Abstract
The genus Acidithiobacillus includes three species that conserve energy from the oxidation of ferrous iron, as well as reduced sulfur, to support their growth. Previous work, based on multi-locus sequence analysis, identified a fourth group of iron- and sulfur-oxidizing acidithiobacilli as a potential distinct species. Eleven strains of ‘Group IV’ acidithiobacilli, isolated from different global locations, have been studied. These were all shown to be obligate chemolithotrophs, growing aerobically by coupling the oxidation of ferrous iron or reduced sulfur (but not hydrogen) to molecular oxygen, or anaerobically by the oxidation of reduced sulfur coupled to ferric iron reduction. All strains were mesophilic, although some were also psychrotolerant. Strain variation was also noted in terms of tolerance to extremely low pH and to elevated concentrations of transition metals. One strain was noted to display far greater tolerance to chloride than reported for other iron-oxidizing acidithiobacilli. All of the strains were able to catalyse the oxidative dissolution of pyrite and, on the basis of some of the combined traits of some of the strains examined, it is proposed that these may have niche roles in commercial mineral bioprocessing operations, such as for low temperature bioleaching of polysulfide ores in brackish waters. The name Acidithiobacillus ferriphilus sp. nov. is proposed to accommodate the strains described, with the type strain being M20T ( = DSM 100412T = JCM 30830T).
The iron-oxidizing acidithiobacilli are the most widely studied of all acidophilic bacteria, due in part to their importance in environmental pollution (generation of acid mine drainage; Blowes et al., 2014) and mineral processing biotechnologies (Brierley & Brierley, 2013). Although it was common practice for many years to regard all Gram-negative, mesophilic chemolithotrophic acidophiles that oxidized both ferrous iron and reduced sulfur as strains of a single species (Acidithiobacillus ferrooxidans; formerly Thiobacillus ferrooxidans; Kelly & Wood, 2000), there are, at the time of writing, three classified species of the genus Acidithiobacillus that have these core characteristics in common: A. ferrooxidans (Temple & Colmer, 1951), Acidithiobacillus ferrivorans (Hallberg et al., 2010) and Acidithiobacillus ferridurans (Hedrich & Johnson, 2013a). While iron- and sulfur-oxidizing Acidithiobacillus species differ in some physiological traits (e.g. optimum and minimum pH and temperature for growth), strain variation within a single species, where reported, has sometimes been found to be as great, or greater, than differences between the type strains of each of these species.
Based on multi-locus sequence analysis, Amouric et al. (2011) reported that 21 strains of iron-oxidizing acidithiobacilli fell into four distinct clusters, each of which was proposed to represent a separate species. ‘Group I’ isolates were confirmed to be strains of A. ferrooxidans and ‘Group III’ as strains of A. ferrivorans, both of which had been previously designated. ‘Group II’ iron-oxidizing acidithiobacilli were later classified as strains of a new species, A. ferridurans (Hedrich & Johnson, 2013a).
The report of Amouric et al. (2011) also included reference to four strains of ‘Group IV’ iron-oxidizing acidithiobacilli. Analysis of the 16S rRNA gene sequences of strains of mesophilic iron- and sulfur-oxidizing chemolithotrophic acidophiles that had been isolated from different global locations and maintained within the Acidophile Culture Collection at Bangor University (BART-ACC; Table 1) showed that these additional strains were also more closely related to the ‘Group IV’ bacteria than to recognized species of the genus Acidithiobacillus. Several of these had been isolated from copper mines and, in one site (the Pyhäsalmi mine in Finland), they were noted to be the dominant iron-oxidizing acidophiles in acidic, metal-rich waters sampled deep within the mine (Kay et al., 2014). Phylogenetic and physiological tests carried out with these isolates [using protocols described by Hedrich & Johnson (2013a), with all experiments replicated] has confirmed that they are strains of a distinct species, for which the binomial Acidithiobacillus ferriphilus sp. nov. is proposed.
