This study provides evidence that hunting and logging can impose stress on animals. Spider monkeys showed elevated fecal glucocorticoid metabolite (FGCM) levels in forest fragments with high levels of human impact, whereas howler monkeys did not. Glucocorticoid measurements can be a useful tool to monitor wildlife populations in disturbed areas.
Keywords: Alouatta seniculus, Ateles hybridus, faecal glucocorticoid metabolites, habitat fragmentation, hunting, logging
Abstract
Habitat fragmentation and anthropogenic disturbances are of major concern to the conservation of endangered species because of their potentially negative impact on animal populations. Both processes can impose physiological stress (i.e. increased glucocorticoid output) on animals, and chronically elevated stress levels can have detrimental effects on the long-term viability of animal populations. Here, we investigated the effect of fragment size and human impact (logging and hunting pressure) on glucocorticoid levels of two sympatric Neotropical primates, the red howler monkey (Alouatta seniculus) and the critically endangered brown spider monkey (Ateles hybridus). These two species have been reported to contrast strongly in their ability to cope with anthropogenic disturbances. We collected faecal samples from eight spider monkey groups and 31 howler monkey groups, living in seven and 10 different forest fragments in Colombia, respectively. We measured faecal glucocorticoid metabolite (FGCM) levels in both species using previously validated methods. Surprisingly, fragment size did not influence FGCM levels in either species. Spider monkeys showed elevated FGCMs in fragments with the highest level of human impact, whereas we did not find this effect in howler monkeys. This suggests that the two species differ in their physiological responsiveness to anthropogenic changes, further emphasizing why brown spider monkeys are at higher extinction risk than red howler monkeys. If these anthropogenic disturbances persist in the long term, elevated FGCM levels can potentially lead to a state of chronic stress, which might limit the future viability of populations. We propose that FGCM measurements should be used as a tool to monitor populations living in disturbed areas and to assess the success of conservation strategies, such as corridors connecting forest fragments.
Introduction
Habitat loss, habitat fragmentation, and anthropogenic disturbances that accompany these processes (e.g. logging, increased hunting pressure) are of major concern to the conservation of endangered species due to their role in population declines (Peres, 2001; Fahrig, 2003). Currently, numerous species from all vertebrate groups are threatened with extinction (IUCN, 2012). The pervasive process of anthropogenic disturbances of natural ecosystems (Hannah et al., 1995; Foley et al., 2005) emphasizes the need to understand the proximate effects that these alterations have on the health and survival of animal populations. Generally, taxa with narrow dietary niches and that occupy few habitat types are at greater extinction risk than taxa that have broader niches and occupy several habitat types (Harcourt et al., 2002). Species respond to fragmentation and disturbances differently depending on factors such as life history, geographical range, ecological niche, and dispersal ability (Purvis et al., 2000; Henle et al., 2004; Cardillo et al., 2005). Some species manage to adjust aspects of their behaviour and social system (Menon and Poirier, 1996; Sumner et al., 1999; González-Solís et al., 2001; Blumstein et al., 2005; Umapathy et al., 2011), whereas species that cannot adjust face local extinction (Cosson et al., 1999; Peres, 2001).
The role that changes in physiological parameters play in population declines and, ultimately, species extinction is not yet well understood. Glucocorticoids (GCs; cortisol and corticosterone, depending on the species) are the frontline hormones of the vertebrate stress response (Sapolsky et al., 2000) and potentially play an important role. Many different stimuli (e.g. predators, aggression from conspecifics, food deprivation) can elicit a stress response that triggers the release of GCs. Such elevations of GC levels are adaptive and important for the survival of the individual (Boonstra, 2005; Monclús et al., 2005). Glucocorticoids mobilize readily available energy that can be used to respond to the stimulus that triggered the release of GCs (Selye, 1956; Breazile, 1987; Stratakis and Chrousos, 1995). During the stress response, other energetically demanding activities, such as digestion and reproduction, are suppressed (Wingfield et al., 1998; Landys et al., 2006). Hence, long-term elevations of GCs can have deleterious effects on reproduction, growth, and immune system activity (Pickering et al., 1991; Cyr and Romero, 2007; Charbonnel et al., 2008; French et al., 2010; Setchell et al., 2010). Anthropogenic disturbances have been associated with GC elevations in many vertebrate taxa (amphibians: Homan et al., 2003; Janin et al., 2011; reptiles: French et al., 2010; birds: Lucas et al., 2006; Wasser et al., 1997; and mammals: Martínez-Mota et al., 2007; Gobush et al., 2008; Rangel-Negrín et al., 2009; Jaimez et al., 2012; Dunn et al., 2013). These GC elevations have been linked to negative effects on reproduction and immune system activity (e.g. Ellenberg et al., 2007; French et al., 2010), suggesting that elevated stress levels caused by human influence can directly affect individual health and, ultimately, population viability.
