Abstract
Background
Recent studies suggest that YKL-40, also called chitinase-3-like-1 protein, has been implicated in the pathogenesis of various inflammatory diseases. It is currently unknown, however, whether YKL-40 plays a role in acute exacerbations of chronic obstructive pulmonary disease (AECOPD) and airway remodeling.
Methods
We evaluated serum YKL-40 levels in patients with AECOPD (n = 37) and stable COPD (n = 44), as well as in controls (n = 47). The association between YKL-40 expression and airway remodeling was analyzed. The effects of YKL-40 on collagen synthesis of primary human lung fibroblasts were also evaluated.
Results
Serum YKL-40 levels were elevated at AECOPD onset as compared to stable disease (median [interquartile range], 78.6 [52.3–122.2] ng/ml versus 46.7 [31.2–75.5] ng/ml; p = 0.0005). The ideal cutoff point for distinguishing patients with AECOPD from those with stable COPD was 64.7 ng/ml (AUC: 0.71; 95%CI: 0.596 to 0.823). YKL-40 expression correlated with airflow obstruction, C-reactive protein, and collagen deposition. Stimulation with YKL-40 promoted collagen production in lung fibroblasts through ERK- and p38-dependent mechanisms.
Conclusions
YKL-40 expression is up-regulated in patients with COPD and correlates with exacerbation attacks and may contribute to airway remodeling by acting on lung fibroblasts. The current data may provide insight into the underlying pathogenesis of COPD, in which YKL-40 has an important pathogenic role.
Trial registration
Electronic supplementary material
The online version of this article (doi:10.1186/s12931-016-0338-3) contains supplementary material, which is available to authorized users.
Keywords: Chronic obstructive pulmonary disease, CHI3L1, YKL-40, Exacerbation, Disease severity
Background
Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are common events that often lead to hospital admissions, increased healthcare costs [1]. During exacerbation, COPD patients experience a worsening of symptoms that coincides with accelerated decline in lung function, resulting in a decrease in quality of life. Airway inflammation plays a pivotal role in the pathogenesis of AECOPD. However, methods used in clinical practice are not appropriate for the evaluation of airway inflammation [2, 3]. For example, spirometry is used to monitor disease activity, but it has been shown that spirometry is not closely associated with the levels of inflammation. Thus, identification of novel biomarkers associated with pathophysiologic changes in COPD is necessary to improve the clinical management of COPD for the benefit of the patients. Recently, several questions have been raised about the role of the chitinase-like protein YKL-40 in chronic bronchial inflammation. YKL-40 (also known as chitinase 3-like 1 (CHI3L1)) binds to the ubiquitously expressed chitin but lacks chitinase activity. Previous studies have demonstrated that YKL-40 is associated with various pathologic conditions that are characterized by aberrant cell growth, tissue inflammation and remodeling, such as asthma, idiopathic pulmonary fibrosis (IPF) and allergic rhinitis [4–15]. However, it is currently unknown whether YKL-40 plays a role in AECOPD.
Airway remodeling is another prominent pathophysiologic feature of COPD, which is characterized by thickening of the airway wall with increased collagen deposition [16]. The mechanisms underlying its development have not been fully elucidated. The extent of airway wall thickening is associated with disease progression, and this thickening is the major cause of decreased lung function in COPD as remodeling reduces airflow and distensibility [17–19]. Previous studies indicated that serum YKL-40 levels were increased in severe asthma patients and were correlated positively with the thickness of the subepithelial basement membrane [8, 20–23]. Furuhashi et al. demonstrated that increased expression of YKL-40 was involved in tissue remodeling and fibrosis in IPF patients [9]. Létuvé et al. suggested that YKL-40 may influence extracellular matrix deposit and turnover by inducing metal matrix proteinase (MMP)-9 production by alveolar macrophages [12]. These data suggest that YKL-40 contributes to tissue remodeling in various human diseases. However, there is no evidence that YKL-40 is involved in airway remodeling in COPD. Lung fibroblasts have been shown to contribute to airway remodeling in airway diseases through synthesis and secretion of the main components of the extracellular matrix (ECM), such as proteoglycans and collagens [17]. Park et al. showed that YKL-40 induced the increased production of transforming growth factor (TGF) beta1, MMP-9 and collagen production in human nasal mucosa fibroblast [24]. Recklies et al. also showed that YKL-40 was preferentially expressed in areas with active fibrogenesis in patients with hepatic fibrosis, where it may act synergistically with insulin-like growth factor I to stimulate the growth of fibroblasts [14, 25]. However, whether YKL-40 participates in the onset of deposition of ECM and fibrosis of the small airways in patients with COPD has not been explored.
In the present study, we hypothesized that the up-regulation of YKL-40 expression is more pronounced in more severe forms of COPD and could induce airway remodeling by acting on human lung fibroblasts. Firstly, we investigated the expression of YKL-40 in patients with COPD and identified its correlation to acute exacerbation, disease severity (e.g., lung function, arterial blood gases) and airway remodeling. In addition, we evaluated the proliferation, transformation and collagen production from primary human lung fibroblasts in vitro after stimulation with YKL-40. Finally, the potential mechanism of YKL-40 action on collagen production in human lung fibroblasts was explored.
Methods
Study population
From October 2013 to November 2014, a total of 81 patients with COPD as defined by the Global Initiative for Chronic Obstructive Lung Disease guidelines (GOLD) guidelines [1], who had a history of chronic respiratory symptoms, such as cough and sputum with or without breathlessness, had a postbronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratio of less than 0.7 were recruited. Exclusion criteria were as follows: any chronic cardiopulmonary disease other than COPD (including asthma); received oral or intravenous corticosteroids or any other anti-inflammatory drugs in the preceding four weeks, given the possibility that the anti-inflammatory drugs may be able to suppress the elevation of pulmonary YKL-40 levels to confound the results [26]; and an inability to give written informed consent or cooperate with the study investigators. We also recruited 47 age-matched healthy subjects with normal spirometry from the communities surrounding our hospital to serve as controls. They were free of respiratory tract infection in the four weeks prior to the study. The characteristic of the patients and controls are shown in Table 1. COPD patients (n = 81) were divided into a stable group (n = 44) and an exacerbation group (n = 37). The division was based upon the status of the patients at the time of the initial visit and those that were experiencing an exacerbation at that time point compared to those that were not. Stable COPD was defined as no change in their treatment course for four or more weeks and also had no evident acute exacerbations of COPD during that same time period [1]. AECOPD was defined as an event in the natural course of the disease characterized by a change in the patient’s baseline dyspnea, cough, and/or sputum that is beyond normal day-to-day variations, is acute in onset, and may warrant a change in regular medication in a patient with underlying COPD [1]. The patients with AECOPD were followed up, and post-exacerbation samples were collected when the patients were on their usual COPD treatment and at their baseline respiratory state.