Table 1. Sites of origin of the various strains of A. ferriphilus used in the present study.
| Strain | Source | Country | Reference |
|---|---|---|---|
| M20T | Galway's Soufriere | Montserrat (West Indies) | Atkinson et al. (2000) |
| Riv13 | White River | ||
| JCM 7812 | Sulfur/iron sulfide mine | Japan | Wakao et al. (1991) |
| Malay | Metal mine drainage water | Malaysia | D. B. Johnson (unpublished data) |
| ST2 | Rio Tinto | Spain | D. B. Johnson (unpublished data) |
| PS102 | |||
| PS104 | Copper/zinc mine | Finland | Kay et al. (2014) |
| PS107 | |||
| KCT10 | |||
| KCT14 | Copper mine, Utah | USA | D. B. Johnson (unpublished data) |
| KCT17 |
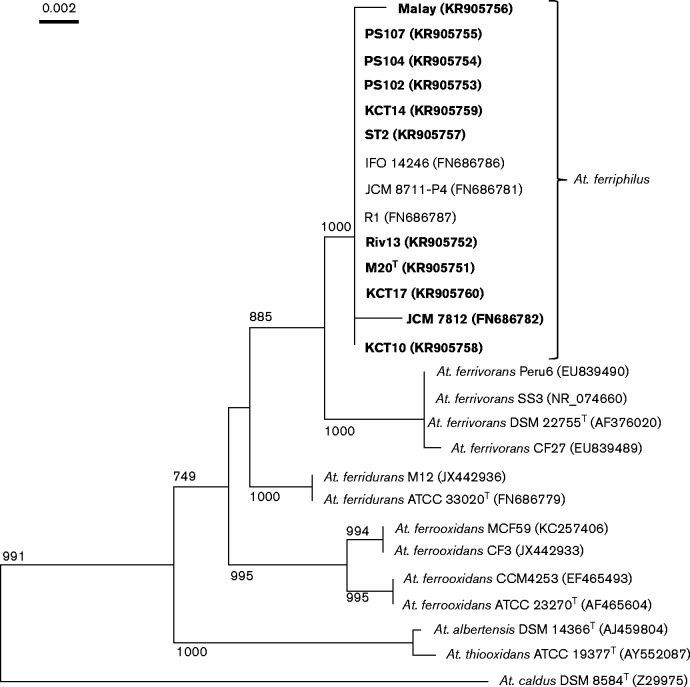
A phylogenetic tree, showing the relationship of strains of A. ferriphilus to other iron-oxidizing acidithiobacilli, is shown in Fig. 1. This confirmed reports (Amouric et al., 2011; Hedrich & Johnson, 2013a) suggesting that ‘Group IV’ and ‘Group III’ (A. ferrivorans) iron-oxidizing acidithiobacilli are more closely related to each other than to ‘Groups I and II’ (A. ferrooxidans and A. ferridurans). The 14 strains of A. ferriphilus shown in Fig. 1 form a tight phylogenetic cluster with >99 % 16S rRNA gene sequence similarity. All the clusters were stable, and bootstrap analysis confirmed that ‘Group IV’ is separate from ‘Group III’, which forms a separate cluster, and that these two groups cluster separately from other Acidithiobacillus species.
Fig. 1.
Neighbour-joining phylogenetic tree derived from 16S rRNA gene sequence data showing the relationship of strain M20T and other A. ferriphilus (‘Group IV’) strains (in bold for strains used in the present study) to other Acidithiobacillus species. Topologies of trees reconstructed by parsimony and maximum-likelihood algorithms were similar. GenBank accession numbers are given in parentheses for each strain, and the tree was rooted with iron-oxidizing acidophile Acidiferrobacter thiooxydans (AF387301; not shown). Bootstrap values are given at the respective nodes; bar, 0.002 % sequence divergence.
All 11 strains of A. ferriphilus (ten BART-ACC strains plus JCM 7812) examined in the present study were shown to catalyse the dissimilatory oxidation of ferrous iron, elemental sulfur and tetrathionate, and also the oxidative dissolution of pyrite, under aerobic conditions. All strains also catalysed the dissimilatory reduction of ferric iron under anoxic conditions, using reduced sulfur as an electron donor. In addition, experiments carried out with the nominated type strain (M20T) confirmed that it was able to grow anaerobically on tetrathionate via ferric iron reduction (Fig. 2), a characteristic it has in common with all other iron- and sulfur-oxidizing Acidithiobacillus species, but not with A. thiooxidans which does not oxidize iron (Hallberg et al., 2001). None of the A. ferriphilus strains examined grew aerobically on hydrogen. This is also the case for most strains of A. ferrivorans, although all strains of A. ferrooxidans and A. ferridurans examined have been shown to grow by coupling the oxidation of hydrogen to the reduction of either molecular oxygen or ferric iron (Ohmura et al., 2002; Hedrich & Johnson, 2013b).
