Abstract
The presence of disease-associated prions in tissues and bodily fluids of chronic wasting disease (CWD)-infected cervids has received much investigation, yet little is known about mother-to-offspring transmission of CWD. Our previous work demonstrated that mother-to-offspring transmission is efficient in an experimental setting. To address the question of relevance in a naturally exposed free-ranging population, we assessed maternal and fetal tissues derived from 19 elk dam–calf pairs collected from free-ranging Rocky Mountain elk from north-central Colorado, a known CWD endemic region. Conventional immunohistochemistry identified three of 19 CWD-positive dams, whereas a more sensitive assay [serial protein misfolding cyclic amplification (sPMCA)] detected CWD prion seeding activity (PrPCWD) in 15 of 19 dams. PrPCWD distribution in tissues was widespread, and included the central nervous system (CNS), lymphoreticular system, and reproductive, secretory, excretory and adipose tissues. Interestingly, five of 15 sPMCA-positive dams showed no evidence of PrPCWD in either CNS or lymphoreticular system, sites typically assessed in diagnosing CWD. Analysis of fetal tissues harvested from the 15 sPMCA-positive dams revealed PrPCWD in 80 % of fetuses (12 of 15), regardless of gestational stage. These findings demonstrated that PrPCWD is more abundant in peripheral tissues of CWD-exposed elk than current diagnostic methods suggest, and that transmission of prions from mother to offspring may contribute to the efficient transmission of CWD in naturally exposed cervid populations.
Introduction
Transmissible spongiform encephalopathies (TSEs) are a class of surreptitious neurodegenerative diseases which have been recognized for almost a century, but only became prominent in the research spotlight during the bovine spongiform encephalopathy and subsequent variant Creutzfeldt–Jakob disease outbreaks in the 1980s and 1990s (Wells et al., 1991; Hill et al., 1997; Brown et al., 2001). Many interesting and confounding aspects of TSEs have been revealed, such as the fact that the causative agent is a prion (Prusiner, 1982), a misfolded, protease-resistant form (PrPres) of a protein with nearly universal expression in the animal kingdom (PrPC), and whose function is still elusive and controversial, despite the enormous body of research undertaken to reveal it (Aguzzi et al., 2008; Sakudo & Onodera, 2014).
TSEs have long incubation periods, ranging from months to years, during which affected hosts harbour and shed infectious particles, despite absence of clinical signs (Gill et al., 2013; Lacroux et al., 2014). Thus, infectious prions can be transmitted from excreta or body fluids (e.g. urine, faeces, saliva, blood) of live animals, as well as carcasses, to infect susceptible animals or contaminate environments and fomites (Brown et al., 2001; Mathiason et al., 2006; Nichols et al., 2009; Notari et al., 2010; Gilch et al., 2011; Saunders et al., 2012). One distinctive feature of prions is that they are extremely resistant to degradation; therefore, environmental contamination is a serious concern.
Chronic wasting disease (CWD) is a TSE with unusually high transmission efficiency among cervids (Miller & Williams, 2004; Sigurdson & Aguzzi, 2007; Gilch et al., 2011). As is the case in all prionopathies, the aberrant, disease-associated protein accumulates in the central nervous system (CNS) and other tissues, causing slow and progressive neurological damage, resulting in behavioral abnormalities, emaciation, loss of bodily functions and ultimately death (Williams & Miller, 2002). Prior to 2000, CWD in free-ranging cervids was localized in Wyoming and Colorado in the USA, but new reports of CWD emerged from more distant states in the ensuing years (Joly et al., 2009). To date, CWD it is the most widespread natural TSE, with prevalence rates of 1–30 % in white-tailed deer (Odocoileus virginianus), 1–13 % in elk (Cervus elaphus nelsoni) and up to 50 % in mule deer (Odocoileus hemionus) populations in certain regions of North America (Miller & Williams, 2004; Gilch et al., 2011; Saunders et al., 2012). In some infected populations, CWD is a leading cause of cervid mortality and may negatively impact population dynamics (Dulberger et al., 2010; Sargeant et al., 2011). Both clinically and preclinically infected cervids harbour sufficient infectious prions to efficiently transmit disease (Hill & Collinge, 2003; Bishop et al., 2013; Elder et al., 2013). CWD can be transmitted by direct contact with infected animals, carcasses, bodily fluids, secretions or excreta (Mathiason et al., 2006; Saunders et al., 2012; Lacroux et al., 2014), or indirectly through uptake of infectious material shed into the environment (Miller & Williams, 2004; Mathiason et al., 2006; Saunders et al., 2012). As a result, CWD prions are in no short supply, cycling between the clinically or latently infected animals and the environment they inhabit. As prions retain infectivity for many years and environmental decontamination is unrealistic, it is unlikely that the CWD reservoir will be depleted once an environment is significantly contaminated (Saunders et al., 2012). Therefore, it is crucial that we prevent movement of infectious prions from contaminated areas and that we understand ecosystem-level effects of having a contaminated environment, as well as the potential implications for public health.
Although TSEs are known as diseases of the CNS, extraneural organs expressing PrPC can also serve as sites of PrPres conversion and/or accumulation (Peralta & Eyestone, 2009), including the peripheral nervous system, lymphatic system, skeletal muscle, reproductive tissues, gastrointestinal tract and excretory system (Race et al., 1998; Notari et al., 2010). Recent advances in methodologies for the sensitive detection of disease-associated prions in tissues and bodily fluids, including serial protein misfolding cyclic amplification (sPMCA) and real-time quaking-induced conversion (RT-QuIC) (Saá et al., 2006; Haley et al., 2012), have enhanced our ability to detect trace amounts of protease-resistant prions more readily than conventional methods such as immunohistochemistry (IHC) (Ironside et al., 2000; Spraker et al., 2009). With the aid of sPMCA, we previously demonstrated that CWD mother-to-offspring transmission occurs in an experimentally infected cervid model (Reeves' muntjac deer) and is associated with a fourfold increase in stillbirth rates (Nalls et al., 2013). Our findings were in agreement with studies confirming maternal transmission in other species, such as sheep, cattle, felids and mouse models (Castilla et al., 2005; Bencsik et al., 2009; Foster et al., 2013). Here, we address two remaining questions regarding the covert prion persistence mechanisms in a naturally infected population: (1) are prions vertically transmitted from CWD naturally exposed elk dams to offspring in utero, and (2) if so, is there an association between vertical transmission and peripheral distribution of prions in dam tissues?
Results
sPMCA
In our efforts to detect the minute amounts of amplifiable prions present in fetal and maternal tissues, we used sPMCA, a highly sensitive and specific method of PrPres detection (Saá et al., 2006), superior to both IHC and Western blotting of unamplified material. We proceeded to evaluate the probability of contamination in our technique, as well as the optimal number of rounds of amplification necessary and sufficient to demonstrate the presence of amplification-competent material in each sample.
