, using a Magnehelic 2000–200 pressure gauge (Dwyer Instruments, Michigan City, IN) and testing protocols as previously published for the Multi-Ethnic Study of Atherosclerosis Lung Study (MESA).21 MIP performance was reviewed by the independent LIFE Study quality control spirometry committee. In particular, to be included in the analytical sample, participants had to achieve a variability of ≤10 cm H2O for the two highest MIP readings at each of the four time points.21 For comparisons between measured and predicted MIP values, we used reference equations from MESA,21 which included the same variability criterion as in the current study. Participants were classified as having respiratory muscle weakness if the highest MIP reading was <LLN (<5th percentile of distribution).21
The MIP was selected as a primary respiratory outcome, for at least three reasons: 1) it is likely to be successfully completed in older persons; 2) it is a predictor of the maximal attainable ventilation during exercise; and 3) it is associated with important health outcomes, including myocardial infarction and cardiovascular death.12,21–23
Respiratory Hospitalizations
Respiratory hospitalizations included exacerbation of obstructive airways disease (EOAD) and pneumonia, ascertained across the 42-month follow-up period. These diagnoses were established by two central adjudicators based on review of relevant medical records; when the two adjudicators were discordant, a final consensus was reached by the LIFE Adjudication Committee.
Adjudication of definite EOAD hospitalization required: 1) diagnosis of asthma, chronic bronchitis, emphysema, or COPD at discharge; and 2) one of the following symptoms on admission—acute increase in sputum volume or purulence, or dyspnea. In addition, one of the following was required at admission or during the hospitalization: recent upper respiratory tract infection, symptoms of cough or fever, wheezing on examination, severe physiologic impairment (FEV1<1.2 liters, hypoxemia, or respiratory acidosis), or treatment with bronchodilators, systemic corticosteroids, antibiotics, or oxygen in a pattern that represented a change from baseline. The adjudication of probable EOAD was considered when the clinical presentation was consistent with EOAD, but all of the above diagnostic criteria were not met.
Adjudication of definite pneumonia hospitalization was based on criteria recorded within 48-hours of admission, including: 1) symptoms of cough, fever, or sputum production, or exam finding of rales or dullness to percussion, and 2) radiographic imaging showing new or progressive infiltrate, consolidation, cavitation, or pleural effusion. The adjudication of probable pneumonia hospitalization was considered when the radiograph was non-diagnostic (e.g. poor quality) but clinical presentation was otherwise consistent with pneumonia, and vice-versa. Because it was based on admission criteria, this outcome included community-acquired and healthcare-associated pneumonia, but not hospital-acquired pneumonia.
Missing Respiratory Data
Of those who completed the baseline respiratory assessments, the percentage of participants with missing respiratory outcomes at each subsequent visit were: at 6 months — Borg Index 6.0%, FEV1 17.1%, and MIP 13.7%; at 18 months — Borg Index 12.7%, FEV1 25.2%, and MIP 23.0%; and at 30 months — Borg Index 19.2%, FEV1 33.9%, and MIP 31.1%. At each visit, for all respiratory outcomes, the absolute difference in percent missing between groups was always <2.8% (range of difference between intervention groups was 0.6% to 2.8%).
The reasons for missing respiratory data included poor test performance or safety concerns regarding testing (spirometry and MIP), study withdrawal, and death. In addition, because the intervention duration was between 24 and 42 months, a portion of the study participants never achieved a 30-month visit due to the timing of their enrollment.
Statistical Analysis
The baseline characteristics were first summarized by intervention group, using means and standard deviations (SD), or counts and percentages. The respiratory outcomes were evaluated as categorical variables (moderate-to-severe dyspnea [Borg >2], low breathing capacity [FEV1 <LLN], respiratory muscle weakness [MIP <LLN], and any definite or probable respiratory hospitalization [EOAD or pneumonia]), and as continuous variables (Borg Index, FEV1, and MIP).
Using GEE marginal logistic regression models appropriate for repeated binary outcomes, the average odds ratios of having a categorical respiratory outcome in the physical activity group relative to health education group were calculated across 30-months of follow-up.35 In this analysis, the respiratory outcomes were moderate-to-severe dyspnea, low breathing capacity, and respiratory muscle weakness. These models included terms for field center and gender (randomization was stratified on these factors). In addition, categorical terms for follow-up visit and the intervention by follow-up visit interaction were included in the models; the interaction term was necessary to allow for separate estimation of intervention effects at each time point. An unstructured covariance matrix was used to account for the within-person correlation between repeated measures. The follow-up visit by intervention term was tested for all models and the average odds ratio across follow-up visits was estimated from a model without this interaction.
