Abstract
Background and Objective
Acute and early human immunodeficiency virus infection (AEH) is accompanied by neuroinflammatory processes as well as impairment in neurocognitive and everyday functions, but little is known about the frequency and clinical correlates of the neurobehavioral disturbances during this period. We compared pre-seroconversion with current levels of apathy, disinhibition, and executive dysfunction; we also examined everyday function and HIV disease correlates of neuropsychiatric impairment in individuals with AEH.
Methods
In this study, 34 individuals with AEH and 39 HIV-seronegative participants completed neuromedical and neuropsychological assessments, a structured psychiatric interview, and the apathy, disinhibition, and executive dysfunction subscales of the Frontal Systems Behavioral Scale.
Results
Independent of any substance use and mood disorders, the AEH group had significantly higher levels of current apathy and executive dysfunction than the controls, but not greater disinhibition. Retrospective ratings of pre-seroconversion levels of apathy, disinhibition, and executive dysfunction were all higher in the AEH group than the controls. After seroconversion, the AEH cohort had increases in current apathy and executive dysfunction, but not disinhibition. In the AEH cohort, higher current global neurobehavioral dysfunction was significantly associated with lower nadir CD4 counts, slowed information processing speed, and more everyday function problems.
Conclusions
These data suggest that individuals who have recently acquired HIV experienced higher-than-normal premorbid levels of neurobehavioral disturbance. Apathy and executive dysfunction are exacerbated during AEH, particularly in association with lower CD4 counts.
Keywords: HIV/AIDS, neuropsychiatry, motivation, neuropsychology
Immune and virologic insults that begin during acute and early human immunodeficiency virus (HIV) infection (AEH) may be related to the development of HIV-associated neurocognitive disorders (HAND). This early association is important because 30% to 50% of chronically HIV-infected individuals have HAND (Schacker et al, 1998).
“Acute HIV infection” is commonly described as the 3- to 4-week period between HIV acquisition and development of detectable antibodies. “Early infection,” the first year after seroconversion, is characterized by rapid viral replication, elevated immune response, and viral diversification (Cohen et al, 2011; Miller et al, 2010). During periods of transient high replication, HIV is entering the central nervous system (CNS) (An et al, 1999; Davis et al, 1992), causing neurovirologic complications in 40% to 90% of infected individuals (eg, Kerndt et al, 2009; Peluso et al, 2013; Schacker et al, 1996).
Several studies have demonstrated brain changes in persons with AEH. Neuroimaging studies have shown alterations in brain structure and function, such as reduced resting cerebral blood flow and elevated levels of myo-inositol and glutamate in the basal ganglia (Ances et al, 2009; Lentz et al, 2009; Young et al, 2014). Further, Lentz and colleagues (2009) found altered levels of N-acetylaspartate, glutamate-glutamine, and choline in the frontal cortex. Using functional magnetic resonance imaging, Wang et al (2011) found reduced functional connectivity in the lateral occipital resting state network. Young and colleagues’ (2014) neuroimaging study of persons with AEH suggested that inflammation and gliosis can be reduced with antiretroviral therapies.
There is a growing awareness that neuroimaging abnormalities in AEH are accompanied by neurocognitive impairment. About 35% to 60% of persons with AEH show neurocognitive difficulties, most of them mild. These impairments fall midway between those of HIV-seronegative and chronically infected populations (eg, Doyle et al, 2013; Moore et al, 2011; Wang et al, 2011; Weber et al, 2013).
Neurocognitive deficits in AEH have been found in the domains of working memory, information processing speed, and learning (Doyle et al, 2013; Moore et al, 2011; Wang et al, 2011). These impairments have clinical implications, as they are a primary risk factor for dependence in several aspects of daily function, including the ability to hold a job (Doyle et al, 2013). Thus, a convergence of recent studies suggests that AEH causes structural and functional CNS changes that have important implications for real-world and health-related outcomes.
The neurobehavioral disturbances that persons display during AEH provide insight into how HIV is affecting the CNS. Here we define neurobehavioral disturbances as a constellation of symptoms that are reliably associated with brain injury, and neuropsychiatric syndromes involving dysregulation of frontostriatal systems (Grace and Malloy, 2001). Neurobehavioral disturbances are separable from neurocognitive impairment, but are nevertheless important determinants of an individual’s real-world function (eg, Gonzalez et al, 2005; Kamat et al, 2012).
Many persons with chronic HIV disease have neural injury of the frontostriatal circuits (eg, Chang et al, 2001; Jernigan et al, 2005), and growing evidence suggests that frontally mediated neurobehavioral disturbances such as apathy, disinhibition, and executive dysfunction are prevalent and clinically impactful in this population (Kamat et al, 2012; Marquine et al, 2014). For example, apathy affects approximately 40% of chronically infected persons and is independently associated with a decline in independence in activities of daily living (Kamat et al, 2012), with medication nonadherence (Barclay et al, 2007; Rabkin et al, 2000), and with lower health-related quality of life (Tate et al, 2003). Indeed, rates of neurobehavioral disturbances such as disinhibition may be elevated before seroconversion and may encourage risk behaviors that can further spread HIV (Atkinson et al, 2009; Vo et al, 2013).
Thus, it is important to understand the prevalence, correlates, and trajectory of neurobehavioral disturbances in the early phases of HIV infection. By studying persons with AEH, we can learn about the extent to which pre-infection neurobehavioral factors evolve during the first year of infection.
METHODS
Participants
We studied 34 people with AEH and 39 HIV-seronegative controls (previously described in Doyle et al, 2013). All participants came from our National Institute on Drug Abuse-funded cohort study investigating the effects of HIV infection and methamphetamine use disorders on CNS structure and function (Doyle et al, 2013). We determined HIV infection using established methods detailed by Le and colleagues (2013). We used one of four methods to determine AEH:
Positive HIV-1 RNA (COBAS® Amplicor, Roche Molecular Diagnostics, Pleasanton, California); negative HIV enzyme immunoassay (EIA) or rapid test
Positive HIV RNA; indeterminate Western blot with no more than three positive bands
Positive HIV RNA; positive EIA or rapid test; less sensitive (detuned) EIA consistent with very recent infection (optical density < 0.3 by Vironostika® [bioMérieux, Durham, North Carolina] or equivalent by VITROS® ECi [Ortho Clinical Diagnostics, Raritan, New Jersey] or OraQuick® detuned [OraSure Technologies, Bethlehem, Pennsylvania])
Last known negative EIA followed within 182 days by a known positive EIA and a neurocognitive assessment
To determine the HIV-positive participants’ duration of infection, when they entered the study we used an algorithm that evaluated their HIV-1 serologic tests, detuned enzyme immunoassay tests, and HIV RNA levels (Le et al, 2013).
