This review focuses on non-invasive endocrinology, which is a key component of amphibian conservation physiology. It enables rapid assessment of reproductive and stress hormones in free-living and captive populations. It also provides a direct physiological measure of population sensitivity to extreme environments and their sub-lethal impacts on reproduction, health and survival.
Keywords: Amphibians, conservation physiology, ecological applications, non-invasive endocrinology, reproduction, stress
Abstract
Non-invasive endocrinology utilizes non-invasive biological samples (such as faeces, urine, hair, aquatic media, and saliva) for the quantification of hormones in wildlife. Urinary-based enzyme immunoassay (EIA) and radio-immunoassay have enabled the rapid quantification of reproductive and stress hormones in amphibians (Anura: Amphibia). With minimal disturbance, these methods can be used to assess the ovarian and testicular endocrine functions as well as physiological stress in captive and free-living populations. Non-invasive endocrine monitoring has therefore greatly advanced our knowledge of the functioning of the stress endocrine system (the hypothalamo–pituitary–interrenal axis) and the reproductive endocrine system (the hypothalamo–pituitary–gonadal axis) in the amphibian physiological stress response, reproductive ecology, health and welfare, and survival. Biological (physiological) validation is necessary for obtaining the excretory lag time of hormone metabolites. Urinary-based EIA for the major reproductive hormones, estradiol and progesterone in females and testosterone in males, can be used to track the reproductive hormone profiles in relationship to reproductive behaviour and environmental data in free-living anurans. Urinary-based corticosterone metabolite EIA can be used to assess the sublethal impacts of biological stressors (such as invasive species and pathogenic diseases) as well as anthropogenic induced environmental stressors (e.g. extreme temperatures) on free-living populations. Non-invasive endocrine methods can also assist in the diagnosis of success or failure of captive breeding programmes by measuring the longitudinal patterns of changes in reproductive hormones and corticosterone within captive anurans and comparing the endocrine profiles with health records and reproductive behaviour. This review paper focuses on the reproductive and the stress endocrinology of anurans and demonstrates the uses of non-invasive endocrinology for advancing amphibian conservation physiology. It also provides key technical considerations for future research that will increase the accuracy and reliability of the data and the value of non-invasive endocrinology within the conceptual framework of conservation physiology.
Introduction
The Earth's biodiversity is experiencing pressure from multiple threatening processes, such as habitat loss, over-harvesting, global climate change, invasive species, and pathogenic diseases, that could be working in synergy to escalate the global extinction crisis (Brook et al., 2008). Comprehensive understanding of the sensitivity of animals, particularly threatened free-living populations, towards critical changes in their surroundings is urgently needed for determining species vulnerability and setting conservation priorities (Hofmann and Todgham, 2010). Conservation physiology is an emerging and important field of conservation science that uses the investigation of complex physiological systems, such as the neuro-endocrine stress axis, to understand how different physical and psychological stressors are affecting the behaviour of study organisms, thus providing an organismic view of the environmental challenges and conservation threats faced (Wikelski and Cooke, 2006). Most recently, Cooke et al. (2013) have defined conservation physiology as ‘an integrative scientific discipline applying physiological concepts, tools, and knowledge to characterizing biological diversity and its ecological implications; understanding and predicting how organisms, populations, and ecosystems respond to environmental change and stressors; and solving conservation problems across the broad range of taxa (i.e. including microbes, plants, and animals)’.
Vertebrate physiologists have been making remarkable contributions to conservation physiology, mainly through investigations of stress and reproductive endocrinology of threatened and rare wildlife (Berger et al., 1999; Gordon and Bartol, 2004; Stevenson et al., 2005; Wikelski and Cooke, 2006). Non-invasive endocrine studies, especially via the development of reproductive and stress hormone assays using excreta (such as faeces or urine), have been successfully used for quantifying the reproductive hormone cycles and physiological stress in numerous vertebrate species, such as big cats, primates, marsupials, birds, reptiles, and amphibians (Janine, 2006; Schwarzenberger, 2007; Heistermann, 2010; Narayan et al., 2010a, b, 2012f). Other non-invasive biological samples, such as hair (Koren et al., 2002), saliva (Kirschbaum and Hellhammer, 1999), and aquatic medium, such as pond water (Gabor et al., 2013), have also been used successfully for monitoring the reproductive and stress endocrine functions in animals. Non-invasive endocrinology provides a direct measure of reproductive hormone cycles and physiological stress responses of wildlife in captivity as well as in situ populations (Ziegler et al., 1997; Dittami et al., 2008; Narayan et al., 2012f, 2013c). Thus, non-invasive endocrinology can provide crucial insight into the relationship between a population and its environment.
Amphibians are a group of animals whose ancestors evolved from a group of fishes to be the first vertebrates to colonize the world's land surface about 270 million years ago (Cogger, 2000). The class Amphibia contains nearly 6000 known species (Stebbins and Cohen, 1995). They have existed on earth for ∼300 million years, yet very recently (over the last several decades) nearly 168 species are believed to have gone extinct and at least 2469 (43%) species are in decline, indicating that the number of threatened species will probably continue to increase (Stuart et al., 2004). There is a rapid and alarming disappearance of amphibian species in seemingly undisturbed ecosystems. This decline of amphibian species is a matter of great concern, mainly because they are important components of many ecosystems and, in some locations, they may constitute the highest fraction of vertebrate biomass in these ecosystems (Blaustein et al., 2010). Several key factors causing global amphibian population declines include global climate change, habitat destruction, contaminants, and pathogenic diseases (such as chytridiomycosis) and invasive species (Stuart et al., 2004; Pounds et al., 2006; Rohr and Raffel, 2010). Interest in amphibian conservation physiology has had a recent resurgence with the global realization that amphibian populations and species are rapidly disappearing (Stuart et al., 2004). While there are several proposed causes for the demise of amphibian populations, they are all based on a common mechanism, namely the inability of amphibian biology to cope with the new and, in many cases, unnatural environmental perturbations. Integration of reproductive and stress hormone assessment with knowledge of the basic biology of anurans can provide a powerful diagnostic tool for both captive and wild populations. As demonstrated in other animals, a fairly mild ecological disturbance, such as eco-tourism, when analysed from a physiological perspective may be found to induce high stress hormone levels (Wasser et al., 1997; Wikelski and Cooke, 2006). Non-invasive endocrinology brings a multifocal approach to solving conservation problems.
This review focuses on amphibian reproductive and stress endocrinology with reference to the recent comprehensive reviews available on these topics. It summarizes the current state of knowledge on the endocrinology of stress in amphibians. Next, it discusses the methodological validation, including physiological (biological) and laboratory validation. It then explores, using field and laboratory-based examples, the contributions of non-invasive endocrinology to amphibian conservation physiology. The review also deliberates technical considerations, including strengths, limitations, and directions for future research that will increase the accuracy, sensitivity, and reliability of the non-invasive endocrine data. Finally, the review provides a conceptual framework that integrates non-invasive endocrinology with other fundamental research areas within conservation physiology.
Amphibian stress (endocrine) physiology
Comprehensive reviews related to the key concepts of the stress theories are available (Romero et al., 2009; McEwen and Wingfield, 2010). Stress is a term that is used across a broad spectrum of scientific research and, as a result, its definition is often ambiguous, and it is sometimes not defined at all (McEwen and Wingfield, 2003; Overli et al., 2007). For the purpose of this review, a stressor (or stress event) will be defined as a noxious stimulant, which exposes the amphibian to energetic costs outside of that required for predictable daily and seasonal requirements. The field of stress physiology was born in 1936, when Hans Selye characterized the stress response as a common set of generalized physiological responses that were experienced by all organisms exposed to a variety of environmental challenges, such as temperature change or exposure to noise. Several decades later, Sterling and Eyer (1988) introduced the term ‘allostasis’, which has been heavily advocated by McEwen and Stellar (1993) as the body's ability to adapt to a changing environment in situations that did not challenge survival. McEwen and Wingfield (2007) have defined the differences between baseline and stress-induced stress hormone levels in three physiological states, termed allostasis, allostatic load, and allostatic overload. Allostasis refers to achieving stability through change, and basal stress hormone levels support allostasis via the regulation of energy distribution to vital systems during predictable environmental changes and life-history stages. Allostatic load refers to the cumulative result of maintaining allostasis and accounts for the seasonal increases in stress hormones caused by predictable increases in energy requirements during more demanding periods, such as winter and breeding. Allostatic overload occurs when an unpredictable event increases energy demands beyond the point at which energy modulation can successfully deal with it, without suppressing other physiological systems. While an acute stress response is necessary to ensure survival and allow adaptation to changes in the environment, it is a problem when the physiological stress response becomes chronic and threatens animal well-being by exerting deleterious effects on the individual's biological state. This has welfare implications during energetically costly periods, such as growth and reproduction, or when animals are suffering from parasites or other diseases (Monfort, 2003; Kindermann et al., 2012).
Stress responses can include a range of physiological systems and behaviours, but the common element is activation of the hypothalamo–pituitary–interrenal (HPI) axis in amphibians, which is homologous to the hypothalamo–pituitary–adrenal axis in higher vertebrates (Rollins-Smith, 2001). The HPI axis is a combination of neural transmitters and hormone responses, and allows the individual to react to a stress event in a way that maximizes survival chances (Hill et al., 2008). The first phase of HPI axis activation involves the central nervous system receiving signals that a stress event has occurred. Within seconds, signals are sent to the hypothalamus, where corticotrophin-releasing hormone is released. Corticotrophin-releasing hormone travels in the hypothalamo–hypophyseal portal system to the anterior pituitary gland, where adrenocorticotrophic hormone (ACTH) is released. The ACTH is released into the general blood circulation, where it travels to the interrenal cortex (adrenal cortex in higher vertebrates) to stimulate the release of glucocorticoids (cortisol in most mammals, but corticosterone in rodents and most other vertebrates, including amphibians, reptiles, birds, and fishes). Glucocorticoid release is considered to be a hallmark of the vertebrate stress endocrine response. Glucocorticoids are metabolic hormones that maintain blood glucose levels during fasting. They also have a wide range of other actions, including effects on intermediary metabolism, immune function, behaviour, electrolyte balance, growth, and reproduction (Guillette et al., 1995). Secretion of glucocorticoids also causes increased heart rate, increased ventilation, vasoconstriction, fat catabolism, and a decrease in digestion and insulin production (Hill et al., 2008; Demas et al., 2011). The glucocorticoids are released when an animal responds to a stressor, so that the animal can adjust to the stressor, but if the stressor is prolonged then chronically elevated glucocorticoid levels may have detrimental effects on the individual (Bliley and Woodley, 2012).
