Abstract
OBJECTIVE
The purpose of this study was to develop an animal model for intrapartum inflammation at term to investigate the interactions between maternal and fetal inflammatory responses and adverse neurologic outcome.
STUDY DESIGN
Lipopolysaccharide (160, 320, or 640 μg/kg) was administered intraperitoneally to day 20 term-pregnant Sprague Dawley rat dams 2, 4, and 6 hours before sample collection. Maternal outcomes included dam core temperature and plasma interleukin 6 (IL-6). Fetal outcomes included plasma IL-6, brain IL-6 messenger RNA expression, and brain IL-6 protein expression. Primary cortical cell cultures were prepared to examine neuronal morphologic condition. Neurite counts were obtained with the use of automated Sholl analysis.
RESULTS
Maternal plasma IL-6 levels peaked 2 hours after lipopolysaccharide stimulus and rapidly resolved, except for an observed low level persistence at 6 hours with 640 μg/kg. Fetal plasma and placental IL-6 expression also peaked rapidly but only persisted in placental samples. Fetal brain IL-6 RNA and protein expression was significantly higher than control litters at 6 hours after the exposure to both 320 μg/kg (P ≤ .05) and 640 μg/kg (P ≤ .01). Cortical cells from fetuses that were exposed for 6 hours to maternal systemic inflammation showed reduced neurite number and neurite length (P < .001) with increasing lipopolysaccharide dose.
CONCLUSION
Our results demonstrate that fetal brain injury follows isolated systemic maternal inflammation and that fetal brain inflammation lags after maternal stimulus, which creates a potential 4-hour clinical window for therapeutic intervention.
Keywords: brain injury, cerebral palsy, chorioamnionitis
Cerebral palsy affects 1-3 of every 1000 newborn infants after term birth, with distinct pathologic conditions that are related to the stage of brain development at the time of insult.1,2 In the immature human brain, the periventricular white matter of the motor cortex is the most susceptible to injury; the basal ganglia and gray matter are more likely to be affected at term.3-4 Intrapartum chorioamnionitis significantly increases the risk of neonatal encephalopathy and subsequent cerebral palsy; this association is most robust at term.5-9 A growing body of evidence suggests that clinical chorioamnionitis at term is largely attributable to maternally driven, noninfectious inflamma-tory causes rather than ascending infection.10-15 Fever at term is associated with low rates of placental infection by detection of bacterial RNA (5.4%).10 Further, 40% of patients with the diagnosis of clinical chorioamnionitis at term do not have evidence of bacteria in the amniotic cavity with the use of cultivation or molecular microbiologic techniques.11 Therefore, it is important that experimental models adequately reproduce the likely maternal-to-fetal transmission of inflammation that occurs with term chorioamnionitis.
Rodent models of fetal inflammation have been used widely to investigate the consequences of maternal inflammation on preterm delivery, fetal development and the mechanisms that are involved in preterm brain damage.16 In addition, 1 model of infectious term chorioamnionitis has been described previously.17,18 Because the clinical cause of inflammation varies with gestational age, the models that are used to study the mechanisms of perinatal brain injury should also vary with respect to inflammatory agent, dose, and delivery route. Lipopolysaccharide commonly is used as a nonspecific inducer of inflammation. In studies that are designed to mimic chorioamnionitis at term, it is critical to model maternal systemic inflammation with subsequent fetal inflammation, rather than the preterm mechanisms of ascending intrauterine infection. Therefore, we used an intraperitoneally administered lipopolysaccharide rodent model to induce maternal systemic to fetal inflammation.
The risk of fetal brain injury that is associated with inflammation at term increases synergistically with other adverse exposures, especially hypoxia and hyperthermia.19-22 Therefore, it is vital to understand the timing of maximal fetal brain inflammation after a maternal inflammatory stimulus so that secondary insults and interventions can be timed effectively in experimental investigations.
Materials and Methods
Animals
Twenty-four timed-pregnant Sprague Dawley rats (Harlan, Indianapolis, IN) were housed in our animal facility with a 12-hour light cycle (6 am to 6 pm) and ad libitum access to food and water during both housing and experimental time. All experiments were performed on day 20 of pregnancy. To avoid any circadian effect, all experimental procedures were started at the same time of the day. The experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committees at Medical University of South Carolina and Temple University. Before each experiment, a baseline sample of maternal blood (0.5 mL) was withdrawn from the tail vein and collected in a microcentrifuge tube that contained a solution of disodium dihydrate EDTA (1.8 mg/mL blood) and centrifuged (1300g, 10 minutes, 4°C). A Lidoderm 5% lidocaine patch (2.5 cm2; ENDO Pharmaceuticals, Malvern, PA) was applied on the dorsum between the shoulder blades 1 hour before the injection of an implantable glass-encapsulated passive transponder that was inserted in a dedicated stainless-steel syringe-type applicator (Implantable Programmable Temperature Transponder, IPTT-300; Bio Medic Data Systems Inc, Seaford, DE). Animals were then returned to their cages for temperature stabilization. After 20 minutes, temperature was recorded with a wireless receiver (DAS-6007; Bio Medic Data Systems Inc) every 2 minutes until at least 5 consecutive recordings were obtained that differed by no >0.3°C.
At this time, either 0.9% sodium chloride (Hospira, Inc, Lake Forest, IL) or increasing doses (0, 160, 320, and 640 μg/kg; n = 6 dams per dose) of Escherichia coli O55:B5 lipopolysaccha-ride (Calbiochem, San Diego, CA) resuspended and sonicated in normal saline (Hospira, Inc) was injected (500 μL) intraperitoneally. Injection time was counted as time 0 hour.
Core body temperature was then recorded every 2 minutes, and 0.5-mL blood samples were collected from the tail vein every 2 hours until the end of the experiment. Euthanasia was performed by decapitation at 2, 4, or 6 hours after intraperitoneal injection, and all the pups were collected by hysterectomy. From each litter, 3 pups were collected aseptically in ice-cold Ca2+ and Mg2+ free Hanks’ balanced salt solution (Gibco, Gaithersburg, MD) to prepare cortical cell cultures. From the remaining pups in each litter, brains and placentas were divided into 2 symmetrical halves; 1 was snap-frozen in liquid nitrogen, and the other was placed in RNAlater at 4°C for 24 hour and stored at −80°C until use. Blood was collected from the vessels of the neck after decapitation with an EDTA-Minivette POCT (Sarstedt, Nümbrecht, Germany).