We studied two primate species that occur sympatrically in Colombia, namely brown spider monkeys (Ateles hybridus) and red howler monkeys (Alouatta seniculus), to investigate whether and how anthropogenic disturbances influence their GC output. The two genera Ateles and Alouatta have been reported to contrast strongly in their ability to cope with anthropogenic disturbances (Bernstein et al., 1976; Bicca-Marques, 2003; Michalski and Peres, 2005). Brown spider monkeys are endemic to Colombia and Venezuela, and are considered to be one of the 25 most endangered primate species worldwide due to severe habitat loss and high hunting pressure (Mittermeier et al., 2012). Habitat fragmentation, slow reproductive cycles, large area requirements, and their dietary niche (for review, see Di Fiore et al., 2010) make this species vulnerable to anthropogenic disturbances. Moreover, spider monkeys exhibit flexible grouping patterns (fission–fusion dynamics) that are likely to reduce intra-group feeding competition and enable them to cope with changes in the availability of ripe fruit, their preferred food resources (e.g. Klein and Klein, 1977; Symington, 1988). Accordingly, the confinement to small fragments might reduce the flexibility of their grouping patterns and thereby lower their potential to minimize competition, which might cause increased physiological stress.
In contrast, red howler monkeys seem less vulnerable to anthropogenic disturbances and are able to persist even in extremely small fragments (Crockett, 1998; Lopez et al., 2005; Michalski and Peres, 2005). They have a broad distribution range, occupy a wide array of ecosystems, and are currently not threatened with extinction (Boubli et al., 2008). Typically, howler monkeys require much smaller home ranges than spider monkeys (reviewed by Di Fiore et al., 2010). They from cohesive groups (Neville, 1972; for review, see Di Fiore and Campbell, 2007) and have a mainly folivorous but flexible diet (Milton, 1980; Julliot and Sabatier, 1993; Bicca-Marques, 2003).
The proximate mechanisms leading to such species-specific differences in the ability to cope with a changing environment have not yet been investigated. Therefore, the aim of this study was to determine how fragment size and level of human impact influence physiological stress levels, measured through faecal glucocorticoid metabolites (FGCMs), in both species. We collected faecal samples from both species in forest fragments that differed in size and level of human impact (hunting and logging activities). We predicted that FGCM levels would increase with both, decreasing fragment size (as a proxy for fragmentation intensity) and increasing level of human impact. Due to differences in diet, area requirements, and adaptation capabilities, we predicted that red howler monkeys would generally react less strongly than brown spider monkeys to these disturbances, and that they would show no elevation in GC levels or a less pronounced elevation compared with brown spider monkeys.
Materials and methods
Study sites
Between April 2010 and April 2012, we collected faecal samples from eight spider monkey and 31 howler monkey groups, living in seven and 10 different fragments, respectively. Fragments differed in size and human impact (Table 1). Four fragments (San Juan, Quinchas, Jamaica, and Juntas) are long-term study sites, and study groups have been habituated to human observers previously (Aldana et al., 2008; Gómez-Posada et al., 2010; Link et al., 2010). We visited all other fragments only for faecal sample collection purposes (for 2–3 weeks). Nine of the study fragments were occupied by both species and the other four (Jamaica, Juntas, LGPM, and Cienaga) were occupied only by howler monkeys. Although most fragments contained both species, sometimes faecal sample collection was feasible only for one. We determined the level of current human impact through observations (e.g. recently cut tree stumps, presence of hunting dogs in fragments, pet primates at farms) and surveys in which we interviewed farm owners and workers. We classified fragments without current human hunting activities and absence of recent logging activity (≤2 months) as level 0, fragments with either hunting or logging activity as level 1, and fragments with both hunting and ongoing logging activity as level 2.