As a sensitivity analysis, the average odd ratios of having an incident respiratory outcome were estimated across 30-months of follow-up, within two subgroups. Subgroup 1 consisted of those who at baseline did not have moderate-to-severe dyspnea, low breathing capacity, and respiratory muscle weakness, respectively, allowing for analysis of “incident cases of new outcomes.” Subgroup 2 consisted of those who at baseline had moderate-to-severe dyspnea, low breathing capacity, and respiratory muscle weakness, respectively, allowing for analysis of “incident cases of resolved outcomes.”
The continuous measures of the respiratory outcomes (dyspnea [Borg Index], breathing capacity [FEV1], and respiratory muscle strength [MIP]) were evaluated using mixed effects analysis of covariance models appropriate for repeatedly measured outcomes. The different post-baseline mean levels of FEV1, MIP, and Borg index were compared between intervention groups using a model containing the same terms as in the previously described logistic regression analyses. Least squares means were obtained for each respiratory outcome and contrasts were used to obtain estimates and test the average intervention effect across follow-up visits.
Time-to-event analyses for the respiratory hospitalizations were performed, using Cox proportional hazards models fit with randomization group as the main effect and stratified by clinical site and gender. The respiratory hospitalizations included outcomes classified as either definite or probable EOAD and pneumonia, respectively, as well as a composite of EOAD or pneumonia (“any” respiratory hospitalization). Similar analyses were performed with respiratory hospitalization classified as definite only.
All statistical comparisons were performed using SAS v9.4 (SAS Institute, Cary, NC). A Type I error rate of 0.05 was assumed for all comparisons.
RESULTS
As shown in Table 1, the baseline characteristics of the physical activity and health education intervention groups were comparable, with an average age of about 79 years; the majority of participants were female, non-Hispanic white, obese, and non-smokers (never or former). Both groups averaged 2 chronic conditions and had similar prevalence for each of the chronic conditions, having a fair-to-poor health status, and use of respiratory medications and oxygen therapy. Both groups averaged an SPPB <8 and included a large proportion of participants with slow gait speed and high sedentary time. Moderate-to-severe dyspnea was reported by nearly one-third of participants in each group, and the rates of low breathing capacity and respiratory muscle weakness were similar, ranging from 15%–20%.
Table 1.
Baseline characteristics by intervention group
| Characteristics | Physical Activity | Health Education | ||
|---|---|---|---|---|
| Na | Mean ± SD or n (%) | Na | Mean ± SD or n (%) | |
| Age | 818 | 78.7 ± 5.2 | 817 | 79.1 ± 5.2 |
| Female | 547 (66.9) | 551 (67.4) | ||
| Non-Hispanic white | 815 | 604 (74.1) | 815 | 635 (77.9) |
| BMI, kg/m2 | 818 | 30.1 ± 5.7 | 817 | 30.3 ± 6.2 |
| BMI ≥ 30 | 818 | 374 (45.7) | 817 | 378 (46.3) |
| Smoking status | ||||
| Never | 807 | 400 (49.6) | 799 | 434 (54.3) |
| Former | 381 (47.2) | 341 (42.7) | ||