Table 1.
Baseline characteristic of subjects
| Patients with COPD | P value | |||||
|---|---|---|---|---|---|---|
| Controls (n = 47) | All (n = 81) | Stable (n = 44) | Exacerbation (n = 37) | Control vs. all patients | Stable vs. exacerbation | |
| Age, yrs | 57.7 ± 1.5 | 58.3 ± 1.0 | 56.8 ± 1.3 | 60.0 ± 1.5 | 0.402 | 0.116 |
| Male/female, n | 28/19 | 54/27 | 27/17 | 27/10 | 0.297 | 0.270 |
| Smoking history, packs/year | 37.0 ± 1.7 | 42.9 ± 1.2 | 41.6 ± 1.1 | 44.3 ± 2.3 | 0.067 | 0.258 |
| Smoking status Never/current/former, n |
25/12/10 | 0/49/32 | 0/28/16 | 0/21/16 | 0.420 | 0.528 |
| FEV1/FVC, % | 82.9 ± 0.8 | 53.1 ± 1.4 | 56.1 ± 1.8 | 49.7 ± 2.2 | <0.001 | 0.027 |
| FEV1, % predicted | 96.6 ± 1.6 | 54.7 ± 2.4 | 61.4 ± 3.2 | 46.7 ± 3.3 | <0.001 | 0.002 |
| PaO2, mmHg | N/D | 74.2 ± 1.0 | 77.5 ± 1.0 | 70.3 ± 1.5 | ― | <0.001 |
| PaCO2, mmHg | N/D | 43.1 ± 0.6 | 40.6 ± 0.7 | 45.9 ± 0.9 | ― | <0.001 |
| CRP, mg/L | 3.5 ± 0.3 | 36.7 ± 3.6 | 14.2 ± 1.1 | 63.5 ± 4.5 | <0.001 | <0.001 |
| Severity of COPDa | N/A | ― | 0.489 | |||
| GOLD I/II, n (%) | 47 (58.0) | 24 (54.5) | 23 (62.2) | |||
| GOLD III/IV, n (%) | 34 (42.0) | 20 (45.5) | 14 (37.8) | |||
| COPD treatments, n (%) | N/A | ― | 0.935 | |||
| ICS/LABA | 58 (71.6) | 32 (72.7) | 26 (70.3) | |||
| SABA or SAMA | 71 (87.7) | 38 (86.4) | 33 (89.2) | |||
| Theophylline | 51 (63.0) | 29 (65.9) | 22 (59.5) | |||
Data are presented as mean ± SEM, unless otherwise stated
aCOPD severity was graded into GOLD I, Mild (FEV1 ≥ 80 % predicted); GOLD II, Moderate (50 % ≤ FEV1 < 80 % predicted); GOLD III, Severe (30 % ≤ FEV1 < 50 % predicted); and GOLD IV, Very severe (FEV1 < 30 % predicted) following the GOLD
FEV 1 forced expiratory volume in 1 s, FVC forced vital capacity, PaO 2 arterial partial oxygen pressure, PaCO 2 arterial partial carbon dioxide pressure, CRP C reactive protein, COPD chronic obstructive pulmonary disease, GOLD Global Initiative for Chronic Obstructive Lung Disease, ICS inhaled corticosteroid, LABA long-acting β agonist, SABA short-acting β agonist, SAMA short-acting muscarinic agonist, N/D not done, N/A not applicable
The study protocol was approved by the Ethics of Research Committee of the Medical College of Guangdong and was registered on the Chinese Clinical Trial Database (ChiCTR-OCC-13003567). Written informed consent was obtained from all participants.
Sample collection
To investigated the expression of YKL-40 in the lung tissue and identified its correlation to airway remodeling, lung tissue specimens were obtained from patients who were undergoing lung lobectomy for localized lung carcinoma (Additional file 1: Table S1). Specimens were dissected at a distance of ≥ 5 cm away from the tumor.
Laboratory measurements
Pulmonary function tests were performed according to American Thoracic Society (ATS) guidelines either on the same day as the bronchoscopy or on the day that the serum samples were collected [27]. Blood gas analysis was performed using a gas analyzer (IL GEM Premier 3000, USA). CRP was performed using ARRAY 360 automatic protein analyzer (BECKMAN, USA). The reference value of serum CRP concentration was 0–10 mg/L. YKL-40 levels were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Uscn Life Science Inc., Wuhan, China). The minimum detection limit of the YKL-40 assay was 13.1 pg/ml.
Immunohistochemistry
Details on the methods used to make these measurements are provided in the Additional file 1. Quantitative measurements of YKL-40 positive cells in the lung tissue were performed according to previously described methods [28]. YKL-40 positive cells were expressed as a percentage of total cells. The intraobserver error was assessed by performing three independent counts on the same section on separate occasions. Sections were examined using a light microscope (BX51; Olympus, Japan) and quantified by the Image Pro 6.1 software (Media Cybernetics).
Quantitation of peribronchial collagen deposition
Peribronchial collagen deposition was detected by Masson trichrome staining. The area of peribronchial Masson trichrome staining (blue color) was visualized and is quantified by the software as a percentage of the total band area as previously described [29].
