Abstract
Background
Resistin-like molecule-β (RELMβ) is a novel secretory protein from intestinal goblet cells and participates in epithelial differentiation, tumor occurrence, and immune response. RELMβ is absent in normal gastric mucosa but is abundantly expressed in gastric carcinoma tissues, and is correlated with tumor invasion and metastasis. Epithelial-mesenchymal transition (EMT) is an important mechanism governing tumor cell invasion. This study thus investigated the modulation of RELMβ in gastric cancer metastasis and its correlation with EMT.
Material/Methods
We used RELMβ-low expression AGS cell line of gastric cancer and normal mucosa cell line GES1 as in vitro models, on which RELMβ0-expressing vector was transfected. The invasion and migration of cells were quantified by Transwell assay. EMT-related protein including E-cadherin, N-cadherin, Snail, and Vimentin were detected by Western blotting in transfected AGS cells.
Results
RELMβ transfection significantly potentiated invasion and migration abilities of AGS cells, whose RELMβ protein level was significantly elevated compared to those in untransfected AGS or GES1 cells. After RELMβ transfection, EMT-related proteins, including N-cadherin, Snail, and Vimentin levels, were elevated, but E-cadherin expression was depressed.
Conclusions
RELMβ-overexpression can facilitate invasion and migration of gastric carcinoma cells and it increases the expression of EMT-related proteins, such as N-cadherin, Snail, Vimentin, but decreases E-cadherin level, thus promoting the progression of EMT.
MeSH Keywords: Activated-Leukocyte Cell Adhesion Molecule; Carcinoma, Acinar Cell; Precursor Cells, B-Lymphoid
Background
The members of the resistin-like molecules (RELMs) family share the same cysteine structure. As an important family member, RELMβ is secreted by intestinal goblet cells [1]. RELMβ has been shown to be abundantly expressed in gastric carcinoma tissues and is closely correlated with biological behaviors and clinical features of tumors [2]. Endothelial-mesenchymal transition (EMT), an important process underlying tumor invasion and metastasis, requires the participation of multiple cytokines for the transformation of epithelial cell genes [3]. This study transfected RELMβ-expressing vector into gastric carcinoma cell line and normal gastric mucosa epithelial cells, on which cell invasion and metastatic abilities, along with EMT-related factors, including E-cadherin, N-cadherin, Snail, Vimentin, were measured.
Material and Methods
Cell culture and transfection
Gastric carcinoma cell line AGS and normal gastric mucosa epithelial cell line GES1 were purchased from Baili Biotech (Shanghai, China). Cells were cultured in RPMI1640 medium (Gibco, USA) in a humidified chamber at 37°C perfused with 5% CO2.
Recombinant vector pClR1- RELMβ and expressing vector peDNA3.1Zeo(+) were digested by EcoRI enzyme, followed by gel purification and de-phosphorylation. Log-phased cells were seeded into 6-well plates in 3 groups: blank control (no transfection), empty vector pcDNA3.1/Zeo(+) transfection, and pcDNA3.1- RELMβ transfected groups. The transfection was performed using the Lipofeoatmine2000 kit (Invitrogen, USA) following the manufacturer’s instructions.
Real-time PCR
Log-phased AGS and GES1 cells were collected, along with transfected AGS cells. Total RNA was extracted by use of the Trizol kit (Invitrogen, USA) following the manual instruction. After quantification by A260/A280 ratio, 200 ng RNA was used as the template for synthesizing cDNA using polyA tail. PCR amplification was performed using a fluorescent quantitative PCR kit (Sangon, China). Specific primers were designed as follows: “RELMβ: 5′-CACCATGAAG CCTACACTGTGTTTCC-3′ (Forward), 5′-TTAAACCATTCGGCAGCAGCGG-3′ (Reverse); GAPDH: 5′-GCCAAGGTCATCCATGACAACTTTGG-3′ (Forward), 5′-GCCTGCTTCACCACC TTCTTGATGTC-3′ (Reverse)”. PCR amplification consisted of 40 cycles each containing 95°C denature for 30 s, 60°C annealing for 30 s and 72°C elongation for 45 s. The relative mRNA expression was calculated by comparative Ct method.