| Current | 26 (3.2) | 24 (3.0) | ||
| Number of chronic conditionsb | 816 | 2.0 ± 1.2 | 815 | 2.0 ± 1.2 |
| Hypertension | 573 (70.2) | 578 (70.9) | ||
| Diabetes mellitus | 199 (24.4) | 216 (26.5) | ||
| Arthritis | 153 (18.8) | 165 (20.2) | ||
| Chronic lung diseasec | 130 (15.9) | 123 (15.1) | ||
| Peripheral artery diseased | 71 (8.7) | 58 (7.1) | ||
| Coronary artery diseasee | 60 (7.4) | 69 (8.5) | ||
| Stroke | 57 (7.0) | 52 (6.4) | ||
| Heart failure | 26 (3.2) | 814 | 45 (5.5) | |
| Respiratory medicationf | 817 | 86 (10.5) | 817 | 92 (11.3) |
| Oxygen therapyg | 818 | 11 (1.3) | 816 | 21 (2.6) |
| Fair-to-poor health status | 816 | 285 (34.9) | 813 | 282 (34.7) |
| 400m walk time (minutes) | 818 | 8.4 ± 1.9 | 817 | 8.5 ± 1.9 |
| Slow gait speed (<0.8 m/sec)h | 333 (40.7) | 309 (37.8) | ||
| SPPB score | 7.4 ± 1.6 | 7.3 ± 1.6 | ||
| SPPB <8i | 465 (56.8) | 439 (53.7) | ||
| Sedentary time (%)j | 656 | 77.1 ± 8.1 | 653 | 77.1 ± 8.2 |
| Respiratory measures | ||||
| Borg dyspnea indexk | 816 | 1.70 ± 1.46 | 816 | 1.72 ± 1.53 |
| Moderate-to-severe dyspnea (Borg>2) | 254 (31.1) | 262 (32.1) | ||
| FEV1 (liters) | 684 | 1.85 ± 0.56 | 679 | 1.86 ± 0.57 |
| Low breathing capacity (FEV1<LLN) | 135 (19.7) | 116 (17.1) | ||
| MIP (cm H2O) | 703 | 59.4 ± 23.0 | 677 | 58.6 ± 22.0 |
| Respiratory muscle weakness (MIP<LLN) | 116 (16.5) | 100 (14.8) | ||
BMI, body mass index; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; LLN, lower limit of normal (Z-score <−1.64); MIP, maximal inspiratory pressure; SD, standard deviation; SPPB, Short Physical Performance Battery.
Varies as a consequence of participants being excluded because of poor testing performance, missing values, or a delayed start to data acquisition (i.e., accelerometry).
Self-reported physician diagnosed.
Asthma or chronic obstructive pulmonary disease (chronic bronchitis or emphysema).
Included self-reported, physician-diagnosis or prior hospitalization for an operation or procedure to improve the blood flow to the legs (angioplasty or stent).
Heart attack, coronary, or myocardial infarction requiring overnight hospitalization.
Included bronchodilators and corticosteroids.
Included intermittent use, at night, or with exercise. Regular use of oxygen therapy was an exclusion criterion in the LIFE Study.
Measured during the 400 meter walk at the participant’s usual walking pace.
SPPB score <8 identified moderate-to-severe mobility impairment.
Percent of accelerometer wear time with activity <100 counts/minute, averaged across days.
Scale of dyspnea severity (0–10), recorded immediately after the 400-m walk test.
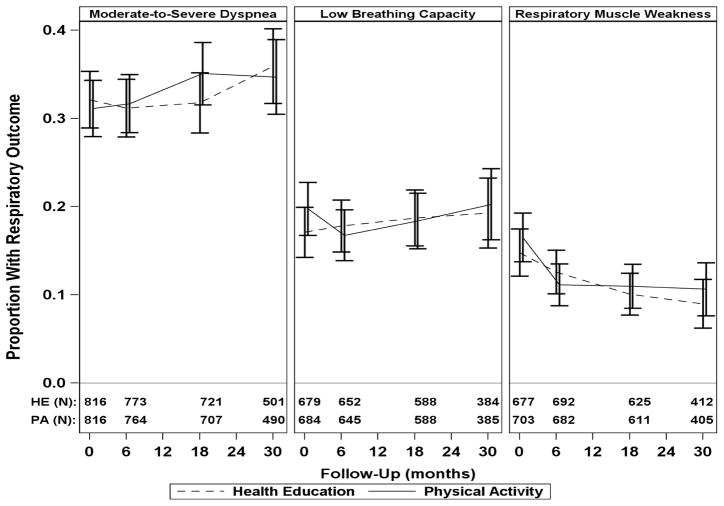
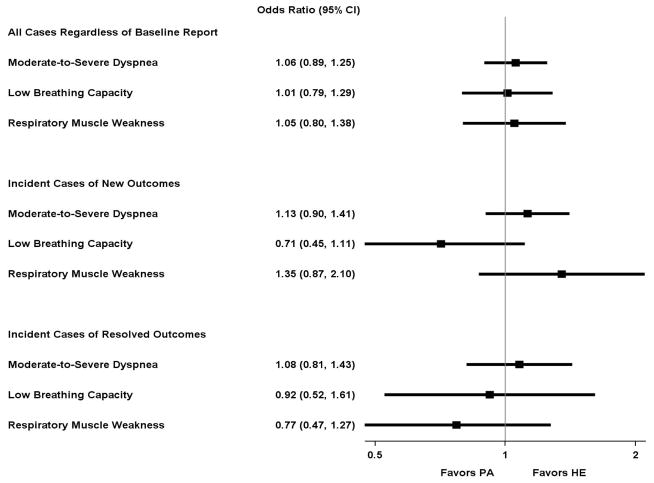
Figure 1 shows the prevalence of the categorical respiratory outcomes over time and by intervention, including moderate-to-severe dyspnea, low breathing capacity, and respiratory muscle weakness. Appendix B, Table B1 provides the same information in tabular format. The average odds ratios for the intervention effect on these categorical respiratory outcomes are shown in Figure 2. No significant intervention effect was observed for moderate-to-severe dyspnea, low breathing capacity, or respiratory muscle weakness.