Table 1:
Number of faecal samples collected for each species in the different forest fragments (n = 13), which varied in size and level of human impact
| No. of samples (groups) |
|||||
|---|---|---|---|---|---|
| Fragment | Size (ha) | Human impact | Ateles hybridus | Alouatta seniculus | Location |
| San Juan | 65 | 0 | 411 (2) | 289 (17) | 6° 43′N, 74° 09′W |
| San Juan3 | 75 | 0 | 10 (1) | 5 (1) | 6° 43′N, 74° 07′W |
| LGPM | 100 | 0 | — | 9 (1) | 6° 41′N, 74° 09′W |
| Quinchas | 250 | 0 | 46 (1) | 21 (3) | 6° 02′N, 74° 16′W |
| Terra Firme1 | 500 | 0 | 3 (1) | — | 6° 41′N, 74° 08′W |
| LGPM2 | 500 | 0 | — | 4 (1) | 6° 41′N, 74° 09′W |
| Jamaica | 4.21 | 1 | — | 12 (1) | 4° 23′N, 75° 48′W |
| Juntas | 25.5 | 1 | — | 13 (1) | 4° 25′N, 75° 47′W |
| India | 500 | 1 | 3 (1) | — | 6° 15′N, 74° 07′W |
| Cienaga | 50 | 2 | — | 6 (2) | 6° 42′N, 74° 08′W |
| Campo Capote | 250 | 2 | 3 (1) | 5 (1) | 6° 34′N, 73° 51′W |
| Remedios | 400 | 2 | 5 (1) | — | 6° 53′N, 74° 34′W |
| San Juan 4 | 500 | 2 | — | 9 (3) | 6° 41′N, 74° 07′W |
| Total no. of samples | 481 (8) | 373 (31) | |||
Faecal sample collection
We collected samples from adult and subadult individuals (mean ± SD: Ateles, 7.9 ± 12.5 samples per individual and Alouatta, 2.3 ± 2.6 samples per individual) and, for every sample, noted sex and age-class, female reproductive state (when identifiable), collection time, and date. The time lag of GC metabolite excretion in faeces is ∼24 h in A. hybridus and ∼46 h in A. seniculus (Rimbach et al., 2013). Thus, we avoided following unhabituated groups on 2–3 consecutive days (depending on the species) to reduce the influence of observer presence on FGCM levels. In unhabituated groups, we did not recognize individuals and, to avoid resampling of individuals, we sampled each group or subgroup (in the case of Ateles) only once. Faecal samples collected in the fragments ‘Jamaica’ and ‘Juntas’ were collected between May and July 2010, and stored at −20°C until extraction in December 2012. Long-term faecal sample storage at −20°C has been shown to preserve immunoreactive faecal GC metabolites reliably (Hunt and Wasser, 2003), and we are therefore confident that this storage method has not affected the FGCM measurements of these samples.
In both species, pregnancy cannot reliably be detected by observation. Thus, to determine the approximate conception date and female reproductive state at sample collection we used parturition date (habituated groups) in combination with average gestation length (Ateles: ∼7.5 months and Alouatta: ∼6.3 months; Di Fiore and Campbell, 2007). We categorized females as lactating for the time in which they nursed dependent offspring. Females that were neither pregnant nor lactating were categorized as non-pregnant, non-lactating. However, in unhabituated groups we were able to categorize females merely as either lactating or non-lactating (pregnant and cycling females). We collected comparative numbers of samples from both sexes (A. hybridus: 51.8% females and 48.2% males; and A. seniculus: 45.6% females and 54.4% males) per fragment and have no reason to assume that the collection was biased towards collecting samples from females in only certain reproductive conditions.
Before placing faecal material into the sample tube, we homogenized the faecal bolus and removed any obvious undigested matter (e.g. large seeds). We placed ∼0.5 g of faeces into a 15 ml pre-weighed polypropylene tube pre-filled with 5 ml of 96% ethanol and shook the tube until the faeces were suspended in the solvent (Shutt et al., 2012; Rimbach et al., 2013). We kept the samples at ambient temperatures until steroid extraction in the evening.
Steroid extraction and faecal glucocorticoid metabolite analysis
We determined faecal wet weight by calculating the difference between the weight of the tube before (tube plus ethanol) and after sample collection (tube, ethanol, and faeces). We shook the tubes firmly for 5 min and thereafter, centrifuged the samples for 1 min using a manually operated centrifuge (Shutt et al., 2012; Rimbach et al., 2013). We poured off ∼2 ml of each faecal extract into 2 ml polypropylene tubes, covered them with parafilm, labelled them, and stored them at ambient temperatures (∼25°C) in a dark place. Every 2 months we transported the extracts to the Universidad de Los Andes, Bogotá and stored them at −20°C until shipment to the Endocrinology Laboratory at the German Primate Center for analysis. We showed in a previous study that storing faecal extracts in this way did not affect FGCM levels (Rimbach et al., 2013).