Table 1 includes the AEH group’s HIV disease characteristics, obtained by certified research staff using a standardized neuromedical evaluation.
TABLE 1.
Demographic, Neurocognitive, Psychiatric, and HIV Disease Characteristics of the Study Participants
| Acute and Early HIV Group (n = 34) | HIV-Negative Group (n = 39) | P | |
|---|---|---|---|
| Demographics | |||
| Age (years; mean [SD]) | 32.7 (10.0) | 35.5 (12.7) | 0.557 |
| Education (years; mean [SD]) | 13.5 (2.1) | 14.2 (1.7) | 0.162 |
| Estimated verbal IQ (WRAT-IV1 reading; mean [SD]) | 103.8 (14.4) | 102.4 (10.6) | 0.324 |
| Sex (% men) | 97.1 | 74.4 | 0.007 |
| Ethnicity (% white) | 55.9 | 59.0 | 0.790 |
| Neuropsychological and functional variables | |||
| Neurocognitively normal (%) | 62.5 | 79 | NA |
| Subsyndromic neurocognitive impairment† (%) | 34.4 | 21 | NA |
| Syndromic neurocognitive impairment‡ (%) | 3.1 | 0 | NA |
| Total real-world functional impairment* | 1.67 (0.15) | 0.53 (0.60) | < 0.001 |
| Psychiatric disorders (lifetime) | |||
| Any mood disorder (%) | 61.8 | 15.4 | < 0.001 |
| Major depressive disorder (%) | 47.1 | 12.8 | 0.001 |
| Bipolar disorder (%) | 17.6 | 2.6 | 0.029 |
| Substance use disorders | |||
| Current dependence | |||
| Alcohol (%) | 0 | 0 | NA |
| Non-alcohol (%) | |||
| Cannabis (%) | 2.94 | 0 | 0.281 |
| Cocaine (%) | 0 | 0 | NA |
| Methamphetamine (%) | 2.94 | 0 | 0.281 |
| Opiates (%) | 0 | 0 | NA |
| Current abuse | |||
| Alcohol (%) | 2.94 | 2.56 | 0.922 |
| Non-alcohol (%) | 5.88 | 0 | 0.125 |
| Cannabis (%) | 5.88 | 0 | 0.125 |
| Cocaine (%) | 0 | 0 | NA |
| Methamphetamine (%) | 0 | 0 | NA |
| Opiates (%) | 0 | 0 | NA |
| Lifetime dependence | |||
| Alcohol (%) | 35.29 | 5.13 | < 0.01 |
| Non-alcohol (%) | 50.0 | 5.13 | < 0.001 |
| Cannabis (%) | 23.53 | 5.13 | 0.022 |
| Cocaine (%) | 8.82 | 0 | 0.060 |
| Methamphetamine (%) | 50 | 0 | < 0.0001 |
| Opiates (%) | 2.94 | 0 | 0.281 |
| Lifetime abuse | |||
| Alcohol (%) | 20.59 | 25.64 | 0.610 |
| Non-alcohol (%) | |||
| Cannabis (%) | 17.65 | 17.95 | 0.973 |
| Cocaine (%) | 14.71 | 0 | 0.0131 |
| Methamphetamine (%) | 0 | 0 | NA |
| Opiates (%) | 5.88 | 2.56 | 0.476 |
| HIV disease characteristics | |||
| Estimated duration of infection (months)* | 2.1 (1.0 to 4.5) | NA | NA |
| Nadir CD4 T-cell count (cells/μL)* | 510 (367 to 710) | NA | NA |
| Current CD4 T-cell count (cells/μL)* | 717 (489 to 868) | NA | NA |
| Plasma HIV RNA (log10 copies/mL)* | 3.9 (2.6 to 4.8) | NA | NA |
| Antiretroviral therapy prescribed (%) | 32.4 | NA | NA |
Data shown as median (interquartile range).
Corresponds to asymptomatic neurocognitive impairment in HIV-positive participants.
Corresponds to mild neurocognitive impairment in HIV-positive participants.
HIV = human immunodeficiency virus. IQ = intelligence quotient. WRAT-IV = Wide Range Achievement Test–Fourth Edition. NA = not applicable.
Exclusion criteria for both groups were a history of a severe psychiatric condition (eg, schizophrenia) or neurologic illness (eg, seizure disorder, active opportunistic CNS infection) unrelated to HIV infection or methamphetamine use, inability to provide informed consent, and an estimated verbal intelligence quotient < 80 based on the Wide Range Achievement Test – Fourth Edition (Wilkinson and Robertson, 2006).
Table 1 also lists the demographic, neurocognitive, and psychiatric characteristics of both study groups. The AEH group had a greater proportion of men than the control group, as well as significantly higher lifetime rates of mood disorders and lifetime alcohol and other substance dependence (all P values < 0.05).
Our study procedures were approved by the Human Research Protections Program at the University of California, San Diego. All participants gave written informed consent.
Procedures
All participants completed the Frontal Systems Behavior Scale (FrSBe) (Grace and Malloy, 2001), along with comprehensive neuropsychological, psychiatric, and functional assessments. For the AEH group we also recorded CD4 cell counts and HIV status. Typically, the participants completed all the assessments during one session lasting up to 7 hours; the participants received as many breaks as needed, including one for lunch. We conducted the assessments at the HIV Neurobehavioral Research Program at the University of California, San Diego.
Frontal Systems Behavior Scale
The FrSBe is 46-item paper-and-pencil questionnaire in which participants rate how often they experience specific neurobehavioral symptoms of apathy (eg, neglecting personal hygiene), disinhibition (eg, engaging in risky behavior), and executive dysfunction (inability to plan ahead). The HIV-positive participants rated the frequency of each neurobehavioral symptom at two time points: before their HIV infection (ie, retrospectively) and at the time of evaluation (ie, currently). The HIV-negative participants also provided both retrospective (before age 20) and current ratings for each symptom.