Transwell chamber assay
For evaluating invasion ability, A Transwell chamber (Millipore, USA) was pre-coated with Matrigel at 4°C overnight, followed by incubation of serum-free medium on the artificial membrane at 37°C for 1 h. Transfected AGS cells were seeded in the upper chamber, while RPMI1640 medium was added into the lower chamber. At the endpoint, cells were stained by Giemsa and observed under an inverted microscope. For evaluating cell migration ability, cells were seeded in the chamber in the same manner without an artificial basal membrane. Cell invasion and migration ability was assessed through counting the numbers of positive-staining cells under a microscope.
Western blotting
Transfected AGS cells were collected for total protein extraction. After quantification, proteins were separated in 8% SDS-PAGE (40 μg per well) and were transferred to the PVDF membrane. The membrane was blocked for 1 h at room temperature. Mouse anti-human RELMβ monoclonal antibody (1:200, Santa Cruz, USA), mouse anti-human vimentin, N-cadherin, E-cadherin polyclonal antibodies (1:1 2000, Santa Cruz, USA), or mouse anti-human Snail monoclonal antibody (1:700, Cell Signaling, USA) was applied for 4°C overnight incubation. After being cultured in secondary antibody and development, the membrane was exposed and the image was captured and analyzed. Intensity of positive bands was quantified using Image J software. Protein expression was quantified as a ratio to loading control (β-actin or GADPH).
Statistical analysis
We used the SPSS 17.0 software package to process all collected data. Measurement data are presented as mean ± standard deviation (SD), while enumeration data were compared by chi-square test. The t test was used for between-group-comparisons. Statistical significance was defined when p<0.05.
Results
RELMβ expression in transfected AGS cells
Using real-time PCR to detect the mRNA level of RELMβ in AGS cells after transfection, we found significantly elevated RELMβ mRNA level after transfection (p<0.05, Table 1, Figure 1).
Table 1.
RELMβ mRNA expression in transfected AGS cells.
p<0.05 compared to AGS cells;
p<0.05 compared to GES1 cells.
Figure 1.
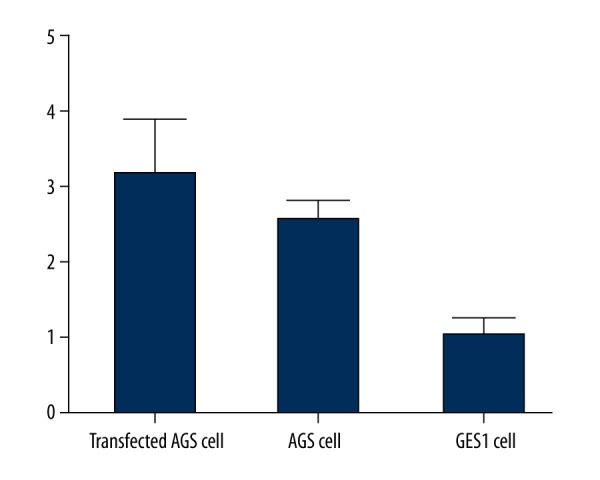
RELMβ mRNA expression by RT-PCR.
Cell migration and invasion assay
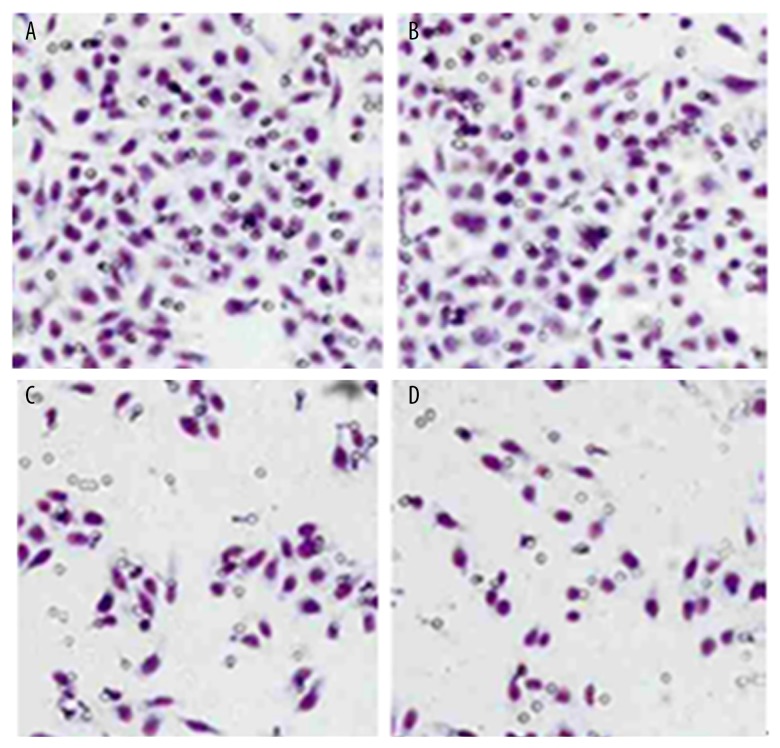
Using Transwell assay to detect migration and invasion ability of RELMβ-transfected AGS cells, results showed significantly potentiated invasion and migration ability of AGS cells after transfection (p<0.05, Figure 2).