Fig. 2.
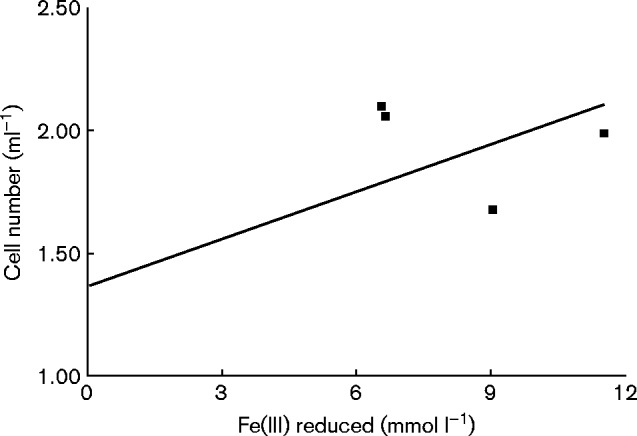
Correlation between cell numbers and ferric iron reduced in cultures of isolate M20T grown anaerobically on tetrathionate as electron donor and ferric iron as electron acceptor (r 2 = 0.71).
As is the case with other iron-oxidizing acidithiobacilli, all strains of A. ferriphilus examined were strict autotrophs. They did not grow heterotrophically on organic substrates (glycerol or yeast extract) and cell numbers were similar in cultures where 20 mM ferrous sulfate medium was supplemented, or not, with either 5 mM glycerol or 0.02 % (w/v) yeast extract, confirming the absence of mixotrophic growth.
The pH and temperature profiles of the 11 strains of A. ferriphilus examined were quite variable (Table S1, available in the online Supplementary Material). Strain M20T had a pH optimum and minimum for growth of 2.0 and 1.5, respectively, and a temperature optimum and maximum of 30 and 33 °C, respectively (Fig. S1). All 11 strains grew at 30 °C, but only eight at 33 °C and one (PS104) at 35 °C. All strains grew at 10 °C (five very slowly) and three strains (including the type strain) at 5 °C. From this it was concluded that A. ferriphilus is mesophilic, but that some strains are psychrotolerant, a feature that has only previously been reported for A. ferrivorans among the iron-oxidizing acidithiobacilli (Hallberg et al., 2010; Liljeqvist et al., 2011). All strains were acidophilic and grew at pH 1.8, although two strains did not grow at pH 1.5, and none at pH 1.25. The most acid-tolerant strain was PS104, which grew in ferrous iron medium at pH 1.35 (Table S1). This physiological characteristic also distinguishes A. ferriphilus from A. ferrivorans, strains of which are more acid-sensitive, with the type strain having a growth pH minimum of 1.9 (Hallberg et al., 2010).
Osmo-tolerance was tested by growing the various strains in 20 mM ferrous iron medium (pH 1.7) containing different concentrations of magnesium sulfate. A similar approach was used to test tolerance to selected transition metals, which were also added as sulfate salts, with the exception of molybdenum where sodium molybdate was used. Oxidation of ferrous iron and increases in cell numbers were used as indicators of positive growth. Salt (NaCl) tolerance was tested using liquid medium (pH 2) containing 1 % (w/v) elemental sulfur, and growth was confirmed by monitoring culture pH (oxidation of elemental sulfur generates sulfuric acid), enumerating cells and streaking cultures identified as positive on ferrous iron overlay medium (Johnson & Hallberg, 2007) to confirm cell viability. The data obtained (Table 2) show that, although there was some variation between isolates, all 11 strains were in general highly tolerant of the cationic transition metals tested, but highly sensitive to the molybdate anion. In this respect, they were more similar to A. ferrooxidans and A. ferridurans than to the more closely related species A. ferrivorans, strains of which were reported to be inhibited by < 50 mM copper and < 100 mM ferric iron (Hallberg et al., 2010). All strains of A. ferriphilus were found to be particularly tolerant of ferrous iron (far more so than to ferric iron); some grew in the presence of 1 M Fe2+, which is greater than values for other iron-oxidizing acidithiobacilli (Hallberg et al., 2010; Hedrich & Johnson, 2013a). Strain variability within this novel species was again illustrated in the case of isolate KCT17, which was far more sensitive to ferric iron than the ten other strains examined (Table 2).