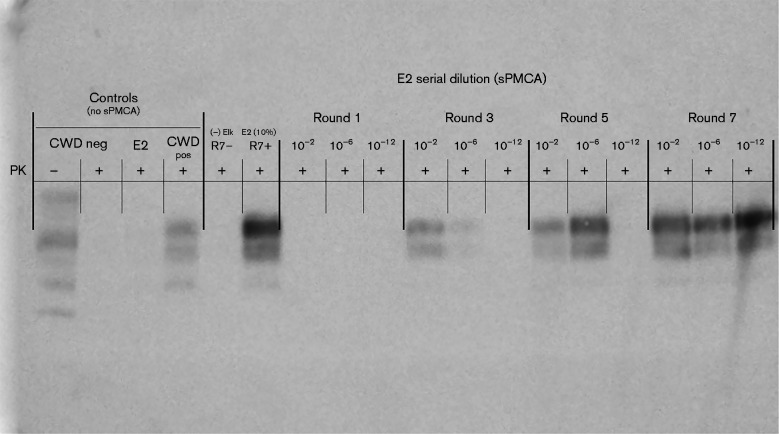
In order to determine the sensitivity of PMCA in our laboratory, we performed PMCA using serial dilution of known CWD-positive elk brain (E2) which has been described in a previous publications (Nichols et al., 2009; Wyckoff et al., 2013). In Fig. 1, dilutions of 10− 2, 10− 6 and 10− 12 of E2 CWD-positive elk brain were sPMCA-amplified for seven rounds, then rounds 1, 3, 5 and 7 were compared by Western blot detection of PrPCWD. PrPCWD at the highest dilution (10− 12) could only be detected after seven, but not after five rounds of amplification. Thus, a 10− 12 dilution of this brain (E2) represents the PMCA seeding dose for seven rounds of PMCA. Subsequently, an estimation of the sample's infectious titre was computed by comparing E2 PMCA and bioassay data. The sPMCA seeding dose (10− 12) corresponds to an extrapolated bioassay infectivity of 0.32 LD50 U (g tissue)− 1 (Fig. 2).
Fig. 1.
PrPCWD detection in dilutions of CWD-positive elk brain following sPMCA. Representative Western blots for detection of PrPCWD in CWD-positive elk brain homogenate (E2) following sPMCA (dilutions 10− 2, 10− 6 and 10− 12, rounds 1, 3, 5 and 7 shown). PrPCWD was not detected in a 10 % homogenate of E2 prior to sPMCA or after one round of sPMCA. After three and five rounds of E2 sPMCA, PrPCWD was detected at 10− 6. PrPCWD was detected at 10− 12 following seven rounds of sPMCA. sPMCA controls (R7– and R7+) showed complete proteinase K (PK) digestion of negative elk brain homogenate (R7–) and proteinase K-resistant PrPCWD in E2 CWD-positive elk brain homogenate (R7+) (10 % homogenates, undiluted, round 7). Western blot controls (no sPMCA) showed complete proteinase K digestion of PrP in negative white-tailed deer brain homogenate and proteinase K-resistant PrPCWD in CWD-positive white-tailed deer brain homogenate (10 % homogenates, undiluted, no sPMCA).
Fig. 2.
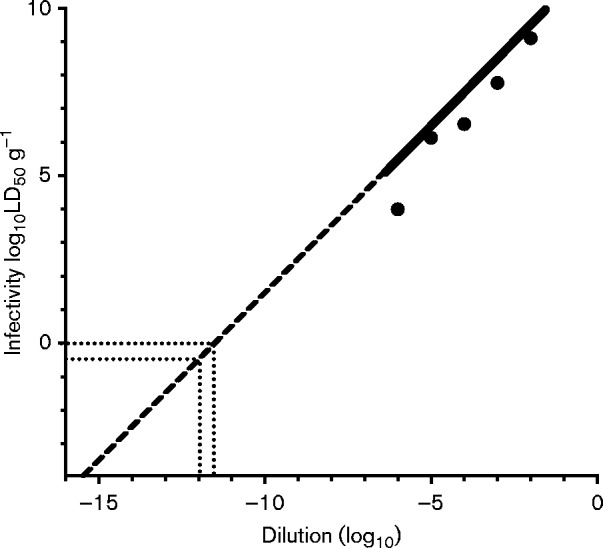
Infectivity titres of serially diluted E2 CWD brain isolate. Groups of 10 mice were inoculated intracranially with the E2 isolate of CWD prions diluted into normal brain homogenate at the indicated serial dilutions. End-point prion titres were determined as described previously (Reed & Muench, 1938). Error bars are smaller than the indicated points for mean infectivity on the graph. Dashed line indicates extrapolated values that lie outside of the dynamic range of the bioassay. Dotted lines converging from y = 100 and x = 10− 11.5 represent the extrapolated dilution of E2 brain homogenate (10− 11.5) containing 1 LD50 U (100) (g tissue)− 1. Dotted lines converging from x = 10− 12 and y = 10− 0.5 represent the extrapolated infectivity titre [0.32 LD50 U (g tissue)− 1] of a 10− 12 dilution of E2 brain homogenate that is the PMCA seeding dose for seven rounds of PMCA.
Specificity was ascertained by performing 75 separate reactions, seven rounds each, using known laboratory-negative and E2-positive 10 % cervid brain homogenate controls. The negative and the positive rounds were blinded and run in parallel with the samples (>500 samples total); representative blots are included the relevant figures. We observed no spontaneous amplification in our negative controls, whereas positive controls always had detectable PrPCWD upon proteinase K digestion. Consequently, we determined that the specificity of sPMCA was 100 % and therefore we considered as positive any tissue with detectable PrPCWD in at least one of the three replicate reactions (ϕ = 0.99 for 0.2 × 10− 12 known positive and known negative brain dilutions).
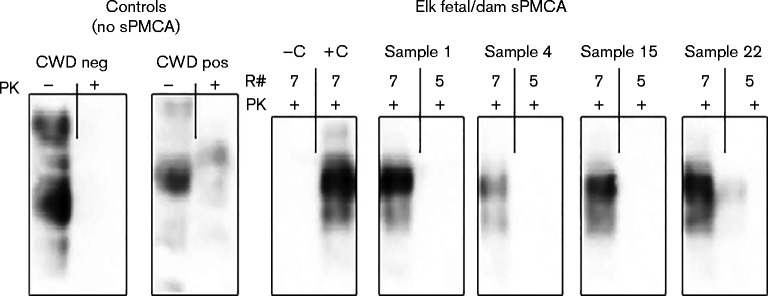
As we determined above that specificity was maintained, but that sensitivity of detection was enhanced by performing seven rather than five round of PMCA (Fig. 1), we therefore deemed it appropriate and necessary to examine our environmentally sourced samples using these parameters, because we anticipated that vertically transmitted prions, as reported elsewhere (Nalls et al., 2013), are present in such minute amounts that detection may be difficult without appropriate amplification. As expected, Western blot detection of PrPCWD in several samples (e.g. umbilicus, intestine and uterus; Fig. 3) was only possible after seven rounds of sPMCA, whereas PrPCWD was not detected in these tissues after five rounds.
Fig. 3.