Human fibroblasts isolation and stimulation
Primary human lung fibroblasts were isolated from lung tissue obtained from donors undergoing resection for localized lung carcinoma who gave informed consent, as described previously [17]. The available clinical characteristics of donors, including age, pack-years, and lung function, are provided in Additional file 1: Table S1. All experiments were carried out using cells between passage 3 and 6. Details on isolation and cultivation of human lung fibroblasts are also provided in the Additional file 1.
Cell viability assays, migration and proliferation
To investigate cell viability, cells were seeded into a 96-well plate at a density of 1 × 104 cells/well. The cells were treated with different doses of recombinant human YKL-40 protein (R&D Systems, Minneapolis, USA) for 48 h. A CCK-8 assay (Liankebio, Hangzhou, China) was used to determine cell viability according to the manufacturer’s instructions. A ‘scratch-wound’ assay was used to assess fibroblast migration fibroblast as described previously [30]. Full details of this method are available in the Additional file 1.
Western blotting assay
Western blot analysis was used to detect changes in collagen type I, collagen type III, α-SMA, ERK, phosphorylated ERK (p-ERK), p38 and phosphorylated (p-p38) (Cell Signaling Technology, USA) as previously described [31].
Statistical analysis
Data were presented as the mean ± SEM, unless otherwise stated. Statistical analysis was performed using SPSS 17.0 (SPSS, Chicago, IL, USA) and GraphPad Prism 5.0 software (GraphPad Software Inc., San Diego, CA, USA). Statistical significance was set at a p value < 0.05. Full details are available in the Additional file 1.
Results
Clinical data
Table 1 shows the main clinical and functional characteristics of the subjects in the study. There was no significant difference in age, gender or smoking status. Compared with patients in the stable group and control group, those in the exacerbation group had lower lung function (p = 0.002) and PaO2 levels (p < 0.001) but higher PaCO2 levels (p < 0.001) and serum CRP levels (p < 0.001). Similar proportions of subjects were receiving medications in both groups (p = 0.935).
Serum YKL-40 levels were elevated during an AECOPD
The serum YKL-40 levels for patients in smokers with COPD were higher than those in smokers without COPD (median [interquartile range], 60.80 [34.7–90.1] ng/ml versus 22.7 [15.6–36.4] ng/ml; p < 0.001) and those in never-smoker individuals (78.60 [52.3–90.11] ng/ml versus 20.9 [15.2–26.3] ng/ml; p < 0.001) (Fig. 1a). The serum YKL-40 and CRP levels of COPD patients in the exacerbation group were higher than those in stable group (YKL-40: 78.6 [52.3–122.2] ng/ml versus 46.7 [31.2–75.5] ng/ml; p = 0.0005; CRP: 60.0 [39.9–93.4] mg/L versus 16.9 [13.0–23.8] mg/L; p < 0.001) (Fig. 1b, c). Moreover, serum levels of YKL-40 were positively correlated with CRP (r = 0.601, p < 0.001) (Fig. 1d). Spearman rank correlation coefficient showed that borderline significance was evident between YKL-40 concentrations and pack-years (r = 0.224, p = 0.045) (Fig. 1e). During the follow-up visit, five AECOPD patients were lost to follow-up because they refused to continue participating. Finally, 32 subjects completed the study and were included in the data analyses. We found that patients in the exacerbation group, after acute exacerbations, demonstrated decreased serum YKL-40 levels compared with those during AECOPD (54.3 [36.4–74.4] ng/ml versus 77.7 [50.9–113.9] ng/ml; p = 0.008) (Fig. 1f). The ideal cutoff point for distinguishing patients with AECOPD from those with stable COPD was 64.7 ng/ml (sensitivity, 64.8 %; specificity, 71.2 %; AUC, 0.71; 95%CI: 0.596 to 0.823) (Fig.1g). YKL-40 levels may be affected by age and gender. Thus, we further analyzed the between-group differences following adjustment for age and gender using multiple regression analyses. We found that the AECOPD patients had significantly higher serum YKL-40 levels than stable COPD patients, even after adjustment for sex, age. In addition, women had lower serum YKL-40 levels, whereas older age was associated with higher serum YKL-40 concentrations (Additional file 1: Table S2).
Fig. 1.
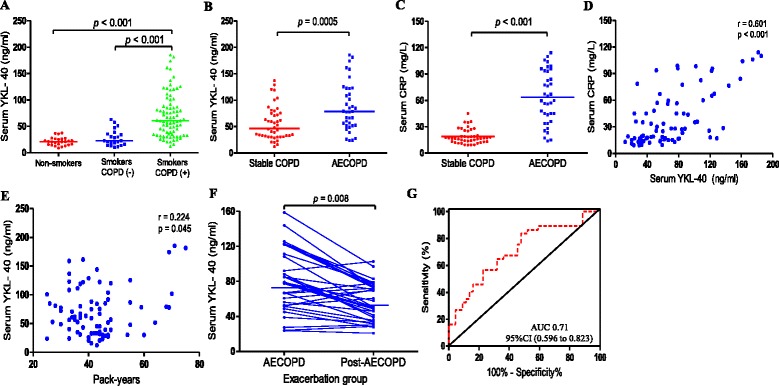
Serum YKL-40 levels in patients with COPD and controls. Serum YKL-40 was increased in smokers with COPD (defined as COPD (+)) compared with smokers without COPD (defined as COPD (−)) and non-smokers (a); When patients were stratified according to exacerbation attacks, the serum YKL-40 and C-reactive protein (CRP) levels in patients in the exacerbation group were higher than those in the stable group (b, c); Spearman rank correlation coefficient showed that YKL-40 was positively associated with CRP (d); Borderline significance was evident between YKL-40 concentrations and pack-years (r = 0.224, p = 0.045) (e); Patients in the exacerbation group, after acute exacerbations, demonstrated decreased serum YKL-40 levels compared with those during AECOPD (f); Receiver operating characteristic (ROC) curve for distinguishing patients with AECOPD from those with stable COPD. The area under the ROC curve was 0.71 (95%CI: 0.596 to 0.823). Horizontal bars represent median values (g)
Serum YKL-40 levels in COPD patients were correlated with clinical parameters
Spearman’s rank correlation analysis showed that serum YKL-40 levels in COPD patients were correlated negatively with FEV1 and PaO2. However, there were no significant correlations between serum YKL-40 levels and other clinical parameters, such as the FEV1/FVC or PaCO2 (Table 2).