Figure 1.
Prevalence of categorical respiratory outcomesa in the physical activity and health education intervention groups across 30 months of follow-up
FEV1, Forced expiratory volume in 1 second; HE, health education; MIP, maximal inspiratory pressure; LLN, lower limit of normal; PA, physical activity.
aModerate-to-severe dyspnea (Borg index>2), low breathing capacity (FEV1<LLN), and respiratory muscle weakness (MIP<LLN).
Figure 2.
Odds ratioa (95% CI) for effect of physical activity intervention on categorical respiratory outcomesb over time
CI, confidence interval; FEV1, Forced expiratory volume in 1 second; HE, health education; MIP, maximal inspiratory pressure; LLN, lower limit of normal; PA, physical activity.
aCalculated as the average odds ratio across the 30-month follow-up, adjusted for field center and gender.
bModerate-to-severe dyspnea (Borg index>2), low breathing capacity (FEV1<LLN), and respiratory muscle weakness (MIP<LLN).
Table 2 shows the average intervention effect on adjusted least squares mean values for continuous respiratory outcomes, including Borg Index, FEV1, and MIP, across 30-months of follow-up. The magnitude of the average treatment effect on the Borg Index, FEV1, and MIP was small and not statistically significant. Appendix B, Table B2 and Figure B1 provide the same information but at 6, 18, and 30 months of follow-up, showing again that the physical activity and health education groups had similar adjusted least squares mean values for the Borg Index, FEV1, and MIP (p-values ranged between .38 and .96).
Table 2.
Average intervention effect on adjusted least squares mean valuesa for continuous respiratory outcomes across 30-months of follow-up
| Intervention Group | Borg Index (Dyspnea Severity)b | FEV1 (Breathing Capacity)c | MIP (Respiratory Muscle Strength)d |
|---|---|---|---|
| Average Intervention Effect Across 30-Month Follow-Up Least Squares Means (95% CI)e | |||
| Physical Activity (PA) | 1.84 (1.69, 1.88) | 1.93 (1.82, 1.85) | 63.21 (61.60, 64.82) |
| Health Education (HE) | 1.79 (1.75, 1.93) | 1.93 (1.81, 1.84) | 62.77 (61.17, 64.37) |
| Difference (PA – HE) | 0.05 (−0.07, 0.17) | 0.00 (−0.05, 0.05) | 0.44 (−1.58, 2.46) |
CI, confidence interval; FEV1, Forced expiratory volume in 1 second; MIP, maximal inspiratory pressure; SD, standard deviation.
Adjusted for field center and gender
Dyspnea scale of 0–10, recorded immediately after the 400-m walk test.
FEV1 in liters.
MIP in cm H2O.
There was no statistical evidence of follow-up visit by intervention interaction (p≥.13 for all).
Table 3A shows hazard ratios for the intervention effect on respiratory hospitalizations, including definite or probable EOAD, pneumonia, and a composite of either diagnosis (“any”). The physical activity intervention was significantly associated with a higher likelihood of hospitalization for EOAD and any respiratory hospitalization, relative to the health education intervention (hazard ratio: 2.34 (1.19, 4.61) (p=.01), and 1.65 (1.11, 2.45) (p=.01), respectively). In addition, the higher likelihood of hospitalization for pneumonia approached significance in the physical activity intervention, relative to the health education intervention (hazard ratio: 1.54 (0.98, 2.42) (p=.06)). When classified as a definite diagnosis (Table 3B), the higher likelihood of hospitalization remained significant for EOAD (hazard ratio: 2.27 [1.12, 4.62], p=.02) but was attenuated for pneumonia and any respiratory hospitalization (hazard ratio: 1.35 (0.79, 2.29) (p=.26) and 1.52 (0.98, 2.35) (p=.06), respectively) in the physical activity intervention, relative to the health education intervention.