Participants rated each question on a Likert-type scale that ranged from 1 (“almost never”) to 5 (“almost always”), with higher ratings indicating more abnormal behavior. The FrSBe yields a total score as well as the three subscale scores for apathy, disinhibition, and executive dysfunction. We used age-, education-, and sex-adjusted T-scores based on the FrSBe manual’s normative sample, in which a cut point of T ≥ 65 indicates a clinical neurobehavioral disturbance.
Neurocognitive Assessment
Participants were administered a comprehensive neurocognitive test battery by trained study staff; the battery was designed to assess the domains of attention and working memory, executive functions, learning, memory, motor skills, information processing speed, and verbal fluency (for details, see Heaton et al, 2010). We used domain T-scores for correlational analyses; we constructed them by converting raw scores from individual tests into demographically adjusted T-scores using published normative standards and then averaging the T-scores by domain (Heaton et al, 2004; Norman et al, 2011; Woods et al, 2004).
To classify the participants by presence and severity of neurocognitive impairment, we applied a published objective algorithm that has excellent inter-rater reliability (Woods et al, 2004). This algorithm conforms to the Frascati criteria for diagnosing HAND, which requires mild impairment in at least two of the seven domains (Antinori et al, 2007). We classified participants as being neurocognitively normal or having asymptomatic neurocognitive impairment or a mild neurocognitive disorder.
Psychiatric Assessment
Trained study staff gave the participants the World Health Organization World Mental Health Composite International Diagnostic Interview Version 2.1 (Wittchen, 1994). This computer-assisted interview provides a cross-culturally relevant assessment of psychiatric disorders using criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (Wittchen, 1994). We used the Interview to elicit current and lifetime mood and substance (including alcohol) use disorders.
Real-World Function Assessment
In this study we used a composite variable that we had created for our previous studies (Blackstone et al, in press; Doyle et al, 2013), to represent impairment in real-world function. The assessment covered five functional domains: cognitive symptoms in daily life, basic activities of daily living, instrumental activities of daily living, employment (all measured using paper-and-pencil questionnaires), and clinician-rated global function. Within each of these domains, we divided participants into “impaired” (score = 1) or “normal” (score = 0) groups according to established procedures. Total scores ranged from 0 to 5, with higher scores indicating more severe functional impairment. For details, see Doyle et al (2013).
Statistical Design
To investigate pre-infection neurobehavioral disturbances, we conducted three multiple linear regression analyses to study the association between AEH and pre-infection levels of apathy, disinhibition, and executive dysfunction. Because sex and lifetime history of mood and substance use disorders differed between our AEH and control groups, we entered these variables as covariates in each of the three models. Next, we performed paired t tests to examine changes in neurobehavioral disturbances following HIV infection. To explore the effect of AEH on neurobehavioral disturbances, we performed separate multiple linear regression analyses, with key demographic and psychiatric variables included as covariates. Finally, we examined the everyday function, neuropsychiatric, and clinical correlates of neurobehavioral disturbance in AEH using Spearman’s rank correlations and t tests. We set the significance threshold at P = 0.05 for all analyses.
RESULTS
Pre-Infection Neurobehavioral Disturbances
The overall model assessing the association between AEH and apathy was significant (adjusted R2 = 0.14, P < 0.01), showing that the AEH group (β = −0.43, P < 0.01) had higher pre-infection apathy ratings than the controls’ retrospective ratings. Although AEH had a simple univariate effect on disinhibition (Cohen d = 0.63, P < 0.01), the multivariable regression including sex and psychiatric factors was not significant (P > 0.10). Finally, the overall model for executive dysfunction was significant (adjusted R2 = 0.12, P = 0.01), revealing that the AEH group had higher pre-infection executive dysfunction ratings than the controls’ retrospective ratings (β = −0.33, P = 0.01).
Sex, mood, and substance use disorders did not emerge as significant factors in any of these pre-infection regressions.
Neurobehavioral Changes After Seroconversion
Within the AEH group, we conducted paired t tests using the participants’ “before” and “after” seroconversion ratings of apathy, disinhibition, and executive dysfunction to examine changes in their neurobehavioral disturbances following HIV infection. For the continuous neurobehavioral scores, self-reported apathy (mean change = 7.55, standard error = 2.49; Cohen d = 0.52) and executive dysfunction (mean change = 4.41, standard error = 2.10; Cohen d = 0.36) both increased after HIV infection (all P values < 0.05). Perceived levels of disinhibition did not change significantly after infection (mean change = 2.06, standard error = 1.47, P = 0.17; Cohen d = 0.24).
The proportions of participants with AEH who experienced incidental clinical elevations in neurobehavioral symptoms following seroconversion (ie, pre-infection FrSBe T-scores < 65 but current ratings ≥ 65) were 28% with apathy, 13% with disinhibition, and 21% with executive dysfunction.
Neurobehavioral Disturbances During AEH
We conducted separate multiple linear regression analyses to investigate the effect of AEH on current apathy, disinhibition, and executive dysfunction, while controlling for sex and lifetime histories of mood and substance use disorders. Table 2 shows that across all three domains, the overall regressions were significant (all P values < 0.001), with AEH emerging as the sole significant independent predictor of higher levels of neurobehavioral disturbances (all P values ≤ 0.01).
TABLE 2.
Multiple Linear Regression Analysis of Predictors of Current Ratings by 73 Study Participants for Apathy, Disinhibition, and Executive Dysfunction
| Model | B | B 95% Confidence Interval | β | P | |
|---|---|---|---|---|---|
| Apathy | |||||
| Adjusted R2 | 0.26 | ||||
| F | 7.40 | < 0.001 | |||
| AEH | − 8.67 | − 12.97 to − 4.37 | − 0.48 | < 0.001 | |
| Sex | 0.85 | − 4.65 to 6.35 | 0.03 | 0.41 | |
| Mood disorder | − 1.76 | − 6.06 to 2.54 | − 0.29 | 0.41 | |
| Substance use disorder | − 1.67 | − 5.74 to 2.40 | − 0.09 | 0.42 | |
| Disinhibition | |||||
| Adjusted R2 | 0.22 | ||||
| F | 6.02 | < 0.001 | |||
| AEH | − 4.71 | − 8.32 to − 1.10 | − 0.32 | 0.01 | |
| Sex | 0.77 | − 3.83 to 5.38 | − 0.17 | 0.73 | |
| Mood disorder | 2.75 | − 6.36 to 0.85 | − 0.17 | 0.13 | |
| Substance use disorder | − 3.06 | − 6.48 to 0.35 | − 0.20 | 0.08 | |
| Executive dysfunction | |||||
| Adjusted R2 | 0.28 | ||||
| F | 7.14 | < 0.001 | |||
| AEH | − 8.10 | − 12.34 to − 3.81 | − 0.45 | < 0.001 | |
| Sex | 0.58 | − 4.91 to 6.07 | 0.02 | 0.83 | |
| Mood disorder | − 0.34 | − 4.6 to 3.94 | − 0.01 | 0.86 | |
| Substance use disorder | −3.23 | −7.30 to 0.83 | − 0.18 | 0.12 | |
AEH = acute and early human immunodeficiency virus. B = unstandardized beta coefficient. β = standardized beta coefficient.