PrPCWD detection in elk fetal and dam tissues following seven versus five rounds (R#) of sPMCA. Representative Western blots for detection of PrPCWD in elk fetal umbilicus (Sample 1), fetal intestine (Sample 4) and dam uterus (Samples 15 and 22) following seven rounds of sPMCA. PrPCWD was not detected in these tissues after five rounds of sPMCA. sPMCA controls (–C and +C) showed complete proteinase K (PK) digestion of negative elk brain homogenate (–C) and proteinase K-resistant PrPCWD in E2 CWD-positive elk brain homogenate (+C) (10 % homogenates, undiluted, round 7). Western blot controls (no sPMCA) showed complete proteinase K digestion of PrP in a negative white-tailed deer brain homogenate and PrPCWD signal post-proteinase K digestion in a CWD-positive white-tailed deer brain homogenate (10 % homogenate, undiluted, no sPMCA).
Maternal results
All 19 animals from the CWD endemic area of Colorado appeared clinically normal at the time of euthanasia, as judged by their body condition and absence of clinical CWD signs. Their CWD status was ascertained by conventional IHC (Monello et al., 2014), which identified three of 19 dams CWD-positive in retropharyngeal lymph nodes (RLNs). Two of these dams were also obex-positive by IHC (Spraker et al., 2009), while the third was IHC obex-negative (Spraker et al., 2009), as shown in Table 1.
Table 1. Elk dam and fetus pairs from CWD endemic area of Colorado: summary of results.
Br, Brain; Ob, obex; Ut, uterus; Pl, placentome; Ct, cotyledon; RLN, retropharyngeal lymph node; Sp, spleen; To, tongue; Ad, adipose tissue; MG, mammary gland; Bl, urinary bladder; K, kidney; Pa, pancreas; NE, nasal epithelium; SG, salivary gland; Ur, ureter; Ov, ovary; RAMALT, rectal mucosa-associated lymphoid tissue. Italics denote sPMCA+ CWD amplification and the five items in bold denote that the IHC and sPMCA data corroborate one another.
| Maternal IHC status | Dam PRNP codon 132 genotype | Maternal sPMCA-positive tissues | Maternal positive/total samples | Gestation trimester | Fetal PRNP codon 132 genotype | Fetal positive/total samples |
|---|---|---|---|---|---|---|
| Negative | MM | None | 0/18 | Second | MM | 0/3 |
| Negative | ML | None | 0/16 | Second | MM | 0/11 |
| Negative | MM | None | 0/18 | First | ML | 0/6 |
| Negative | ML | None | 0/18 | First | MM | 0/5 |
| Positive (Ob, RLN)* | MM | Br, Ob, RAMALT, RLN, Sp, Ut, Ov, Ur, To, Bl, K, Pa, NE, SG | 14 (C+L + R + S)/18 | Second | MM | 1 (colon)/10 |
| Positive (Ob, RLN)* | MM | Ob, RLN, Um, Ov, To, Bl, K, Pa, SG, Ad | 6 (C+L + R + S)/17 | Second | ML | 1 (lung)/6 |
| Negative | ML | Ob, RAMALT, Um, Ov, Ur, Ad | 10 (C+L + R + S)/18 | Second | ML | 5 (vagus nerve, spleen, colon, thymus, liver)/11 |
| Negative | ML | Br, To, Bl, K, Ad | 5 (C+S)/19 | Second | MM | 2 (RLN, thymus)/10 |
| Negative | MM | Br, Ob, SG | 3 (C+S)/19 | Second | MM | 2 (brain, ileum)/6 |
| Positive (RLN)† | MM | RLN, Pl, Ct Um, NE, Ur | 6(L+R + S)/15 | Second | MM | 2 (RLN, liver)/9 |
| Negative | ML | RAMALT, Spl Ut, Ct, Um, K | 6(L+R + S)/18 | Second | ML | 1 (spleen)/7 |
| Negative | MM | Ut, To, K, SG, MG | 5 (R+S)/11 | Second | MM | 1 (rectum)/8 |
| Negative | MM | Pl, Bl, NE, Ur | 4(R+S)/16 | Second | MM | 1 (popliteal lymph node)/7 |
| Negative | ML | Pl, Bl, NE, Ur | 4 (R+S)/16 | Second | MM | 0/7 |
| Negative | MM | Pl, Bl | 2 (R+S)/18 | Second | ML | 0/11 |
| Negative | ML | Ob, Um, NE | 3(C+R + S)/19 | First | MM | 1 (vagus nerve)/6 |
| Negative | MM | Ut, Pl, To, K, NE, Ad, MG | 7 (R+S)/17 | First | MM | 1 (ileum)/8 |
| Negative | ML | Sp, Ov | 2 (L+R)/18 | First | ML | 1 (thymus)/8 |
| Negative | ML | Br, Ob, Sp, Um | 4(C+L + R)/18 | First | MM | 0/3 |
IHC: obex-positive, RLN-positive.
IHC: obex-negative, RLN-positive.
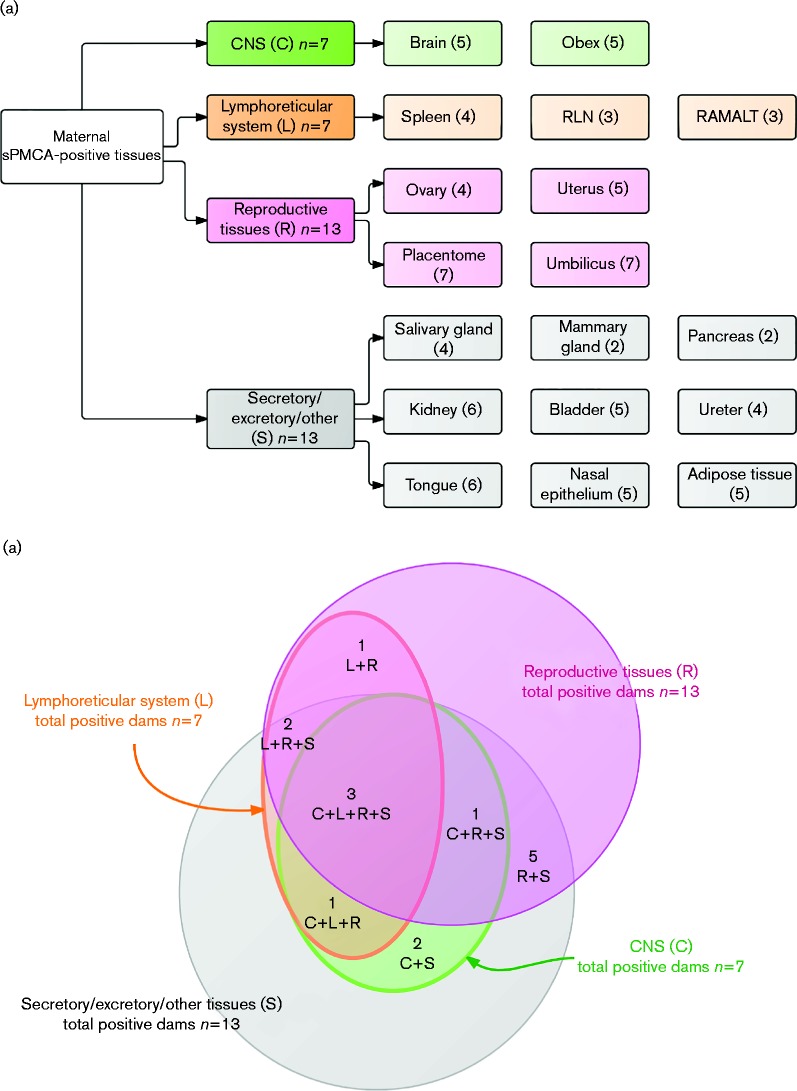
To comprehensively map PrPCWD presence and distribution in free-ranging elk, we analysed by blinded sPMCA a variety of maternal tissues, which we classified into four main categories (Fig. 4a):
CNS (C): brain at the level of the obex.