Measurement of stress hormones
Blood plasma or serum has been used widely for assessing amphibian stress endocrine function. In 1961, Carstensen and colleagues demonstrated in vitro that corticosterone and aldosterone were the primary steroids formed in incubates of the interrenal glands of the American bullfrog (Rana catesbeiana) by stimulating their production using mammalian ACTH (Carstensen et al., 1961). Dale (1962) probably provided the earliest work on glucocorticoid measurement in anuran excreta through his study on the leopard frog [Rana pipiens (Schreber)] and the northern wood frog [Rana sylvatica (Le Conte)] larvae. In another study, Jungreis et al. (1970) identified corticosterone and aldosterone (3:1 ratio) as major hormonal products of the interrenal tissues of R. pipiens. Interestingly, earlier studies have also reported that fully aquatic amphibians produce cortisol as their primary glucocorticoid, while terrestrial or semi-terrestrial amphibians produce corticosterone as their primary glucocorticoid (Bern and Nandi, 1964; Nandi, 1967). In their study, Jungreis et al. (1970) suggested a potential ontogenetic shift in the steroidogenic pathway from cortisol in the aquatic larvae to corticosterone in the semi-terrestrial or terrestrial adult. A few years later, Leboulenger et al. (1977) measured corticosterone in plasma samples of the edible frog (Rana esculenta) via a competitive protein-binding radio-immunoassay (RIA) method using baboon plasma as the source of corticosterone-binding globulin (CBG). Their study most possibly provided the first demonstration of a seasonal pattern of plasma corticosterone in anurans. Two years later, Dupont and colleagues provided the first evidence of circadian and circannual variation in corticosterone in mature male and female free-living R. esculenta (Dupont et al., 1979). Thereafter, Leboulenger and colleagues developed two RIA techniques for the direct measurement of frog plasma concentrations of corticosterone (Leboulenger et al., 1982). Several studies went on to use the RIA technique to measure plasma corticosterone in anurans. For example, Pancak and Taylor (1983) studied the daily and seasonal rhythms of plasma corticosterone in American toads (Bufo americanus). Their study also provided insights into the role of corticosterone in amphibian locomotion. Following on, Licht et al. (1983) demonstrated the effects of capture and captivity on plasma levels of gonadal steroids and on corticosterone in the bullfrog (Rana catesbeiana). Two years later, Mendonça et al. (1985) reported the relationships between amphibian reproductive steroids (such as plasma levels of testosterone and luteinizing hormone) and plasma corticosterone in R. esculenta. Numerous studies based on the plasma RIA methods went on to explore the relationships between stress and reproduction in amphibians (see detailed reviews on this topic by Moore et al., 2005; Wilczynski et al., 2005). The limitations of the RIA technique are mainly associated with its use of radioactive labels, which requires a special laboratory for protection from exposure to radiation, and also due to the short half-life of many radioisotopes, such as 125I (60 days), 131I (8 days), and 3H (12 years; Vasudevan et al., 2011). Immunological techniques, such as enzyme immunoassays (EIAs), are used today because they are cheap and use non-radioactive labels. Direct enzyme-linked immunosorbent assay, also known as single-antibody EIA, is as sensitive as RIA and is gaining in popularity for the measurement of reproductive and stress hormone metabolites in amphibians (Germano et al., 2009; Narayan et al., 2010a, b; Janin et al., 2011; Kindermann et al., 2012).
Quantification of acute physiological stress hormone responses of amphibians
Manual capture and some form of restraint or confinement have often been used for assessing the acute (short-term) physiological stress responses in laboratory model animals, such as rats and mice (Markham et al., 2006; Zamudio et al., 2009), as well as birds (Muller et al., 2006) and reptiles (Cree et al., 2003; Klukowski, 2011). In amphibians, several earlier studies have demonstrated the effect of capture and handling on plasma corticosterone. For example, in the whistling frog (Litoria ewingii) plasma corticosterone increased at 30 min and was higher at 3 h after capture (Coddington and Cree, 1995). Plasma concentrations of corticosterone also increased within 30 min in the spotted salamander (Ambystoma maculatum) and in the Alleghney dusky (Desmognathus ochrophaeus) after capture in studies conducted by Homan et al. (2003) and Ricciardella et al. (2010), respectively. Sequential blood sampling following physical restraint or anaesthesia is not easy for amphibians, and the physiological stress generated by the sampling procedure limits the use of blood for the assessment of acute stress responses in amphibians. A minimally invasive method for the delivery of corticosterone (to induce elevated plasma corticosterone) using a dermal patch has also been developed for red-legged (Plethodon shermani), Ocoee (Desmognathus ocoee), and Allegheny dusky salamanders (Desmognathus ochrophaeus), as well as for Coqui frogs (Eleutherodactylus coqui; Wack et al., 2010). This minimally invasive method of corticosterone delivery has recently been used to demonstrate the impacts of elevated plasma corticosterone on amphibian metabolic responses (Wack et al., 2012).
Non-invasive urine sampling provides a simple method of assessing baseline and short-term corticosterone stress responses in anurans with minimal disturbance. Mild capture and handling (for a maximum of 5 min on each sampling occasion) causes increased urinary corticosterone metabolite concentrations in anurans (Narayan et al., 2010a), which enables individual baseline and short-term corticosterone stress responses to be assessed over time periods (Narayan et al., 2011a). Recently, it was demonstrated using cane toads (Rhinella marina) that the magnitude of the short-term urinary corticosterone stress response was affected by the intensity and the duration of manual restraint (Narayan et al., 2012a), and also by acclimation temperature (15 vs. 25 or 35°C) in laboratory conditions (Narayan et al., 2012b). Urinary corticosterone metabolite EIA and short-term capture handling protocols are also well suited for assessing individual variation in anuran corticosterone responses within both captive and natural populations (Narayan et al., 2012c, d, 2013a). Acute capture and handling has been used for assessing the short-term corticosterone stress responses of anurans to different types of physical and psychological stressors, such as marking techniques (toe clipping), transportation and captive adjustment, natural altitudinal gradients, and extreme temperatures (see summary Table 1). This type of data will be needed in order to increase our understanding of the role of corticosterone in relationship to amphibian fitness and the environment.
Table 1:
Summary of the studies in non-invasive amphibian endocrinology
| Species | Population | Assay | Reagent source | Hormone | Stressor | Outcome | References |
|---|---|---|---|---|---|---|---|
| Bell frogs (Litoria raniformis) | Free-living | Urinary | R156/7, R522-2, CL425 | T, EC, P | T, EC, and P levels increased during breeding. | Germano et al. (2009) | |
| EIA | EC sexing hormone (98%) | ||||||
| Fijian ground frog (Platymantis vitiana) | Free-living | Urinary | R156/7, R522-2, CL425 | T, EC, P | T, EC, and P levels increased during breeding in both captive and free-living populations. | Narayan et al. (2010b) | |
| Captive | EIA | EC sexing hormone (80%) | |||||
| Fijian ground frog (P. vitiana) | Free-living | Urinary | CJM006 | CORT | ACTH challenge Capture handling | CORT increased in frog urine at 6 h, 1–2 days after ACTH. | Narayan et al. (2010a) |
| Captive | EIA | CORT was elevated 3–4 h after capture handling. | |||||
| No seasonal pattern in CORT. | |||||||
| Cane toad (Rhinella marina) | Captive | Urinary | RIA kit (MP Biomedicals, Fountain Parkway Solon, OH, USA) | CORT | ACTH challenge | CORT increased 1–2 days after ACTH | Narayan et al. (2011a) |
| RIA | Short-term capture | CORT was elevated 2 h after capture handling | |||||
| Captivity | No sex difference | ||||||
| Stony Creek Frog (Litoria wilcoxii) | Free-living | Urinary | CJM006 | CORT | Chytrid fungus | CORT levels high for chytrid fungus-positive frogs | Kindermann et al. (2012) |
| EIA | |||||||
| Great Barred Frog (Mixophyes fasciolatus) | Free-living | Urinary | CJM006 | CORT | Natural altitudinal gradients | Frogs living at high elevation (660–790 m) had much higher baseline CORT than frogs living lowland (60 m) | (Graham C, Narayan E, McCallum H, Hero J-M unpublished data) |
| EIA | |||||||
| Fijian tree frog (Platymantis vitiensis) | Free-living | Urinary | CJM006 | CORT | Capture handling | CORT was elevated 4–5 h after capture handling | Narayan et al. (2012c) |
| EIA | |||||||
| Fijian ground frog (P. vitiana) | Captive | Urinary | CJM006 | CORT | Transportation | CORT was higher 6 h after transportation and after 5 and 15 days in captivity. Returned to baseline after 25 days of captive enrichment | Narayan and Hero (2011) |
| EIA | Captivity | ||||||
| Cane toad (R. marina) | Free-living | Urinary | CJM006, R156/7 | CORT, T | Toe clipping | CORT increased 6 h after clipping and remained elevated at 72 h. T decreased 6 h after clipping and remained low at 72 h | Narayan et al. (2011c) |
| EIA | |||||||
| Cane toad (R. marina) | Captive | Urinary | CJM006, R156/7 | CORT, T | Manual restraint for 5, 15, or 30 min | CORT was much higher after 15 or 30 min restraint than after 5 min restraint. T was low after 5, 15, or 30 min restraint | Narayan et al. (2012a) |
| EIA | |||||||
| Fijian ground frog (P. vitiana) | Captive | Urinary | CJM006 | CORT | Cane toad (R. marina) | CORT was elevated after 6 h exposure to sight of a cane toad | (Narayan E, Cockrem JF, Hero J-M unpublished data) |
| EIA | |||||||
| Common toad (Bufo bufo) | Free-living | Urinary | EIA kit no. 500651 (Cayman Chemical, Ann Arbor, Michigan, USA) | CORT | Habitat availability and fragmentation | CORT was significantly altered by habitat availability and fragmentation | Janin et al. (2011) |
| EIA | |||||||
| Common toad (B. bufo) | Outdoor enclosure | Saliva | EIA kit no. 500651 (Cayman Chemical) | CORT | Matrix resistance (substrate choice experiments) | Adult toads had higher CORT on ploughed soil than on forest litter or meadow substrates | Janin et al. (2012) |
| Common midwife toad (Alytes obstetricans) | Laboratory | Aquatic media | EIA kit no. 500651 (Cayman Chemical) | CORT | Chytrid fungus | CORT release rates were higher in infected populations of two species of tadpoles than in an uninfected population for both species | Gabor et al. (2013) |
| Mallorcan midwife toad (Alytes muletensis) | |||||||
| Maud Island frog (Leiopelma pakeka) | Free-living | Urinary | R156/7, R522-2, CL425 | T, EC, P | T, EC, and P peaking during winter breeding. EC 90% success as the sexing hormone | Germano et al. (2012) | |
| EIA | |||||||
| American toad (Bufo americanus) | Captive | Faecal EIA | Sigma Chemical Company (St Louis, MO, USA) | T, EC, P | First report of faecal hormone metabolite analysis in amphibians. | Szymanski et al. (2006) | |
| Boreal toads (Bufo boreas boreas) | T better sexing hormone than EC and P | ||||||
Abbreviations: ACTH, adrenocorticotrophic hormone; CORT, corticosterone; EC, estrone conjugate; EIA, enzyme immunoassay; P, progesterone; RIA, radio-immunoassay; and T, testosterone.