Table 2.
Correlation between serum YKL-40 levels and clinical parametersa
| Parameters | Serum YKL-40 | |||||
|---|---|---|---|---|---|---|
| Stable (n = 44) | Exacerbation (n = 37) | All (n = 81) | ||||
| r s | P value | r s | P value | r s | P value | |
| FEV1, % of predicted | −0.399 | 0.007 | −0.440 | 0.006 | −0.442 | <0.001 |
| FEV1/FVC, % | −0.156 | 0.356 | −0.01 | 0.944 | −0.201 | 0.072 |
| CRP, mg/L | 0.507 | <0.001 | 0.624 | <0.001 | 0.601 | <0.001 |
| PaO2, mmHg | −0.349 | 0.020 | −0.60 | <0.001 | −0.556 | <0.001 |
| PaCO2, mmHg | 0.018 | 0.917 | 0.087 | 0.573 | 0.185 | 0.098 |
aSpearman’s rank order method. FEV 1 forced expiratory volume in 1 s, FVC forced vital capacity, CRP C reactive protein, PaO 2 arterial partial oxygen pressure, PaCO 2 arterial partial carbon dioxide pressure
Increased YKL-40 expression was positively correlated with collagen deposition
Examination of lung tissue sections from patients who were undergoing lung lobectomy for peripheral carcinoma showed that smokers with COPD, the percentage of YKL-40 positive cells (27.1 [21.9–34.2]%) was significantly increased than those without COPD (16.8 [13.9–20.8]%; p = 0.002) and non-smokers (14.2 [9.4–17.9]%; p < 0.001) (Fig. 2a–c, e). No immunostaining was observed in control isotype IgG-treated tissue sections (D). Detailed examination of the cellular sources of YKL-40 in lung tissue revealed that a high level of expression of YKL-40 in macrophages and neutrophils (Fig. 2f–m). Collagen deposition in the lungs was increased in smokers with COPD (56.5 [45.6–68.5]%) compared to smokers without COPD (33.2 [21.2–44.9]%; p = 0.001) and non-smokers (25.9 [22.3–32.7]%; p < 0.001) (Fig. 3a-d). Furthermore, collagen deposition correlated with YKL-40 expression in lung tissues (r = 0.57; p < 0.001) (Fig. 3e).
Fig. 2.

YKL-40 was expression in lung tissues and localized within macrophages and neutrophils. Very faint staining for YKL-40 was observed in the non-smokers (a); In the smokers without COPD, there were more YKL-40 positive cells in the lung parenchyma (b); Smokers with COPD had considerably more YKL-40 positive cells staining in the lung parenchyma (expressed as a percentage of total cells) (c); No immunostaining was observed in control isotype IgG-treated tissue sections (d); Quantification of YKL-40 expression in lung tissues (e); Confocal microscopy showed localization of YKL-40 (green fluorescence) within CD68 or CD45 positive cells (red fluorescence) of the tissue sections (f-i and j-m, respectively). (all images are × 400 magnification). Horizontal bars represent median values
Fig. 3.

Collagen deposition in small airways from non-smokers, smokers without COPD and smokers with COPD. Photomicrographs of blue staining showing collagen expression around the small airway walls from non-smokers (a), smokers without COPD (b), and smokers with COPD (c) (all images are × 400 magnification); Measurement of collagen deposition in small airways. Smokers with COPD showed increased collagen deposition compared to both the non-smokers, smokers without COPD (d); Collagen deposition was positively correlated with serum YKL-40 levels (r = 0.57; p < 0.001) (e). Horizontal bars represent median values
YKL-40 can stimulate the proliferation, differentiation and collagen synthesis in human lung fibroblast
The migration of human lung fibroblast cells was evaluated by a scratch wound assay. Treatment with YKL-40 significantly increased the number of human lung fibroblast cells as compared to the untreated control (Fig. 4a-b). Treatment with YKL-40 also increased the migration capacity of human lung fibroblast cells compared with the untreated control (Fig. 4c-d). The upregulation of α-SMA expression is a characteristic marker for differentiation of lung fibroblasts to myofibroblasts [23]. Using immunofluorescence and western blotting, we detected the expression of α-SMA in human lung fibroblast cells after 48 h of treatment with YKL-40 (10 and 100 ng/ml) and found that it significantly increased α-SMA protein expression in a concentration-dependent manner compared with the untreated control cells (Fig. 5). Treatment with YKL-40 (100 ng/ml) also significantly increased the expression of collagen I and III production compared with untreated control fibroblast cells (Fig. 6).
Fig. 4.
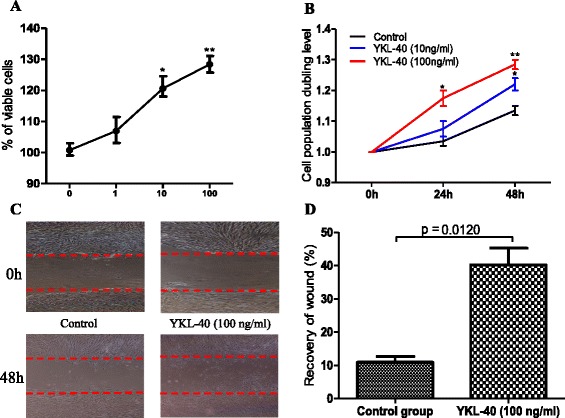
The effect of YKL-40 treatment on the proliferation and migration of human lung fibroblast cells. The cells were treated with 0, 10 or 100 ng/ml YKL-40 for 48 h, then resuspended and counted (a); The cells were treated with 0, 10 or 100 ng/ml YKL-40 for 0 h, 24 h and 48 h, then cell viability was measured with CCK-8 assay. Increased number of YKL-40-treated fibroblasts versus control fibroblasts was observed (b). Representative images of human lung fibroblast cells treated with YKL-40 or untreated, at time 0 and after 48 h of incubation were shown. Increased fibroblast migration was observed in YKL-40-treated fibroblasts versus control fibroblasts (c). Results are expressed as percentage of recovered wound area (d). Results were expressed as mean ± SEM (n = 4 per group) of three independent experiments. *p < 0.05, **p < 0.01 compared with basal
Fig. 5.