Lymphoreticular system (L): spleen, RLNs and rectal mucosa-associated lymphoid tissue (RAMALT).
Reproductive system (R): uterus, ovary, placentome (caruncle/cotyledon interface), umbilical cord.
Secretory/excretory/other (S): salivary gland, mammary gland, pancreas, kidney, urinary bladder, ureter, nasal epithelium, tongue and adipose tissue.
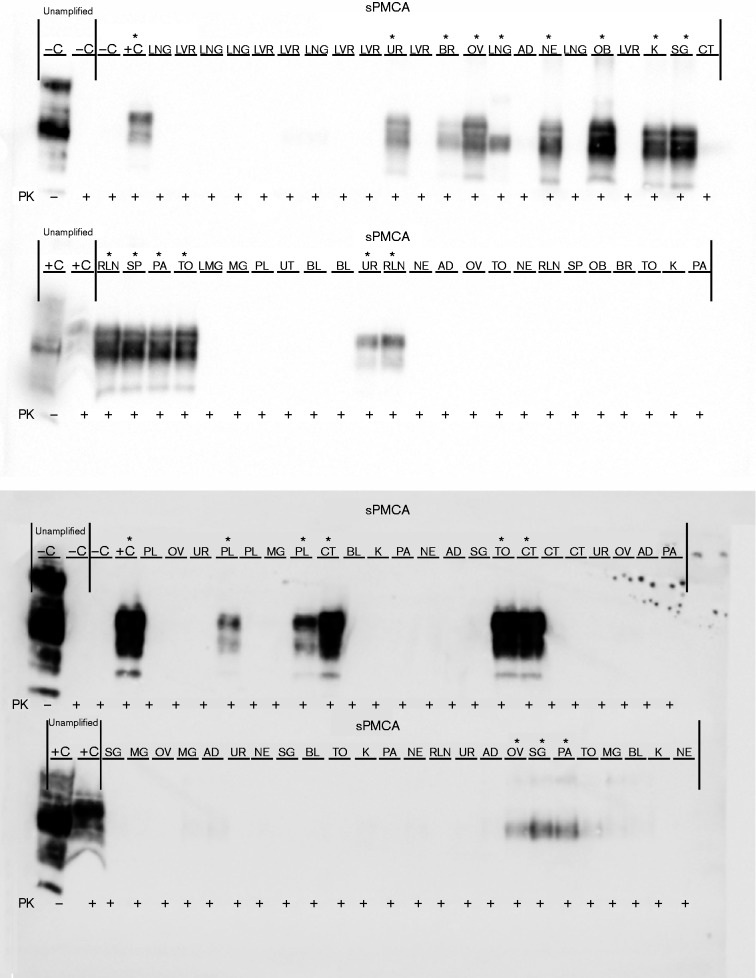
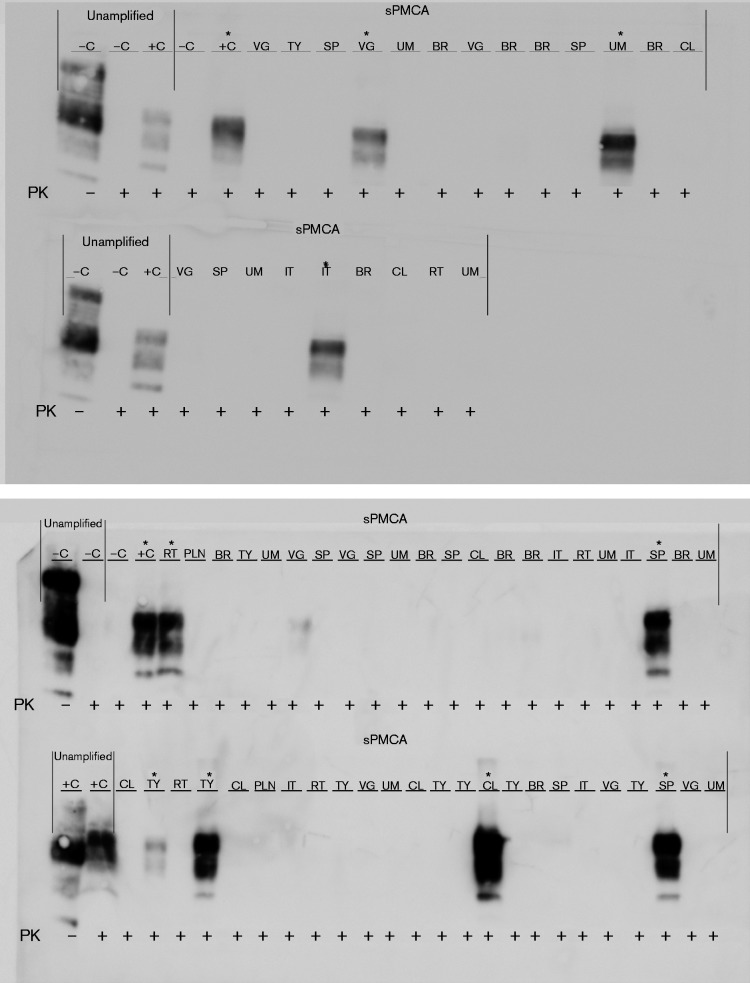
Our blinded sPMCA analysis corroborates the CWD status of CWD-positive elk determined by Monello et al. (2013) (Table 1). Strikingly, we identified 12 additional positive dams, which had been classified as CWD-negative by conventional IHC in the previous study. The total number of sPMCA PrPCWD-positive dams was 15 of 19; seven positive in brain/obex sections (C), seven positive in one or more of the lymphoid tissues (L), 13 positive in reproductive tissues (R) and 13 positive in one or more secretory/excretory or other tissues (S) (Fig. 4a). Representative Western blots of various blinded dam tissues after seven rounds of sPMCA are presented in Fig. 5.
Fig. 4.
(a) Category classification and numbers of tissues found PrPCWD-positive by sPMCA in elk dams. Numbers represent the numbers of dams found positive in that tissue or category of tissues. (b) PrPCWD distribution in sPMCA-positive adult elks. Total dams found positive by sPMCA in either category (n = 15) of total elk dams studied from the endemic area (n = 19). Total dams with at least one C tissue positive, n = 7; total dams with at least one L tissue positive, n = 7; total dams with at least one R tissue positive, n = 13; and total dams with at least one S tissue positive, n = 13.
Fig. 5.