Table 3.
Hazard ratio (95% confidence interval) for effect of physical activity intervention on respiratory hospitalization over timeb
| A. Definite or probable: | ||||
|---|---|---|---|---|
| Respiratory Hospitalization | Physical Activity (N=818) | Health Education (N=817) | HR (95% CI) | P-value |
| n (%) | ||||
| Exacerbation of obstructive airways disease | 28 (3.4) | 12 (1.5) | 2.34 (1.19, 4.61) | .01 |
| Pneumonia | 47 (5.7) | 31 (3.8) | 1.54 (0.98, 2.42) | .06 |
| Anyc | 65 (7.9) | 40 (4.9) | 1.65 (1.11, 2.45) | .01 |
| B. Definite: | ||||
|---|---|---|---|---|
| Respiratory Hospitalization | Physical Activity (N=818) | Health Education (N=817) | HR (95% CI) | P-value |
| n (%) | ||||
| Exacerbation of obstructive airways disease | 25 (3.1) | 11 (1.3) | 2.27 (1.12, 4.62) | .02 |
| Pneumonia | 32 (3.9) | 24 (2.9) | 1.35 (0.79, 2.29) | .26 |
| Anyc | 51 (6.2) | 34 (4.2) | 1.52 (0.98, 2.35) | .06 |
HR, hazard ratio; CI, confidence interval.
Pneumonia or exacerbation of obstructive airways disease.
Across the entire follow-up of the main trial (up to 42 months), stratified by field center and gender.
Includes exacerbation of obstructive airways disease or pneumonia.
DISCUSSION
In the largest and longest randomized trial of structured physical activity in sedentary older persons with mobility limitations (LIFE Study), there was no treatment effect of the physical activity intervention on dyspnea severity, FEV1, and MIP (using continuous or categorical variables), when compared with a health education intervention. However, we also found that structured physical activity, relative to health education, was associated with a higher likelihood of respiratory hospitalization, significantly for EOAD and marginally for pneumonia.
The lack of a treatment effect on dyspnea and measures of respiratory impairment (FEV1 and MIP), whether analyzed as continuous or categorical variables, is in contrast to the main results from the LIFE Study, showing that the physical activity intervention yielded a significant 18% reduction in the risk of developing major mobility disability, defined as loss of ability to walk 400 meters, and a 28% reduction in the risk of persistent mobility disability when compared with a health education intervention.15 These contrasting results suggest that an intervention focused primarily on walking and lower extremity function, although improving mobility outcomes, may not address the mechanisms that underlie dyspnea and respiratory impairments. As discussed earlier, these mechanisms include an age-related decline in lung function and respiratory muscle strength, and an age-related onset and progression of cardiopulmonary disease.1–4
The lack of improvement in dyspnea severity in our highly sedentary study population was especially surprising, given that this was evaluated in response to a submaximal exercise workload (400MWT was performed at the participant’s usual walking pace). We had postulated that gains in lower extremity function and endurance in the physical activity group would reduce deconditioning and, in turn, improve exertional dyspnea. Our results suggest instead that the lack of improvement in dyspnea severity may have been due to preexisting cardiopulmonary disease, as the latter and related risk factors were highly prevalent in the LIFE Study. Future work should therefore evaluate whether preexisting cardiopulmonary disease, including objective measures of disease (i.e., echocardiography, spirometry, oximetry) and an assessment of pathophysiologic mechanisms (e.g. dynamic hyperinflation, with expiratory and inspiratory flow-limitation),12 attenuates the treatment effect of physical activity on dyspnea severity and respiratory impairments in sedentary older persons.