The effect of YKL-40 treatment on α-SMA protein expression in lung fibroblast cells. Lung fibroblast cells were treated with 0, 10 or 100 ng/ml YKL-40 for 48 h. Measurements of α-SMA expression upon stimulation by YKL-40 as determined by immunofluorescence staining (a), and Western blot analysis (b); Analysis by densitometry of immunodetection of α-SMA (c). Results were expressed as mean ± SEM of three independent experiments
Fig. 6.

The effect of YKL-40 on collagen synthesis in lung fibroblast cells. Lung fibroblast cells were treated with 0, 10 or 100 ng/ml YKL-40 for 48 h. Collagen I and collagen III production were determined by immunofluorescence staining (a), and Western blot analysis (b); Analysis by densitometry of immunodetection of collagen I and collagen III (c, d). Results were expressed as mean ± SEM of three independent experiments
Increased phosphorylation of p-38 and ERK in YKL-40-induced collagen production
The mitogen-activated protein kinase (MAPK) family is well known to play a key role in mediating inflammatory responses [32]. To assess whether MAPK pathways are involved in YKL-40-induced collagen production, the effect of YKL-40 on activation of ERK and p38 was evaluated by western blotting. As shown in Fig. 7, stimulation of human lung fibroblast cells with YKL-40 resulted in a transient phosphorylation of ERK and p38. In addition, YKL-40 activated ERK and p38 phosphorylaton in a dose-dependent manner.
Fig. 7.
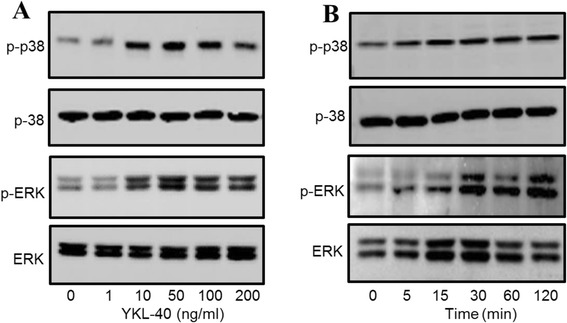
YKL-40 induced phosphorylation of extracellular signal related kinase (ERK), and p38 in lung fibroblast cells. Recombinant human YKL-40 protein activated ERK and p38 phosphorylaton in a dose-dependent manner (0, 1, 10, 50, 100 and 200 ng/ml for 2 h) (a); Stimulation of lung fibroblast cells with 50 ng/ml of recombinant human YKL-40 protein also induced phosphorylation of ERK and p38 in a time-dependent manner (0, 5, 15, 30, 60, 120 min) (b)
Discussion
In the present study, we further explored the potential role of YKL-40 measurement in the management of COPD. Our study indicated that YKL-40 levels were increased in patients with COPD during exacerbation, and the elevated YKL-40 was associated positively with CRP and negatively with FEV1 and PaO2. Importantly, our findings revealed that the expression of YKL-40 in lung tissues of COPD patients was correlated with deposition of collagen in the airway walls and induced lung fibroblast activation, suggesting that a potential mechanism of small airway remodeling in COPD.
COPD is accompanied by systemic inflammation that occurs as a result of many mechanisms, particularly airway inflammation and smoking [33–35]. Previous studies indicated that YKL-40 was increased in many inflammatory diseases that were accompanied by tissue destruction. TNF-α stimulated YKL-40 synthesis in alveolar macrophages, and exposure of these cells to YKL-40 promoted the release of IL-8, monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1α, and metalloproteinase-9 [12]. We found that serum YKL-40 levels were increased in COPD compared with smokers without COPD and non-smokers. When the COPD subjects were stratified, serum YKL-40 levels in the exacerbation group were higher than those in the stable group, which suggested that serum levels of YKL-40 correlated with COPD exacerbation attacks. Previous study by Nordenbaek et al. has shown that serum YKL-40 reflects a different aspect of the inflammatory pulmonary process than conventional acute-phase proteins [36]. CRP is increased during bacterial infections which could be the cause of the exacerbations in the patients with COPD. CRP levels are useful in evaluating COPD exacerbation [37]. Consistent with previous studies, we found that the serum YKL-40 levels and CRP were elevated in patients with AECOPD. Moreover, the serum YKL-40 levels correlated positively with serum CRP levels. These findings indicated that YKL-40 has a potential effect on the pathogenesis of inflammation of COPD and may serve as a specific serologic marker of granulocyte function at the site of tissue inflammation as a supplement to conventional acute-phase proteins [36]. Gumus et al. showed that high serum YKL-40 level is related to hypoxemia and hypoxia-related mediators may cause systemic inflammation in COPD [38]. We also found that serum YKL-40 levels correlated inversely with PaO2 as well as FEV1. However, borderline significance was evident between YKL-40 concentrations and pack-years. These findings are consistent with those reported by Matsuura and colleagues [10]. These results suggested that concentrations of circulatory YKL-40 in patients with COPD are profoundly affected by other local or systemic factors in addition to cigarette smoke (CS) exposure [10].