We analysed all faecal samples using a previously validated (Rimbach et al., 2013) enzyme-immunoassay for 11β-hydroxyetiocholanolone, a group-specific measurement of 5β-reduced GC metabolites (Ganswindt et al., 2003) with a 3α,11β-dihydroxy structure. The enzyme-immunoassay was performed as described in detail by Heistermann et al. (2004). Prior to hormone measurement, we diluted extracts 1:250–1:2000 (depending on original concentration) in assay buffer and thereafter, took duplicate aliquots to assay. Intra- and inter-assay coefficients of variation of were 6.1 and 7.8%, respectively, for high-value and 7.4 and 13.0%, respectively, for low-value quality controls. All hormone concentrations are expressed as nanograms per gram faecal wet weight.
Statistical analyses
To assess the influence of fragment size and level of human impact on FGCM levels, we used linear mixed models (LMM; Baayen, 2010). We fitted all models with the lmer function from the lme4 R-package (Bates and Maechler, 2010) in R2.15.1 (R Development Core Team 2012). We used restricted maximal likelihood methods to estimate the models, because they are robust against unequal sample sizes (Keselman et al., 2001). We used two LMMs per species, and group and fragment ID were used as random factors in all models. Glucocorticoid excretion often shows a diurnal rhythm, and GC levels can be affected by a variety of potentially confounding factors (for review, see Millspaugh and Washburn, 2004; Keay et al., 2006; Goymann, 2012). To account for these factors, we used sex, age, female reproductive state, group size, and sample collection time as control variables in the models (because some of these variables have been shown to affect FGCM levels in the study species; Rimbach et al., 2013). We tested for interactions between fragment size and human impact, and between group size and fragment size. These were not significant (P ≥ 0.05) and are not included in the final models. For each study species we used two models, one using the size of each fragment and one comparing fragments with ‘control sites’. In the second set of models, we defined ‘control sites’ as fragments that were larger than a certain, species-specific, size. Due to differences in area requirements between the study species (Di Fiore et al., 2010), we defined fragments larger than 50 ha as ‘control sites’ (or as a proxy for continuous forest) for red howler monkeys, whereas for brown spider monkeys we defined fragments larger than 200 ha as ‘control sites’.
We log transformed the response variable (FGCM levels) to achieve normal distribution and checked that the assumptions of normally distributed and homogeneous residuals were fulfilled by visually inspecting Q–Q plots and the residuals plotted against the fitted values for each model. To assess model stability, we ran diagnostics (dfbetas) that did not suggest the existence of influential cases. We used the function vif of the R-package car (Fox and Weisberg, 2011) applied to a standard linear model, excluding the random effects, to derive variance inflation factors (Field, 2005). We used a likelihood ratio test (R function ‘anova’) to determine the significance of the full model (all fixed and random effects) compared with the corresponding null model (only random effects). We used the functions pvals.fnc of the R-package ‘language R’ (Baayen, 2010) to determine P values based on Markov Chain Monte Carlo (MCMC) sampling (Baayen, 2011). All statistical tests were two tailed, and the statistical threshold was set at P ≤ 0.05.