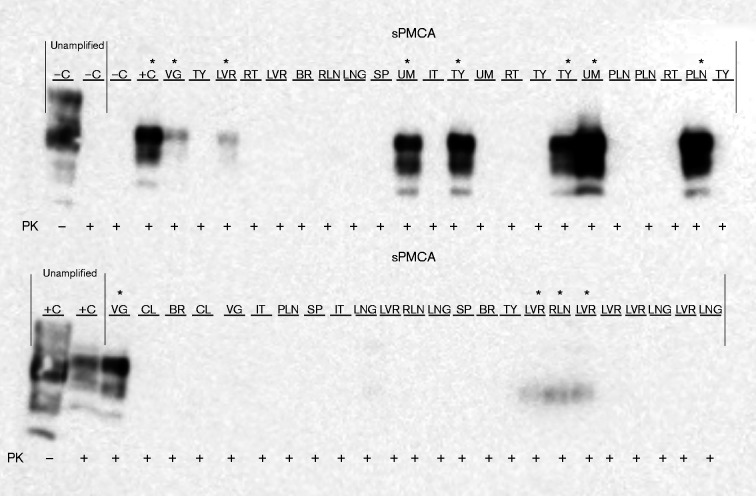
PrPCWD detection in elk dam tissues following seven rounds of sPMCA. Representative Western blots for detection of PrPCWD in dam tissues. sPMCA controls showed the lack of amplification for a CWD-negative sample and the successful amplification of a known CWD-positive white-tailed deer. Western blot controls (no sPMCA) showed complete proteinase K (PK) digestion of PrP in a negative white-tailed deer brain homogenate and PrPCWD signal post-proteinase K digestion in a CWD-positive white-tailed deer brain homogenate (10 % homogenate, undiluted, no sPMCA). Asterisks denote sPMCA + CWD amplification. Tissue codes: C, control. (+, positive; –, negative); AD, adipose tissue; BL, urinary bladder; BR, brain; CT, cotyledon; K, kidney; LNG, lung; LVR, liver; MG, mammary gland; NE, nasal epithelium; OB, obex; OV, ovary; PA, pancreas; PL, placenta; RLN, retropharyngeal lymph node; SG, salivary gland; SP, spleen; TO, tongue; UR, ureter; UT, uterus.
We found dams with deposition in all tissue categories analysed (C+L+R+S, n = 3), dams with peripheral distribution only (L+R+S, n = 2), and dams with CNS and secretory deposits only (C+S, n = 2) (Fig. 4b). Additionally, we found one dam with deposition in all tissue categories except lymphoid organs (C+R+S, n = 1), one with deposition only in CNS, lymphoid and reproductive organs (C+L+R, n = 1), and another with deposition in lymphoid and reproductive tissues only (L+R, n = 1). All positive dams had either positive R or S tissues – one-third being positive in tissues exclusively outside the nervous and lymphatic systems (R+S, n = 5). The latter result is remarkable in that these five dams lacked detectable sPMCA PrPCWD in any of the C or L tissues, which would typically be assessed for diagnostic purposes. Moreover, three of these dams had foetuses with one positive tissue, suggesting that lymphoid or nervous system deposition may not be required for in utero transmission.
Fetal results
None of the fetal tissues collected from five elk fetuses from the south-western portion of North Dakota tested positive for CWD using blinded sPMCA as described above. In fetal tissues collected from the CWD endemic region of Colorado, seven of 19 showed no evidence of CWD using blinded sPMCA. Four were collected from sPMCA-negative dams, while the other three were collected from dams with at least one sPMCA-positive tissue. All PrPCWD-positive elk fetuses (12 of 19) were carried by sPMCA-positive dams. Three of the positive fetuses were in early gestation, whilst the remaining nine were in mid-gestation. While most fetuses had only one or two tissues positive (Table 1), one fetus had five (vagus nerve, spleen, colon, thymus and liver). Its dam was sPMCA-positive in all four categories (C+L+R+ S, 10 of 18 tissues positive), but was negative by conventional IHC in the previous study (Monello et al., 2013). Conversely, another elk dam – positive in all categories by sPMCA (C+L+ R+S, 14 of 18 tissues positive) and who had also been diagnosed as advanced CWD by IHC – had a mid-gestation fetus with only one tissue (colon) positive by sPMCA. The dam with no CNS positivity by either IHC or sPMCA, but with IHC-positive RLNs and sPMCA-positive L+R+S, was carrying a mid-gestation fetus with positive liver and RLNs. We also detected sPMCA PrPCWD in the umbilicus (n = 7) and placentomes (n = 7) of free-ranging elk. Representative Western blots of various blinded fetal tissues after seven rounds of sPMCA are presented in Fig. 6.
Fig. 6.
PrPCWD detection in elk fetal tissues following seven rounds of sPMCA. Representative Western blots for detection of PrPCWD in fetal tissues. sPMCA controls showed the lack of amplification for a CWD-negative sample and the successful amplification of a known CWD-positive white-tailed deer. Western blot controls (no sPMCA) showed complete proteinase K (PK) digestion of PrP in a negative white-tailed deer brain homogenate and PrPCWD signal post-proteinase K digestion in a CWD-positive white-tailed deer brain homogenate (10 % homogenate, undiluted, no sPMCA). Tissue codes: C, control (+, positive; –, negative); BR, brain; CL, colon; IT, intestine; LNG, lung; LVR, liver; PLN, popliteal lymph node; RLN, retropharyngeal lymph node; RT, rectum; SP, spleen; TY, thymus; UM, umbilicus; VG, vagus nerve. Asterisks denote sPMCA+ CWD amplification.
Discussion
The prevalence of CWD in elk in this study area was previously estimated at 12.9 % based on IHC results of RLNs and obex (Monello et al., 2013), but 18.90 % by sPMCA (Wyckoff et al., 2015). Monello et al. (2014) predicted that, under current population conditions, declines in this herd would occur if CWD prevalence exceeded 13 %. As IHC proved highly specific in the above studies, but insufficiently sensitive to detect CWD in subclinical individuals (Haley et al., 2012; Wyckoff et al., 2015), we attempted to evaluate the previously unobservable CWD burden in these animals by examining additional tissues and also to determine the potential for in utero transmissibility.
Overall, blinded sPMCA was capable of detecting fivefold more PrPCWD-positive dams, bringing the percentage of subclinical carriers in our sample to 79 %. Had we decided to include only nervous system or lymphoid tissue samples in our investigation, we would have only found an additional seven positive dams (i.e. 3.3 times more) for a total of 10 positive dams of 19 tested (53 %). These data suggest that asymptomatic carriers can harbour PrPCWD in tissues outside of – and even without – the involvement of the nervous or lymphatic systems.
Additionally, as CWD affects elk of reproductive age, we aimed to investigate whether the pathogenic protein could be found in the fetuses of the euthanized cows. We show here that the majority of the positive dams (13 of 15) had positive reproductive tissues and 12 of 15 positive dams had positive fetuses. Seven positive dams had detectable PrPCWD in the umbilical cord. Spiropoulos et al. (2014) found that in utero infected domestic sheep lambs harbour most infectious material in the umbilical cord. In our study, all fetuses with positive umbilical cords harboured PrPCWD in at least one tissue; however, only five of seven elk dams with positive placentomes had positive fetuses. This latter observation is perplexing, but not without precedent; for example, in some pregnant Creutzfeldt–Jakob disease patients, PrPCJD was demonstrated in placental tissue, but not in the neonate (Di Gangi et al., 2015). The seemingly arbitrary PrPCWD distribution pattern in elk fetuses may be more closely related to PrPC expression subtleties, including timing of PrPC expression during normal developmental biology (Peralta et al., 2012), rather than to the dynamics of prion infection and dissemination as seen in adults. As the fetus may be exposed throughout gestation to maternal influx of prions via blood, placental transport or recycling of amniotic fluid (Wooding et al., 1997), and because PrPC isoforms expressed on most fetal cells (Peralta et al., 2012) can serve as docking or conversion scaffolds for prions (Makzhami et al., 2014), it is possible for PrPCWD to be found on virtually any tissue once the fetal-maternal barrier is crossed. Whether these prions had the potential to replicate efficiently in the fetal tissues cannot be ascertained nor whether they would have led to clinical disease at any time post-birth. A definitive pattern of transmissibility or tissue susceptibility cannot be deduced at this time due to sample limitations; however, it seems plausible that vertical transmission of CWD in free-ranging animals is an additional mechanism of prion persistence within cervid populations. An interesting further study would be to determine whether subclinical infections can occur across generations without eventually developing clinical disease.