Figure 2.
Migration and invasion ability of RELMβ-transfected AGS cells. (A, C) show cell invasion assay; (B, D) show cell migration assay. (A, B) are RELMβ-transfected AGS cells; (C, D) are untransfected AGS cells. Magnification of all images, 200×.
RELMβ protein expression in transfected AGS cells
Western blot assay showed significantly elevated RELMβ protein levels in RELMβ-transfected AGS cells compared to blank AGS cells or GES1 cells (p<0.05, Table 2, Figure 3).
Table 2.
RELMβ protein expression.
p<0.05 compared to AGScells;
p<0.05 compared to GES1 cells.
Figure 3.
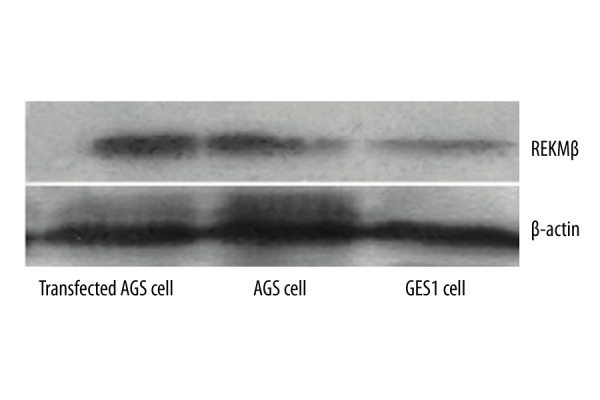
RELMβ protein expressions. β-actin was used as a loading control.
EMT-related protein levels
We further tested the expression of EMT-related proteins, including E-cadherin, N-cadherin, Snail, and Vimentin, in those AGS cells transfected with RELMβ. Results showed significantly elevated expression of N-cadherin, Snail, and Vimentin in post-transfected cells, while E-cadherin expression was depressed (p<0.05, Table 3, Figure 4).
Table 3.
EMT-related protein expression levels.
| Group | RELMβ transfected | AGS cell | GES1 cell |
|---|---|---|---|
| E-cadherin | 0.14±0.03*,# | 0.26±0.05 | 0.44±0.07 |
| N-cadherin | 0.76±0.09*,# | 0.46±0.05 | 0.15±0.07 |
| Snail | 0.65±0.04*,# | 0.37±0.08 | 0.18±0.06 |
| Vimentin | 1.16±0.12*,# | 0.28±0.02 | 0.13±0.07 |
p<0.05 compared to AGS cells;
p<0.05 compared to GES1 cells.
Figure 4.
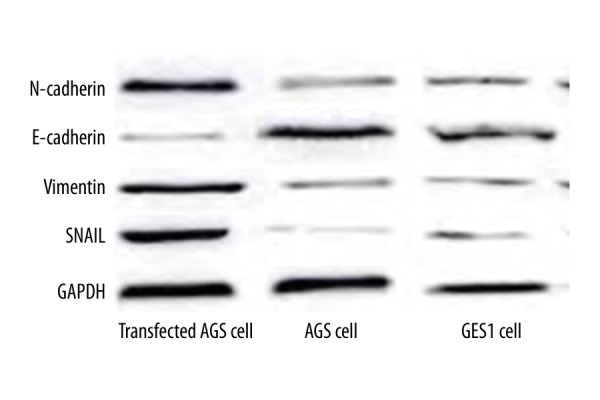
EMT-related protein expressions in RELMβ-transfected AGS cells, AGS cells, and GES1 cells.