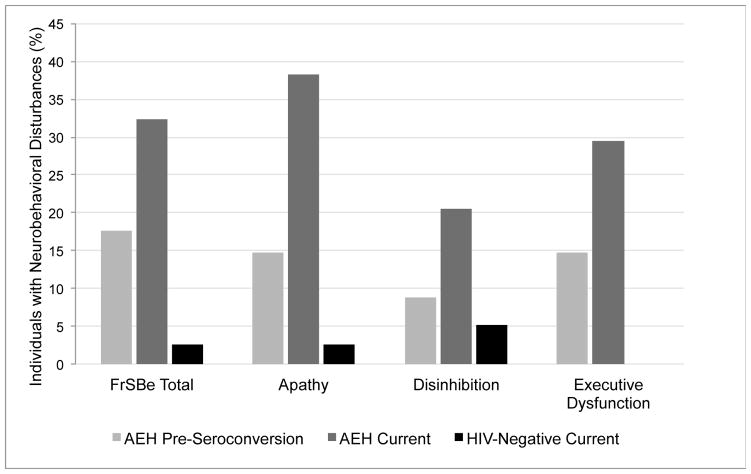
Figure 1 shows the percentages of participants in each study group whom we classified as having clinical neurobehavioral symptoms (FrSBe T score ≥ 65) based on their overall level of self-reported current symptoms as well as their self-reported levels of current apathy, disinhibition, and executive dysfunction. In the AEH group, 32% of participants reported current symptoms, specifically 38% apathy, 21% disinhibition, and 29% executive dysfunction. These percentages were significantly higher than those in the seronegative group, in which only 3% reported clinically elevated levels of apathy (χ2 = 14.52, P < 0.001; odds ratio = 22.90, 95% confidence interval = 2.79 to 187.65), 5% disinhibition (χ2 = 3.53, P = 0.04; odds ratio = 4.66, 95% confidence interval = 0.89 to 24.26), and none executive dysfunction (χ2 = 12.9, P < 0.001; odds ratio = 33.01, 95% confidence interval = 4.96 to −1.14).
FIGURE 1.
Percentage of participants in the acute and early HIV infection (AEH) group (n = 34) and HIV-seronegative control group (n = 39) with clinical global neurobehavioral disturbance, apathy, disinhibition, or executive dysfunction on the Frontal Systems Behavior Scale (FrSBe) (Grace and Malloy, 2001). The lighter gray bars show the AEH group’s retrospective self-reports of their symptoms before they seroconverted; the darker gray bars show their self-reported symptoms at the time of evaluation. The black bars show the control group’s self-reported symptoms at the time of evaluation. The control group reported no executive dysfunction.
Clinical and Neuropsychiatric Correlates of Neurobehavioral Disturbances in AEH
Our next analyses investigated the HIV disease and neuropsychiatric correlates of neurobehavioral disturbances in the AEH group. For these analyses, we used the total current FrSBe T-score in an effort to reduce type I error (the pattern of correlations did not differ systematically across individual subscales). In the AEH group, higher ratings of neurobehavioral disturbances correlated strongly with more severe impairment in everyday function (Spearman rho = 0.72, P < 0.001) and lower nadir CD4 cell counts (Spearman rho = −0.42, P = 0.01).
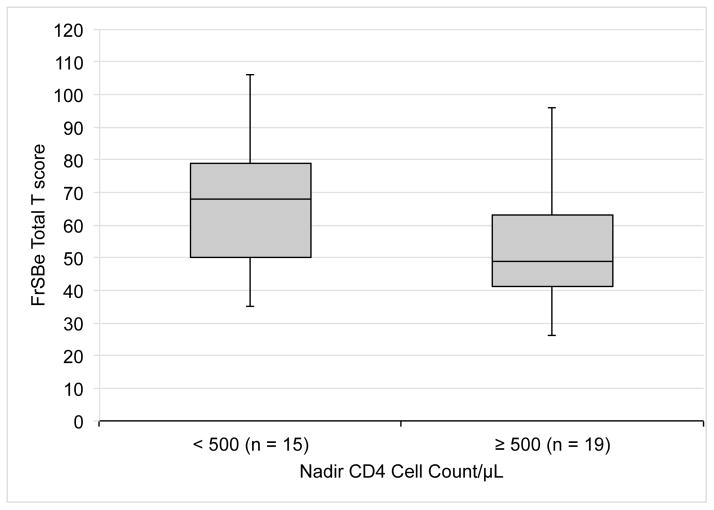
To characterize further the relationship between nadir CD4 cell count and neurobehavioral disturbance, we split our HIV-positive participants into two groups using the standard cut point of 500/μL. As shown in Figure 2, the 15 individuals with nadir CD4 cell counts < 500/μL reported significantly more neurobehavioral disturbances than did the 19 whose counts were ≥ 500/μL (Cohen d = 0.69, P = 0.02). FrSBe ratings did not show a relationship with current CD4 cell count or plasma viral load.
FIGURE 2.
Levels of neurobehavioral disturbance, determined by the Frontal Systems Behavior Scale (FrSBe) (Grace and Malloy, 2001) in participants with acute and early HIV infection whose nadir CD4 cell counts were < 500/μL versus ≥ 500/μL. Box plots represent medians and interquartile ranges for the two subgroups.
We also examined the cognitive correlates of neurobehavioral disturbances in the AEH group. We saw no statistically significant differences in neurobehavioral disturbances across the groups with HAND. At the domain level, higher neurobehavioral ratings were moderately associated with slower information processing (ρ = −0.37, P = 0.031), but we found no significant correlations between total FrSBe ratings and the cognitive domains of attention, learning, memory, motor skills, executive function, or verbal fluency (all P values > 0.1).