Peribronchiolar fibrosis is an important feature of COPD and is resulted from the increased extracellular matrix deposition [12]. Collagens are the classical components of the extracellular matrix. Collagens are synthetized primarily by fibroblasts as precursor molecules with the propeptides being cleaved during the process of secretion of the newly formed collagens [39]. YKL-40 has been shown to be a growth factor for mesenchymal cells that contributes to degradation of extracellular matrix and tissue remodeling [20–24]. Moreover, YKL-40 acts a chemoattractant for endothelial cells, and modulates vascular endothelial cell morphology by promoting the formation of branching tubules [9]. Previous studies indicated that YKL-40 promoted reticular basement membrane (RBM) thickening in severe asthma and contributed to tissue remodeling and fibrosis in IPF patients [9, 20–22]. Collectively, these data suggest that increased levels of YKL-40 contribute to the pathologic process of human diseases with tissue remodeling. Therefore, we further hypothesized that YKL-40 might promote collagen desposition and airway remodeling in COPD. We examined the expression of YKL-40 in small airways from smokers with COPD and controls by immunohistochemistry. There was a significant increase in YKL-40 expression in small airways of smokers with COPD and correlated closely with deposition of collagen. These data suggest that YKL-40 may be associated with small airway remodeling, a finding that could help elucidate the mechanism of small airway remodeling in COPD.
Recent studies have shown that YKL-40 is preferentially expressed in areas with active fibrogenesis in patients with hepatic fibrosis, where it may act synergistically with insulin-like growth factor I to stimulate the growth of fibroblasts [12]. YKL-40 may also contribute to fibrosis by modulating the rate of type I collagen fibril formation [40]. However, whether YKL-40 participates in the onset of fibrosis of the small airways in patients with COPD remains to be determined. On the basis of the above findings, we were prompted to further explore the role of YKL-40 in collagen production in human lung fibroblasts in vitro. As expected, we found that treatment with YKL-40 increased proliferation, migration, collagen secretion and α-SMA expression in human lung fibroblasts. Our results were consistent with previous work demonstrating that YKL-40 are able to increase ECM, such as proteoglycans and collagens in nasal mucosa fibroblast [24]. Given the role of YKL-40 in collagen production in human lung fibroblasts, we sought to determine the key signaling mechanisms by which this occurs. We demonstrated that MAPK signaling was required for YKL-40-induced collagen production in human lung fibroblasts. Taken together, we presume that YKL-40 could activate lung fibroblasts and its downstream MAPK pathway, and promote proliferation and collagen production, which may enhance the progression of small airway remodeling in COPD.
There are some limitations to this study that need to be considered. First, exposure to YKL-40 significantly up-regulated proliferation from fibroblasts obtained from non-smokers, smokers without COPD and smokers with COPD. Although there was a trend to up-regulated proliferation from fibroblasts obtained from smokers with COPD, there were no significantly difference among these different patient groups (Additional file 1: Figure S1). Due to the relatively small sample size, we could not precisely analyze the fibroblasts from these different patient groups behaved differently regarding their responses towards YKL-40. Future studies with larger sample sizes as well as animal experiments should be performed to clarify this issue as it may suggest a target for therapeutic intervention in future. Second, it is well known that tumors attract macrophages which are critical in influencing tumor growth and metastasis [41, 42]. Previous study showed that YKL-40 is synthesized by activated macrophages [43]. Although resected normal tissues more than 5 cm away from the tumor in our study, it should be noted that tumors attract macrophages which are critical in influencing tumor growth and metastasis and YKL-40 is synthesized by activated macrophages. Therefore, the expression of YKL-40 in lung tissues should be interpreted cautiously due to this limitation.
Conclusions
In summary, the current findings demonstrate that elevated YKL-40 levels are associated with acute exacerbations and airway remodeling in patients with COPD. Moreover, the in vitro data show that YKL-40 may promote airway remodeling in COPD by acting on human lung fibroblasts. Overall, the current data may provide insight into the underlying pathogenesis of COPD, in which YKL-40 has an important pathogenic role.
Acknowledgements
We are grateful to all of the patients for agreeing to take part in our study. This work was supported by grant of the Medical Scientific Research Project of Guangdong Province, China (No. A2012430).
Abbreviations
- COPD
chronic obstructive pulmonary disease
- FEV1
forced expiratory volume in one second
- FVC
forced vital capacity
- GOLD
Chronic Obstructive Lung Disease guidelines
Additional file
YKL-40 expression in chronic obstructive pulmonary disease: relation to acute exacerbations and airway remodeling. Table S1.
Characteristics of patients undergoing lung resection. Table S2. A multivariable linear regression model predicting the YKL-40 levels in patients with AECOPD and patients with stable COPD adjusted for sex, age. Figure S1. The effect of YKL-40 treatment on the proliferation of human lung fibroblast cells from non-smokers, smokers without COPD and smokers with COPD. (DOCX 60.7 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
TWL contributed to study design, performed the statistical analysis, contributed to the interpretation of data and drafted the manuscript; MC and YYL contributed to the acquisition of data and to the critical review of the manuscript; XNZ and DML contributed to study design, to the acquisition of data and to the critical review of the manuscript; HHS and BW contributed to study design, to the interpretation of data and to the critical review of the manuscript; ZLJ and TWL contributed to pathology image analysis. YJW, XNZ, CC and QCL contributed to sample collection. All authors read and approved the final manuscript.
Contributor Information
Tianwen Lai, Email: laitianwen2011@163.com.
Dong Wu, Email: wudong98@126.com.
Min Chen, Email: colbygdmc@163.com.
Chao Cao, Email: caocdoctor@163.com.
Zhiliang Jing, Email: 150079294@qq.com.
Li Huang, Email: 63577387@qq.com.
Yingying Lv, Email: 352768539@qq.com.
Xuanna Zhao, Email: 792665234@qq.com.
Quanchao Lv, Email: 1029948027@qq.com.
Yajun Wang, Email: 251042033@qq.com.
Dongming Li, Email: 1335887835@qq.com.
Bin Wu, Phone: 86-759-2387-413, Email: wubin621011@126.com.
Huahao Shen, Phone: 86-759-2387-413, Email: huahaoshen@163.com.
References
- 1.GOLD Committee. Global strategy for the diagnosis, management, and prevention of COPD. http://www.goldcopd.org/guidelines-pocket-guide-to-copd-diagnosis.html. Accessed 11 Jan 2015.