Another important point for consideration is the time course of the serum corticosterone response to acute stressors, such as capture handling in amphibians, which is particularly pertinent to field studies. With birds, a short very small time window (<3 min) between initial capture and blood sampling is available, because it is known that in birds the stress responses occur fairly quickly (Romero and Reed, 2005). In contrast, in amphibians and other poikilothermic animals, this time window between initial capture and sampling should be quite large because of their slower metabolisms (Bennett, 1978). Thus, the non-invasive urinary method provides an extra advantage in field studies by allowing a larger time window during the sampling before urinary tests will be confounded, and it also provides the most appropriate index of baseline corticosterone.
Non-invasive assessment of reproductive hormones
Guimarães (2003) stated that reproduction is one of the keys, besides environmental protection, for the successful conservation of an endangered species. Hormones are the essence of reproduction, and understanding the intricacies of how animals reproduce is fundamental to managing or conserving wildlife (Pukazhenthi and Wildt, 2004). There are many scientific papers describing how urine and/or faecal hormone analyses reveal the mysteries of reproductive status, function, and fitness of rare species in zoos and research institutions (see comprehensive reviews by Wildt and Wemmer, 1999; Schwarzenberger, 2007). Although most studies are more descriptive rather than being conservation oriented, there are a growing number of studies using reproductive hormone metabolite assessment in the field. For example, Creel et al. (1993) conditioned dwarf mongooses (Helogale parvula) in the Serengeti National Park to urinate on a rubber pad during the course of normal scent-marking. The result was hundreds of urine samples that were analysable for reproductive hormone metabolites, allowing elegant examinations of behavioural and endocrine mechanisms of reproductive suppression. The evaluation of reproductive hormone metabolites in either urine or faeces permits the long-term study of reproductive patterns in individuals, populations, or species, all without perturbing the animal (Monfort et al., 1993). Whitten et al. (1998) pointed out that endocrine data complement the traditional behavioural data collected by field biologists, thus providing quantitative measures for the examination of basic reproductive processes. For ex situ programmes, it enables predictions about, for example, why the species fail to breed in captivity, the success of an assisted breeding programme, the point when available resources for housing animals will become limiting, or the impact of the environment on an animal's physiology (Carlstead et al., 1993). The non-invasive assessment of reproductive hormone metabolites in anuran faeces is supported by two recent publications (Szymanski et al., 2006; Hogan et al., 2013). Earlier studies of amphibian hormones have focused on androgens, with testosterone or di-hydrotestosterone being described as the major plasma androgen measured in different amphibian species (Kime and Hews, 1978; Canosa and Ceballos, 2002). As there are many possible ways in which amphibians could process steroid hormones, it is difficult to predict the identities of specific metabolites they excrete, and one may anticipate species differences in the identity of the secreted hormone metabolites. Nevertheless, antibodies and EIA protocols that are well established in mammalian and bird species have been used for amphibians (Munro and Stabenfeldt, 1984; Szymanski et al., 2006). To date, only three studies (Germano et al., 2009, 2012; Narayan et al., 2010b) have demonstrated the patterns of reproductive hormone cycles in free-living populations of anurans using urinary-based testosterone, estrone conjugate and progesterone EIAs (see summary Table 1).
Interactions between stress and reproductive hormones
Comprehensive reviews on the amphibian reproductive endocrine system and its interaction with the stress endocrine system are available (Moore et al., 2005; Carr, 2011; Eikenaar et al., 2012). In addition, recent reviews on the roles of the reproductive and stress endocrine systems in the ecological context of animal reproduction, behaviour, and survival are available (Wilczynski and Lynch, 2011; Creel et al., 2013). Glucocorticoids are widely accepted to have inhibitory effects on the reproductive system in mammals, altering mating behaviours and suppressing gonadal hormone secretion (Moore et al., 1991). This relationship, however, has been shown to be equivocal in amphibians, and the relationship between the reproductive endocrine system (hypothalamo–pituitary–gonadal axis) and the stress endocrine system (HPI axis) is complex (Moore and Jessop, 2003; Wingfield and Sapolsky, 2003). The productivity of the environment may also affect this relationship (Wilczynski et al., 2005). For example, short-term increases in corticosterone and testosterone are facultative to breeding behaviours (Orchinik et al., 1988). In a recent study, Narayan et al. (2012e) showed that in adult male cane toads (R. marina), a non-traditional model of an explosive breeding anuran species, urinary testosterone metabolite levels increased when male toads in breeding condition were exposed to a repeated capture handling stressor. In another study, using non-breeding male toads, there was an inverse relationship between urinary testosterone and urinary corticosterone metabolites during exposure to a manual restraint stressor (Narayan et al., 2012a). It has also been shown that a chronic stress event that increases corticosterone above seasonal cycle levels may also result in the suppression of testosterone production (Orchinik et al., 2000). Species with limited breeding opportunities will often have a low sensitivity to stress, maximizing their chances of reproduction, whereas species with several breeding opportunities will suppress breeding in the face of stress in order to maximize survival (Bears et al., 2003). Suppression of breeding for even one season may have detrimental effects on vulnerable populations. Population survival is a delicate balance between recruitment and death, and anything that disrupts this balance can have damaging effects on long-term population/species survival (Hayes et al., 2010). Thus, interactions between the gonadal (reproductive) and stress endocrine axes should be important topics of investigation for conservation physiology because they can affect the recruitment rates of populations.
Biological/physiological validation
It is crucial to demonstrate that non-invasive hormone measures accurately reflect biological events of interest. For example, an ovarian cycle can be validated by comparing two independent measures of the same reproductive hormone in matched samples (such as faecal vs. urinary estradiol E2), or by comparing temporal excretion patterns of hormones (e.g. urinary estrone and progesterone) with external signs of reproductive status (e.g. visual inspection of underbelly oocytes is often used as an indirect measure of the vitellogenic status of female anurans; Narayan et al., 2010b). Likewise, administration of a drug known to stimulate hormone production is useful for demonstrating a cause-and-effect relationship between its exogenous administration and the subsequent excretion of the targeted hormone. Typically, hormonal challenges include gonadotrophin-releasing hormone to study pituitary hormones, luteinizing hormone, human chorionic gonadotrophin, and follicle-stimulating hormone and subsequent androgen production. Most importantly, a biological validation determines the excretory lag time between stimulation of an endocrine gland and the appearance of its hormone metabolites in excreta. Urinary testosterone, estrone conjugate and progesterone EIAs were validated physiologically for the endangered Fijian ground frog (Platymantis vitiana) using human chorionic gonadotrophin challenge. The results demonstrated ‘raise and return to baseline’ profiles in urinary testosterone metabolites in male frogs and urinary progesterone and estrone conjugate metabolite profiles in female frogs over 2–3 days, which reflected the experimental stimulation of the hypothalamo–pituitary–gonadal axis. Administration of ACTH mimics a natural interrenal stress response by causing a rapid rise in circulating corticosterone, followed by a return to baseline within a few hours. The same pattern should also occur in urine or faeces, with the onset of peak excretion being delayed by the species-specific excretory lag time (Wasser et al., 2000; Preest et al., 2005). Urinary corticosterone metabolite EIA has been validated physiologically for two anuran species, the Stony Creek Frog (Litoria wilcoxii) and the Fijian ground frog (P. vitiana), using an ACTH challenge that demonstrated ‘raise and return to baseline’ profiles in urinary corticosterone within 2–3 days, thus reflecting the experimental stimulation of the HPI axis (Narayan et al., 2010a; Kindermann et al., 2012). The relatively slow (several hours to a few days) lag time of urinary corticosterone metabolites means that this can be used to measure the physiological stress responses to both short-term and chronic stressors. According to Queyras and Carosi (2004), lag time in metabolic excretion of urine could also be determined by the injection of radioactive hormones and measurement of the fluctuations in urine concentration. This method would be difficult when working with a threatened species, because it would require special animal ethics permits and other necessary permits prior to experimental manipulations.
Considerations of hormone metabolism and laboratory validation
A large percentage (∼80%) of hormones in the blood circulation are bound to CBG or sex hormone-binding globulin. It is only the ‘free fraction’ (1–10% of total plasma hormone concentration) that is usually considered to be the biologically active fraction (i.e. a hormone that is directly available for biological action). It has been suggested that CBG and/or sex hormone-binding globulin transport hormones to target tissues (Pemberton et al., 1988). Quantification of free hormone concentrations and binding globulin levels can be technically challenging (Cook, 2012). The liver is the major site for steroid metabolism, and it is assumed that only the free glucocorticoids (i.e. not bound to CBG) are degraded by the liver (Palme et al., 2005). Accordingly, urinary hormone measurements reflect free hormone in blood over the time that urine collects between micturition, because CBG-bound hormones are excluded from the kidney filtrate. Recently, Sheriff et al. (2010) tested this assumption by comparing plasma (free cortisol) with the concentrations of bile and faecal cortisol metabolites. Their study found strong correlations between plasma free cortisol levels with bile and faecal cortisol metabolite levels. Hormones are conjugated to highly charged side-chain moieties (e.g. glucuronide or sulfate molecules) before excretion that improve solubility in water [see review by Brown et al. (2003) for a detailed description of steroid biosynthesis and metabolism]. Whether these hormone metabolites are primarily passed in urine or faeces is species dependent (Pukazhenthi and Wildt, 2004). Urinary steroids are excreted as conjugates (e.g. estradiol sulfate and estrone glucuronide), and these are end-products of complex metabolic processes involving large families of steroids. For instance, estrone glucuronide molecules are the ultimate result of the conversion and the breakdown of a variety of oestrogens (Pukazhenthi and Wildt, 2004).