Associations between FrSBe scores and lifetime substance use disorders and major depressive disorder were all non-significant (all P values > 0.1). Individuals with a lifetime history of bipolar disorder had higher ratings for current but not pre-infection neurobehavioral disturbances (Cohen d = 1.17, P = 0.01).
At the suggestion of a reviewer, we conducted further analyses examining whether participants with AEH and a history of bipolar disorder had a greater risk of worsening neurobehavioral disturbances after HIV infection. We calculated the difference between pre-infection and current FrSBe T-scores to provide a continuous index of change in self-reported neurobehavioral disturbances. The participants with a lifetime history of bipolar disorder showed a greater increase in neurobehavioral disturbances after infection than those without bipolar disorder (Cohen d = 1.17, P = 0.004).
DISCUSSION
Despite growing evidence of HIV-associated CNS abnormalities early in the course of HIV infection, little is known about the neurobehavioral sequelae of AEH. Our results in this study suggest that individuals in the acute and early stages of HIV infection experience prominent neurobehavioral disturbances. Our HIV-infected participants were 22 times more likely than our seronegative controls to report having clinically elevated levels of current apathy, and 33 times more likely to report elevated current executive dysfunction.
Although similar results are found in persons with chronic HIV disease, our data show at least mild neurobehavioral disturbances even before seroconversion and exacerbated during AEH. Notably, we found that these disturbances were largely independent of psychiatric factors (mood and substance use disorders) and demographic characteristics. Altogether, our data are consistent with the literature on neurobehavioral disturbances in chronic HIV infection (eg, Kamat et al, 2012; Marquine et al, 2014), and extend the findings to a unique, high-risk subset of the HIV population whose neuropsychiatric symptoms have received little study.
The prevalence and trajectory of different aspects of our participants’ neurobehavioral disturbances appeared to vary during the year after HIV infection. Our participants with AEH had elevations in apathy and executive dysfunction before infection, and both disturbances worsened significantly during the first year of HIV disease. Indeed, about one quarter of our participants with AEH had clinical exacerbations, possibly related to early HIV-associated neuropathologic changes.
Findings for disinhibition were more complicated. Before infection, the participants with AEH reported higher levels of disinhibition, but when we included psychiatric factors in the model, this effect was fully dampened. Moreover, the severity of disinhibition did not change notably after seroconversion. This pattern suggests that disinhibition may precede HIV infection and remain stable over the course of the disease (see Vo et al, 2013). In fact, disinhibition, which is prevalent in at-risk populations such as individuals with substance use disorders and bipolar disorder, may be a risk factor for acquiring and transmitting HIV (Kalichman, 2006).
In chronically HIV-infected groups, neural abnormalities in the nucleus accumbens and frontal cortex are associated with aspects of neurobehavioral disturbances (Kamat et al, 2014; Paul et al, 2005; Hoare et al, 2010). For example, our research group recently reported that higher apathy ratings in a group of chronically HIV-infected persons were associated with greater white matter abnormalities in the anterior corona radiata, genu, and orbital medial prefrontal cortex (Kamat et al, 2014). The associations between white matter alterations and apathy were independent of depression and were stronger among participants with lower current CD4 cell counts. White matter abnormalities in the corona radiata also correlated moderately with self-reported executive dysfunction. There were no associations with disinhibition. Thus, since frontostriatal regions appear to be particularly vulnerable to neural damage in persons with early AEH (Ances et al, 2009; Lentz et al, 2009, 2011; Young et al, 2014), early HIV-associated neural abnormalities might underlie these individuals’ neurobehavioral disturbances (eg, Bonelli and Cummings, 2007; Kamat et al, 2014).
In chronically HIV-infected persons, behavioral disturbances (eg, apathy, executive dysfunction, sensation seeking) have been linked to everyday function difficulties, such as needing help to perform instrumental activities of daily living, as well as medication non-adherence and risky sexual practices (eg, Barclay et al, 2007; Gonzalez et al, 2005; Kamat et al, 2012). Preliminary evidence suggests higher rates of functional dependence in individuals with AEH (Doyle et al, 2013). This increase may be partly driven by a neurobehavioral disturbance such as apathy. Our data from the present study show that within the AEH group, higher global ratings of neurobehavioral dysfunction were associated with more cognitive symptoms in daily life, dependence in activities of daily living, and unemployment. Neurobehavioral disturbances such as loss of motivation, perseverative thinking, lack of insight, poor judgment, and impulsivity may compromise many critical aspects of interpersonal functions and instrumental activities of daily living.
Thus, early behavioral dysfunction in persons with AEH may predict future difficulties with everyday function. Our data support the utility of asking persons with AEH about their behavioral disturbances as a supplement to standardized neuropsychological assessments. Future studies may extend our findings by exploring whether AEH-associated behavioral disturbances could also confer risk for medication non-adherence, engagement in HIV transmission risk behaviors, and poor health decision making.
Previous studies suggest that lifetime psychiatric (eg, substance use and mood) disorders, which can also produce neurobehavioral disturbances, are prevalent during AEH (Doyle et al, 2013; Plankey et al, 2007; Weber et al, 2013). Our data suggest that individuals with a history of bipolar disorder are more likely than individuals without bipolar disorder to suffer a worsening of their neurobehavioral problems after contracting HIV infection; the mechanism of this relationship remains to be characterized, but may reflect involvement of frontostriatal pathways that are affected in both syndromes. In AEH, bipolar disorder also appears to be associated with adverse outcomes in instrumental activities of daily living, routine cognitive function, and employment (Doyle et al, 2013). The impact of comorbid bipolar disorder and neurobehavioral disturbances may interfere with safer sexual behaviors, thus increasing the risk of HIV infection and transmission. This is a potentially important target for future research. Together, these findings highlight the clinical utility of early detection of behavioral disturbances in at-risk and acutely infected individuals.
With regard to the neurocognitive correlates of neurobehavioral disturbances in AEH, we did not observe an association with HAND, although we did find that neurobehavioral disturbances were related to slowed information processing. This pattern is consistent with prior data suggesting that many individuals with AEH are impaired on measures of information processing speed (eg, Wang et al, 2011; Weber et al, 2013). Chronically HIV-infected persons with neuropsychiatric disturbances have also been shown to perform worse on tasks of rapid, effortful information processing (Castellon et al, 2000).