Enzyme-linked immunosorbent assay
Quantification of interleukin-6 (IL-6) from plasma samples was performed with Quantikine rat IL-6 immunoassay kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. Because of the limited volumes that were obtained, plasma that was collected from the pups was pooled for IL-6 quantification. Sample absorbance was quantified by a microplate reader (Bio-Tek Instruments, Winooski, VT) at 450 nm with a correction wavelength set at 570 nm. Values were calculated by nonlinear regression with the use of the GraphPad Prism (version 6; GraphPad Software Inc, La Jolla, CA). The sensitivity of the assay was 21 pg/mL. The coefficient of variation was 8.1%.
Quantitative polymerase chain reaction
Total RNA was extracted from brain and placenta samples by homogenization in TRIzol reagent (1 mL/≤100 mg tissue; Ambion Life Technologies, Austin, TX) according to the manufacturer instructions. Extracted RNA was resus-pended in 100 μL of nuclease-free water with the addition of RNasin ribonuclease inhibitor (Promega Corp, Madison, WI) and stored at −80°C. RNA was further purified with the RNeasy Mini Kit combined with the RNAse-Free DNase Set (Qiagen, Valencia, CA) according to manufacturer's protocol. RNA was analyzed by spectrophotometry (NanoVue Plus; GE Healthcare, Piscataway, NJ) and by gel electrophoresis (6 V/cm). Each sample (2-4 μg) was loaded onto a 1.2% agarose gel with 0.7% formaldehyde added as a denaturing agent.
One-step quantitative polymerase chain reaction (qPCR) was performed with StepOne Plus (Applied Biosystems, Foster City, CA) reverse transcription PCR system with the GoTaq 1-Step RT-qPCR System (Promega Corp). One hundred nanograms of RNA and 500 nmol/L of carboxy-X-rhodamine reference dye (Promega Corp) were added to each 20 μL reaction. The housekeeping gene primer pair (β actin) was diluted to 300 nmol/L, and the IL-6 primer pair was added according to manufacturer instructions. QPCR cycles were set as recommended by the manufacturer, with minor modifications (15 minutes at 37.5°C; 10 minutes at 95°C, and 40 cycles of 15 seconds at 95°C and 1 minute at 60°C). Final melting curves were run by 0.5°C increments (15 seconds at 95°C; 1 minute at 60°C) to 95°C (15 seconds). Single peak melting curves were obtained from all qPCR reactions, which is indicative of specific product amplification (data not shown).
Primer sequences for β actin were obtained from NCBI Blast (F: 5′-AGC CAT GTA CGT AGC CAT CC -3′; R: 5′- CTC TCA GCT GTG GTG GTG AA -3′). The primer pair for rat IL-6 was a RT2 qPCR Primer Assay (Qiagen). For each experimental group, 8 samples from 2 litters were run in triplicate. Relative quantification of gene expression was calculated by 2−ΔΔCt and are shown as folds of increased messenger RNA transcription after normalization over the control values.23
Electrophoresis
QPCR product size was confirmed by gel electrophoresis on Criterion (Bio-Rad Laboratories, Hercules, CA) 15% tris/boric acid/ethylenediaminetetraacetic acid precast gels run for 3 hours in a Criterion Cell (Bio-Rad Laboratories), according to manufacturer instructions. Diluted β-actin samples (1:2) and undiluted IL-6 reactions were run together with a GeneRuler Ultra Low Range DNA ladder (Thermo Scientific, Waltham, MA) were used as reference, according to manufacturer protocol. Gels were poststained for 45 minutes on a rocker in a ×3 solution of RedGel (Biotium, Inc, Haywood, CA) diluted in water. Images were acquired with a Gel Doc EZ system (Bio-Rad Laboratories).
Quantitative western blot
Protein extracts were prepared from rat brain tissues. Cellular debris was removed by centrifugation for 5 minutes at 4°C; the supernatant was assayed for protein content by Bradford analysis (Bio-Rad Laboratories). Fifty micrograms of proteins were diluted with Laemmli sodium dodecylsulfate sampleereducing buffer, (6X), heated at 95°C for 10 minutes, and separated by gradient sodium dodecylsulfate sample–polyacrylamide gel electrophoresis in 1X Tris-Glycine-Sodium Dodecyl Sulfate buffer and transferred to reinforced supported nitro-cellulose membranes (Whatman, Piscataway, NJ) for 2 hours at 4°C. Membranes were blocked with Odyssey Blocking Buffer (LI-COR, Inc, Lincoln, NE) for 1 hour at room temperature and incubated with specific primary antibodies for 2 hours in Odyssey Blocking Buffer with 0.1% Tween-20. The blots subsequently were washed 3 times and incubated with IRDye 800CW goat anti-mouse and IRDye 680RD goat anti-rabbit secondary antibodies and visualized with an Odyssey CLx Imaging System (LI-COR, Inc), with the use of Odyssey software (LI-COR Biosciences). Anti–IL-6 mouse monoclonal antibody (ab9324) was purchased from Abcam (Cambridge, MA). Anti-GAPDH rabbit polyclonal (# PA1-987), which was purchased from Pierce Chemical Co (Rockford, IL), served as a loading control.
Cortical cell cultures
Cultures were prepared as previously described.17 Briefly, brains were removed from the skull and sectioned into ice-cold Hank's Balanced Salt Solution (Gibco) to discard olfactory bulbs, brain stem, cerebellum, and meninges. Cortices from each litter were stored separately until being processed in ice-cold Hybernate-E complete media (5 mL/pair; Gibco) supplemented as indicated by manufacturer with GlutaMAX-I and B27 supplement (Gibco). Tissues were trypsinized for 15 minutes in 0.05% Trypsin-EDTA (Gibco) diluted in Neurobasal media (Gibco) (1:80; 4 mL/pair of cortices) in a cell culture incubator (37°C; 5% CO2; 95% humidity). Fragments of tissue were then added to neurobasal media that were enriched with 10% heat-inactivated fetal bovine serum (Sigma Chemical Company, St. Louis, MO). Samples were pooled together and further dissociated by mechanical trituration for 1 minute. The cell suspension was collected for cell counting and seeding.