The higher rates of respiratory hospitalizations with structured physical activity, relative to health education, were not accompanied by differences in dyspnea severity, FEV1, or MIP. Although the mechanisms underlying the higher likelihood of respiratory hospitalization are uncertain, it is possible that clinical monitoring and more frequent participant contact during exercise visits at the LIFE field centers led to early diagnosis based on symptoms suggestive of EOAD and pneumonia. Upon enrollment, LIFE participants were vulnerable for adverse respiratory outcomes, as evidenced by their high prevalence of dyspnea and cardiopulmonary disease, including related risk factors and impairments.10 Importantly, established risk factors for having a respiratory hospitalization, as defined in the LIFE Study, include symptomatic cardiopulmonary disease, but not increased physical activity.36–39
The null results of the present study suggest that a multidisciplinary strategy may be needed to improve respiratory outcomes in older persons.1–4,40 As a proven multidisciplinary intervention that improves respiratory outcomes, pulmonary rehabilitation programs include lower and upper limb exercises, inspiratory muscle training, and extensive education on disease pathophysiology, medication and symptom management, nutrition, health-enhancing behaviors, and coping strategies.40–42 Prior work has shown that pulmonary rehabilitation programs improve dyspnea severity, exercise capacity, quality of life, and mental health, as well as reduce hospitalization for EOAD.40–44 Similarly, when managing the increased risk of dyspnea and respiratory hospitalization that is known to be associated with older age,1–4,39 the most effective intervention is likely to be a combination of physical activity and health education. As in the present study, however, pulmonary rehabilitation programs have not achieved improvements in lung function, particularly FEV1, reflecting the absence of a direct effect on pulmonary disease (severity and progression), and suggesting instead alternate mechanisms for the therapeutic benefit (health-enhancing behaviors and improved physical function).40,42–44
We acknowledge at least four potential study limitations. First, our respiratory outcomes included dyspnea, FEV1, MIP, and respiratory hospitalizations. Although not exhaustive, this represented a fairly comprehensive respiratory assessment, including longitudinal evaluations and involving older persons having a mean age of 79 years at baseline. Second, participants in the LIFE Study were not enrolled on the basis of respiratory impairments, and the physical activity intervention did not specifically target respiratory outcomes. We hypothesize that respiratory outcomes are more likely to improve in older persons who have a respiratory impairment and are managed with a multidisciplinary approach.40–44 Third, the LIFE Study only included participants with mobility limitations, potentially decreasing the intensity of the physical activity intervention and, in turn, its beneficial effect on the aerobic capacity of the muscles of ambulation and respiration.12 Fourth, because older age is associated with changes in symptom awareness,45–48 the intensity of the physical activity intervention may have been misclassified (i.e., exercise effort was calibrated to self-perceived exertion, instead of a participant’s predetermined maximum heart rate).49
In conclusion, among sedentary older persons with mobility limitations (LIFE Study), we found that structured physical activity had no effect on dyspnea severity, FEV1, and MIP, but was associated with a higher likelihood of having a respiratory hospitalization, when compared with a health education intervention. We therefore posit that, in sedentary older persons with mobility limitations, structured physical activity that is focused primarily on walking and lower extremity function, while improving mobility, is limited in its capacity to improve respiratory outcomes, and requires monitoring of symptoms related to EOAD and pneumonia.
Supplementary Material
Acknowledgments
Funding source: This work was supported by a National Institutes of Health/National Institute on Aging Cooperative Agreement #UO1 AG22376 and a supplement from the National Heart, Lung and Blood Institute 3U01AG022376-05A2S, and sponsored in part by the Intramural Research Program, National Institute on Aging, NIH. The following field centers are funded, in part, by Claude D. Pepper Older Americans Independence Center—Administrative Coordinating Center, University of Florida, Gainesville, FL (1 P30 AG028740); Tufts University, Boston, MA (1P30AG031679); University of Florida, Gainesville, FL (1 P30 AG028740); University of Pittsburgh, Pittsburgh, PA (P30 AG024827); Yale University, New Haven, CT (P30AG021342) —and the NIH/NCRR CTSA at Stanford University (UL1 RR025744), University of Florida (U54RR025208) and Yale University (UL1 TR000142). Tufts University is also supported by the Boston Rehabilitation Outcomes Center (1R24HD065688-01A1). Dr. Vaz Fragoso was supported by a VA Merit Award. Dr. Stafford was supported by a Mid-Career Mentoring Award (K24 HL086703). Dr. Gill is the recipient of an Academic Leadership Award (K07AG3587) from the National Institute on Aging. Clinicaltrials.gov identifier NCT01072500.
Research investigators for the LIFE Study group are listed in Appendix A.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Dr. Vaz Fragoso had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors made substantial contributions to study concept and design, to data acquisition, analysis and interpretation, and to drafting the submitted article.
Sponsor’s Role: The investigators retained full independence in the conduct of this research and report no conflicts of interest.