Our finding that inefficient information processing was the only cognitive impairment related to our participants’ neurobehavioral disturbances provides preliminary evidence for the specificity of the association between these neuropsychiatric constructs. Impairments in planning, initiation, regulation, inhibition, and monitoring of behavior are key symptoms of apathy, disinhibition, and executive dysfunction. Efficient information processing is closely tied to these five abilities. Given this overlap in symptoms, it is not surprising that the slower processing speed in our participants with AEH was associated with elevated neurobehavioral disturbances. The concurrence of these related but distinct cognitive and behavioral deficits lends support to CNS involvement early in AEH.
Together with prior data showing the relationship between everyday function and the domains of information processing speed and learning in AEH (Doyle et al, 2013), our results from this study suggest that efficient information processing is fundamental to a broad range of neurobehavioral functions that can be impaired in AEH, while learning is specifically important to general activities of daily living. For example, symptoms of neurobehavioral dysfunction such as impulsivity, sensation seeking, and lack of motivation, combined with slowed information processing, may manifest as risky decision making in quickly evolving social situations (eg, involving substance use or high-risk sexual behavior).
Evidence is mixed regarding the relationship between markers of HIV disease severity and neuropsychiatric impairment in cohorts with AEH. For example, both Marcotte and colleagues (2003) and Weber and colleagues (2013) reported that individuals with AEH and neurocognitive deficits had higher plasma viral loads than cognitively intact individuals with AEH, suggesting that viremia might be associated with worse neuropsychological function. By contrast, Moore et al (2011) found no association between cognitive function and HIV disease markers in their AEH sample.
In our current study, lower nadir CD4 cell counts correlated with worse neurobehavioral disturbances. This finding complements prior research in chronically HIV-infected persons suggesting that lower nadir CD4 cell are a risk factor for greater neurocognitive impairment and neuropsychiatric disturbance (eg, Ellis et al, 2011; Valcour et al, 2006). Individuals with AEH typically have higher nadir CD4 levels than do chronically infected cohorts (Valcour et al, 2012). However, variability in the higher range of nadirs may be associated with greater risk of cognitive impairment (eg, Ellis et al, 2011; Munoz-Moreno et al, 2008). Consistent with these data, our participants with AEH and nadir CD4 values of ≤ 500/μL had worse self-reported neurobehavioral function.
Our data indicate that even individuals who have relatively high nadir CD4 cell counts during AEH may be at increased risk of irreversible neural injury. Previous studies suggested that starting antiretroviral therapy early may slow the trajectory of inflammatory CNS changes (Young et al, 2014) and may prevent further immunosuppression, thereby reducing risk of neuropsychiatric impairment (Ellis et al, 2011). Our data indicating that neurobehavioral disturbances arise early in HIV infection may bolster the argument for beginning antiretroviral therapy soon after diagnosis.
Our investigation had several limitations. One was our sample size. The AEH population is smaller and more difficult to recruit than the chronically infected HIV population. A larger, well-characterized AEH cohort would improve future studies.
A second limitation was that a cross-sectional study like ours does not permit examination of the relationships among neuropsychiatric disturbance, cognition, and function over time. Because these factors likely have diverse trajectories, a well-controlled longitudinal study could tease them apart.
A final limitation was the study’s partial reliance on self-reported data. The accuracy of our self-report measure of neurobehavioral dysfunction may have been affected by bias, depression, and/or mild anosognosia (Blackstone et al, 2012). The same could be true for our measures of functional impairment, which were also based on self-report. Because our participants were relatively young, few had caregivers who could have provided collateral information for informant reports. Still, the construct validity of the FrSBe in HIV-infected cohorts is supported by its relationships with other measures of neurobehavioral disturbance (Marquine et al, 2014) and neuroimaging (Kamat et al, 2014). Moreover, FrSBe data from other groups with frontostriatal system injury show agreement between self- and informant-reported neurobehavioral disturbances (Barrett et al, 2013; Chiaravalloti and DeLuca, 2003; Grace et al, 1997).
Future studies may benefit from assessing behavioral disturbance with objective measures of everyday function (eg, performance-based assessments and electronic medication adherence tracking) and clinical interviews like the Neuropsychiatric Inventory (Cummings et al, 1994).
Acknowledgments
TMARC is supported by Center award P50DA026306 (I.G.) from the National Institute on Drug Abuse. This research was also supported by National Institutes of Health grants P30-MH62512 (I.G.), R25-MH081482 (Principal Investigator: M. Cherner), T32-DA31098 (Principal Investigator: R.K. Heaton), L30 DA034362 (J.E.I.), K23 DA037793 (J.E.I.), and L30 DA032120 (E.E.M.), as well as California HIV Research Program grant RN07-SD-702 (S.J.L.).
TMARC Group members: Director: Igor Grant, MD. Co-Directors: Ronald J. Ellis, MD, PhD, Scott L. Letendre, MD, Cristian L. Achim, MD, PhD. Center Manager: Mariana Cherner, PhD. Associate Center Manager: Erin E. Morgan, PhD. Assistant Center Manager: Aaron M. Carr, BA. Behavioral Assessment and Medical (BAM) Core, Neuromedical and Laboratory Unit (NLU): Scott L. Letendre, MD (Core Co-Director/NLU Chief), Ronald J. Ellis, MD, PhD; BAM Core, Neuropsychiatric Unit (NPU): Robert K. Heaton, PhD (Core Co-Director/NPU Chief), J. Hampton Atkinson, MD, Thomas D. Marcotte, PhD, Erin E. Morgan, PhD, Matthew Dawson (NPU Manager). Neuroimaging Core: Gregory G. Brown, PhD (Core Director), Thomas T. Liu, PhD, Miriam Scadeng, PhD, Christine Fennema-Notestine, PhD, Sarah L. Archibald, MA, John R. Hesselink, MD, Mary Jane Meloy, PhD, Craig E. L. Stark, PhD. Neuroscience and Animal Models Core: Cristian L. Achim, MD, PhD (Core Director), Marcus Kaul, PhD, Virawudh Soontornniyomkij, MD. Pilot and Developmental Core: Mariana Cherner, PhD (Core Director), Stuart A. Lipton, MD, PhD; Pilot Study Leaders: Jennifer E. Iudicello, PhD, Assawin Gongvatana, PhD, Rachel D. Schrier, PhD, Virawudh Soontornniyomkij, MD. Administrative Coordinating Core (ACC), Data Management and Information Systems Unit: Anthony C. Gamst, PhD (Unit Chief), Clint Cushman, BA (Unit Manager); ACC Statistics Unit: Florin Vaida, PhD (Unit Chief), Ian S. Abramson, PhD, Reena Deutsch, PhD, Anya Umlauf, MS; ACC Participant Unit: J. Hampton Atkinson, MD (Unit Chief), Jennifer Marquie-Beck, MPH (Unit Manager). Project 1: Arpi Minassian, PhD (Project Director), William Perry, PhD, Mark A. Geyer, PhD, Jared W. Young, PhD. Project 2: Amanda B. Grethe, PhD (Project Director), Susan F. Tapert, PhD, Assawin Gongvatana, PhD. Project 3: Erin E. Morgan, PhD (Project Director), Igor Grant, MD. Project 4: Svetlana Semenova, PhD (Project Director), Athina Markou, PhD, James Kesby, PhD. Project 5: Marcus Kaul, PhD (Project Director).