Results
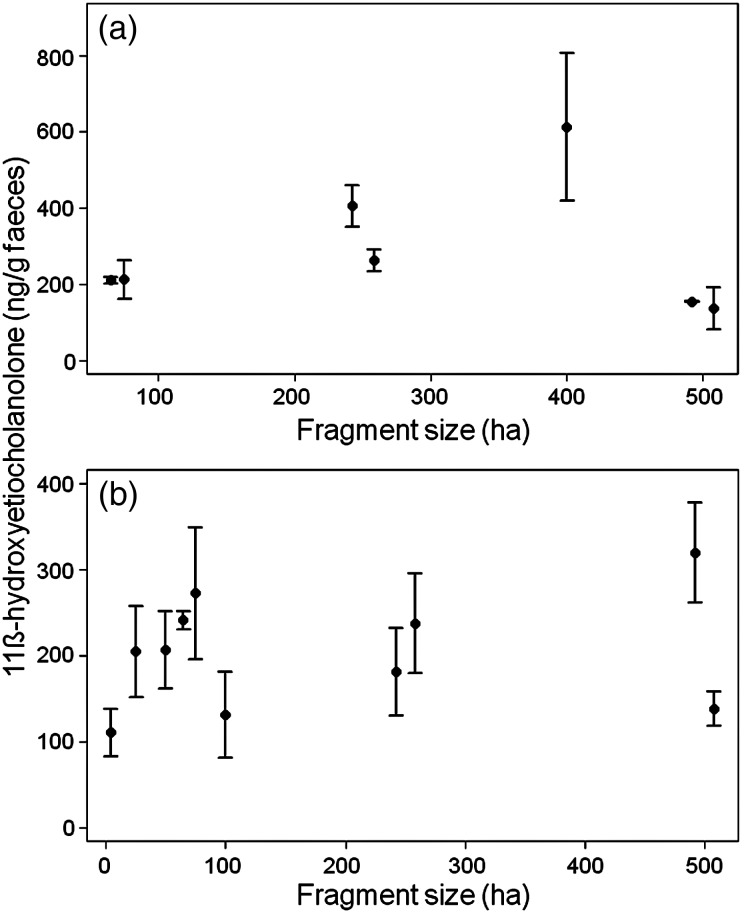
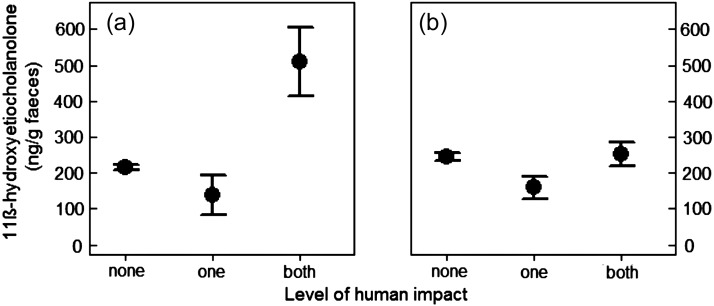
In both species, FGCM levels varied substantially between different fragments (Figure 1). The fragment size did not affect FGCM levels in spider monkeys (n = 481 samples; LMM: χ2 = 2.06, d.f. = 1, P = 0.15; Figure 1a), whereas human impact had an influence (LMM: χ2 = 17.5, d.f. = 2, P = 0.0001; Figure 2a). Specifically, spider monkeys living in fragments with both kinds of human influence had FGCM levels (mean ± SEM = 496.7 ± 90.0 ng/g) that were more than twice as high as those found in animals living in fragments with no disturbance (mean ± SEM = 206.74 ± 7.5 ng/g; PMCMC = 0.01; Table 2) or only one type of disturbance (mean ± SEM = 137.16 ± 45.5 ng/g; PMCMC = 0.04). There was no difference between the FGCM levels of spider monkeys living in fragments with no human impact or only one type (PMCMC = 0.48, Table 2).
Figure 1:
Mean ± SEM faecal glucocorticoid metabolite levels of Ateles hybridus (a) and Alouatta seniculus (b) in relationship to forest fragment size.
Figure 2:
Mean ± SEM faecal glucocorticoid metabolite levels of Ateles hybridus (a) and Alouatta seniculus (b) in relationship to level of human impact (none: no hunting or logging, one: either hunting or logging, both: both hunting and logging).
Table 2:
Results of the linear mixed models examining the influence of forest fragment size and level of human impact on log-transformed faecal glucocorticoid metabolite levels in Ateles hybridus
| d.f. | χ2 | P value | |
|---|---|---|---|
| Null vs. full model | 9 | 113.38 | <0.001* |
| Variable | Estimate ± SEM | t | PMCMC |
| Intercept | 5.76 ± 0.33 | 17.52 | 0.0001* |
| Fragment size | 0.09 ± 0.05 | 1.81 | 0.1694 |
| Impact: both–none | −0.93 ± 0.32 | −2.88 | 0.0134* |
| Impact: both–one | −1.37 ± 0.57 | −2.39 | 0.0400* |
| Impact: one–none | 0.43 ± 0.59 | 0.72 | 0.4820 |
| Group size | 0.03 ± 0.04 | 0.83 | 0.7428 |
| Time | −0.29 ± 0.03 | −8.72 | 0.0001* |
| Sex | 0.14 ± 0.09 | 1.48 | 0.1532 |
| Age | −0.33 ± 0.11 | −2.84 | 0.0062* |
| Overall effect | d.f. | χ2 | P |
|---|---|---|---|
| Female reproductive state | 3 | 21.15 | <0.001* |
| Fragment size | 1 | 2.06 | 0.1565* |
| Human impact | 2 | 17.5 | 0.0001* |
*Variables that significantly influenced faecal glucocorticoid metabolite levels.