Immunofluorescence
Cells were seeded (40,000 cells/well) into 12-well plates on 0.01% poly-L-lysine coated (Sigma Chemical Company) 18-mm glass coverslips in neurobasal media that were supplemented with GlutaMAX and B27 supplement (neuronal selective media; Gibco). On in vitro day 3, the cells were rinsed with warm phosphate-buffered saline solution (PBS) and fixed with ice-cold 4% paraformaldehyde for 30 minutes at room temperature.
Cultures were costained with DAPI (Sigma-Aldrich, St. Louis, MO) and anti-MAP2 antibody. Briefly, cell membranes were permeabilized with 0.5% Triton X-100 in PBS (Sigma) and nonspecific binding sites were saturated with 2% BSA (Fisher Scientific, Waltham, MA) in PBS. Samples were incubated overnight at 4°C with the primary antibody (ab11267, 1:500) in 1% goat serum (Sigma-Aldrich) in PBS. A secondary antibody solution that was prepared in the aforementioned manner (ab96882, 1:100) was incubated for 1.5 hours in the dark at room temperature. Nuclei were stained for 5 minutes at room temperature with 1 μg/mL DAPI in PBS. Coverslips were mounted with Prolong Gold anti-fade reagent (Invitrogen, Carlsbad, CA), according to manufacturer instructions. Images were acquired with a Nikon i80 microscope (Nikon Instruments Inc, Melville, NY) equipped with a Roper Scientific camera (Q Imaging, Surrey, Canada) with a ×100 objective.
Three brains from 2 litters were used to prepare cell cultures; from each culture, 3 coverslips were stained. From each coverslip, the images of at least 10 single nontouching neurons were acquired randomly (n = 60 per experimental condition). The total number of neurites and the length of each neurite were obtained with the use of automated software for Sholl analysis (ImageJ; National Institutes of Health, Bethesda, MD).
Statistical analysis
Ordinal-by-ordinal testing or 1-way analysis of variance with Least Signifi-cant Difference post-hoc testing was performed (SPSS Statistics for Windows, version 20.0; IBM, Armonk, NY). A probability value of < .05 was considered statistically significant. Forty neurons were counted from 6 pooled cortical cultures for each experimental condition (80% power to detect a 33% decrease in mean number of neurites compared with the control group in pairwise analysis). Eight brains were prepared for analysis of inflammatory cytokines for each experimental condition (80% power to detect a 60% increase in mean IL-6 compared with the control group in pairwise analysis).
Results
Lipopolysaccharide reduces maternal core temperature
The lowest dose of lipopolysaccharide that was administered (160 μg/kg) did not have any effect on maternal core temperature. At 320 μg/kg and 640 μg/kg, lipopolysaccharide significantly reduced body core temperature with dose-dependent latency, duration, and magnitude (Figure 1). At the median dose, the effect lasted up to 4 hours after a latency of 1.5 hours; at the higher dose, the effect lasted up to 5.5 hours after a latency of 1 hour. The mean decrease in temperature was −1.0 ± 0.3°C (1.5 hours to 4 hours) after 320 μg/kg and −1.6 ± 0.3°C (1.5 hours to 5.5 hours) after 640 μg/kg.
FIGURE 1. Dose-dependent effect of lipopolysaccharide on dam core temperature.
Lipopolysaccharide dose effect on dam core temperature was monitored up to 6 hours at 640 μg/kg (dotted/-dashed line), 320 μg/kg (dashed line), 160 μg/kg (dotted line), and 0 μg/kg (continuous line). Data are expressed as mean ± SD (n = 2-6 animals per time point). One-way analysis of variance with Least Significant Difference Post-Hoc test was performed with repeated measures taken into account; a probability value of ≤ .05 was set as a significance threshold. The single asterisk indicates a probability value of ≤ .05; the double asterisk indicates a probability value of ≤ .01 vs control. The apostrophe indicates minutes.
Maternal and fetal plasma IL-6 concentrations after lipopolysaccharide
Peak maternal plasma IL-6 cytokine response occurred within the first 2 hours, and the magnitude of response did not differ with dose of lipopolysaccharide, except for a persistent low-level maternal response at 6 hours after the highest dose (Figure 2, A). Maternal plasma levels declined rapidly to baseline by 6 hours at lower doses. Control group dams had constant undetectable IL-6 plasma levels. Fetal plasma IL-6 levels also peaked at 2-4 hours, but the magnitude of the response did not appear to be dose dependent (Figure 2, B). Fetal plasma IL-6 levels returned to baseline by 6 hours at all doses.
FIGURE 2. Time-course of maternal and fetal plasma IL-6 levels after exposure to increasing doses of LPS.
LPS was injected at time 0 hours at the doses of 640 μg/kg (squared bars), 320 μg/kg (vertical line bars), 160 μg/kg (horizontal line bars), and 0 μg/kg (open bars). Data are expressed as mean ± SD. One-way analysis of variance with Least Significance Difference Post-Hoc test was performed; a probability value of ≤ .05 was set as a significance threshold. A, Values that were obtained at time 0 hours before LPS administration were below the lowest limit of detection of the assay in maternal plasma (mean sensitivity = 21 pg/mL). At 2 hours, 320 μg/kg and 640 μg/kg was associated with increased IL-6 compared with control (P < .05). At 4 and 6 hours, only 640 μg/kg significantly increased compared with control (P < .05). Control group dams had constant basal IL-6 levels at all time points. B, Values at 2 and 4 hours increased at all doses compared with controls (P<.05); there was no significant difference in fetal response by dose. At 6 hours, fetal levels were comparable with controls at all doses. Control fetuses had constant basal IL-6 levels at all time points.