Discussion
The RELMs family is a group of secretory proteins containing abundant cysteine. It consists of 4 major family members: RELMα/HIMF, RELMβ/FIZZ2, Resistin/FIZZ3, and RELMγ/FIZZ4 [4,5]. RELMβ was first discovered in epithelial cells of the mouse intestine, while human RELMβ is mainly distributed in pulmonary vessels, bronchial mucosa epithelial cells, intestinal epithelial cells, kidney, and adrenal glands [6]. Studies have confirmed the elevated RELMβ secretion from intestinal goblet cells in mice infected with nematodes, and such secretion was mediated by cytokines, including IL-4, IL-13, and STAT6 for specific mucosal immunity and gastrointestinal defense [7]. People with elevated plasma RELMβ levels normally have smoking history or deficits of exercise, both of which are independent risk factors for gastric carcinoma [8].
In this study we transfected RELMβ-expressing vector into gastric cancer AGS cells, in parallel with blank AGS and normal gastric mucosal epithelial GES1 cells as controls. Real-time PCR and Western blotting showed significantly elevated expression level of RELMβ in those cells after transfection. Transwell assay demonstrated increased migration and invasion abilities of transfected AGS cells. A further comparison showed that, even in intact AGS cells, RELMβ expression level was still higher than in GES1 cells. These data collectively show that over-expression of RELMβ can facilitate the migration and invasion of gastric carcinoma cells. It has been discovered that RELMβ is up-regulated in colorectal cancer tissues compared to those in normal intestine epithelial cells. Moreover, serum RELMβ in gastrointestinal tumor patients was positively correlated with tumor stage and malignancy [9,10]. A systematic study of 156 gastric cancer patients also revealed the correlation between higher RELMβ level and unfavorable prognosis, possibly due to the elevated expression of RELMβ in gastric carcinoma tissues and further induction of matrix metalloproteinase-2 (MMP-2) and MMP-9 from mononuclear cells for tumor progression [11]. Esophagus carcinoma and Barrett esophageal atypical proliferation cells also had elevated serum RELMβ levels with advancement of TNM stage, making it a potential marker for esophageal squamous carcinoma. RELMβ has also been suggested to facilitate mitosis, proliferation, and migration of fibroblasts [12]. The binding of CDX-2 onto human RELMβ can facilitate proliferation of intestinal epithelial cells, modulate cell cycle, and potentiate cell-to-cell adhesion, making the RELMβ level negatively correlated with tumor differentiation stage [13,14].
EMT has been shown to be closely related with invasion and migration of malignant tumors, mainly to due to the acquirement of mesenchymal cells in epithelial-derived cells [15]. As a tumor-suppressor gene, E-cadherin can limit the peripheral infiltration of tumor cells, along with the invasive migration [16]. N-cadherin, however, mainly inhibits cell apoptosis and is positively expressed in the formation and differentiation of embryonic tissues [17]. Vimentin is expressed in mesenchymal tissues and is related with tumor invasion and metastasis, making it an important cytokine in aggravating tumor malignancy [18]. The incidence of gastric carcinoma in China is increasing. Due to its insidious onset, gastric cancer is usually diagnosed at its late stage. With the progression of disease, there will be aggravated tumor cell invasion, which can be reflected by EMT as decreased epithelial marker E-cadherin and increased mesenchymal markers N-cadherin, Snail, and Vimentin [19,20]. In this study, we found elevated protein levels of N-cadherin, Snail, and Vimentin in RELMβ-transfected AGS cells, in addition to decreased expression of E-cadherin, all of which suggest the progression of EMT. We thus propose that the down-regulation of RELMβ may impede the occurrence of EMT, although further studies are required for substantiation.
Conclusions
RELMβ is abundantly expressed in gastric carcinoma cells, and can facilitate the invasion and migration of tumor cells via facilitating EMT, as it increased expression of EMT-related proteins, including N-cadherin, Snail, and Vimentin, in addition to inhibiting E-cadherin expression. The early and timely detection of RELMβ, therefore, may provide a novel diagnostic index and/or drug target for gastric cancer.