Peripheral metabolism occurs mainly in the liver and to some extent in the kidneys, which delivers steady-state levels of plasma androgens and oestrogens. Oestrogens and androgens are broken down into the same class of metabolites by sulfatases, dehydrogenases, and glucuronide transferases to enhance their solubility and to facilitate their elimination. Steroid inactivation could also take place in target tissues, mainly upon completion of the specific biological response. As highlighted earlier, inactive androgens and oestrogens are mainly eliminated as urinary (mostly conjugated) metabolites. This conversion to hydrophilic compounds ensures their solubility in biological fluids at rather high concentrations (see comprehensive texts on steroidal metabolism by Norman and Litwack, 1997; Salway, 2004; Lehninger, 2005).
The EIA reagents used in non-invasive endocrinology studies are polyclonal, such as the corticosterone antiserum (CJM006) and the conjugated horseradish peroxidase label that were produced in rabbits using protocols that were standardized for a standard direct competitive EIA system by Munro and Stabenfeldt (1984) and described elsewhere (Kurstak, 1985). Thus, the EIA measurement provides the total concentration of the conjugated metabolites of plasma free hormone in anuran urine. Most recently, Watson et al. (2013) provided a detailed technical description regarding the development and optimization of the CJM006 EIA system for assessing stress hormones non-invasively in a variety of taxa, including amphibians.
Laboratory validation and standardization of each assay system is an important step towards establishing a reliable non-invasive endocrinology laboratory. Parallelism determines whether the assay system is detecting the hormone metabolites of interest. The parallelism curve also gives the sample dilution factor based on the 50% binding point of the sample on the standard curve (Narayan et al., 2010b). Recovery represents the degree to which the measured hormone concentration corresponds to the true concentration of a hormone. It tests for potential interference caused by moieties contained within the biological sample that are independent of specific antigen–antibody binding. Recovery is tested by addition of standard hormone in aliquots to pooled anuran urine samples. For this purpose, urine samples with low hormone concentrations are selected, such as samples collected from outside the breeding period for testing recovery of reproductive steroids and control treatment samples that have low levels of stress hormone metabolites when testing for stress hormones. Other potential means of assessing recovery are by initially stripping the urine of the steroidal metabolites and then adding known quantities of steroids to the stripped urine to test how accurately they are detected relative to the standards. Recovery is expressed as the mean ± SEM (Narayan et al., 2010b). Excellent recovery values of >90% for commercially available (Sigma Aldrich) hormone standards have been reported for non-invasive urinary corticosterone and reproductive hormone EIAs (Narayan et al., 2010b).
Urinary steroid metabolite concentrations are standardized to creatinine levels to control for water content and are normally reported as picograms of hormone metabolite per microgram of creatinine (Narayan et al., 2010a). Amphibians rely on filling the bladder for osmotic needs, and this results in them having large volumes of easily obtained fluid at any given time. Creatinine is a by-product of muscle metabolism, and it has been shown to provide an effective measure of glomerular filtration in anurans (Forster, 1938). Creatinine is filtered out of the blood by the kidneys (glomerular filtration and proximal tubular secretion). It is excreted at a constant rate in individuals with normal kidney function and therefore provides a good index of the amount of time over which hormones have been metabolized into the urine regardless of the volume of the sample. Creatinine index, also known as the ‘Jaffe Reaction’ after Max Jaffe, has been used widely for correcting urinary hormone metabolite measures (e.g. Kindermann et al., 2013; Narayan et al., 2013b).
Urine sampling
Amphibians should always be handled by wearing new non-powdered gloves or disposable freezer bags for each individual to avoid the spread of chytrid fungus (Narayan et al., 2011b). Urine can be obtained upon initial capture and by gentle massage of the underbelly abdomen over a sterile cup. This method works well for large anurans (such as cane toads, R. marina; snout–vent length = 100 mm). Another method, using sterile pipette tips (200 μl) works reasonably well for large to medium sized species, such as the Stony Creek Frog (L. wilcoxii; snout–vent length = 45–70 mm), red-eyed tree frog (Litoria chloris; snout–vent length = 65 mm) and for the Great Barred Frog (Mixophyes fasciolatus; snout–vent length = 80 mm). Typically, the sterile tip of the pipette is inserted at a very gentle pace inside (5 mm) the anuran's cloaca while the frog is manually held (this can be done by the same person manually holding the anuran), and urine is obtained by capillary action. For medium to small sized species, such the Philoria species, a non-heparinized micro-capillary tube (20–50 μl) could be used for obtaining urine. Sigma-Aldrich (#P2174) micro-capillary tubes have been widely used for the purpose of collecting urine in small frog species, such as Pseudophryne and Crinia species (Phil Bryne, personal communication). The time required for urine collection from initial capture to sampling is normally between 30 s and 5 min.
Reproductive and stress hormone assessment in captivity
Non-invasive assessment of corticosterone and reproductive hormones in amphibians should be considered for rare and threatened species undergoing captive breeding programmes. In a recent study, urinary concentrations of reproductive hormones, such as estrone conjugate, in female frogs showed poor cycling of estrone conjugate during the breeding season, which also accounted for the retention of vitellogenic oocytes (follicles undergoing atresia; Narayan et al., 2010a). Urinary corticosterone metabolites were also measured in captive Fijian ground frogs (Narayan et al., 2010a), and the changes in baseline levels of urinary corticosterone metabolites were correlated with the reproductive condition of the frogs in captivity. Non-invasive reproductive and stress hormone methods could be used for verifying the reproductive cycle in females, and for assessing the potential impacts of captive husbandry conditions and nutritional deficiencies in captive anurans. Non-invasive reproductive hormone assessment can be used to track the reproductive performance of individuals and also for making predictions about the breeding events. It can also provide useful information for making appropriate management decisions aimed towards establishment of a sustainable captive breeding colony for future release into the wild once the in situ threats have been eliminated.
Another important consideration is sexing, which could be a major concern for amphibian captive breeding, especially when working with monomorphic species. Recent studies have successfully used non-invasive urinary EIAs for assessing sex in captive anurans. For example, the urinary estrone conjugate assessment was 80% successful in sexing P. vitiana (Narayan et al., 2010b). In sexually dimorphic Southern Bell frogs (Litoria raniformis), this same measure was found to be 98% reliable for distinguishing between known males and females (Germano et al., 2009). Recently, Germano et al. (2012) established that urinary estrone conjugate had 94% success in identifying the sex of the IUCN endangered Maud Island frog (Leiopelma pakeka). Szymanski et al. (2006) utilized non-invasive faecal hormone methods to distinguish sex in American toads (B. americanus) and boreal toads (Bufo boreas boreas). Their study reported that faecal testosterone metabolite concentrations provided the most useful measure of sex in the American toads (they found no overlap in the 95% confidence interval surrounding mean testosterone concentrations for males and females; Szymanski et al., 2006). Most recently, Hogan et al. (2013) reported sex determination in the monomorphic Geocrinia frogs using faecal reproductive hormone metabolite EIAs. Their study found promising results showing higher faecal testosterone-to-estrone conjugate metabolite ratios in mature males in comparison to mature females. Thus, non-invasive endocrine techniques could provide a more accurate sexing tool, in comparison to morphometrics, for selecting mating pairs for captive breeding programmes.
Non-invasive endocrine methods could also be applied to artificial reproductive technology, which is necessary for species that are difficult to reproduce in captivity (Kouba and Vance, 2009). The first step in developing artificial reproductive technology for any amphibian species is to characterize seasonal hormone profiles and develop exogenous hormone administration techniques that induce spermiation and ovulation. Thus, non-invasive endocrine assessment provides a valuable method to study the breeding cycle in anurans that are undergoing intensive captive breeding programmes.
Ecological applications of non-invasive endocrinology
Many challenges confront biologists studying the behaviour of amphibians in the wild, such as remoteness of natural habitat and technological limitations. Most of the behavioural studies on amphibians necessitate their maintenance in captivity. Stress is a vital factor to consider when assessing animal welfare both in captivity and in the wild. Captive amphibians are faced with a variety of stressors, such as capture, transportation to release sites, and captivity, and these factors may affect the settling of species into their new environment. Short holding periods can cause significant short-term stress hormone responses, which may last for up to a month after release or subside within a few weeks depending on the adaptive capacity of the species (Narayan et al., 2011a). Thus, the non-invasive endocrinology tool has useful implications for amphibian conservation and management, especially for examining stress with respect to translocation and captive breeding programmes.
Recently, non-invasive urinary corticosterone EIA was used to demonstrate the stress associated with transportation and captive transfer of the endangered Fijian ground frog (P. vitiana; Narayan and Hero, 2011). The physiological acclimation into captivity has also been demonstrated using the cane toad (R. marina; Narayan et al., 2011a). Most recently, Janin et al. (2011) measured urinary corticosterone metabolites in the common toad (Bufo bufo) and used this as a physiological index, in combination with body condition, of habitat quality and fragmentation. Their study used manual shaking as a psychological stressor to test the acute stress hormone response of the toads, and they found that low body condition and elevated baseline urinary corticosterone levels were associated with low forest availability and high forest fragmentation within a 500 m radius around the ponds. They highlighted that non-invasive stress endocrine measures should be used as a complementary approach that can enable early detection of population health status within changing environments.
Non-invasive stress endocrinology has also been used to assess the suitability of mark–recapture techniques in amphibian ecology (Parris and McCarthy, 2001). Recently, Narayan et al. (2011c) demonstrated, using urinary corticosterone EIA, that toe clipping generates a physiological stress response in male cane toads (R. marina) in field conditions and suggested that toe clipping could have detrimental sublethal impacts on reproductive hormones and behaviour. This sort of data is important, especially when debates are on-going regarding the welfare of amphibians and whether toe clipping should be completely prohibited in countries that have huge amphibian diversity (Décio, 2013).