- 2.Debley JS, Cochrane ES, Redding GJ, Carter ER. Lung function and biomarkers of airway inflammation during and after hospitalization for acute exacerbations of childhood asthma associated with viral respiratory symptoms. Ann Allergy Asthma Immunol. 2012;109:114–120. doi: 10.1016/j.anai.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menzies D, Jackson C, Mistry C, Houston R, Lipworth BJ. Symptoms, spirometry, exhaled nitric oxide, and asthma exacerbations in clinical practice. Ann Allergy Asthma Immunol. 2008;101:248–255. doi: 10.1016/S1081-1206(10)60489-9. [DOI] [PubMed] [Google Scholar]
- 4.De Ceuninck F, Gaufillier S, Bonnaud A, Sabatini M, Lesur C, Pastoureau P. YKL-40 (Cartilagegp-39) induces proliferative events in cultured chondrocytes and synoviocytes and increases glycosaminoglycan synthesis in chondrocytes. Biochem Biophys Res Commun. 2001;285:926–931. doi: 10.1006/bbrc.2001.5253. [DOI] [PubMed] [Google Scholar]
- 5.Johansen JS, Jensen BV, Roslind A, Nielsen D, Price PA. Serum YKL-40, a new prognostic biomarker in cancer patients? Cancer Epidem Biomarkers Prev. 2006;15:194–202. doi: 10.1158/1055-9965.EPI-05-0011. [DOI] [PubMed] [Google Scholar]
- 6.Kim SH, Das K, Noreen S, Coffman F, Hameed M. Prognostic implications of immunohistochemically detected YKL-40 expression in breast cancer. World J Surg Oncol. 2007;5:17. doi: 10.1186/1477-7819-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rathcke CN, Johansen JS, Vestergaard H. YKL-40, a biomarker of inflammation, is elevated in patients with type 2 diabetes and is related to insulin resistance. Inflamm Res. 2006;55:53–59. doi: 10.1007/s00011-005-0010-8. [DOI] [PubMed] [Google Scholar]
- 8.Chupp GL, Lee CG, Jarjour N, Shim YM, Holm CT, He S, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007;357:2016–2027. doi: 10.1056/NEJMoa073600. [DOI] [PubMed] [Google Scholar]
- 9.Furuhashi K, Suda T, Nakamura Y, Inui N, Hashimoto D, Miwa S, et al. Increased expression of YKL-40, a chitinase-like protein, in serum and lung of patients with idiopathic pulmonary fibrosis. Respir Med. 2010;104:1204–1210. doi: 10.1016/j.rmed.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 10.Matsuura H, Hartl D, Kang MJ, Dela Cruz CS, Koller B, Chupp GL, et al. Role of breast regression protein-39 in the pathogenesis of cigarette smoke-induced inflammation and emphysema. Am J Respir Cell Mol Biol. 2011;44:777–786. doi: 10.1165/rcmb.2010-0081OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otsuka K, Matsumoto H, Niimi A, Muro S, Ito I, Takeda T, et al. Sputum YKL-40 levels and pathophysiology of asthma and chronic obstructive pulmonary disease. Respiration. 2012;83:507–519. doi: 10.1159/000330840. [DOI] [PubMed] [Google Scholar]
- 12.Létuvé S, Kozhich A, Arouche N, Grandsaigne M, Reed J, Dombret MC, et al. YKL-40 is elevated in patients with chronic obstructive pulmonary disease and activates alveolar macrophages. J Immunol. 2008;181:5167–5173. doi: 10.4049/jimmunol.181.7.5167. [DOI] [PubMed] [Google Scholar]
- 13.Lai T, Chen M, Deng ZLY, Wu D, Li D, et al. YKL-40 is correlated with FEV1 and the asthma control test (ACT) in asthmatic patients: influence of treatment. BMC Pulm Med. 2015;15:1. doi: 10.1186/1471-2466-15-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansen JS, Christoffersen P, Møller S, Price PA, Henriksen JH, Garbarsch C, et al. Serum YKL-40 is increased in patients with hepatic fibrosis. J Hepatol. 2000;32:911–920. doi: 10.1016/S0168-8278(00)80095-1. [DOI] [PubMed] [Google Scholar]
- 15.Tang H, Fang Z, Sun Y, Li B, Shi Z, Chen J, et al. YKL-40 in asthmatic patients, and its correlations with exacerbation, eosinophils and immunoglobulin E. Eur Respir J. 2010;35:757–760. doi: 10.1183/09031936.00034409. [DOI] [PubMed] [Google Scholar]
- 16.Sun C, Zhu M, Yang Z, Pan X, Zhang Y, Wang Q, et al. LL-37 secreted by epithelium promotes fibroblast collagen production: a potential mechanism of small airway remodeling in chronic obstructive pulmonary disease. Lab Invest. 2014;94:991–1002. doi: 10.1038/labinvest.2014.86. [DOI] [PubMed] [Google Scholar]
- 17.Krimmer DI, Burgess JK, Wooi TK, Black JL, Oliver BG. Matrix proteins from smoke-exposed fibroblasts are pro-proliferative. Am J Respir Cell Mol Biol. 2012;46:34–9. doi: 10.1165/rcmb.2010-0426OC. [DOI] [PubMed] [Google Scholar]
- 18.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 19.James AL, Wenzel S. Clinical relevance of airway remodelling in airway diseases. Eur Respir J. 2007;30:134–155. doi: 10.1183/09031936.00146905. [DOI] [PubMed] [Google Scholar]
- 20.Lee CG, Dela Cruz CS, Herzog E, Rosenberg SM, Ahangari F, Elias JA. YKL-40, a chitinase-like protein at the intersection of inflammation and remodeling. Am J Respir Crit Care Med. 2012;185:692–694. doi: 10.1164/rccm.201202-0203ED. [DOI] [PubMed] [Google Scholar]
- 21.Konradsen JR, James A, Nordlund B, Reinius LE, Söderhäll C, Melén E, et al. The chitinase-like protein YKL-40: a possible biomarker of inflammation and airway remodeling in severe pediatric asthma. J Allergy Clin Immunol. 2013;132:328–335. doi: 10.1016/j.jaci.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Tang H, Sun Y, Shi Z, Huang H, Fang Z, Chen J, et al. YKL-40 induces IL-8 expression from bronchial epithelium via MAPK (JNK and ERK) and NF-κB pathways, causing bronchial smooth muscle proliferation and migration. J Immunol. 2013;190:438–446. doi: 10.4049/jimmunol.1201827. [DOI] [PubMed] [Google Scholar]