In vitro diagnostic tests can be confusing because results may not always equal clinical disease. A similar puzzling phenomenon has been recognized for variant Creutzfeldt–Jakob disease, for which disease exposure estimates using appendix and tonsil IHC of exposed population samples were strikingly higher than the clinical variant Creutzfeldt–Jakob disease cases recorded (Gill et al., 2013). One explanation for this discrepancy is that the prion infectivity threshold for the CNS is higher than that for the lymphoreticular system or peripheral organs (Béringue et al., 2012). Thus, an outstanding question remains: are there obscure mechanisms by which latent prions are peripherally maintained in the susceptible host population but never develop into clinical disease?
In the case of CWD, current conventional detection methods such as IHC identify a small fraction of carriers in free-ranging elk populations. By contrast, we show that peripheral PrPCWD in free-ranging animals classified as negative by conventional means is demonstrable using a highly sensitive and specific detection assay (sPMCA). As 15 of the 19 dams analysed were positive in at least one tissue, we calculated the estimated PrPCWD prevalence in the CWD endemic group to be 70.5 %. This number may seem alarming, because the previously reported disease prevalence was close to 13 % (Monello et al., 2013). Worthy of note here is that, even if we had applied a highly stringent filter to our data and considered as positive only samples which had detectable PrPCWD at least half the times run, and from which at least two homogenates were made and proved positive, 12 of the 19 dams would still have been positive and of these half were carrying fetuses which harboured proteinase K-resistant prions (Table S1, available in the online Supplementary material). The fact that minute amounts of amplifiable and proteinase K-resistant material can be found in various organs, bodily fluids and secretions of free-ranging elk (current work and Saunders et al., 2012) suggests that CWD prions are harboured by these animals in more tissues and in patterns more unpredictable than anticipated. However, we must stress that this overwhelming proportion of PrPCWD positivity in the CWD endemic cohort reflects merely the presence of amplifiable protease-resistant material in various tissues and does not necessarily indicate these animals will develop clinical disease. Therefore, the significance of this discovery in relation to definitive disease is limited to speculation. Additional longitudinal studies in experimental animals need to be performed in order to address the correlation between PrPCWD accumulation in tissues of asymptomatic elk and the resulting clinical outcomes.
We also performed sequencing analysis of the PRNP gene to investigate the role of codon 132 polymorphism genetics in vertical transmissibility of CWD (Table 1). However, unlike for sheep (Andreoletti et al., 2002), we have not found a genetic determinant or correlation between detection of amplifiable prions and the genetic profile of each dam–calf pair.
Detection of sPMCA PrPCWD in elk that test IHC-negative reiterates concerns that currently used diagnostic tests for CWD, whilst useful research tools, cannot be relied upon to detect all animals harbouring CWD prions (Monello et al., 2013; Wyckoff et al., 2015). These subclinically infected animals may serve as a mode of transmission of CWD across the landscape. Our current discovery lends a better understanding of the extent of PrPCWD distribution in tissues of elk from a CWD endemic area and to the possibility of vertical transmission in asymptomatic cervids. Furthermore, it underscores that IHC results should not be interpreted as a safety test for food for human consumption. Additional applications of highly sensitive and specific assays such as sPMCA in experimental and natural environments will help refine our understanding of prion disease epidemiology, ecology and management.
Methods
Elk tissue origin and handling
This study was performed in parallel with two other studies investigating the effects of CWD in an elk population in north-central Colorado with a long history of CWD exposure and clinical disease (Monello et al., 2013, 2014). Nineteen whole elk fetuses were collected from elk dams euthanized during early ( < 12 weeks) and mid- (12–25 weeks) gestation. Corresponding tissues were collected from the dams. For comparison, control tissues were collected from the fetuses of pregnant elk culled in the south-western part of North Dakota, a region where CWD is not known to occur. Fetuses were bagged and frozen shortly after the time of death, and then transported to Colorado State University. After thawing, tissues were harvested using single-use, animal- and tissue-specific blades and forceps to prevent cross-contamination, as previously described (Nalls et al., 2013). The following tissues were collected from each fetus: brain, vagus nerve, spleen, colon, ileum, rectum, RLN, popliteal lymph node, thymus, liver and lung. Similarly, the following maternal tissues were collected: brain at the level of the obex (CNS: ‘C’); spleen, RAMALT and RLNs (lymphoreticular system: ‘L’); uterus, ovary, placentome and umbilical cord (reproductive system: ‘R’); salivary gland, mammary gland, pancreas, kidney, urinary bladder, ureter, nasal epithelium, tongue and adipose tissue (secretory/excretory/other: ‘S’). Tissues were then homogenized at 2–10 % (w/v) in cold, sterile 0.1 M PBS containing 0.1 % Triton-X, using 0.5 mm zirconium oxide beads in a BBX24B Bullet Blender Blue homogenizer (NextAdvance). All samples were coded, double-blinded and then subjected to sPMCA as described previously (Nalls et al., 2013). Sample identities were not revealed until after all analyses were completed.
sPMCA
A 10 % normal brain homogenate (NBH) in 0.1 M PBS buffer (pH 7.5, with 1 % Triton X-100) was prepared from whole brains collected from clinically healthy cervidized transgenic 5037 mice expressing normal levels of brain PrPcervid-elk (TgCerPrP-E2265037, up to 4 months old) to serve as a substrate for the PrPC-to-PrPCWD seeded conversion reaction in sPMCA. Slight modifications were made to the previously described procedure. Briefly, 30 μl elk tissue homogenate was added to 50 μl 10 % (w/v) NBH and subjected to seven 24 h rounds of sonication. Each round of sPMCA equals 288 cycles of 10 s sonication/5 min incubation at 37 °C (Misonix). After each round, 30 μl amplified material was transferred to 0.2 ml PCR tubes containing 50 μl 10 % NBH, and two 2.38 mm and three 3.15 mm Teflon beads (McMaster-Carr). Whenever tissue availability permitted it, each tissue was sampled three times and the homogenates thus prepared were individually amplified by sPMCA. When the quantity of tissue was limited (i.e. fetal tissues), one homogenate was obtained and subjected to sPMCA three separate times. PrPres presence was ascertained upon proteinase K digestion and subsequent visualization by Western blotting using an anti-cervid PrP antibody BAR-224 (Cayman Chemical) conjugated to HRP.