Placental and fetal IL-6 messenger RNA expression
Placental IL-6 RNA expression peaked at 2 hours (Figure 3, A). At 6 hours, placental IL-6 RNA expression remained elevated compared with controls at all doses. Fetal brain IL-6 RNA expression was increased significantly only at the 6-hour time point and only at doses of 320 μg/kg and 640 μg/kg (Figure 3, B). Fetal brain IL-6 RNA expression for individual pups is shown in Figure 3, C. These data indicate that fetal brain response is largely uniform in this intraperitoneal administration model.
FIGURE 3. Time-course of placental and fetal brain IL-6 RNA levels after dam exposure to increasing doses of LPS.
Representative gel of amplicons that were obtained from A, placenta tissue or B, fetal brain 2 hours after maternal LPS injections. In each gel, products that were obtained from amplification reactions with IL-6 primers are shown on the left; products that were obtained with β-actin primers are shown on the right. Lanes are labeled c(controls) and i, ii, iii (160, 320 and 640 μg/kg LPS), respectively. Graphs depict IL-6 RNA expression levels in placenta and fetal brain relative to expression at 0 hours with LPS doses of 640 μg/kg (squared bars), 320 μg/kg (vertical line bars), 160 μg/kg (horizontal line bars) and 0 μg/kg (open bars). Data are expressed as mean ± SD. One-way analysis of variance with Least Significant Difference Post-Hoc test was performed; a probability value of ≤ .05 was set as a significance threshold. A, The single asterisk indicates a probability value of ≤ .05; the double asterisk indicates a probability value of ≤ .01 vs saline solution; the triple asterisk indicates no significance compared with 640 μg at 2 hours or 160 μg at 6 hours. B, The single asterisk indicates a probability value of ≤ .05 vs saline solution. C, Distribution of fold IL-6 expression normalized to β-actin in individual pup brains from the 640 μg and control groups is shown.
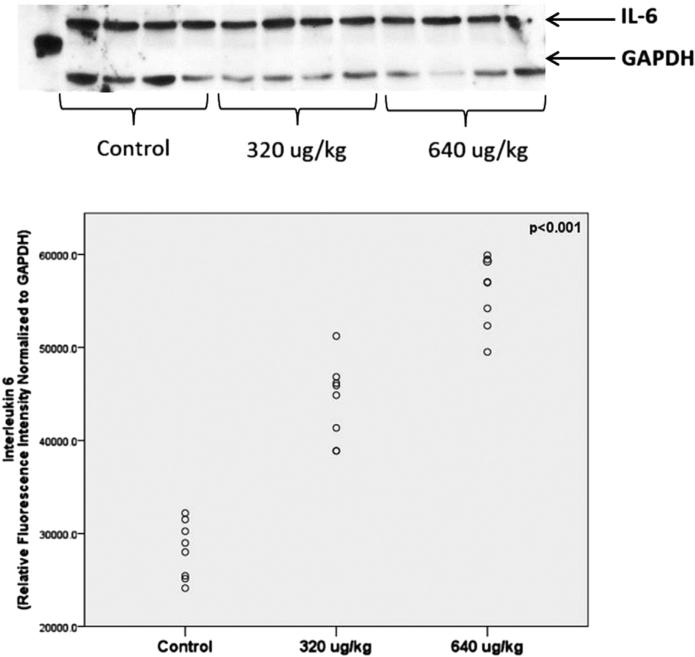
IL-6 protein in fetal brain
Fetal brain IL-6 protein expression was quantified in fetal brain at 6 hours (Figure 4). Given that IL-6 RNA expression was not increased at 160 μg/kg, only specimens from pups that were exposed to 320 or 640 μg/kg were assayed. Protein data confirmed our gene data. IL-6 protein expression was increased significantly at both 320 μg/kg and 640 μg/kg but was highest in the 640 μg/kg group. Further, the data spread again suggests a relatively uniform pup brain inflammatory response to maternal lipopolysaccharide treatment.
FIGURE 4. IL-6 protein expression in fetal brains 6 hours after maternal LPS injection.
Representative protein blots from control, 320 μg/kg, and 640 μg/kg with control (GAPDH). Relative fluorescent intensity normalized to GAPDH shown from individual pup brains. One-way analysis of variance with Least Significant Difference Post-Hoc test was performed; a probability value of ≤ .05 was set as a significance threshold.
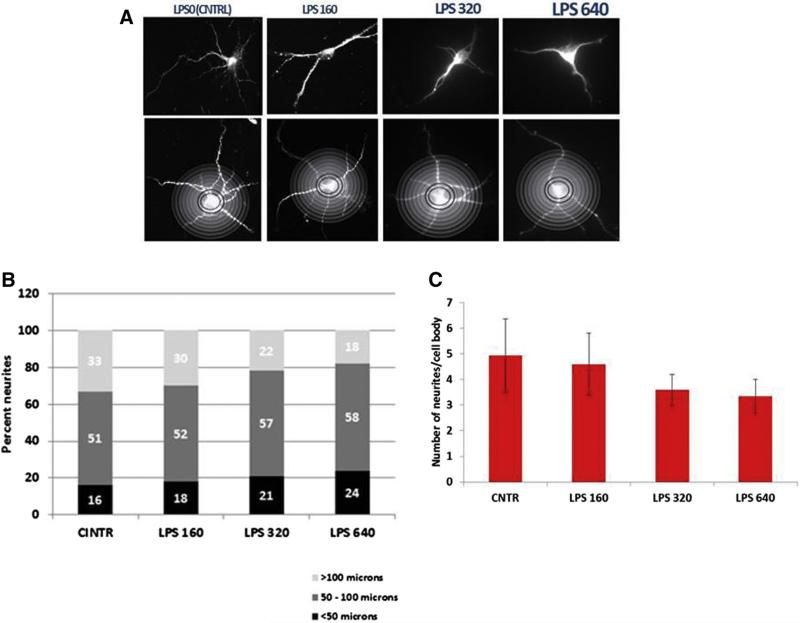
Neuronal cell damage assessed by neurite sprouting
To assess fetal brain injury, we used an established method to quantify neuronal cell damage in cortical primary cultures.16 A representative image from each maternal dose is shown with a superimposed Sholl template (Figure 5, A). The number of neurites per cell body and the length of each individual neurite was quantified by automated Sholl analysis in 40 neurons. There was a significant decrease in both neurite length with an increasing lipopolysaccharide dose (Figure 5, B) and the number of neurites per cell body after the 320 μg/kg and 640 μg/kg doses (Figure 5, C).