The results of the second model (comparing fragments with ‘control sites’) were similar to the model using fragment size. The FGCM levels of spider monkeys did not differ between fragments (mean ± SEM = 217.28 ± 8.12 ng/g) and ‘control sites’ (mean ± SEM = 378.51 ± 106.42 ng/g; n = 481 samples; LMM, χ2 = 0.27, d.f. = 1, P = 0.60; Table 3). As in the first model, the level of human impact influenced FGCM levels of spider monkeys (LMM, χ2 = 16.21, d.f. = 2, P = 0.0003; Table 3).
Table 3:
Results of the linear mixed models examining the influence of forest type (fragment or ‘control sites’) and level of human impact on log-transformed faecal glucocorticoid metabolite levels in Ateles hybridus
| d.f. | χ2 | P value | |
|---|---|---|---|
| Null vs. full model | 9 | 72.52 | <0.001* |
| Variable | Estimate ± SEM | t | PMCMC |
| Intercept | 6.33 ± 0.32 | 19.58 | 0.0001* |
| Forest type | −0.20 ± 0.39 | −0531 | 0.5708 |
| Impact: both–none | −1.06 ± 0.34 | −3.05 | 0.0168* |
| Impact: both–one | 1.04 ± 0.57 | 1.81 | 0.0868 |
| Impact: one–none | −1.06 ± 0.34 | −3.05 | 0.0172* |
| Group size | 0.10 ± 0.03 | 2.61 | 0.3718 |
| Time | −0.23 ± 0.03 | −6.30 | 0.0001* |
| Sex | −0.01 ± 0.10 | −0.15 | 0.9004 |
| Age | −0.36 ± 0.12 | −2.95 | 0.0030* |
| Overall effect | d.f. | χ2 | P value |
|---|---|---|---|
| Female reproductive state | 3 | 8.38 | 0.0386* |
| Forest type | 1 | 0.27 | 0.6033* |
| Human impact | 2 | 16.21 | 0.0003* |
*Variables that significantly influenced faecal glucocorticoid metabolite levels.
In howler monkeys, neither fragment size (Figure 1b) nor human impact (none: mean ± SEM = 245.9 ± 10.6 ng/g; one: 159.4 ± 31.0 ng/g; and both: 251.2 ± 32.9 ng/g; Figure 2b) influenced FGCM levels (n = 373 samples, full vs. null model χ2 = 13.76, d.f. = 9, P = 0.13). The second model showed similar results. Neither human impact nor forest type (fragments: mean ± SEM = 168.61 ± 26.89 ng/g; and ‘control sites’: mean ± SEM = 246.94 ± 10.36 ng/g) influenced FGCM levels of howler monkeys (n = 373 samples, full vs. null model, χ2 = 11.02, d.f. = 9, P = 0.27).
Discussion
Consistent with our predictions, spider monkeys had higher FGCM levels in fragments with the highest level of human impact compared with less impacted fragments. We did not find such an effect in howler monkeys. In contrast to our predictions, neither fragment size nor forest type (fragments compared to ‘control sites’) influenced FGCM levels of either species. Time of sample collection, age, and female reproductive state influenced FGCM levels in A. hybridus (possible explanations are discussed elsewhere; see Rimbach et al., 2013), but we controlled for these factors in data analyses. This study reinforces previous results concerning species-specific differences in the ability to cope with anthropogenic disturbances and strengthens the assumption that brown spider monkeys are more susceptible to human alterations than red howler monkeys.