The use of non-invasive stress endocrinology is also gaining popularity for study of the complexities of amphibian decline, especially within the context of interactions between the host, the environment, and the pathogenic disease chytridiomycosis (Lips et al., 2008; Rohr and Raffel, 2010). Recently, Gabor et al. (2013) developed a non-invasive waterborne hormone EIA technique to measure corticosterone metabolite levels in two species of tadpoles, the common midwife toad (Alytes obstetricans) and the Mallorcan midwife toad (Alytes muletensis). Their study demonstrated that tadpoles with high chytrid fungus loads had high concentrations of corticosterone metabolites. In another study, Kindermann et al. (2012) demonstrated, using non-invasive urinary corticosterone metabolite EIA in the Stony Creek Frogs (L. wilcoxii), that adult frogs with high chytrid fungus loads expressed much higher baseline urinary corticosterone metabolite levels in comparison to the frogs that were negative for the chytrid fungus.
In Australia and globally, amphibian declines have shown a strong association with altitude, which is used as a climatic gradient by field ecologists (Kriger and Hero, 2008). Non-invasive urine sampling has been used to measure baseline urinary corticosterone metabolite levels in native anurans, such as the Great Barred Frog (M. fasciolatus), in an attempt to test whether sublethal high levels of stress are suppressing other key physiological systems, especially immunocompetence, and making high altitude amphibian populations more vulnerable to chytrid fungus (Graham C, Narayan E, McCallum H, Hero J-M unpublished data). Results have shown that species living at higher elevations also have much higher levels of baseline urinary corticosterone metabolites in comparison to their counterparts living at lower elevations (sampling was done at the same time of the year). Thus, non-invasive reproductive and stress endocrinology provides amphibian ecologists with a useful tool to understand the physiological sensitivity of anurans to environmental stressors and their sublethal impacts on amphibian reproductive ecology and fitness. Overall, non-invasive endocrinology tools should be used to gain a holistic understanding of the factors associated with chytridiomycosis-driven amphibian declines.
Strengths, limitations, and technical considerations for future research
Urinary EIA has a number of advantages that make it an excellent tool for studying the anuran hypothalamo–pituitary–gonadal axis and HPI axis functions. Enzyme immunoassay techniques are relatively cheap, and hormone metabolites can be measured in small volumes (120 μl of anuran urine required per hormone assay). Anurans tend to urinate frequently and, once collected, urine can be preserved indefinitely by freezing at −20 or −80°C. Faecal sampling could be very difficult in field conditions due to the need to find discrete scat samples in the wild that can be linked to an individual; however, urinary methods do not have this problem. Furthermore, collection of faecal samples from an elusive species, such as a frog, would be time consuming and could require the capture and captivity of individuals.
The polyclonal antibodies being employed in urinary-based EIAs detect the total content of the steroid metabolites of interest (a combination of free as well as conjugated forms). These are the metabolic end-products of biologically active hormones that take part in the endocrine response and are metabolized in the kidney prior to excretion. Therefore, it is important to test whether there are significant differences in the levels of conjugated metabolic end-products, which can also be affected by bacterial degradation in the gut and differences in metabolic rates between sexes and individuals (Goymann, 2012).
As highlighted in the latest review by Goymann (2012), the variation in hormone metabolism can be quantified in animals by injecting them with a radiolabelled hormone and using high-performance liquid chromatography methods to determine the different types of metabolites that are produced after metabolism and bacterial degradation of the hormones. It is also important to validate the assays by extracting the biological sample in various solvents or enzymes that remove the conjugated moieties and then comparing the levels of steroids measured in the extracted vs. unextracted sample. This will verify whether or not the conjugated metabolites are the major end-products or if there is little conjugation effect. If possible, the various metabolites of specific hormones that are excreted in the urine or faeces of anurans should be determined. This may require amphibian ecology laboratories to collaborate with biochemistry laboratories that have access to the necessary technical equipment, such as high-performance liquid chromatography. Other factors, such as the effects temperature and sample storage time on steroid metabolites, stability, and decay rates should also be considered in future studies (Millspaugh and Washburn, 2004).
It should be made pre-requisite that appropriate biological validation through exogenous hormone challenge (such as ACTH or human chorionic gonadotrophin challenge) is done for each anuran species for which non-invasive reproductive or stress hormone methods are being developed for the first time. This will provide useful information regarding excretory lag times and also demonstrate that the assay system is in fact detecting the hormones of interest. Another consideration is the need for the quantification of creatinine references when measuring hormone metabolites in urine, which requires sufficient volume of sample, and this could be a limitation when working with tiny frogs. Creatinine is a useful urinary marker to estimate filtration rates, because the conjugated conjugated hormones possibly gain entry into the bladder through active secretion through the proximal tubule. Therefore, it is important for studies utilizing urinary hormone measurements to use creatinine assay as a reference value.
Furthermore, the cross-reactivity of the antibody employed for non-invasive reproductive and stress hormone measurements should be readily available to confirm that the antibody cross-reacts only with metabolites of the original hormone. For example, cross-reactivity of the CJM06 anti-corticosterone antiserum was 100% with corticosterone, 14.25% with desoxycorticosterone, and 0.9% with tetrahydrocorticosterone (C. J. Munro, personal communication). Goymann (2012) also reviewed potential differences in the concentration of metabolite hormones between sexes and recommended that biological validation should be done separately for each sex. Additionally, Szymanski et al. (2006) demonstrated that the extract of cricket (or cricket plus supplement) food sources showed no detected hormone metabolites that could interfere with the EIA for reproductive steroids in the American toads.
In my view, urinary measurements of reproductive and stress hormone metabolites in amphibians should not have issues such as the effect of diet because urinary measures do not require correction by the dry faecal mass. It is important to measure the plasma free hormone, because only 1–10% of the total hormone in the plasma possesses biological activity. According to the free hormone theory, while a hormone is bound to a binding globulin it is not biologically active (Mendel, 1989). Corticosteroid-binding globulin should be measured because it regulates the access of hormone to tissues (Ward et al., 2007). It is possible that the nature of the stressors themselves could also influence the structure and the binding capacity of the CBG, which could also lead to differences in the concentrations of excretory metabolites between sexes, breeding events, and seasons. Therefore, it is highly recommended that future studies should consider the quantification of CBG [see Ward et al. (2007) for a detailed methodology for measuring amphibian CBG], and future studies should compare the plasma free hormone levels with the urinary hormone metabolite levels in response to experimental stressors. Future research should consider these limitations and conduct additional laboratory validations to increase the accuracy and reliability of non-invasively obtained hormone data.
Conclusion
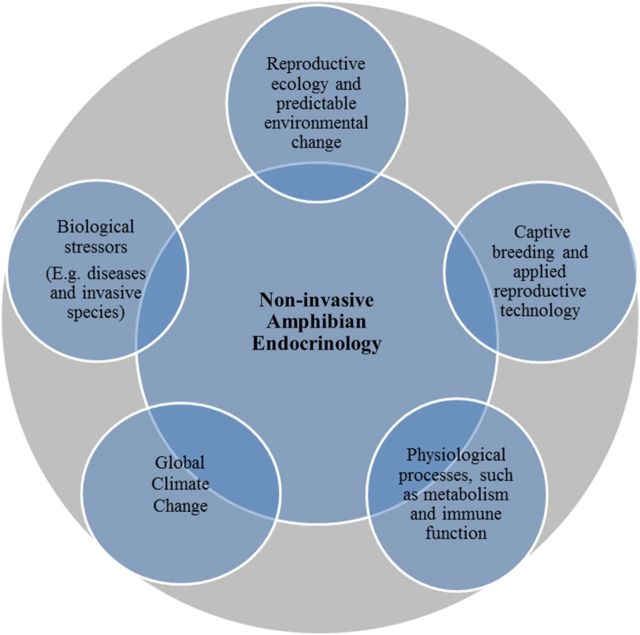
This review clearly demonstrates that non-invasive endocrinology plays a fundamental role in advancing amphibian conservation physiology. As shown in the conceptual framework (Fig. 1), this physiological method integrates perfectly with the other important research areas of conservation physiology, including reproductive ecology and predictable environmental change, biological stressors (such as diseases and invasive species), global climate change, captive breeding and applied reproductive technology, and other key physiological processes (such as immune system responses and whole animal metabolism).
Figure 1:
Conceptual model presenting non-invasive amphibian endocrinology (innermost circle) as a key component of amphibian conservation physiology (outermost circle) through its integration with five key areas of research (smaller circles).
Non-invasive endocrinology contributes directly to the advancement of amphibian conservation physiology. A non-invasive endocrinology approach can be used to evaluate the success or failure of captive breeding programmes by assessing the longitudinal changes in reproductive and stress hormone metabolites of captive individuals and comparing these hormone changes with the reproductive status and behaviour data. The EIA systems employed these days are readily available and inexpensive. The urine hormone analysis goes beyond captive populations. It is a useful tool for studying populations in the wild, particularly translocated individuals, reintroduced populations, or areas where some sort of management intervention has been implemented. Stress maintenance plays an integral role in the reproductive success of amphibians, because high stress levels could lead to reproductive failures both in the wild and in captive situations. Through the analysis of urine samples, this type of tool can provide invaluable management information about the physiological stress responses and reproductive status of diverse amphibian species.
In conclusion, given that amphibian populations worldwide are declining rapidly as a result of disturbances to natural habitats, it will become essential to utilize non-invasive physiological measures of stress and reproductive health, because these physiological measures can provide objective and quantitative measures of the impacts of disturbance. Non-invasive physiological indices are particularly useful for the assessment of the relative impacts of disturbance, delineating the pressures that most need mitigation as well as tracking the effectiveness of such mitigation efforts. As demonstrated in this review, there are numerous ways by which this novel method is assisting in the conservation physiology of amphibians. Reproductive and stress hormone evaluation in field conditions could be merged with behavioural ecology studies to help improve our understanding of the complex interplay between stress and reproductive hormones and the environment in maintaining reproductive fitness and survival of threatened species. Ultimately, through regular technical updating of biological and laboratory validations, this non-invasive endocrine tool will provide a long-lasting contribution to conservation physiology.