FIGURE 5. LPS dose-dependent reduction in neurite sprouting by automated Sholl analysis.
A, A representative neuron is shown from primary neuronal cultures at each dose with an overlay, B, to demonstrate quantification of neurite length. Categories of neurite length by LPS dose are shown. C, Ordinal-by-ordinal analysis was performed for categories of neurite length (P = .0006). The number of neurites per cell body is shown. One-way analysis of variance with Least Significant Difference Post-Hoc test was performed; a probability value of ≤ .05 was set as a significance threshold. The number of neurites per cell body was significantly related to dose (overall analysis of variance: P < .001). Post-hoc pairwise analysis compared with control: P < .06 vs 160 μg/kg; P < .001 vs 320 μg/kg; P < .001 vs 640 μg/kg.
Comment
Principal study findings
Our data suggest a rapid and consistent maternal, placental, and fetal in-flammatory response after maternal intraperitoneal lipopolysaccharide administration. The mechanism of the fetal response to maternal inflammation is not well understood either in animal models or in humans in vivo. Lipopolysaccharide does not appear to cross the placenta24,25; direct placental transfer of maternal cytokines such as IL-6 is limited.26-28 We and others have demonstrated that the placenta can be a source of inflammatory cytokines and may be the link that propagates the fetal inflammatory response.27,29
After a fetal plasma cytokine response, it is unknown to what extent cytokines permeate the fetal blood-brain barrier (BBB). In adult rodents, cytokines are transported through the BBB in low amounts.30,31 In rat fetuses, contrasting opinions have been reported regarding the maturity of BBB that may permit for more permissive transfer.32-34 An other potential source of inflammatory cytokines is the brain itself; neurons, astrocytes, microglial, and endothelial cells are all capable of producing cytokines. It is still unclear from available data whether the damaging fetal neuroinflammatory cascade originates from the fetal,35-37 placental,29,38,39 or maternal40 compartment or a combination of these sources.
The effect of systemic inflammation on maternal temperature
Previous investigations in rat models after lipopolysaccharide administration have reported altered thermoregulatory responses. Core temperature depression appears to follow a decrease in the central nervous system thermoregulatory set-point.41 Brain nitric oxide42 and endogenous glucocorticoids43 have been shown to act as mediators. Because lipopolysaccharide passage across the BBB does not occur in adult rats,25 the changes induced on dam core temperature are likely indirect effects of lipopolysaccharide and involve cytokines and second messengers such as prostaglandins.
Harre et al44 showed no gestation-related changes in the activation of signal transducer and activator of transcription 3, which is an IL-6 activated transcription factor, and suggests that signal transducer and activator of transcription 3 is not involved in the hypothermic response. Fofie et al45 hypothesized that hypothermia that occurs in term pregnant rats is due to an imbalance of cryogenic cytokines (IL-1 receptor antagonist) compared with pyrogenic cytokines (IL-6 and interleukin 1 beta). Fofie et al45 based this hypothesis on findings that lipopolysaccharide O111:B4 induced IL-1 receptor antagonist and tumor necrosis factore–α in all rats, but that IL-6 and IL-1β increased only in nonpregnant rats. In contrast, our data with lipopolysaccharide O55:B5 demonstrate a robust increase in IL-6 after lipopolysaccharide administration in pregnant dams. Further, peak maternal serum IL-6 levels correspond to the nadir in maternal temperature (2 hours).
The effect of systemic maternal lipopolysaccharide on maternal and fetal cytokines and fetal IL-6
Our data suggest a rapid and consistent maternal, placental, and fetal inflammatory response after maternal intraperitoneal lipopolysaccharide administration. The timeline in maternal plasma, placenta, and fetal plasma suggests that the inflammatory response in these compartments is rapid and essentially synchronous. Of note, neonatal cytokine production is biased towards IL-6 (compared with other inflamma-tory cytokines such as tumor necrosis factore–α),46 and IL-6 predominates in the fetal inflammatory response syndrome.47 In the fetal brain compartment, the inflammatory response is delayed until 6 hours after maternal stimulus. Fetal brain levels lag 4 hours behind fetal plasma levels; therefore, permissive transfer across an immature BBB is not a compelling mechanism. Taken together, these data suggest a fetal neuroinflammatory response with in situ cytokine production.
The effect of systemic lipopolysaccharide on fetal neuronal injury
Our data demonstrate that there is dose-dependent fetal neuronal injury after isolated maternal lipopolysaccharide injection. The injury we demonstrate would be expected primarily to affect communications between neurons and other surrounding cells through a decrease in length of communicating neurites or a decrease in their number. Our data are consistent with that of Gilmore et al,48 who found that inflammatory cytokines affect the development of primary dendrites and the complexity of developing cortical neurons prepared from embryonic Sprague-Dawley rats. These findings suggest a possible impairment consequent to maternal in-flammation in the absence of infection, hyperthermia, or hypoxia. The functional significance of these findings is not understood fully at this time. It is not known whether neuronal plasticity can compensate for early injury and/or to what degree.
Research implications
The current investigation establishes a reliable model of chorioamnionitis at term and delineates several important variables. First, we have determined that the ideal dose of E coli O55:B5 lipopolysaccharide is 640 μg/kg, which induces maximal fetal brain inflammation without precipitating parturition. This is critical to allow investigators a sufficient window to administer either additional intrauterine insults or targeted protective interventions. The lipopolysaccharide serotype, the dose within the O55:B5 lipopolysaccharide serotype, and the timing of sampling can have a profound effect on experimental results.49-53 We acknowledge that lipopolysaccharide mimics an infectious stimulus for inflammation. Although much of term chorioamnionitis is now believed to be sterile, the signature maternal and fetal cytokine of term fever is IL-6.54
Lipopolysaccharide O55:B5 appears to reproduce the time course and elevation of IL-6 that has been observed in human serum and cord blood after term chorioamnionitis. We chose not to assess multiple other cytokines because the maternal/fetal cytokine patterns of infectious vs sterile chorioamnionitis overlap and have not been reported discretely. Until these patterns are better understood, it is impossible to assess whether the lipopolysaccharide sero-type O55:B5 is the best stimulus to mimic term chorioamnionitis. However, the key take-away point is that sterile maternal inflammation, with or without fever, results in inflammation in the fetal brain and placenta and increased measures of fetal brain injury and may affect the fetal immune system.