Table 2. Comparison of tolerance of strains of A. ferriphilus to elevated concentrations (mmol l− 1) of selected transition metals, magnesium and NaCl.
Numbers indicate MICs and (in parentheses) the maximum concentrations at which growth was observed.
| Strain | Fe(II) | Fe(III) | Co | Cu | Mo | Ni | Zn | Mg | NaCl* |
|---|---|---|---|---|---|---|---|---|---|
| M20T | 1000 (900) | 500 (300) | 600 (400) | 500 (300) | < 0.1 | 500 (300) | 800 (700) | 1000 (900) | 500 (250) |
| JCM 7812 | 700 (500) | 500 (300) | 600 (400) | 300 (100) | < 0.1 | 500 (300) | 800 (700) | 900 (800) | 500 (250) |
| Malay | 1200 (1000) | 500 (300) | 600 (400) | 300 (100) | < 0.1 | 500 (300) | 800 (700) | 1200 (1000) | 500 (250) |
| Riv13 | 1200 (1000) | 500 (300) | 600 (400) | 300 (100) | < 0.1 | 500 (300) | 700 (600) | 1200 (1000) | 500 (250) |
| ST2 | 1200 (1000) | 500 (300) | 600 (400) | 500 (300) | < 0.1 | 500 (300) | 800 (700) | 1200 (1000) | 1000 (800) |
| PS102 | 900 (700) | 300 (100) | 600 (400) | 500 (300) | < 0.1 | 500 (300) | 800 (700) | 900 (800) | 500 (250) |
| PS104 | 1200 (1000) | 500 (300) | 800 (600) | 500 (300) | < 0.1 | 500 (300) | 800 (700) | 1200 (1000) | 500 (250) |
| PS107 | 1000 (900) | 500 (300) | 400 (200) | 300 (100) | < 0.1 | 300 (100) | 800 (700) | 1200 (1000) | 500 (250) |
| KCT10 | 1000 (900) | 300 (100) | 600 (400) | 500 (300) | < 0.1 | 500 (300) | 800 (700) | 1000 (900) | 700 (500) |
| KCT14 | 1200 (1000) | 500 (300) | 600 (400) | 500 (300) | < 0.1 | 500 (300) | 800 (700) | 1200 (1000) | 500 (250) |
| KCT17 | 700 (500) | 100 (50) | 600 (400) | 500 (300) | < 0.1 | 300 (100) | 600 (400) | 1200 (1000) | 500 (250) |
Grown on elemental sulfur; all other data refer to cultures grown on ferrous iron.
Comparison with data from magnesium sulfate-amended cultures shows that, in many cases, tolerance of ferrous iron was limited by osmotic stress rather than by ferrous iron per se, with growth being observed and inhibited by the presence of similar concentrations of both magnesium sulfate and ferrous sulfate (Table 2). All 11 strains were able to grow in sulfur medium containing 250 mM NaCl, and two (ST2 and KCT10) in the presence of 500 mM salt, a similar concentration of chloride to that of seawater. The most salt-tolerant strain (ST2) grew in the presence of 800 mM (but not 1 M) NaCl in sulfur medium. However, neither strain ST2 nor strain KCT10 grew in ferrous iron liquid medium containing 500 mM salt, even though strain ST2 had originally been isolated from the Rio Tinto on a ferrous iron overlay plate (Johnson & Hallberg, 2007) containing 500 mM NaCl (D. B. Johnson, unpublished data). Even so, the tolerance of these two strains of A. ferriphilus to chloride greatly exceeded values reported for other species of iron-oxidizing acidithiobacilli.