Acknowledgements
I wish to thank Ketan Christi, Craig Morley and Frank Molinia, Jean-Marc Hero and John Cockrem for their support during my early career PhD and Postdoctoral research. I also thank my Honours and PhD students who have contributed to this field of non-invasive endocrinology under my guidence, including Alex Simic, Nicole Evans, Clara Graham, Billy Ross, Christina Kindermann, and Tempe Parnell. Thanks to three anonymous reviewers who provided critical comments and suggestions. Funding for this activity was supported by the Griffith University Postdoctoral Fellowship.
References
- 1.Bears H, Smith JNM, Wingfield JC. (2003) Adrenocortical sensitivity to stress in dark-eyed juncos (Junco hyemalis oregonus) breeding in low and high elevation habitat. Ecoscience 10: 127–133. [Google Scholar]
- 2.Bennett AF. (1978) Activity metabolism of the lower vertebrates. Annu Rev Physiol 40: 447–469. [DOI] [PubMed] [Google Scholar]
- 3.Berger J, Testa JW, Roffe T, Monfort SL. (1999) Conservation endocrinology: a noninvasive tool to understand relationships between carnivore colonization and ecological carrying capacity. Conserv Biol 13: 980–989. [Google Scholar]
- 4.Bern H, Nandi J. (1964) Endocrinology of poikelothermic vertebrates. In Pincus G, Thimann KV, Astwood FB, eds, The Hormones, Vol IV Academic Press, New York, pp. 199–293. [Google Scholar]
- 5.Blaustein AR, Walls SC, Bancroft BA, Lawler JJ, Searle CL, Gervasi SS. (2010) Direct and indirect effects of climate change on amphibian populations. Diversity 2: 281–313. [Google Scholar]
- 6.Bliley JM, Woodley SK. (2012) The effects of repeated handling and corticosterone treatment on behavior in an amphibian (Ocoee salamander: Desmognathus ocoee). Physiol Behav 105: 1132–1139. [DOI] [PubMed] [Google Scholar]
- 7.Brook BW, Sodhi NS, Bradshaw CJA. (2008) Synergies among extinction drivers under global change. Trends Ecol Evol 23: 453–460. [DOI] [PubMed] [Google Scholar]
- 8.Brown J, Walker S, Steinman K. (2003) Endocrine manual for reproductive assessment of domestic and non-domestic species. Conservation and Research Center, Smithsonian National Zoological Park, (internal publication). [Google Scholar]
- 9.Canosa LF, Ceballos NR. (2002) Seasonal changes in testicular steroidogenesis in the toad Bufo arenarum H. Gen Comp Endocrinol 125: 426–434. [DOI] [PubMed] [Google Scholar]
- 10.Carlstead K, Brown JL, Strawn W. (1993) Behavioral and physiological correlates of stress in laboratory cats. Appl Anim Behav Sci 38: 143–158. [Google Scholar]
- 11.Carr JA. (2011) Stress and reproduction in amphibians. In DO Norris, KH Lopez, eds, Hormones and Reproduction of Vertebrates, pp. 99–116. London: Academic Press. [Google Scholar]
- 12.Carstensen H, Burgers ACJ, Li CH. (1961) Demonstration of aldosterone and corticosterone as the principal steroids formed in incubates of adrenals of the American bullfrog (Rana catesbeiana) and stimulation of their production by mammalian adrenocorticotropin. Gen Comp Endocrinol 1: 37–50. [DOI] [PubMed] [Google Scholar]
- 13.Coddington EJ, Cree A. (1995) Effect of acute captivity stress on plasma concentrations of corticosterone and sex steroids in female whistling frogs, Litoria ewingi. Gen Comp Endocrinol 100: 33–38. [DOI] [PubMed] [Google Scholar]
- 14.Cogger HG. (2000) Reptiles and Amphibians of Australia. Reed New Holland, Sydney. [Google Scholar]
- 15.Cook NJ. (2012) Review: minimally invasive sampling media and the measurement of corticosteroids as biomarkers of stress in animals. Can J Anim Sci 92: 227–259. [Google Scholar]
- 16.Cooke SJ, Sack L, Franklin CE, Farrell AP, Beardall J, Wikelski M, Chown SL. (2013) What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conserv Physiol 1: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cree A, Tyrrell CL, Preest MR, Thorburn D, Guillette LJ. (2003) Protecting embryos from stress: corticosterone effects and the corticosterone response to capture and confinement during pregnancy in a live-bearing lizard (Hoplodactylus maculatus). Gen Comp Endocrinol 134: 316–329. [DOI] [PubMed] [Google Scholar]
- 18.Creel SR, Wildt DE, Monfort SL. (1993) Aggression, reproduction and androgens in wild dwarf mongooses: a test of the challenge hypothesis. Am Nat 141: 816–825. [DOI] [PubMed] [Google Scholar]
- 19.Creel S, Dantzer B, Goymann W, Rubenstein DR. (2013) The ecology of stress: effects of the social environment. Funct Ecol 27: 66–80. [Google Scholar]
- 20.Dale E. (1962) Steroid excretion by larval frogs. Gen Comp Endocrinol 2: 171–176. [DOI] [PubMed] [Google Scholar]
- 21.Décio TC. (2013) Population declines: toe-clipping vital to amphibian research. Nature 493: 305. [DOI] [PubMed] [Google Scholar]
- 22.Demas GE, Adamo SA, French SS. (2011) Neuroendocrine-immune crosstalk in vertebrates and invertebrates: implications for host defence. Funct Ecol 25: 29–39. [Google Scholar]
- 23.Dittami J, Katina S, Möstl E, Eriksson J, Machatschke IH, Hohmann G. (2008) Urine androgens and cortisol metabolites in field-sampled bonobos (Pan paniscus). Gen Comp Endocrinol 155: 552–557. [DOI] [PubMed] [Google Scholar]
- 24.Dupont W, Bourgeois P, Reinberg A, Vaillant R. (1979) Circannual and circadian rhythms in the concentration of corticosterone in the plasma of the edible frog (Rana esculenta L.). J Endocrinol 80: 117–125. [DOI] [PubMed] [Google Scholar]
- 25.Eikenaar C, Husak J, Escallón C, Moore IT. (2012) Variation in testosterone and corticosterone in amphibians and reptiles: relationships with latitude, elevation, and breeding season length. Am Nat 180: 642–654. [DOI] [PubMed] [Google Scholar]
- 26.Forster RP. (1938) The use of inulin and creatinine as glomerular filtrate measuring substances in the frog. J Cell Comp Physiol 12: 213–222. [Google Scholar]
- 27.Gabor CR, Fisher MC, Bosch J. (2013) A non-invasive stress assay shows that tadpole populations infected with Batrachochytrium dendrobatidis have elevated corticosterone levels. PLoS One 8: pe56054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Germano JM, Molinia FC, Bishop PJ, Cree A. (2009) Urinary hormone analysis assists reproductive monitoring and sex identification of bell frogs (Litoria raniformis). Theriogenology 72: 663–671. [DOI] [PubMed] [Google Scholar]
- 29.Germano JM, Molinia FC, Bishop PJ, Bell BD, Cree A. (2012) Urinary hormone metabolites identify sex and imply unexpected winter breeding in an endangered, subterranean-nesting frog. Gen Comp Endocrinol 175: 464–472. [DOI] [PubMed] [Google Scholar]
- 30.Gordon M, Bartol S. (2004) Experimental Approaches to Conservation Biology. University of California Press, Berkeley and Los Angeles, California, 343 pages. [Google Scholar]
- 31.Goymann W. (2012) On the use of non-invasive hormone research in uncontrolled, natural environments: the problems with sex, diet, metabolic rate and the individual. Methods Ecol Evol 3: 757–765. [Google Scholar]
- 32.Guillette JLJ, Cree A, Rooney AA. (1995) Biology of stress: interactions with reproduction, immunology and intermediary metabolism. In FLFC Warwick, JB Murphy, eds, Health and Welfare of Captive Reptiles. Chapman & Hall, London, pp. 32–81. [Google Scholar]
- 33.Guimarães MV. (2003) The importance of the application of non-invasive techniques in the study of wild animal reproduction. Annu Rev Biomed Sci 5: 29–37. [Google Scholar]
- 34.Hayes TB, Falso P, Gallipeau S, Stice M. (2010) The cause of global amphibian declines: a developmental endocrinologist's perspective. J Exp Biol 213: 921–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heistermann M. (2010) Non-invasive monitoring of endocrine status in laboratory primates: methods, guidelines and applications. Adv Sci Res 5: 1–9. [Google Scholar]
- 36.Hill RW, Wyse GA, Anderson M. (2008) Animal Physiology. Sinauer Associates, Sunderland, MA, USA. [Google Scholar]
- 37.Hofmann GE, Todgham AE. (2010) Living in the now: physiological mechanisms to tolerate a rapidly changing environment. Annu Rev Physiol 72: 127–145. [DOI] [PubMed] [Google Scholar]
- 38.Hogan LA, Lisle AT, Johnston SD, Goad T, Robertston H. (2013) Adult and juvenile sex identification in threatened monomorphic geocrinia frogs using fecal steroid analysis. J Herpetol 47: 112–118. [Google Scholar]
- 39.Homan RN, Reed JM, Romero LM. (2003) Corticosterone concentrations in free-living spotted salamanders (Ambystoma maculatum). Gen Comp Endocrinol 130: 165–171. [DOI] [PubMed] [Google Scholar]
- 40.Janin A, Léna J-P, Deblois S, Joly P. (2012) Use of Stress-Hormone Levels and Habitat Selection to Assess Functional Connectivity of a Landscape for an Amphibian. Conserv Biol 26: 923–931. [DOI] [PubMed] [Google Scholar]
- 41.Janin A, Léna J-P, Joly P. (2011) Beyond occurrence: body condition and stress hormone as integrative indicators of habitat availability and fragmentation in the common toad. Biol Conserv 144: 1008–1016. [Google Scholar]
- 42.Janine LB. (2006) Comparative endocrinology of domestic and nondomestic felids. Theriogenology 66: 25–36. [DOI] [PubMed] [Google Scholar]