Glossary
- AEH
acute and early human immunodeficiency virus
- CNS
central nervous system
- EIA
enzyme immunoassay
- FrSBe
Frontal Systems Behavior Scale
- HAND
human immunodeficiency virus-associated neurocognitive disorders
- HIV
human immunodeficiency virus
Footnotes
The authors report no conflicts of interest.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States Government.
The Translational Methamphetamine AIDS Research Center (TMARC) is affiliated with the University of California, San Diego; the Sanford-Burnham Medical Research Institute; and the University of California, Irvine.
References
- An SF, Groves M, Gray F, et al. Early entry and widespread cellular involvement of HIV-1 DNA in brains of HIV-1 positive asymptomatic individuals. J Neuropathol Exp Neurol. 1999;58:1156–1162. doi: 10.1097/00005072-199911000-00005. [DOI] [PubMed] [Google Scholar]
- Ances BM, Sisti D, Vaida F, et al. Resting cerebral blood flow: a potential biomarker of the effects of HIV in the brain. Neurology. 2009;73:702–708. doi: 10.1212/WNL.0b013e3181b59a97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson JH, Higgins JA, Vigil O, et al. Psychiatric context of acute/early HIV infection. The NIMH Multisite Acute HIV Infection Study: IV. AIDS Behav. 2009;13:1061–1067. doi: 10.1007/s10461-009-9585-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay TR, Hinkin CH, Castellon SA, et al. Age-associated predictors of medication adherence in HIV-positive adults: health beliefs, self-efficacy, and neurocognitive status. Health Psychol. 2007;26:40–49. doi: 10.1037/0278-6133.26.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett RD, McLellan TL, McKinlay A. Self versus family ratings of the frontal systems behaviour scale and measured executive functions: adult outcomes following childhood traumatic brain injury. PloS One. 2013;8(10):e76916. doi: 10.1371/journal.pone.0076916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone K, Moore DJ, Heaton RK, et al. Diagnosing symptomatic HIV-associated neurocognitive disorders: self-report versus performance-based assessment of everyday functioning. J Int Neuropsych olSoc. 2012;18:79–88. doi: 10.1017/S135561771100141X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone K, Weber E, Iudicello JE, et al. Real-world impact of HIV-associated neurocognitive impairment. In: Chiaravalloti N, Goverover Y, editors. Changing Brain, Changes in Daily Life. New York, New York: Springer; In press. [Google Scholar]
- Bonelli RM, Cummings JL. Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci. 2007;9:141–151. doi: 10.31887/DCNS.2007.9.2/rbonelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellon SA, Hinkin CH, Myers HF. Neuropsychiatric disturbance is associated with executive dysfunction in HIV-1 infection. J Int Neuropsychol Soc. 2000;6:336–347. doi: 10.1017/s1355617700633088. [DOI] [PubMed] [Google Scholar]
- Chang L, Speck O, Miller EN, et al. Neural correlates of attention and working memory deficits in HIV patients. Neurology. 2001;57:1001–1007. doi: 10.1212/wnl.57.6.1001. [DOI] [PubMed] [Google Scholar]
- Chiaravalloti ND, DeLuca J. Assessing the behavioral consequences of multiple sclerosis: an application of the Frontal Systems Behavior Scale (FrSBe) Cogn Behav Neurol. 2003;16:54–67. doi: 10.1097/00146965-200303000-00007. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Shaw GM, McMichael AJ, et al. Acute HIV-1 infection. N Engl J Med. 2011;364:1943–1954. doi: 10.1056/NEJMra1011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Davis LE, Hjelle BL, Miller VE, et al. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–1739. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- Doyle KL, Morgan EE, Morris S, et al. Real-world impact of neurocognitive deficits in acute and early HIV infection. J Neurovirol. 2013;19:565–573. doi: 10.1007/s13365-013-0218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Badiee J, Vaida F, et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25:1747–1751. doi: 10.1097/QAD.0b013e32834a40cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Vassileva J, Bechara A, et al. The influence of executive functions, sensation seeking, and HIV serostatus on the risky sexual practices of substance-dependent individuals. J Int Neuropsychol Soc. 2005;11:121–131. doi: 10.1017/s1355617705050186. [DOI] [PubMed] [Google Scholar]
- Grace J, Malloy PF. Frontal Systems Behavior Scale. Professional Manual. Lutz, Florida: Psychological Assessment Resources; 2001. [Google Scholar]
- Grace J, Malloy PF, Stout J. Assessing frontal behavioral syndromes: reliability and validity of the Frontal Lobe Personality Scale. Arch Clin Neuropsychol. 1997;12:327. [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, et al. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults Scoring Program. Odessa, Florida: Psychological Assessment Resources; 2004. [Google Scholar]
- Hoare J, Fouche JP, Spottiswoode B, et al. White matter correlates of apathy in HIV-positive subjects: a diffusion tensor imaging study. J Neuropsychiatry Clin Neurosci. 2010;22:313–320. doi: 10.1176/jnp.2010.22.3.313. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, et al. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Kalichman SC. HIV transmission risk behaviors of men and women living with HIV-AIDS: prevalence, predictors, and emerging clinical interventions. Clinical Psychology: Science and Practice. 2000;7:32–47. [Google Scholar]