Biomass of strain M20T was obtained by growing 10-litre batch cultures in 100 mM ferrous sulfate medium at 30 °C in a bioreactor vessel (Electrolab) that was stirred and aerated at ∼1.5 l min− 1. The initial pH of the batch cultures was ∼1.45, and this increased to ∼1.75 by the time that all of the iron had been oxidized (100 mM magnesium sulfate was added to the medium to provide increased buffering from the bisulfate/sulfate couple). Cells were harvested, and pellets from several batch cultures were combined and sent to the DSMZ (Deutsche Sammlung von Mikrooganismen und Zellkulturen, Braunschweig, Germany) for analysis of fatty acids, polar lipids, respiratory quinones and chromosomal base composition.
The major fatty acids found in strain M20T grown on ferrous iron were C18 : 1ω7c, C18 : 1 2-OH, C16 : 0 and C12 : 0. With the exception of C18 : 1 2-OH, the fatty acids found and their relative abundances were similar to those reported for the (iron-grown) type strain of A. ferrooxidans and (hydrogen-grown) type strain of A. ferridurans (Table 3; no published data are available for A. ferrivorans). The major polar lipids of strain M20T were aminolipid, phospholipid and phosphatidylglycerol, and the major quinone present (94 %) was Q8 (as also reported for A. ferridurans; Hedrich & Johnson, 2013a) with smaller amounts of Q9 (3 %) and Q7 (2 %). The mean base composition of the chromosomal DNA of strain M20T was 57.4 mol% G+C; values reported for the type strains of other iron-oxidizing acidithiobacilli are 58–59 mol% for A. ferrooxidans (Kelly & Wood, 2000), 58 ± 0.02 mol% for A. ferridurans (Hedrich & Johnson, 2013a) and 55–56 mol% for A. ferrivorans (Hallberg et al., 2010).
Table 3. Cellular fatty acids (%) in A. ferriphilus strain M20T grown on ferrous iron at pH 1.45–1.75 and 30 °C, and comparison with values reported for the type strains of A. ferrooxidans and A. ferridurans .
Strains: 1, M20T; 2, A. ferrooxidans ATCC 23270T (grown on ferrous iron at pH 1.5 and 25 °C; Mykytczuk et al., 2010); 3, A. ferridurans ATCC 33020T (grown on hydrogen at pH 2 and 30 °C; Hedrich & Johnson, 2013a). No published data are available for A. ferrivorans. –, Absent.
| Fatty acid | 1 | 2* | 3 |
|---|---|---|---|
| C12 : 0 | 5.7 | 8 | 6.6 |
| C13 : 1 AT 12–13 | 0.4 | − | − |
| C14 : 0 | 0.2 | 11 | 0.3 |
| C15 : 0 | – | – | 0.7 |
| C15 : 0 3-OH | – | – | 0.5 |
| C16 : 0 | 7.5 | 18a | 15.6 |
| C16 : 0 2-OH | 0.5 | – | 1.2 |
| C16 : 0 3-OH | 2.7 | – | 0.9 |
| C16 : 1 | – | 21b | – |
| C16 : 1ω5c | 0.4 | – | – |
| C17 : 0 | 0.5 | 6c | 1.9 |
| C17 : 0 cyclo | – | – | 6.7 |
| C17 : 0 2-OH | 0.1 | – | – |
| C17 : 1ω6c | 0.4 | – | – |
| C17 : 1ω8c | 0.6 | 0.5d | 0.7 |
| C18 : 0 | 0.9 | 0.5e | 1.5 |
| C18 : 0 2-OH | 0.5 | – | – |
| C18 : 0 3-OH | 0.1 | – | – |
| C18 : 1ω5c | – | – | 0.6 |
| C18 : 1ω7c | 33.8 | 21.5f | 16.6 |
| C18 : 1 2-OH | 10.3 | – | 0.9 |
| 11-methyl C18 : 1ω7c | – | – | 0.3 |
| 10-methyl C19 : 0 1.0 | |||
| C19 : 0 cyclo ω8c | – | 14.5 | 17.5 |
| C20 : 2ω6,9c | – | – | 0.4 |
| Summed feature 1† | – | – | 0.3 |
| Summed feature 2† | 10.14 | – | 9.9 |
| Summed feature 3† | 21.57 | – | 14.9 |
Values represent: a, C16 : 0, C16 : 0 2-OH, C16 : 0 3-OH; b, C16 : 1ω6c, C16 : 1 2-OH, C16 : 1ω5c; c, C17 : 0, C17 : 0 cyclo, C17 : 0 2-OH; d, C17 : 1ω8c, C17 : 1ω6c, anteiso-C17 : 1; e, C18 : 0, C18 : 0 2-OH, C18 : 0 3-OH; f, C18 : 1ω7c, C18 : 1 2-OH.