Footnotes
Source of support: Natural Science Fund project in Shandong province (ZR2013HQ031)
References
- 1.Zheng LD, Yang CL, Qi T, et al. Effects of resistin-like molecule beta over-expression on gastric cancer cells in vitro. World J Gastroenterol. 2012;18(8):754–66. doi: 10.3748/wjg.v18.i8.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng LD, Tong QS, Weng MX, et al. Enhanced expression of resistin-like molecule beta in human colon cancer and its clinical significance. Dig Dis Sci. 2009;54(2):274–81. doi: 10.1007/s10620-008-0355-2. [DOI] [PubMed] [Google Scholar]
- 3.Polk DB, Peek RM., Jr Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10(6):403–14. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angelini DJ, Su Q, Kolosova IA, et al. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELM alpha) recruits bone marrow-derived cells to the murine pulmonary vasculature. PLoS One. 2010;5(6):e11251. doi: 10.1371/journal.pone.0011251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holcomb IN, Kabakoff RC, Chan B, et al. FIZZ1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. Embo J. 2000;19(15):4046–55. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbert DR, Yang JQ, Hogan SP, et al. Intestinal epithelial cell secretion of RELM-beta protects against gastrointestinal worm infection. J Exp Med. 2009;206(13):2947–57. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Panhuys N, Camberis M, Yamada M, et al. Mucosal trapping and degradation of Nippostrongylus brasiliensis occurs in the absence of STAT6. Parasitology. 2013;140(7):833–43. doi: 10.1017/S0031182012002260. [DOI] [PubMed] [Google Scholar]
- 8.Neilson AP, Djuric Z, Land S, Kato I. Plasma levels of resistin-like molecule beta in humans. Cancer Epidemiol. 2011;35(5):485–89. doi: 10.1016/j.canep.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mavi P, Niranjan R, Dutt P, et al. Allergen-induced resistin-like molecule-alpha promotes esophageal epithelial cell hyperplasia in eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol. 2014;307(5):G499–507. doi: 10.1152/ajpgi.00141.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaji-Kegan K, Su Q, Angelini DJ, et al. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMalpha) increases lung inflammation and activates pulmonary microvascular endothelial cells via an IL-4-dependent mechanism. J Immunol. 2010;185(9):5539–48. doi: 10.4049/jimmunol.0904021. [DOI] [PubMed] [Google Scholar]
- 11.Zheng L, Weng M, He J, et al. Expression of resistin-like molecule beta in gastric cancer: its relationship with clinicopathological parameters and prognosis. Virchows Arch. 2010;456(1):53–63. doi: 10.1007/s00428-009-0861-4. [DOI] [PubMed] [Google Scholar]
- 12.Angelini DJ, Su Q, Yamaji-Kegan K, et al. Resistin-like molecule-beta in scleroderma-associated pulmonary hypertension. Am J Respir Cell Mol Biol. 2009;41(5):553–61. doi: 10.1165/rcmb.2008-0271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamaji-Kegan K, Takimoto E, Zhang A, et al. Hypoxia-induced mitogenic factor (FIZZ1/RELMalpha) induces endothelial cell apoptosis and subsequent interleukin-4-dependent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2014;306(12):L1090–103. doi: 10.1152/ajplung.00279.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kushiyama A, Sakoda H, Oue N, et al. Resistin-like molecule beta is abundantly expressed in foam cells and is involved in atherosclerosis development. Arterioscler Thromb Vasc Biol. 2013;33(8):1986–93. doi: 10.1161/ATVBAHA.113.301546. [DOI] [PubMed] [Google Scholar]
- 15.Szczepanik AM, Scislo L, Scully T, et al. IL-6 serum levels predict postoperative morbidity in gastric cancer patients. Gastric Cancer. 2011;14(3):266–73. doi: 10.1007/s10120-011-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liew PL, Hsu CS, Liu WM, et al. Prognostic and predictive values of Nrf2, Keap1, p16 and E-cadherin expression in ovarian epithelial carcinoma. Int J Clin Exp Pathol. 2015;8(5):5642–49. [PMC free article] [PubMed] [Google Scholar]
- 17.Mrozik KM, Cheong CM, Hewett D, et al. Therapeutic targeting of N-cadherin is an effective treatment for multiple myeloma. Br J Haematol. 2015;171(3):387–99. doi: 10.1111/bjh.13596. [DOI] [PubMed] [Google Scholar]
- 18.Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest. 2011;121(9):3375–83. doi: 10.1172/JCI57158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lukaszewicz-Zając M, Mroczko B, Gryko M, et al. Comparison between clinical significance of serum proinflammatory proteins (IL-6 and CRP) and classic tumor markers (CEA and CA 19-9) in gastric cancer. Clin Exp Med. 2011;11(2):89–96. doi: 10.1007/s10238-010-0114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grant JL, Fishbein MC, Hong LS, et al. A novel molecular pathway for Snail-dependent, SPARC-mediated invasion in non-small cell lung cancer pathogenesis. Cancer Prev Res (Phila) 2014;7(1):150–60. doi: 10.1158/1940-6207.CAPR-13-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]