- 43.Jungreis AM, Huibregtse WH, Ungar F. (1970) Corticosteroid identification and corticosterone concentration in serum of Rana pipiens during dehydration in winter and summer. Comp Biochem Physiol 34: 683–689. [DOI] [PubMed] [Google Scholar]
- 44.Kime DE, Hews EA. (1978) Androgen biosynthesis in vitro by testes from amphibia. Gen Comp Endocrinol 35: 280–288. [DOI] [PubMed] [Google Scholar]
- 45.Kindermann C, Narayan E, Hero J-M. (2012) Urinary corticosterone metabolites and chytridiomycosis disease prevalence in a free-living population of male Stony Creek frogs (Litoria wilcoxii). Comp Biochem Physiol A Mol Integr Physiol 162: 171–176. [DOI] [PubMed] [Google Scholar]
- 46.Kindermann C, Narayan EJ, Wild F, Wild CH, Hero J-M. (2013) The effect of stress and stress hormones on dynamic colour-change in a sexually dichromatic Australian frog. Comp Biochem Physiol A Mol Integr Physiol 165: 223–227. [DOI] [PubMed] [Google Scholar]
- 47.Kirschbaum C, Hellhammer DH. (1999) Noise and stress – salivary cortisol as a non-invasive measure of allostatic load. Noise Health 1: 57–66. [PubMed] [Google Scholar]
- 48.Klukowski M. (2011) Effects of breeding season, testosterone and ACTH on the corticosterone response of free-ranging male fence lizards (Sceloporus undulatus). Gen Comp Endocrinol 173: 295–302. [DOI] [PubMed] [Google Scholar]
- 49.Koren L, Mokady O, Karaskov T, Klein J, Koren G, Geffen E. (2002) A novel method using hair for determining hormonal levels in wildlife. Anim Behav 63: 403–406. [Google Scholar]
- 50.Kouba AJ, Vance CK. (2009) Applied reproductive technologies and genetic resource banking for amphibian conservation. Reprod Fertil Dev 21: 719–737. [DOI] [PubMed] [Google Scholar]
- 51.Kriger KM, Hero J-M. (2008) Altitudinal distribution of chytrid (Batrachochytrium dendrobatidis) infection in subtropical Australian frogs. Austral Ecol 33: 1022–1032. [Google Scholar]
- 52.Kurstak E. (1985) Progress in enzyme immunoassays: production of reagents, experimental design, and interpretation. Bull World Health Organ 63: 793–811. [PMC free article] [PubMed] [Google Scholar]
- 53.Leboulenger F, Trochard MC, Morin JP, Dupont W, Vaudry H, Vaillant R. (1977) [Competitive protein-binding radioassay of corticosterone in the plasma of the frog, Rana esculenta L. (author's transl)]. J Physiol (Paris) 73: 73–83. [PubMed] [Google Scholar]
- 54.Leboulenger F, Delarue C, Belanger A, Perroteau I, Netchitailo P, Leroux P, Jegou S, Tonon MC, Vaudry H. (1982) Direct radioimmunoassays for plasma corticosterone and aldosterone in frog. I. Validation of the methods and evidence for daily rhythms in a natural environment. Gen Comp Endocrinol 46: 521–532. [DOI] [PubMed] [Google Scholar]
- 55.Lehninger AL. (2005) Lehninger Principles of Biochemistry. W.H. Freeman, New York. [Google Scholar]
- 56.Licht P, McCreery BR, Barnes R, Pang R. (1983) Seasonal and stress related changes in plasma gonadotropins, sex steroids, and corticosterone in the bullfrog, Rana catesbeiana. Gen Comp Endocrinol 50: 124–145. [DOI] [PubMed] [Google Scholar]
- 57.Lips KR, Diffendorfer J, Mendelson JR, III, Sears MW. (2008) Riding the wave: reconciling the roles of disease and climate change in amphibian declines. PLoS Biol 6: pe72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McEwen BS, Stellar E. (1993) Stress and the individual. Arch Intern Med 153: 2093–2101. [PubMed] [Google Scholar]
- 59.McEwen BS, Wingfield JC. (2003) The concept of allostasis in biology and biomedicine. Horm Behav 43: 2–15. [DOI] [PubMed] [Google Scholar]
- 60.McEwen BS, Wingfield JC. (2007) Allostasis and allostatic load. In Fink G., ed. Encyclopedia of Stress, Ed 2 Academic Press, New York, pp. 135–141. [Google Scholar]
- 61.McEwen BS, Wingfield JC. (2010) What is in a name? Integrating homeostasis, allostasis and stress. Horm Behav 57: 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Markham JA, Beckel-Mitchener AC, Estrada CM, Greenough WT. (2006) Corticosterone response to acute stress in a mouse model of Fragile X syndrome. Psychoneuroendocrinology 31: 781–785. [DOI] [PubMed] [Google Scholar]
- 63.Mendel CM. (1989) The free hormone hypothesis: a physiologically based mathematical model. Endocr Rev 10: 232–274. [DOI] [PubMed] [Google Scholar]
- 64.Mendonça MT, Licht P, Ryan MJ, Barnes R. (1985) Changes in hormone levels in relation to breeding behavior in male bullfrogs (Rana catesbeiana) at the individual and population levels. Gen Comp Endocrinol 58: 270–279. [DOI] [PubMed] [Google Scholar]
- 65.Millspaugh JJ, Washburn BE. (2004) Use of fecal glucocorticoid metabolite measures in conservation biology research: considerations for application and interpretation. Gen Comp Endocrinol 138: 189–199. [DOI] [PubMed] [Google Scholar]
- 66.Monfort SL, Schwartz CC, Wasser SK. (1993) Monitoring reproduction in captive moose using urinary and fecal steroid metabolites. J Wildl Manage 57: 400–407. [Google Scholar]
- 67.Monfort SL. (2003) Non-invasive endocrine measures of reproduction and stress in wild populations. In WV Holt, AR Pickard, JC Rodger, DE Wildt, eds, Reproductive Science and Integrated Conservation. Cambridge University Press, Cambridge, pp. 147–165. [Google Scholar]
- 68.Moore FL, Boyd SK, Kelley DB. (2005) Historical perspective: hormonal regulation of behaviors in amphibians. Horm Behav 48: 373–383. [DOI] [PubMed] [Google Scholar]
- 69.Moore IT, Jessop TS. (2003) Stress, reproduction, and adrenocortical modulation in amphibians and reptiles. Horm Behav 43: 39–47. [DOI] [PubMed] [Google Scholar]
- 70.Moore MC, Thompson CW, Marler CA. (1991) Reciprocal changes in corticosterone and testosterone levels following acute and chronic handling stress in the tree lizard, Urosaurus ornatus. Gen Comp Endocrinol 81: 217–226. [DOI] [PubMed] [Google Scholar]
- 71.Muller C, Jenni-Eiermann S, Blondel J, Perret P, Caro SP, Lambrechts M, Jenni L. (2006) Effect of human presence and handling on circulating corticosterone levels in breeding blue tits (Parus caeruleus). Gen Comp Endocrinol 148: 163–171. [DOI] [PubMed] [Google Scholar]
- 72.Munro C, Stabenfeldt G. (1984) Development of a microtitre plate enzyme-immunoassay for the determination of progesterone. J Endocrinol 101: 41–49. [DOI] [PubMed] [Google Scholar]
- 73.Nandi J. (1967) Comparative endocrinology of steroid hormones in vertebrates. Am Zool 7: 115–133. [DOI] [PubMed] [Google Scholar]
- 74.Narayan E, Molinia F, Christi K, Morley C, Cockrem J. (2010a) Urinary corticosterone metabolite responses to capture, and annual patterns of urinary corticosterone in wild and captive endangered Fijian ground frogs (Platymantis vitiana). Aust J Zool 58: 189–197. [Google Scholar]
- 75.Narayan E, Molinia F, Christi K, Morley C, Cockrem J. (2010b) Annual cycles of urinary reproductive steroid concentrations in wild and captive endangered Fijian ground frogs (Platymantis vitiana). Gen Comp Endocrinol 166: 172–179. [DOI] [PubMed] [Google Scholar]
- 76.Narayan E, Hero J-M. (2011) Urinary corticosterone responses and haematological stress indicators in the endangered Fijian ground frog (Platymantis vitiana) during transportation and captivity. Aust J Zool 59: 79–85. [Google Scholar]
- 77.Narayan EJ, Cockrem JF, Hero J-M. (2011a) Urinary corticosterone metabolite responses to capture and captivity in the cane toad (Rhinella marina). Gen Comp Endocrinol 173: 371–377. [DOI] [PubMed] [Google Scholar]
- 78.Narayan E, Molinia F, Hero J-M. (2011b) Absence of invasive Chytrid fungus (Batrachochytrium dendrobatidis) in native Fijian ground frog (Platymantis vitiana) populations on Viwa-Tailevu, Fiji Islands. Acta Herpetologica 6: 261–266. [Google Scholar]
- 79.Narayan E, Molinia F, Christina K, Cockrem J, Hero J-M. (2011c) Urinary corticosterone responses to capture and toe-clipping in the cane toad (Rhinella marina) indicate that toe-clipping is a stressor for amphibians. Gen Comp Endocrinol 174: 238–245. [DOI] [PubMed] [Google Scholar]
- 80.Narayan E, Hero J-M, Cockrem JF. (2012a) Inverse urinary corticosterone and testosterone responses to different durations of restraint in the cane toad (Rhinella marina). Gen Comp Endocrinol 179: 345–349. [DOI] [PubMed] [Google Scholar]
- 81.Narayan E, Cockrem JF, Hero JM. (2012b) Effects of temperature on urinary corticosterone metabolite responses to short-term capture and handling stress in the cane toad (Rhinella marina). Gen Comp Endocrinol 178: 301–305. [DOI] [PubMed] [Google Scholar]
- 82.Narayan E, Cockrem J, Hero J-M. (2012c) Individual variation in urinary corticosterone metabolite responses in two closely related species of Fijian frogs. Gen Comp Endocrinol 177: 55–61. [DOI] [PubMed] [Google Scholar]
- 83.Narayan E, Molinia F, Cockrem J, Hero J-M. (2012d) Individual variation and repeatability in urinary corticosterone metabolite responses to capture in the cane toad (Rhinella marina). Gen Comp Endocrinol 175: 284–289. [DOI] [PubMed] [Google Scholar]
- 84.Narayan E, Molinia FC, Cockrem JF, Hero J-M. (2012e) Changes in urinary testosterone and corticosterone metabolites during short-term confinement with repeated handling in wild male cane toads (Rhinella marina). Aust J Zool 59: 264–269. [Google Scholar]