Western blotting
The seventh round of the sPMCA reaction was subjected to Western blotting as described previously (Nalls et al., 2013). Briefly, known laboratory controls, and unamplified and amplified samples from CWD endemic and non-endemic areas were mixed with proteinase K (Invitrogen) for a 20 μg ml− 1 final concentration and incubated at 37 °C for 30 min, followed by an additional 10 min at 45 °C with shaking. Samples were mixed with Reducing Agent (10 × )/LDS Sample Buffer (4 × ) (Invitrogen) as per the manufacturer's instructions, heated to 95 °C for 5 min and then run through a 12 % Bistris gel at 100 V for 2 h. Proteins were transferred to a PVDF membrane in a Trans-Blot Turbo Transfer System (Bio-Rad). The membrane was blocked with Casein TBS Blocking buffer (Thermo Scientific) and probed with BAR-224 antibody as described above, and then developed with ECL Plus Western blotting Detection Reagents (GE) and viewed with an ImageQuant LAS-4000 (GE). Sample identities were revealed after blotting.
Prion inoculation into mice
Mice were inoculated intracranially as described previously (Meyerett et al., 2008). Briefly, isofluorane anaesthetized mice were inoculated with 30 μl brain homogenate intracerebrally 3 mm deep through the coronal suture 3–5 mm lateral of the sagittal suture. Onset of clinical disease was measured by scoring mice from normal (0) to exhibiting terminal clinical signs (>9) as described previously (Wyckoff et al., 2013).
Acknowledgements
We thank Drs. Nathaniel Denkers and Carmela Irene for critically reading the manuscript. We would like to thank the staff from the National Park Service Biological Resources Division, Rocky Mountain National Park and Theodore Roosevelt National Park for assistance with sample collection and preservation. This study was supported by the National Institutes of Health (RO1 AI093634).
Supplementary Data
Supplementary Data
References
- Aguzzi A., Baumann F., Bremer J. (2008). The prion's elusive reason for being Annu Rev Neurosci 31 439–477 10.1146/annurev.neuro.31.060407.125620 . [DOI] [PubMed] [Google Scholar]
- Andréoletti O., Lacroux C., Chabert A., Monnereau L., Tabouret G., Lantier F., Berthon P., Eychenne F., Lafond-Benestad S., other authors (2002). PrPSc accumulation in placentas of ewes exposed to natural scrapie: influence of foetal PrP genotype and effect on ewe-to-lamb transmission J Gen Virol 83 2607–2616 10.1099/0022-1317-83-10-2607 . [DOI] [PubMed] [Google Scholar]
- Bencsik A., Debeer S., Petit T., Baron T. (2009). Possible case of maternal transmission of feline spongiform encephalopathy in a captive cheetah PLoS One 4 e6929 10.1371/journal.pone.0006929 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béringue V., Herzog L., Jaumain E., Reine F., Sibille P., Le Dur A., Vilotte J.L., Laude H. (2012). Facilitated cross-species transmission of prions in extraneural tissue Science 335 472–475 10.1126/science.1215659 . [DOI] [PubMed] [Google Scholar]
- Bishop M.T., Diack A.B., Ritchie D.L., Ironside J.W., Will R.G., Manson J.C. (2013). Prion infectivity in the spleen of a PRNP heterozygous individual with subclinical variant Creutzfeldt-Jakob disease Brain 136 1139–1145 10.1093/brain/awt032 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P., Will R.G., Bradley R., Asher D.M., Detwiler L. (2001). Bovine spongiform encephalopathy and variant Creutzfeldt-Jakob disease: background, evolution, and current concerns Emerg Infect Dis 7 6–16 10.3201/eid0701.010102 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilla J., Brun A., Díaz-San Segundo F., Salguero F.J., Gutiérrez-Adán A., Pintado B., Ramírez M.A., del Riego L., Torres J.M. (2005). Vertical transmission of bovine spongiform encephalopathy prions evaluated in a transgenic mouse model J Virol 79 8665–8668 10.1128/JVI.79.13.8665-8668.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gangi S., Bertin M., Noventa M., Cagnin A., Cosmi E., Gizzo S. (2015). Obstetric dilemma on the most appropriate management of Creutzfeldt–Jakob disease in pregnancy: seventh case presentation, literature review and new insight J Matern Fetal Neonatal Med 28 254–261 10.3109/14767058.2014.916678 . [DOI] [PubMed] [Google Scholar]
- Dulberger J., Hobbs N.T., Swanson H.M., Bishop C.J., Miller M.W. (2010). Estimating chronic wasting disease effects on mule deer recruitment and population growth J Wildl Dis 46 1086–1095 10.7589/0090-3558-46.4.1086 . [DOI] [PubMed] [Google Scholar]
- Elder A.M., Henderson D.M., Nalls A.V., Wilham J.M., Caughey B.W., Hoover E.A., Kincaid A.E., Bartz J.C., Mathiason C.K. (2013). In vitro detection of prionemia in TSE-infected cervids and hamsters PLoS One 8 e80203 10.1371/journal.pone.0080203 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J.D., Goldmann W., Hunter N. (2013). Evidence in sheep for pre-natal transmission of scrapie to lambs from infected mothers PLoS One 8 e79433 10.1371/journal.pone.0079433 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilch S., Chitoor N., Taguchi Y., Stuart M., Jewell J.E., Schätzl H.M. (2011). Chronic wasting disease Top Curr Chem 305 51–77 10.1007/128_2011_159 . [DOI] [PubMed] [Google Scholar]
- Gill O.N., Spencer Y., Richard-Loendt A., Kelly C., Dabaghian R., Boyes L., Linehan J., Simmons M., Webb P., other authors (2013). Prevalent abnormal prion protein in human appendixes after bovine spongiform encephalopathy epizootic: large scale survey BMJ 347 f5675 10.1136/bmj.f5675 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley N.J., Mathiason C.K., Carver S., Telling G.C., Zabel M.D., Hoover E.A. (2012). Sensitivity of protein misfolding cyclic amplification versus immunohistochemistry in ante-mortem detection of chronic wasting disease J Gen Virol 93 1141–1150 10.1099/vir.0.039073-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A.F., Collinge J. (2003). Subclinical prion infection in humans and animals Br Med Bull 66 161–170 10.1093/bmb/66.1.161 . [DOI] [PubMed] [Google Scholar]
- Hill A.F., Desbruslais M., Joiner S., Sidle K.C., Gowland I., Collinge J., Doey L.J., Lantos P. (1997). The same prion strain causes vCJD and BSE Nature 389 448–450, 526 10.1038/38925 . [DOI] [PubMed] [Google Scholar]
- Ironside J.W., Head M.W., Bell J.E., McCardle L., Will R.G. (2000). Laboratory diagnosis of variant Creutzfeldt-Jakob disease Histopathology 37 1–9 10.1046/j.1365-2559.2000.00946.x . [DOI] [PubMed] [Google Scholar]
- Joly D.O., Samuel M.D., Langenberg J.A., Rolley R.E., Keane D.P. (2009). Surveillance to detect chronic wasting disease in white-tailed deer in Wisconsin J Wildl Dis 45 989–997 10.7589/0090-3558-45.4.989 . [DOI] [PubMed] [Google Scholar]