Summed features represent groups of two or three fatty acids that could not be separated by GLC with the MIDI system. Summed feature 1 contains iso-C15 : 1 and/or iso-C13 : 0 3-OH.; summed feature 2 contains C14 : 0 3-OH and/or iso-C16 : 1; summed feature 3 contains C16 : 1ω7c, C16 : 1ω6c and/or iso-C15 : 0 2-OH.
In summary, the 11 strains of iron- and sulfur-oxidizing chemolithotrophic acidophiles described herein, together with four other strains included as ‘Group IV’ acidithiobacilli by Amouric et al. (2011), are representatives of a novel species, A. ferriphilus sp. nov. Although more closely related (from multi-locus sequence analysis) to A. ferrivorans than to either A. ferrooxidans or A. ferridurans, strains of A. ferriphilus share some traits with the former, and others with the latter two species. Some physiological characteristics suggest that some strains of A. ferriphilus could play a significant role in commercial mineral bio-processing operations, such as low temperature bioleaching of polysulfide ores in brackish waters, where they would, in theory, be superior to other species due to the unique combination of transition metal tolerance, salt tolerance and psychro-tolerance.
Description of Acidithiobacillus ferriphilus sp. nov.
Acidithiobacillus ferriphilus [fer.ri′phi.lus. L. n. ferrum iron; N.L. adj. philus -a -um (from Gr. adj. philos -ê -on) friend, loving; N.L. masc. adj. ferriphilus iron-loving, referring to its ability to grow in the presence of elevated concentrations of ferrous iron].
Gram-stain-negative, motile, straight rods (1–2 μm long) that do not form endospores. Forms small, ferric-iron-stained colonies on acidic ferrous iron overlay media. Obligate chemolithoautotroph, capable of growth using ferrous iron or reduced sulfur (elemental sulfur or tetrathionate) as electron donors. Facultative anaerobe, capable of coupling the oxidation of ferrous iron and reduced sulfur to the reduction of molecular oxygen, and the oxidation of reduced sulfur to the reduction of ferric iron. Mesophilic and extremely acidophilic, although some strains are psychrotolerant (and grow at 5 °C). The type strain has pH and temperature growth optima of pH 2.0 and 30 °C.
The type strain, M20T ( = DSM 100412T = JCM 30830T) was isolated from an acidic pool in a geothermal area of Montserrat (West Indies). The G+C content of the chromosomal DNA of the type strain is 57.4 mol%. Other strains of A. ferriphilus have been isolated from acidic iron-rich waters at metal mine sites.
Acknowledgements
This work was supported in part by the Natural Environment Research Council, UK (Grant ref. NE/L014076/1).