Proximity to humans, hunting, and logging activities are likely to be perceived as threatening by many animals. Red deer (Cervus elaphus) that were chased by humans (Bateson and Bradshaw, 1997) and female African elephants (Loxodonta africana) that ranged in areas with high poaching risk (Gobush et al., 2008) had elevated GC levels compared to conspecifics that did not experience the disturbance. The presence of humans can lead to elevated GC levels in several animal taxa (birds: Fowler, 1999; Müllner et al., 2004; Thiel et al., 2011; reptiles: French et al., 2010; mammals: Creel et al., 2002; Barja et al., 2007; Behie et al., 2010; Muehlenbein et al., 2012; Piñeiro et al., 2012; Zwijacz-Kozica et al., 2012). Moreover, proximity to humans can impair the breeding success of animals (Ellenberg et al., 2006; Hinam and St Clair, 2008; Strasser and Heath, 2013), which might be caused by increased GC levels (Ellenberg et al., 2007; Charbonnel et al., 2008). Furthermore, logging activities can result in elevated GC levels (Wasser et al., 1997). Concordant with previous studies, we found elevated FGCM levels of brown spider monkeys in fragments where logging and hunting occurred. Whether this elevation of FGCM levels indicates a state of chronic stress with potential negative consequences on health and fitness is difficult to assess, especially in such long-lived animals, and is beyond the scope of this paper (for a discussion of chronic stress, see e.g. Boonstra, 2013). In contrast to spider monkeys, we did not find an effect of logging and hunting on FGCM levels of red howler monkeys, which suggests that this species might have a lower sensitivity to react to anthropogenic disturbances with an activation of the hypothalamic–pituitary–adrenal axis than brown spider monkeys. Likewise, the intensity of human presence (number of tourists) did not influence the physiological stress levels of another howler monkey species (Alouatta palliata mexicana; Aguilar-Melo et al., 2013, but see Behie et al., 2010).
Fruit availability often declines in small and established (>10 years) fragments (Putz et al., 1990; Cordeiro and Howe, 2001; Arroyo-Rodríguez and Mandujano, 2006; Dunn et al., 2010), and low food availability can cause elevated GC levels in primates (Cavigelli, 1999; Muller and Wrangham, 2004; Chapman et al., 2007; Behie et al., 2010). Surprisingly, spider monkeys living in small fragments did not show elevated FGCM levels compared with those living in larger ones, although they potentially experience low levels of food availability and high resource competition. Although some fragments included in this study are very small, drastic changes in food availability might not have occurred yet because most of these fragments have been created rather recently (<10 years). This might explain why fragment size did not influence FGCM levels of either species. Alternatively, it could be that elevated GC levels were associated with low food availability in some fragments, but we are lacking data on resource availability for most fragments and thus, cannot test this assumption. However, two very small fragments (Jamaica and Juntas) were isolated about 100 years ago, and food availability is extremely low (Gómez-Posada et al., 2010). Nevertheless, howler monkeys are able to persist in these fragments and seem to maintain relatively low GC levels.
In contrast to frugivores, folivores are often able to persist in moderately disturbed areas (Johns and Skorupa, 1987), probably because leaf quantity and quality are often higher in disturbed areas, especially at edges, where light exposure is high (Johns, 1988; Ganzhorn, 1995; Irwin, 2008). In the case of howler monkeys, altered leaf availability and quality might compensate for negative effects associated with small fragments and human impact because of their mainly folivorous diet. This supports the notion that they are capable of habituating to human activities, which is likely to explain why we did not find increased FGCM levels in individuals that live in small and disturbed fragments.
The observed inter-specific differences in responsiveness to human impact could also be the result of a different ‘perception’ of stressful factors. Differences in population densities between fragments might be a crucial factor determining GC levels of animals, and howler monkeys have been reported to live at very high densities in fragments (Rumiz, 1990; Ostro et al., 2001; Gómez-Posada et al., 2010; Link et al., 2010). Owing to the lack of data on population densities for most fragments, we were not able to include this variable. Future research should include this factor and thus help to determine whether the species shows variation in FGCM levels according to different population density levels.
Generally, our results support previous findings emphasizing a species-specific effect of human disturbance and habitat fragmentation on adrenocortical activity. This specificity might be the reason for inconsistent results revealed by previous studies that used GC levels as markers of physiological stress in a conservation context (reviewed by Busch and Hayward, 2009). While several studies report elevated GC levels in response to anthropogenic disturbances (Wasser et al., 1997; Lucas et al., 2006; Martínez-Mota et al., 2007; Rangel-Negrín et al., 2009; Janin et al., 2011; Jaimez et al., 2012), others found the reverse, or no effect at all. For instance, red-bellied lemurs (Eulemur rubriventer; Tecot, 2008) and African forest elephants (Loxodonta africana cyclotis; Munshi-South et al., 2008) show higher GC levels in undisturbed areas than conspecifics in disturbed habitats. Canadian grizzly bears (Ursus arctos) exhibit lower GC levels in areas with high poaching activity compared with less disturbed areas (Wasser et al., 2004), whereas Alaskan brown bears (Ursus arctos horribilis), a closely related subspecies, show no effect of human presence on GCs (von der Ohe et al., 2004). This demonstrates that species probably differ in their sensitivity to disturbances and that not all species respond with a predicable change in GC levels, or (not mutually exclusive) that such a physiological response depends on the degree of the threat perceived.