- 85.Narayan E, Hero JM, Evans N, Nicolson V, Mucci A. (2012f) Non-invasive evaluation of physiological stress hormone responses in a captive population of the greater bilby (Macrotis lagotis). Endang Species Res 18: 279–289. [Google Scholar]
- 86.Narayan E, Cockrem JF, Hero J-M. (2013a) Are baseline and short-term corticosterone stress responses in free-living amphibians repeatable? Comp Biochem Physiol A Mol Integr Physiol 164: 21–28. [DOI] [PubMed] [Google Scholar]
- 87.Narayan EJ, Cockrem JF, Hero J-M. (2013b) Repeatability of baseline corticosterone and short-term corticosterone stress responses, and their correlation with testosterone and body condition in a terrestrial breeding anuran (Platymantis vitiana). Comp Biochem Physiol A Mol Integr Physiol 165: 304–312. [DOI] [PubMed] [Google Scholar]
- 88.Narayan EJ, Webster K, Nicolson V, Mucci A, Hero J-M. (2013c) Non-invasive evaluation of physiological stress in an iconic Australian marsupial: the Koala (Phascolarctos cinereus). Gen Comp Endocrinol 187C: 39–47. [DOI] [PubMed] [Google Scholar]
- 89.Norman AW, Litwack G. (1997) Hormones. Academic Press, San Diego. [Google Scholar]
- 90.Orchinik M, Licht P, Crews D. (1988) Plasma steroid concentrations change in response to sexual behavior in Bufo marinus. Horm Behav 22: 338–350. [DOI] [PubMed] [Google Scholar]
- 91.Orchinik M, Matthews L, Gasser PJ. (2000) Distinct specificity for corticosteroid binding sites in amphibian cytosol, neuronal membranes, and plasma. Gen Comp Endocrinol 118: 284–301. [DOI] [PubMed] [Google Scholar]
- 92.Øverli Ø, Sørensen C, Pulman KGT, Pottinger TG, Korzan W, Summers CH, Nilsson GE. (2007) Evolutionary background for stress-coping styles: relationships between physiological, behavioral, and cognitive traits in non-mammalian vertebrates. Neurosci Biobehav Rev 31: 396–412. [DOI] [PubMed] [Google Scholar]
- 93.Palme R, Rettenbacher S, Touma C, El-Bahr SM, Möstl E. (2005) Stress hormones in mammals and birds. Comparative aspects regarding metabolism, excretion, and noninvasive measurement in fecal samples. Ann NY Acad Sci 1040: 162–171. [DOI] [PubMed] [Google Scholar]
- 94.Pancak MK, Taylor DH. (1983) Seasonal and daily plasma corticosterone rhythms in American toads, Bufo americanus. Gen Comp Endocrinol 50: 490–497. [DOI] [PubMed] [Google Scholar]
- 95.Parris KM, McCarthy MA. (2001) Identifying effects of toe clipping on anuran return rates: the importance of statistical power. Amphibia-Reptilia 22: 275–289. [Google Scholar]
- 96.Pemberton PA, Stein PE, Pepys MB, Potter JM, Carrell RW. (1988) Hormone binding globulins undergo serpin conformational change in inflammation. Nature 336: 257–258. [DOI] [PubMed] [Google Scholar]
- 97.Pounds AJ, Bustamante MR, Coloma LA, Consuegra JA, Fogden MPL, Foster PN, La Marca E, Masters KL, Merino-Viteri A, Puschendorf R. et al. (2006) Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439: 161–167. [DOI] [PubMed] [Google Scholar]
- 98.Preest MR, Cree A, Tyrrell CL. (2005) ACTH-induced stress response during pregnancy in a viviparous gecko: no observed effect on offspring quality. J Exp Zool A Comp Exp Biol 303A: 823–835. [DOI] [PubMed] [Google Scholar]
- 99.Pukazhenthi BS, Wildt DE. (2004) Which reproductive technologies are most relevant to studying, managing and conserving wildlife? Reprod Fertil Dev 16: 33–46. [DOI] [PubMed] [Google Scholar]
- 100.Queyras A, Carosi M. (2004) Non-invasive techniques for analyzing hormonal indicators of stress. Ann Ist Super Sanita 40: 211–221. [PubMed] [Google Scholar]
- 101.Ricciardella LF, Bliley JM, Feth CC, Woodley SK. (2010) Acute stressors increase plasma corticosterone and decrease locomotor activity in a terrestrial salamander (Desmognathus ochrophaeus). Physiol Behav 101: 81–86. [DOI] [PubMed] [Google Scholar]
- 102.Rohr JR, Raffel TR. (2010) Linking global climate and temperature variability to widespread amphibian declines putatively caused by disease. Proc Natl Acad Sci USA 107: 8269–8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rollins-Smith LA. (2001) Neuroendocrine-immune system interactions in amphibians: implications for understanding global amphibian declines. Immunol Res 23: 273–280. [DOI] [PubMed] [Google Scholar]
- 104.Romero LM, Reed JM. (2005) Collecting baseline corticosterone samples in the field: is under 3 min good enough? Comp Biochem Physiol A Mol Integr Physiol 140: 73–79. [DOI] [PubMed] [Google Scholar]
- 105.Romero LM, Dickens MJ, Cyr NE. (2009) The reactive scope model — a new model integrating homeostasis, allostasis, and stress. Horm Behav 55: 375–389. [DOI] [PubMed] [Google Scholar]
- 106.Salway JG. (2004) Metabolism at a Glance. Blackwell Publishers, Oxford, UK. [Google Scholar]
- 107.Schwarzenberger F. (2007) The many uses of non-invasive faecal steroid monitoring in zoo and wildlife species. Int Zoo Yearb 41: 52–74. [Google Scholar]
- 108.Sheriff MJ, Krebs CJ, Boonstra R. (2010) Assessing stress in animal populations: do fecal and plasma glucocorticoids tell the same story? Gen Comp Endocrinol 166: 614–619. [DOI] [PubMed] [Google Scholar]
- 109.Stebbins RC, Cohen NW. (1995) A Natural History of Amphibians. Princeton University Press, Princeton, NJ. [Google Scholar]
- 110.Sterling P, Eyer J. (1988) Allostasis: a new paradigm to explain arousal pathology. In Fisher S, Reason J, eds, Handbook of Life Stress, Cognition and Health. John Wiley, New York, pp. 629–647. [Google Scholar]
- 111.Stevenson RD, Tuberty SR, deFur PL, Wingfield JC. (2005) Ecophysiology and conservation: the contribution of endocrinology and immunology– introduction to the symposium. Integr Comp Biol 45: 1–3. [DOI] [PubMed] [Google Scholar]
- 112.Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, Waller RW. (2004) Status and trends of amphibian declines and extinctions worldwide. Science 306: 1783–1786. [DOI] [PubMed] [Google Scholar]
- 113.Szymanski DC, Gist DH, Roth TL. (2006) Anuran gender identification by fecal steroid analysis. Zoo Biol 25: 35–46. [Google Scholar]
- 114.Vasudevan DM, Sreekumari S, Vaidyanathan K. (2011) Textbook of Biochemistry for Medical Students. Jaypee Brothers Medical Publishers (P) Ltd, New Delhi. [Google Scholar]
- 115.Wack CL, Lovern MB, Woodley SK. (2010) Transdermal delivery of corticosterone in terrestrial amphibians. Gen Comp Endocrinol 169: 269–275. [DOI] [PubMed] [Google Scholar]
- 116.Wack CL, DuRant SE, Hopkins WA, Lovern MB, Feldhoff RC, Woodley SK. (2012) Elevated plasma corticosterone increases metabolic rate in a terrestrial salamander. Comp Biochem Physiol A Mol Biochem Integr Physiol 161: 153–158. [DOI] [PubMed] [Google Scholar]
- 117.Ward CK, Fontes C, Breuner CW, Mendonça MT. (2007) Characterization and quantification of corticosteroid-binding globulin in a southern toad, Bufo terrestris, exposed to coal-combustion-waste. Gen Comp Endocrinol 152: 82–88. [DOI] [PubMed] [Google Scholar]
- 118.Wasser SK, Bevis K, King G, Hanson E. (1997) Non-invasive physiological measures of disturbance in the northern spotted owl. Conserv Biol 11: 1019–1022. [Google Scholar]
- 119.Wasser SK, Hunt KE, Brown JL, Cooper K, Crockett CM, Bechert U, Millspaugh JJ, Larson S, Monfort SL. (2000) A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen Comp Endocrinol 120: 260–275. [DOI] [PubMed] [Google Scholar]
- 120.Watson R, Munro C, Edwards KL, Norton V, Brown JL, Walker SL. (2013) Development of a versatile enzyme immunoassay for non-invasive assessment of glucocorticoid metabolites in a diversity of taxonomic species. Gen Comp Endocrinol 186: 16–24. [DOI] [PubMed] [Google Scholar]
- 121.Whitten PL, Stavisky R, Aureli F, Russell E. (1998) Response of fecal cortisol to stress in captive chimpanzees (Pan troglodytes). Am J Primatol 44: 57–69. [DOI] [PubMed] [Google Scholar]
- 122.Wikelski M, Cooke SJ. (2006) Conservation physiology. Trends Ecol Evol 21: 38–46. [DOI] [PubMed] [Google Scholar]
- 123.Wilczynski W, Lynch KS, O'Bryant EL. (2005) Current research in amphibians: studies integrating endocrinology, behavior, and neurobiology. Horm Behav 48: 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wilczynski W, Lynch KS. (2011) Female sexual arousal in amphibians. Horm Behav 59: 630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wildt DE, Wemmer C. (1999) Sex and wildlife: the role of reproductive science in conservation. Biodivers Conserv 8: 965–976. [Google Scholar]
- 126.Wingfield JC, Sapolsky RM. (2003) Reproduction and resistance to stress: when and how. J Neuroendocrinol 15: 711–724. [DOI] [PubMed] [Google Scholar]
- 127.Zamudio SR, Quevedo-Corona L, Garcés L, De La Cruz F. (2009) The effects of acute stress and acute corticosterone administration on the immobility response in rats. Brain Res Bull 80: 331–336. [DOI] [PubMed] [Google Scholar]
- 128.Ziegler TE, Santos CV, Pissinatti A, Strier KB. (1997) Steroid excretion during the ovarian cycle in captive and wild muriquis, Brachyteles arachnoids. Am J Primatol 42: 311–321. [DOI] [PubMed] [Google Scholar]