- Kamat R, Brown GG, Bolden K, et al. Apathy is associated with white matter abnormalities in anterior, medial brain regions in persons with HIV infection. J Clin Exp Neuropsychol. 2014;36:854–866. doi: 10.1080/13803395.2014.950636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat R, Woods SP, Marcotte TD, et al. Implications of apathy for everyday functioning outcomes in persons living with HIV infection. Arch Clin Neuropsychol. 2012;27:520–531. doi: 10.1093/arclin/acs055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerndt PR, Dubrow R, Aynalem G, et al. Strategies used in the detection of acute/early HIV infections. The NIMH Multisite Acute HIV Infection Study: I. AIDS Behav. 2009;13:1037–1045. doi: 10.1007/s10461-009-9580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T, Wright EJ, Smith DM, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med. 2013;368:218–230. doi: 10.1056/NEJMoa1110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz MR, Kim WK, Kim H, et al. Alterations in brain metabolism during the first year of HIV infection. J Neurovirol. 2011;17:220–229. doi: 10.1007/s13365-011-0030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz MR, Kim WK, Lee V, et al. Changes in MRS neuronal markers and T cell phenotypes observed during early HIV infection. Neurology. 2009;72:1465–1472. doi: 10.1212/WNL.0b013e3181a2e90a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotte TD, Deutsch R, McCutchan JA, et al. Prediction of incident neurocognitive impairment by plasma HIV RNA and CD4 levels early after HIV seroconversion. Arch Neurol. 2003;60:1406–1412. doi: 10.1001/archneur.60.10.1406. [DOI] [PubMed] [Google Scholar]
- Marquine MJ, Iudicello JE, Morgan EE, et al. “Frontal systems” behaviors in comorbid human immunodeficiency virus infection and methamphetamine dependency. Psychiatry Res. 2014;215:208–216. doi: 10.1016/j.psychres.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WC, Rosenberg NE, Rutstein SE, et al. The role of acute and early HIV infection in the sexual transmission of HIV. Curr Opin HIV AIDS. 2010;5:277–282. doi: 10.1097/COH.0b013e32833a0d3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, Letendre SL, Morris S, et al. Neurocognitive functioning in acute or early HIV infection. J Neurovirol. 2011;17:50–57. doi: 10.1007/s13365-010-0009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Moreno JA, Fumaz CR, Ferrer MJ, et al. Nadir CD4 cell count predicts neurocognitive impairment in HIV-infected patients. AIDS Res Hum Retroviruses. 2008;24:1301–1307. doi: 10.1089/aid.2007.0310. [DOI] [PubMed] [Google Scholar]
- Norman MA, Moore DJ, Taylor M, et al. Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. J Clin Exp Neuropsychol. 2011;33:793–804. doi: 10.1080/13803395.2011.559157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul RH, Brickman AM, Navia B, et al. Apathy is associated with volume of the nucleus accumbens in patients infected with HIV. J Neuropsychiatry Clin Neurosci. 2005;17:167–171. doi: 10.1176/appi.neuropsych.17.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso MJ, Meyerhoff DJ, Price RW, et al. Cerebrospinal fluid and neuroimaging biomarker abnormalities suggest early neurological injury in a subset of individuals during primary HIV infection. J Infect Dis. 2013;207:1703–1712. doi: 10.1093/infdis/jit088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plankey MW, Ostrow DG, Stall R, et al. The relationship between methamphetamine and popper use and risk of HIV seroconversion in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2007;45:85–92. doi: 10.1097/QAI.0b013e3180417c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabkin JG, Ferrando SJ, van Gorp W, et al. Relationships among apathy, depression, and cognitive impairment in HIV/AIDS. J Neuropsychiatry Clin Neurosci. 2000;12:451–457. doi: 10.1176/jnp.12.4.451. [DOI] [PubMed] [Google Scholar]
- Schacker T, Collier AC, Hughes J, et al. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257–264. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- Schacker TW, Hughes JP, Shea T, et al. Biological and virologic characteristics of primary HIV infection. Ann Intern Med. 1998;128:613–620. doi: 10.7326/0003-4819-128-8-199804150-00001. [DOI] [PubMed] [Google Scholar]
- Tate D, Paul RH, Flanigan TP, et al. The impact of apathy and depression on quality of life in patients infected with HIV. AIDS Patient Care STDS. 2003;17:115–120. doi: 10.1089/108729103763807936. [DOI] [PubMed] [Google Scholar]
- Valcour V, Chalermchai T, Sailasuta N, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206:275–282. doi: 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Yee P, Williams AE, et al. Lowest ever CD4 lymphocyte count (CD4 nadir) as a predictor of current cognitive and neurological status in human immunodeficiency virus type 1 infection-The Hawaii Aging with HIV Cohort. J Neurovirol. 2006;12:387–391. doi: 10.1080/13550280600915339. [DOI] [PubMed] [Google Scholar]
- Vo QT, Cox C, Li X, et al. Neuropsychological test performance before and after HIV-1 seroconversion: the Multicenter AIDS Cohort Study. J Neurovirol. 2013;19:24–31. doi: 10.1007/s13365-012-0136-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Foryt P, Ochs R, et al. Abnormalities in resting-state functional connectivity in early human immunodeficiency virus infection. Brain Connect. 2011;1:207–217. doi: 10.1089/brain.2011.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E, Morgan EE, Iudicello JE, et al. Substance use is a risk factor for neurocognitive deficits and neuropsychiatric distress in acute and early HIV infection. J Neurovirol. 2013;19:65–74. doi: 10.1007/s13365-012-0141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson GJ. Wide Range Achievement Test-4: Professional Manual. Lutz, Florida: Psychological Assessment Resources; 2006. [Google Scholar]
- Wittchen HU. Reliability and validity studies of the WHO-Composite International Diagnostic Interview (CIDI): a critical review. J Psychiatr Res. 1994;28:57–84. doi: 10.1016/0022-3956(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Frol AB, et al. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol. 2004;26:759–778. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- Young AC, Yiannoutsos CT, Hegde M, et al. Cerebral metabolite changes prior to and after antiretroviral therapy in primary HIV infection. Neurology. 2014;83:1592–1600. doi: 10.1212/WNL.0000000000000932. [DOI] [PMC free article] [PubMed] [Google Scholar]