References
- 1.Vaz Fragoso CA, Gill T. Respiratory impairment and the aging lung: A novel paradigm for assessing pulmonary function. J Gerontol Med Sci. 2012;67:264–275. doi: 10.1093/gerona/glr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaz Fragoso CA, Gill TM, McAvay G, et al. Respiratory impairment in older persons: When less means more. Am J Med. 2013;126:49–57. doi: 10.1016/j.amjmed.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaz Fragoso CA, Enright PL, McAvay G, et al. Frailty and respiratory impairment in older persons. Am J Med. 2012;125:79–86. doi: 10.1016/j.amjmed.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Alawi M, Hassan T, Chotirmall SH. Advances in the diagnosis and management of asthma in older adults. Am J Med. 2014;127:370–378. doi: 10.1016/j.amjmed.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Buchner DM, Beresford SA, Larson EB, et al. Effects of physical activity on health status in older adults. II. Intervention studies. Annu Rev Public Health. 1992;13:469–488. doi: 10.1146/annurev.pu.13.050192.002345. [DOI] [PubMed] [Google Scholar]
- 6.Stuck AE, Walthert JM, Nikolaus T, et al. Risk factors for functional status decline in community-living elderly people: A systematic literature review. Soc Sci Med. 1999;48:445–469. doi: 10.1016/s0277-9536(98)00370-0. [DOI] [PubMed] [Google Scholar]
- 7.Guralnik JM, Ferrucci K, Simonnick EM, et al. Lower extremity function over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guralnik JM, Simonsick EM, Ferucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality in nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 10.Vaz Fragoso CA, Beavers DP, Hankinson JL, et al. Respiratory impairment and dyspnea and their associations with physical inactivity and mobility in sedentary community-dwelling older persons. J Am Geriatr Soc. 2014;62:622–628. doi: 10.1111/jgs.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis. Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Thoracic Society/American College of Chest Physicians (ATS/ACCP) statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 13.Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13–64. doi: 10.1164/rccm.201309-1634ST. [DOI] [PubMed] [Google Scholar]
- 14.Physical Activity Guidelines for Americans. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Office of Disease Prevention and Health Promotion; 2008. [Google Scholar]
- 15.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: The LIFE Study randomized clinical trial. JAMA. 2014;311:2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fielding RA, Rejeski WJ, Blair S, et al. The lifestyle interventions and independence for elders study: Design and methods. J Gerontol A Biol Sci Med Sci. 2011;66A:1226–1237. doi: 10.1093/gerona/glr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsh AP, Lovato LC, Glynn NW, et al. Lifestyle interventions and independence for elders study: Recruitment and baseline characteristics. J Gerontol A Biol Sci Med Sci. 2013;68:1549–1558. doi: 10.1093/gerona/glt064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 19.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 20.Sin DD, Jones RL, Mannino DM, et al. Forced expiratory volume in 1 second and physical activity in the general population. Am J Med. 2004;117:270–273. doi: 10.1016/j.amjmed.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 21.Sachs MC, Enright PL, Stukovsky KDH, et al. Performance of maximum inspiratory pressure tests and maximum inspiratory pressure reference equations for 4 race/ethnic groups. Respir Care. 2009;54:1321–1328. [PMC free article] [PubMed] [Google Scholar]
- 22.van der Palen J, Rea TD, Manolio TA, et al. Respiratory muscle strength and the risk of incident cardiovascular events. Thorax. 2004;59:1063–1067. doi: 10.1136/thx.2004.021915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enright PL, Kronmal RA, Manolio TA, et al. Respiratory muscle strength in the elderly: Correlates and reference values. Am J Respir Crit Care Med. 1994;149:430–438. doi: 10.1164/ajrccm.149.2.8306041. [DOI] [PubMed] [Google Scholar]