Second, we delineate the time course for fetal brain inflammation after maternal systemic inflammation to allow subsequent studies to time secondary insults accurately (ie, ischemia-hypoxia and hyperthermia). Given the relatively small number of fetal brain samples in each subgroup, we may have had limited power to detect smaller increases in fetal brain IL-6 before the peak at 6 hours. Therefore, we cannot rule out some in-flammatory insult at 2 or 4 hours. Reduced maternal core temperature during systemic inflammation may have a neuroprotective effect on fetal brain that is supported by the clinical use of hypothermia for infants who are born with complications such as perinatal asphyxia and ischemic encephalopathy. Hypothermia has been shown to improve outcomes if administered within the first 6 hours of life.54-57 Therefore, lipopolysaccharide-based rat models that seek to investigate in utero fetal brain injury after term chorioamnionitis should stabilize explicitly and/or increase dam temperature to avoid inadvertent neuroprotection. We have developed and tested a heated air system to increase dam core temperature (data not shown) to mimic fully term chorioamnionitis. Despite this limitation, our results underscore that neuronal injury can result from the maternal-to-fetal transmission of inflammation without the additional insult of hyperthermia.
Clinical implications of this research
Our findings may have important clinical implications. If human responses are similar, the onset of clinical chorioamnionitis (maternal fever) coincides with maternal and placental inflammation and follows shortly after maternal inflammatory stimuli (eg, epidural analgesia). There may be a 4-hour therapeutic window between the inflammatory stimulus and the onset of significant fetal brain inflammation. During this window, the potential deleterious effects on the fetal brain may be limited to hyperthermia. It is critical to note that our results do not suggest that cesarean delivery for chorioamnionitis is beneficial; indeed, available data suggest no benefit of a shortened duration of neonatal exposure.58 More encouraging is the concept of intrauterine neuroprotection. The ideal neuroprotective agent could be given after the identification of maternal fever and rapidly cross the placenta and fetal BBB before the onset of fetal brain inflammation. One proposed neuroprotectant with adequate and rapid placental transfer is N-acetyl cysteine59; however, no adequate clinical trials have been reported. In addition, several promising neonatal interventions have been described and are under development, which include dendrimer-based N-acetyl cysteine therapy.60 The experimental model we describe can enhance the investigation of new, clinically relevant, therapeutic windows to reduce the incidence of neonatal encephalopathy and cerebral palsy back to background levels from the approximately 9-fold increased risk that has been observed after inflammation/hyperthermia at term.5-9
Acknowledgments
Supported by seed accounts from the Medical University of South Carolina and Temple University.
Footnotes
The authors report no conflict of interest.
Presented at the 60th Annual Scientific Meeting of the Society for Reproductive Investigation, Orlando, FL, March 20-23, 2013.
REFERENCES
- 1.Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol. 2007;109(suppl):8–14. [PubMed] [Google Scholar]
- 2.Himmelmann K, Uvebrant P. The panorama of cerebral palsy in Sweden: XI, changing patterns in the birth-year period 2003-2006. Acta paediatr. 2014;103:618–24. doi: 10.1111/apa.12614. [DOI] [PubMed] [Google Scholar]
- 3.Fairhurst C. Cerebral palsy: the whys and hows. Arch Dis Child Educ Pract Ed. 2012;97:122–31. doi: 10.1136/edpract-2011-300593. [DOI] [PubMed] [Google Scholar]
- 4.Soleimani F, Vameghi R, Biglarian A. Ante-natal and intrapartum risk factors for cerebral palsy in term and near-term newborns. Arch Iran Med. 2013;16:213–6. [PubMed] [Google Scholar]
- 5.Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. JAMA. 1997;278:207–11. [PubMed] [Google Scholar]
- 6.Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA. 2000;284:1417–24. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 7.Impey L, Greenwood C, MacQuillan K, Reynolds M, Sheil O. Fever in labour and neonatal encephalopathy: a prospective cohort study. BJOG. 2001;108:594–7. doi: 10.1111/j.1471-0528.2001.00145.x. [DOI] [PubMed] [Google Scholar]
- 8.Glass HC, Bonifacio SL, Sullivan J, et al. Magnetic resonance imaging and ultrasound injury in preterm infants with seizures. J Child Neurol. 2009;24:1105–11. doi: 10.1177/0883073809338328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu CS, Nohr EA, Bech BH, Vestergaard M, Catov JM, Olsen J. Health of children born to mothers who had preeclampsia: a population-based cohort study. Am J Obstet Gynecol. 2009;201269:e1–10. doi: 10.1016/j.ajog.2009.06.060. [DOI] [PubMed] [Google Scholar]
- 10.Riley LE, Celi AC, Onderdonk AB, et al. Association of epidural-related fever and noninfectious inflammation in term labor. Obstet Gynecol. 2011;117:588–95. doi: 10.1097/AOG.0b013e31820b0503. [DOI] [PubMed] [Google Scholar]
- 11.Romero R, Miranda J, Kusanovic JP, et al. Clinical chorioamnionitis at term: I, microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med. 2015;43:19–36. doi: 10.1515/jpm-2014-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma SK, Rogers BB, Alexander JM, McIntire DD, Leveno KJ. A randomized trial of the effects of antibiotic prophylaxis on epidural-related fever in labor. Anesth Analag. 2014;118:604–10. doi: 10.1213/ANE.0b013e3182a5d539. [DOI] [PubMed] [Google Scholar]