- 23.Bara I, Ozier A, Girodet PO, Carvalho G, Cattiaux J, Begueret H, et al. Role of YKL-40 in bronchial smooth muscle remodeling in asthma. Am J Respir Crit Care Med. 2012;185:715–722. doi: 10.1164/rccm.201105-0915OC. [DOI] [PubMed] [Google Scholar]
- 24.Park SJ, Jun YJ, Kim TH, Jung JY, Hwang GH, Jung KJ, et al. Increased expression of YKL-40 in mild and moderate/severe persistent allergic rhinitis and its possible contribution to remodeling of nasal mucosa. Am J Rhinol Allergy. 2013;27:372–380. doi: 10.2500/ajra.2013.27.3941. [DOI] [PubMed] [Google Scholar]
- 25.Recklies AD, White C, Ling H. The chitinase 3-like protein human cartilage glycoprotein 39 (HC-gp39) stimulates proliferation of human connective-tissue cells and activates both extracellular signal-regulated kinase- and protein kinase B-mediated signaling pathways. Biochem J. 2002;365:119–126. doi: 10.1042/bj20020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shuhui L, Mok YK, Wong WS. Role of mammalian chitinases in asthma. Int Arch Allergy Immunol. 2009;149:369–377. doi: 10.1159/000205583. [DOI] [PubMed] [Google Scholar]
- 27.American Thoracic Society Standardization of spirometry (1994 update) Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 28.Xanthou G, Alissafi T, Semitekolou M, Simoes DC, Economidou E, Gaga M, et al. Osteopontin has a crucial role in allergic airway disease through regulation of dendritic cell subsets. Nat Med. 2007;13:570–578. doi: 10.1038/nm1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fattouh R, Midence NG, Arias K, Johnson JR, Walker TD, Goncharova S, et al. Transforming growth factor-beta regulates house dust mite-induced allergic airway inflammation but not airway remodeling. Am J Respir Crit Care Med. 2008;177:593–603. doi: 10.1164/rccm.200706-958OC. [DOI] [PubMed] [Google Scholar]
- 30.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protocols. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 31.Hou C, Kong J, Liang Y, Huang H, Wen H, Zheng X, et al. HMGB1 contributes to allergen-induced airway remodeling in a murine model of chronic asthma by modulating airway inflammation and activating lung fibroblasts. Cell Mol Immunol. 2015;12:409–423. doi: 10.1038/cmi.2014.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manetsch M, Che W, Seidel P, Chen Y, Ammit AJ. MKP-1: a negative feedback effector that represses MAPK-mediated pro-inflammatory signaling pathways and cytokine secretion in human airway smooth muscle cells. Cell Signal. 2012;24:907–913. doi: 10.1016/j.cellsig.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Liu S, Zhou Y, Wang X, Wang D, Lu J, Zheng J, et al. Biomass fuels are the probable risk factor for chronic obstructive pulmonary disease in rural South China. Thorax. 2007;62:889–897. doi: 10.1136/thx.2006.061457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.She J, Yang P, Wang Y, Qin X, Fan J, Wang Y, et al. Chinese water-pipe smoking and the risk of COPD. Chest. 2014;146:924–931. doi: 10.1378/chest.13-1499. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann RF, Zarrintan S, Brandenburg SM, Kol A, de Bruin HG, Jafari S, et al. Prolonged cigarette smoke exposure alters mitochondrial structure and function in airway epithelial cells. Respir Res. 2013;2:14–97. doi: 10.1186/1465-9921-14-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nordenbaek C, Johansen JS, Junker P, et al. YKL-40, a matrix protein of specific granules in neutrophils, is elevated in serum of patients with community-acquired pneumonia requiring hospitalization. J Infect Dis. 1999;180(5):1722–6. doi: 10.1086/315050. [DOI] [PubMed] [Google Scholar]
- 37.Hurst JR, Donaldson GC, Perera WR, et al. Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;174:867–74. doi: 10.1164/rccm.200604-506OC. [DOI] [PubMed] [Google Scholar]
- 38.Gumus A, Kayhan S, Cinarka H, Kirbas A, Bulmus N, Yavuz A, et al. High serum YKL-40 level in patients with COPD is related to hypoxemia and disease severity. Tohoku J Exp Med. 2013;229:163–170. doi: 10.1620/tjem.229.163. [DOI] [PubMed] [Google Scholar]
- 39.Harju T, Kinnula VL, Pääkkö P, Salmenkivi K, Risteli J, Kaarteenaho R. Variability in the precursor proteins of collagen I and III in different stages of COPD. Respir Res. 2010;30:165. doi: 10.1186/1465-9921-11-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bigg HF, Wait R, Rowan AD, Cawston TE. The mammalian chitinase-like lectin, YKL-40, binds specifically to type I collagen and modulates the rate of type I collagen fibril formation. J Biol Chem. 2006;281:21082–21095. doi: 10.1074/jbc.M601153200. [DOI] [PubMed] [Google Scholar]
- 41.Bingle L, Brown NJ, Lewis CE. The role of tumor associated macrophages in tumor progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 42.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 43.Renkema GH, Boot RG, Au FL, Donker-Koopman WE, Strijland A, et al. Chitotriosidase, a chitinase, and the 39-kDa human cartilage glycoprotein, a chitin-binding lectin, are homologues of family 18 glycosyl hydrolases secreted by human macrophages. Eur J Biochem. 1998;251:504–509. doi: 10.1046/j.1432-1327.1998.2510504.x. [DOI] [PubMed] [Google Scholar]