- Lacroux C., Comoy E., Moudjou M., Perret-Liaudet A., Lugan S., Litaise C., Simmons H., Jas-Duval C., Lantier I., other authors (2014). Preclinical detection of variant CJD and BSE prions in blood PLoS Pathog 10 e1004202 10.1371/journal.ppat.1004202 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makzhami S., Passet B., Halliez S., Castille J., Moazami-Goudarzi K., Duchesne A., Vilotte M., Laude H., Mouillet-Richard S., other authors (2014). The prion protein family: a view from the placenta Front Cell Dev Biol 2 35 10.3389/fcell.2014.00035 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiason C.K., Powers J.G., Dahmes S.J., Osborn D.A., Miller K.V., Warren R.J., Mason G.L., Hays S.A., Hayes-Klug J., other authors (2006). Infectious prions in the saliva and blood of deer with chronic wasting disease Science 314 133–136 10.1126/science.1132661 . [DOI] [PubMed] [Google Scholar]
- Meyerett C., Michel B., Pulford B., Spraker T.R., Nichols T.A., Johnson T., Kurt T., Hoover E.A., Telling G.C., Zabel M.D. (2008). In vitro strain adaptation of CWD prions by serial protein misfolding cyclic amplification Virology 382 267–276 10.1016/j.virol.2008.09.023 . [DOI] [PubMed] [Google Scholar]
- Miller M.W., Williams E.S. (2004). Chronic wasting disease of cervids Curr Top Microbiol Immunol 284 193–214 . [DOI] [PubMed] [Google Scholar]
- Monello R.J., Powers J.G., Hobbs N.T., Spraker T.R., O'Rourke K.I., Wild M.A. (2013). Efficacy of antemortem rectal biopsies to diagnose and estimate prevalence of chronic wasting disease in free-ranging cow elk (Cervus elaphus nelsoni) J Wildl Dis 49 270–278 10.7589/2011-12-362 . [DOI] [PubMed] [Google Scholar]
- Monello R.J., Powers J.G., Hobbs N.T., Spraker T.R., Watry M.K., Wild M.A. (2014). Survival and population growth of a free-ranging elk population with a long history of exposure to chronic wasting disease J Wildl Manage 78 214–223 10.1002/jwmg.665. [DOI] [Google Scholar]
- Nalls A.V., McNulty E., Powers J., Seelig D.M., Hoover C., Haley N.J., Hayes-Klug J., Anderson K., Stewart P., other authors (2013). Mother to offspring transmission of chronic wasting disease in Reeves' muntjac deer PLoS One 8 e71844 10.1371/journal.pone.0071844 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T.A., Pulford B., Wyckoff A.C., Meyerett C., Michel B., Gertig K., Hoover E.A., Jewell J.E., Telling G.C., Zabel M.D. (2009). Detection of protease-resistant cervid prion protein in water from a CWD-endemic area Prion 3 171–183 10.4161/pri.3.3.9819 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notari S., Moleres F.J., Hunter S.B., Belay E.D., Schonberger L.B., Cali I., Parchi P., Shieh W.J., Brown P., other authors (2010). Multiorgan detection and characterization of protease-resistant prion protein in a case of variant CJD examined in the United States PLoS One 5 e8765 10.1371/journal.pone.0008765 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta O.A., Eyestone W.H. (2009). Quantitative and qualitative analysis of cellular prion protein (PrPC) expression in bovine somatic tissues Prion 3 161–170 10.4161/pri.3.3.9772 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta O.A., Huckle W.R., Eyestone W.H. (2012). Developmental expression of the cellular prion protein (PrPC) in bovine embryos Mol Reprod Dev 79 488–498 10.1002/mrd.22057 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S.B. (1982). Novel proteinaceous infectious particles cause scrapie Science 216 136–144 10.1126/science.6801762 . [DOI] [PubMed] [Google Scholar]
- Race R., Jenny A., Sutton D. (1998). Scrapie infectivity and proteinase K-resistant prion protein in sheep placenta, brain, spleen, and lymph node: implications for transmission and antemortem diagnosis J Infect Dis 178 949–953 10.1086/515669 . [DOI] [PubMed] [Google Scholar]
- Reed J., Muench H. (1938). A simple method of estimating fifty per cent end points Am J Hygiene 27 493–497. [Google Scholar]
- Saá P., Castilla J., Soto C. (2006). Ultra-efficient replication of infectious prions by automated protein misfolding cyclic amplification J Biol Chem 281 35245–35252 10.1074/jbc.M603964200 . [DOI] [PubMed] [Google Scholar]
- Sakudo A., Onodera T. (2014). Prion protein (PrP) gene-knockout cell lines: insight into functions of the PrP Front Cell Dev Biol 2 75 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargeant G.A., Weber D.C., Roddy D.E. (2011). Implications of chronic wasting disease, cougar predation, and reduced recruitment for elk management J Wildl Manage 75 171–177 10.1002/jwmg.27. [DOI] [Google Scholar]
- Saunders S.E., Bartelt-Hunt S.L., Bartz J.C. (2012). Occurrence, transmission, and zoonotic potential of chronic wasting disease Emerg Infect Dis 18 369–376 10.3201/eid1803.110685 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdson C.J., Aguzzi A. (2007). Chronic wasting disease Biochim Biophys Acta 1772 610–618 10.1016/j.bbadis.2006.10.010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiropoulos J., Hawkins S.A., Simmons M.M., Bellworthy S.J. (2014). Evidence of in utero transmission of classical scrapie in sheep J Virol 88 4591–4594 10.1128/JVI.03264-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spraker T.R., VerCauteren K.C., Gidlewski T., Schneider D.A., Munger R., Balachandran A., O'Rourke K.I. (2009). Antemortem detection of PrPCWD in preclinical, ranch-raised Rocky Mountain elk (Cervus elaphus nelsoni) by biopsy of the rectal mucosa J Vet Diagn Invest 21 15–24 10.1177/104063870902100103 . [DOI] [PubMed] [Google Scholar]
- Wells G.A., Wilesmith J.W., McGill I.S. (1991). Bovine spongiform encephalopathy: a neuropathological perspective Brain Pathol 1 69–78 10.1111/j.1750-3639.1991.tb00642.x . [DOI] [PubMed] [Google Scholar]
- Williams E.S., Miller M.W. (2002). Chronic wasting disease in deer and elk in North America Rev Sci Tech 21 305–316 . [DOI] [PubMed] [Google Scholar]
- Wooding F.B., Morgan G., Adam C.L. (1997). Structure and function in the ruminant synepitheliochorial placenta: central role of the trophoblast binucleate cell in deer Microsc Res Tech 38 88–99 . [DOI] [PubMed] [Google Scholar]
- Wyckoff A.C., Lockwood K.L., Meyerett-Reid C., Michel B.A., Bender H., VerCauteren K.C., Zabel M.D. (2013). Estimating prion adsorption capacity of soil by BioAssay of Subtracted Infectivity from Complex Solutions (BASICS) PLoS One 8 e58630 10.1371/journal.pone.0058630 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff A.C., Galloway N., Meyerett-Reid C., Powers J., Spraker T., Monello R.J., Pulford B., Wild M., Antolin M., other authors (2015). Prion amplification and hierarchical Bayesian modeling refine detection of prion infection Sci Rep 5 8358 10.1038/srep08358 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data