- 13.Goetzl L, Zighelboim I, Badell M, et al. Maternal corticosteroids to prevent intrauterine exposure to hyperthermia and inflammation; a randomized, double-blind, placebo-controlled trial. Am J Obstet Gynecol. 2006;195:1031–7. doi: 10.1016/j.ajog.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Park HS, Romero R, Lee SM, Park CW, Jun JK, Yoon BH. Histologic chorioamnionitis is more common after spontaneous labor than after induced labor at term. Placenta. 2010;31:792–5. doi: 10.1016/j.placenta.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts DJ, Celi AC, Riley LE, et al. Acute histologic chorioamnionitis at term: nearly always noninfectious. PLoS ONE. 2012;7:1. doi: 10.1371/journal.pone.0031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burd I, Balakrishnan B, Kannan S. Models of fetal brain injury, intrauterine inflammation, and preterm birth. Am J Reprod Immunol. 2012;67:287–94. doi: 10.1111/j.1600-0897.2012.01110.x. [DOI] [PubMed] [Google Scholar]
- 17.Burd I, Brown A, Gonzalez JM, Chai J, Elovitz MA. A mouse model of term chorioamnionitis: unraveling causes of adverse neurological outcomes. Reprod Sci. 2011;18:900–7. doi: 10.1177/1933719111398498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elovitz MA, Brown AG, Breen K, Anton L, Maubert M, Burd I. Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int Journal Dev Neurosci. 2011;29:663–71. doi: 10.1016/j.ijdevneu.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Impey LW, Greenwood CE, Black RS, Yeh PS, Sheil O, Doyle P. The relationship between intrapartum maternal fever and neonatal acidosis as risk factors for neonatal encephalopathy. Am J Obstet Gynecol. 2008;198:49, e1–6. doi: 10.1016/j.ajog.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Peebles DM, Wyatt JS. Synergy between antenatal exposure to infection and intrapartum events in causation of perinatal brain injury at term. BJOG. 2002;109:737–9. doi: 10.1111/j.1471-0528.2002.01019.x. [DOI] [PubMed] [Google Scholar]
- 21.Malaeb S, Dammann O. Fetal inflammatory response and brain injury in the preterm newborn. J Child Neurol. 2009;24:1119–26. doi: 10.1177/0883073809338066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Hagberg H, Nie C, Zhu C, Ikeda T, Mallard C. Dual role of intrauterine immune challenge on neonatal and adult brain vulnerability to hypoxia-ischemia. J Neuropathol Exp Neurol. 2007;66:552–61. doi: 10.1097/01.jnen.0000263870.91811.6f. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Goto M, Yoshioka T, Ravindranath T, Battelino T, Young RI, Zeller WP. LPS injected into the pregnant rat late in gestation does not induce fetal endotoxemia. Res Commun Mol Pathol Pharmacol. 1994;85:109–12. [PubMed] [Google Scholar]
- 25.Ashdown H, Dumont Y, Ng M, Poole S, Boksa P, Luheshi GN. The role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophrenia. Mol Psychiatry. 2006;11:47–55. doi: 10.1038/sj.mp.4001748. [DOI] [PubMed] [Google Scholar]
- 26.Dahlgren J, Samuelsson AM, Jansson T, Holmäng A. Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatr Res. 2006;60:147–51. doi: 10.1203/01.pdr.0000230026.74139.18. [DOI] [PubMed] [Google Scholar]
- 27.Aaltonen R, Heikkinen T, Hakala K, Laine K, Alanen A. Transfer of proinflammatory cytokines across term placenta. Obstet Gynecol. 2005;106:802–7. doi: 10.1097/01.AOG.0000178750.84837.ed. [DOI] [PubMed] [Google Scholar]
- 28.Samuelsson A, Jennische E, Hansson H, Holmang A. Prenatal exposure to interleukin-6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABAA dysregulation and impaired spatial learning. Am J Physiol. 2006;290:R1345–56. doi: 10.1152/ajpregu.00268.2005. [DOI] [PubMed] [Google Scholar]
- 29.Hsiao EY, Patterson PH. Placental regulation of maternal-fetal interactions and brain development. Dev Neurobiol. 2012;72:1317–26. doi: 10.1002/dneu.22045. [DOI] [PubMed] [Google Scholar]
- 30.Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2:241–8. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- 31.Banks WA, Plotkin SR, Kastin AJ. Permeability of the blood-brain barrier to soluble cytokine receptors. Neuroimmunomodulation. 1995;2:161–5. doi: 10.1159/000096887. [DOI] [PubMed] [Google Scholar]
- 32.Adinolfi M, Beck SE, Haddad SA, Seller MJ. Permeability of the blood-cerebrospinal fluid barrier to plasma proteins during foetal and perinatal life. Nature. 1976;259:140–1. doi: 10.1038/259140a0. [DOI] [PubMed] [Google Scholar]
- 33.Olsson Y, Klatzo I, Sourander P, Steinwall O. Blood-brain barrier to albumin in embryonic new born and adult rats. Acta Neuropathologica. 1968;10:117–22. doi: 10.1007/BF00691305. [DOI] [PubMed] [Google Scholar]
- 34.Saunders NR, Knott GW, Dziegielewska KM. Barriers in the immature brain. Cell Mol Neurobiol. 2000;20:29–40. doi: 10.1023/A:1006991809927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roncarolo MG, Bigler M, Ciuti E, Martino S, Tovo PA. Immune responses by cord blood cells. Blood Cells. 1994;20:573–6. [PubMed] [Google Scholar]
- 36.Sautois B, Fillet G, Beguin Y. Comparative cytokine production by in vitro stimulated mononucleated cells from cord blood and adult blood. Exp Hematol. 1997;25:103–8. [PubMed] [Google Scholar]
- 37.Müller K, Zak M, Nielsen S, Pedersen FK, de Nully P, Bendtzen K. In vitro cytokine production and phenotype expression by blood mono-nuclear cells from umbilical cords, children and adults. Pediatr Allergy Immunol. 1996;7:117–24. doi: 10.1111/j.1399-3038.1996.tb00118.x. [DOI] [PubMed] [Google Scholar]