Supplementary Data
Supplementary Data
References
- Amouric A., Brochier-Armanet C., Johnson D. B., Bonnefoy V., Hallberg K. B. (2011). Phylogenetic and genetic variation among Fe(II)-oxidizing acidithiobacilli supports the view that these comprise multiple species with different ferrous iron oxidation pathways Microbiology 157 111–122 10.1099/mic.0.044537-0 . [DOI] [PubMed] [Google Scholar]
- Atkinson T., Cairns S., Cowan D. A., Danson M. J., Hough D. W., Johnson D. B., Norris P. R., Raven N., Robinson C., other authors (2000). A microbiological survey of Montserrat Island hydrothermal biotopes Extremophiles 4 305–313 10.1007/s007920070018 . [DOI] [PubMed] [Google Scholar]
- Blowes D. W., Ptacek C. J., Jambor J. L., Weisener C. G., Paktunc D., Gould W. D., Johnson D. B. (2014). In Treatise on Geochemistry, 11, 2nd edn pp. 131–190. Edited by Holland H. D., Turekian K. K. Oxford: 10.1016/B978-0-08-095975-7.00905-0 Elsevier. [DOI] [Google Scholar]
- Brierley C. L., Brierley J. A. (2013). Progress in bioleaching: part B: applications of microbial processes by the minerals industries Appl Microbiol Biotechnol 97 7543–7552 10.1007/s00253-013-5095-3 . [DOI] [PubMed] [Google Scholar]
- Hallberg K. B., Thomson H. E. C., Boeselt I., Johnson D. B. (2001). Aerobic and anaerobic sulfur metabolism by acidophilic bacteria. In Process Metallurgy 11A: Biohydrometallurgy: Fundamentals, Technology and Sustainable Development, pp. 423–431. Edited by Ciminelli V. S. T., Garcia O., Jr Amsterdam: Elsevier. [Google Scholar]
- Hallberg K. B., González-Toril E., Johnson D. B. (2010). Acidithiobacillus ferrivorans, sp. nov.; facultatively anaerobic, psychrotolerant iron-, and sulfur-oxidizing acidophiles isolated from metal mine-impacted environments Extremophiles 14 9–19 10.1007/s00792-009-0282-y . [DOI] [PubMed] [Google Scholar]
- Hedrich S., Johnson D. B. (2013a). Acidithiobacillus ferridurans sp. nov., an acidophilic iron-, sulfur- and hydrogen-metabolizing chemolithotrophic gammaproteobacterium Int J Syst Evol Microbiol 63 4018–4025 10.1099/ijs.0.049759-0 . [DOI] [PubMed] [Google Scholar]
- Hedrich S., Johnson D. B. (2013b). Aerobic and anaerobic oxidation of hydrogen by acidophilic bacteria FEMS Microbiol Lett 349 40–45 . [DOI] [PubMed] [Google Scholar]
- Johnson D. B., Hallberg K. B. (2007). Techniques for detecting and identifying acidophilic mineral-oxidizing microorganisms. In Biomining, pp. 237–261. Edited by Rawlings D. E., Johnson D. B. Berlin: 10.1007/978-3-540-34911-2_12 Springer. [DOI] [Google Scholar]
- Kay C. M., Haanela A., Johnson D. B. (2014). Microorganisms in subterranean acidic waters within Europe's deepest metal mine Res Microbiol 165 705–712 10.1016/j.resmic.2014.07.007 . [DOI] [PubMed] [Google Scholar]
- Kelly D. P., Wood A. P. (2000). Reclassification of some species of Thiobacillus to the newly designated genera Acidithiobacillus gen. nov., Halothiobacillus gen. nov. and Thermithiobacillus gen. nov Int J Syst Evol Microbiol 50 511–516 10.1099/00207713-50-2-511 . [DOI] [PubMed] [Google Scholar]
- Liljeqvist M., Valdes J., Holmes D. S., Dopson M. (2011). Draft genome of the psychrotolerant acidophile Acidithiobacillus ferrivorans SS3 J Bacteriol 193 4304–4305 10.1128/JB.05373-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mykytczuk N. C. S., Trevors J. T., Ferroni G. D., Leduc L. G. (2010). Cytoplasmic membrane fluidity and fatty acid composition of Acidithiobacillus ferrooxidans in response to pH stress Extremophiles 14 427–441 10.1007/s00792-010-0319-2 . [DOI] [PubMed] [Google Scholar]
- Ohmura N., Sasaki K., Matsumoto N., Saiki H. (2002). Anaerobic respiration using Fe3+, S0, and H2 in the chemolithoautotrophic bacterium Acidithiobacillus ferrooxidans J Bacteriol 184 2081–2087 10.1128/JB.184.8.2081-2087.2002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple K. L., Colmer A. R. (1951). The autotrophic oxidation of iron by a new bacterium, Thiobacillus ferrooxidans J Bacteriol 62 605–611 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakao N., Hanada K., Takahashi A., Sakurai Y., Shiota H. (1991). Morphological, physiological, and chemotaxonomical characteristics of iron- and sulfur-oxidizing bacteria isolated from acid mine drainage waters J Gen Appl Microbiol 37 35–48 10.2323/jgam.37.35. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data