- 24.Stewart AL, Verboncoeur CJ, McLellan BY, et al. Physical activity outcomes of CHAMPS II: A physical activity promotion program for older adults. J Gerontol A Biol Sci Med Sci. 2001;56:M465–M470. doi: 10.1093/gerona/56.8.m465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 26.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167:875–881. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthews CE, Ainsworth BE, Thompson RW, et al. Sources of variance in daily physical activity levels as measured by an accelerometer. Med Sci Sports Exerc. 2002;34:1376–1381. doi: 10.1097/00005768-200208000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Allen SC, Yeung P. Inability to draw intersecting pentagons as a predictor of unsatisfactory spirometry technique in elderly hospital inpatients. Age Ageing. 2006;35:304–316. doi: 10.1093/ageing/afj090. [DOI] [PubMed] [Google Scholar]
- 30.Enright P, Kronmal RA, Higgins MW, et al. Prevalence and correlates of respiratory symptoms and disease in the elderly. Chest. 1994;106:827–834. doi: 10.1378/chest.106.3.827. [DOI] [PubMed] [Google Scholar]
- 31.Vaz Fragoso CA, Concato J, McAvay G, et al. Staging the severity of chronic obstructive pulmonary disease in older persons based on spirometric Z-scores. J Am Geriatr Soc. 2011;59:1847–1854. doi: 10.1111/j.1532-5415.2011.03596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaz Fragoso CA, Concato J, McAvay G, et al. Respiratory impairment and COPD hospitalization: A competing risk analysis. Eur Respir J. 2012;40:37–44. doi: 10.1183/09031936.00128711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95 year age range: The global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stanojevic S, Wade A, Stocks J, et al. Reference ranges for spirometry across all ages. Am J Respir Crit Care Med. 2008;177:253–260. doi: 10.1164/rccm.200708-1248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 36.Dougherty RH, Fahy JV. Acute exacerbations of asthma: Epidemiology, biology and the exacerbation-prone phenotype. Clin Exp Allergy. 2009;39:193–202. doi: 10.1111/j.1365-2222.2008.03157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Müllerova H, Maselli DJ, Locantore N, et al. Hospitalized exacerbations of COPD: Risk factors and outcomes in the ECLIPSE cohort. Chest. 2015;147:999– 1007. doi: 10.1378/chest.14-0655. [DOI] [PubMed] [Google Scholar]
- 38.Loeb M. Pneumonia in older persons. Clin Infect Dis. 2003;37:1335–1339. doi: 10.1086/379076. [DOI] [PubMed] [Google Scholar]
- 39.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 40.Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;8:e13–e64. doi: 10.1164/rccm.201309-1634ST. [DOI] [PubMed] [Google Scholar]
- 41.Burtin C, Decramer M, Gosselink R, et al. Rehabilitation and acute exacerbations. Eur Respir J. 2011;38:702–712. doi: 10.1183/09031936.00079111. [DOI] [PubMed] [Google Scholar]
- 42.Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation. Chest. 2007;131:4S–42S. doi: 10.1378/chest.06-2418. [DOI] [PubMed] [Google Scholar]
- 43.O’Brien K, Geddse EL, Reid WD, et al. Inspiratory muscle training compared with other rehabilitation interventions in chronic obstructive pulmonary disease: A systematic review update. J Cardiopulm Rehabil Prev. 2008;28:128–141. doi: 10.1097/01.HCR.0000314208.40170.00. [DOI] [PubMed] [Google Scholar]
- 44.Berry MJ, Rejeski WJ, Adair NE, et al. A randomized controlled trial comparing long-term and short-term exercise in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2003;23:60–68. doi: 10.1097/00008483-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Connolly MJ, Crowley JJ, Charan NB, et al. Reduced subjective awareness of bronchoconstriction provoked by methacholine in elderly asthmatic and normal subjects as measured on a simple awareness scale. Thorax. 1992;47:410–413. doi: 10.1136/thx.47.6.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alexander KP, Newby LK, Canno CP, et al. Acute coronary care in the elderly, part I: Non–ST-segment–elevation acute coronary syndromes. Circulation. 2007;115:2549–2569. doi: 10.1161/CIRCULATIONAHA.107.182615. [DOI] [PubMed] [Google Scholar]
- 47.Zammitt NN, Frier BM. Hypoglycemia in type 2 diabetes: Pathophysiology, frequency, and effects of different treatment modalities. Diabetes Care. 2005;28:2948–2961. doi: 10.2337/diacare.28.12.2948. [DOI] [PubMed] [Google Scholar]
- 48.Vaz Fragoso CA, Van Ness PH, Araujo KLB, et al. Age-related differences in sleep-wake symptoms among adults undergoing polysomnography. J Am Geriatr Soc. 2015 doi: 10.1111/jgs.13632. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson PD. Exercise prescription and proscription for patients with coronary artery disease. Circulation. 2005;112:2354–2363. doi: 10.1161/CIRCULATIONAHA.104.502591. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.