- 38.Thiex NW, Chames MC, Loch-Caruso RK. Tissue-specific cytokine release from human extra-placental membranes stimulated by lipopolysaccharide in a two-compartment tissue culture system. Reprod Biol Endocrinol. 2009;7:117. doi: 10.1186/1477-7827-7-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keelan JA, Wong PM, Bird PS, Mitchel MD. Innate inflammatory responses of human decidual cells to periodontopathic bacteria. Am J Obstet Gynecol. 2010;202:471, e1–11. doi: 10.1016/j.ajog.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 40.Liechty KW, Kim HB, Adzick NS, Crombleholme TM. Fetal wound repair results in scar formation in interleukin-10-deficient mice in a syngeneic murine model of scarless fetal wound repair. J Pediatr Surg. 2000;35:866–73. doi: 10.1053/jpsu.2000.6868. [DOI] [PubMed] [Google Scholar]
- 41.Tsang M, Fewell JE, Moore SL. LPS induced hypothermia in pregnant rats: a regulated thermoregulatory response. Physiol Behav. 2006;89:235–40. doi: 10.1016/j.physbeh.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Begg DP, Kent S, McKinley MJ, Mathai ML. Suppression of endotoxin-induced fever in near-term pregnant rats is mediated by brain nitric oxide. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2174–8. doi: 10.1152/ajpregu.00032.2007. [DOI] [PubMed] [Google Scholar]
- 43.Moore SL, Fewell JE. Mifepristone (RU38486) influences the core temperature response of term pregnant rats to intraperito-neal lipopolysaccharide. Exp Physiol. 2006;91:741–6. doi: 10.1113/expphysiol.2006.033688. [DOI] [PubMed] [Google Scholar]
- 44.Harré EM, Mouihate A, Pittman QJ. Attenuation of fever at near term: is interleukin-6-STAT3 signalling altered? J Neuroendocrinol. 2006;18:57–63. doi: 10.1111/j.1365-2826.2005.01393.x. [DOI] [PubMed] [Google Scholar]
- 45.Fofie AE, Fewell JE, Moore SL. Pregnancy influences the plasma cytokine response to intraperitoneal administration of bacterial endotoxin in rats. Exp Physiol. 2005;90:95–101. doi: 10.1113/expphysiol.2004.028613. [DOI] [PubMed] [Google Scholar]
- 46.Angelone D, Wessels MR, Coughlin M, et al. Innate immunity of the human newborn is polarized toward a high ratio of IL-6/TNF-a production in vitro and in vivo. Pediatr Res. 2006;60:205–9. doi: 10.1203/01.pdr.0000228319.10481.ea. [DOI] [PubMed] [Google Scholar]
- 47.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 48.Gilmore JH, Fredrik Jarskog L, Vadlamudi S, Lauder JM. Prenatal infection and risk for schizophrenia: IL-1beta, IL-6, and TNFalpha inhibit cortical neuron dendrite development. Neuropsychopharmacology. 2004;29:1221–9. doi: 10.1038/sj.npp.1300446. [DOI] [PubMed] [Google Scholar]
- 49.Cai Z, Pan ZL, Pang Y, Evans OB, Rhodes PG. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr Res. 2000;47:64–72. doi: 10.1203/00006450-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 50.Urakubo A, Jarskog LF, Lieberman JA, Gilmore JH. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr Res. 2001;47:27–36. doi: 10.1016/s0920-9964(00)00032-3. [DOI] [PubMed] [Google Scholar]
- 51.Wang W, Dow KE, Flavin MP. Hyperthermia amplifies brain cytokine and reactive oxygen species response in a model of perinatal inflammation. Neurosci Lett. 2008;445:233–5. doi: 10.1016/j.neulet.2008.08.088. [DOI] [PubMed] [Google Scholar]
- 52.Hirsch E, Saotome I, Hirsh D. Differential fetal and maternal contributions to the cytokine milieu in a murine model of infection-induced preterm birth. Am J Obstet Gynecol. 1995;172:1598–603. doi: 10.1016/s0002-9378(99)70227-9. [DOI] [PubMed] [Google Scholar]
- 53.Hirsch E, Blanchard R, Mehta SP. Differential fetal and maternal contributions to the cytokine milieu in a murine model of infection-induced preterm birth. Am J Obstet Gynecol. 1999;1800:429–34. doi: 10.1016/s0002-9378(99)70227-9. [DOI] [PubMed] [Google Scholar]
- 54.Goetzl L, Evans T, Rivers J, Suresh MS, Lieberman E. Elevated maternal and fetal serum interleukin-6 levels are associated with epidural fever. Am J Obstet Gynecol. 2002;187:834–8. doi: 10.1067/mob.2002.127135. [DOI] [PubMed] [Google Scholar]
- 55.Battin MR, Dezoete JA, Gunn TR, Gluckman PD, Gunn AJ. Neurodevelopmental outcome of infants treated with head cooling and mild hypothermia after perinatal asphyxia. Pediatrics. 2001;107:480–4. doi: 10.1542/peds.107.3.480. [DOI] [PubMed] [Google Scholar]
- 56.Gunn AJ, Gluckman PD, Gunn TR. Selective head cooling in newborn infants after perinatal asphyxia: a safety study. Pediatrics. 1998;102:885–92. doi: 10.1542/peds.102.4.885. [DOI] [PubMed] [Google Scholar]
- 57.Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. CochraneDatabase Syst Rev. 2013;1:CD003311. doi: 10.1002/14651858.CD003311.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rouse DJ, Landon M, Leveno KJ, et al. The Maternal-Fetal Medicine Units cesarean registry: chorioamnionitis at term and its duration-relationship to outcomes. Am J Obstet Gynecol. 2004;191:211–6. doi: 10.1016/j.ajog.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 59.Wiest DB, Chang E, Fanning D, Garner S, Cox T, Jenkins DD. Antenatal Pharmacokinetics and placental transfer of N-acetylcysteine in chorioamnionitis for fetal neuroprotection. J Pediatrics. 2014;165:672–7. doi: 10.1016/j.jpeds.2014.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kannan S, Dai H, Navath RS, et al. Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci Transl Med. 2012;4:130ra46. doi: 10.1126/scitranslmed.3003162. [DOI] [PMC free article] [PubMed] [Google Scholar]