One important limitation of our study is the lack of faecal samples from extensive and continuous forests and uneven sample sizes between fragments. The small sample size in fragments where primates are being hunted reflects the challenge of encountering and following arboreal animals that are wary and fearful of humans. Although we have only few samples of A. hybridus from two fragments with both types of human impact, the FGCM levels of all those samples are much higher than for those collected in other fragments. Thus, it is conceivable that these differences in FGCM levels reflect true differences, although additional studies should be conducted to confirm these results. Another impeding factor for sample collection is the high degree of fragmentation of the remaining habitat of A. hybridus (Urbani et al., 2008) that exacerbated the access to large forests. Our sample size of fragments with only one type of human impact is also small, and future research (with a larger number of fragments) may clarify which of the two factors (logging or hunting) drives the observed elevation in FGCM levels in brown spider monkeys. Moreover, the intensity of human activities is likely to be another important factor influencing FGCM levels. Unfortunately, we were not able to quantify the intensity of hunting and logging activities during this study (e.g. how many people worked in the fragments per day, how many primates were killed in a defined time period). One way to overcome problems concerning the acquisition of data on the intensity of human activities may be to collect samples of the same population before, during, and after logging or hunting activities occur and investigate how FGCM levels change accordingly.
In the absence of data on fitness and health parameters, the results of this study should be interpreted conservatively and they should be confirmed by additional studies. Nevertheless, by controlling for many variables that can potentially confound GC levels (of which some have been neglected previously) and by comparing GC levels of two species that occur (at least partly) in the same fragments, we provide important evidence for species-specific differences in physiological responsiveness and susceptibility to anthropogenic disturbances.
This study reveals that some species (e.g. howler monkeys) may not be negatively influenced by a moderate level of human activity and suggests that agricultural ecosystems could be of use to conserve them. However, our results also demonstrate that GC levels of some species can be elevated in response to anthropogenic disturbances. To what extent these GC elevations reflect a situation of chronic stress with potentially negative fitness consequences or are merely a reflection of an acute adrenocortical reaction to ongoing human activities and, as such, might be adaptive to cope with a short-term challenge (without consequences on fitness) is impossible to assess in the absence of longer-term investigations on fitness and health parameters. It is conceivable, however, that if anthropogenic disturbances persist in the long term, this can potentially lead to a state of chronic stress, which might limit the future viability of animal populations. This study emphasizes the need for the active protection of continuous forests for the conservation of species with low coping abilities (e.g. spider monkeys). Measurements of physiological stress levels should be used to monitor populations living in disturbed areas and to assess the success (concerning amelioration or minimization of stress) of conservation strategies such as corridors connecting fragments and the promotion of alternative sources of animal protein for the human population (e.g. to decrease hunting pressure).
Acknowledgements
We thank the Lalinde and De Greiff families for permission to work at ‘Hacienda San Juan de Carare’ and all the persons who allowed us to visit their farms and forests. We thank Professor Pablo R. Stevenson and the Laboratorio de Ecología de Bosques Tropicales y Primatología for logistic support with sample storage. For their help with sample collection, we thank Felipe Alfonso, Natalia Alvis, Nathalia Fuentes, Miguel García, David Gongora, Claudia Lodoño, Natalia Mejía, Jennifer Rey-Goyeneche, Germán Ríos, and especially Andres Montes. For their support in the laboratory, we acknowledge Andrea Heistermann and Petra Kiesel. We thank Cedric Girard-Buttoz for his statistical advice. Fundación ProAves provided all logistic support for work at Reserva El Paujil (Quinchas). We also thank the Ministerio de Medio Ambiente y Desarrollo Sostenible in Colombia for the permits needed for this study. Fundación Proyecto Primates provided all logistics. The study was conducted completely non-invasively under the research permit 4120-E1-110897. This work was supported by the German Primate Center; Margot Marsh Biodiversity Foundation's Primate Action Fund; Rufford Small Grants Foundation; and Idea Wild.
References
- 1.Aguilar-Melo AR, Andresen E, Cristóbal-Azkarate J, Arroyo-Rodríguez V, Chavira R, Schondube J, Serio-Silva JC, Cuarón AD. (2013) Behavioral and physiological responses to subgroup size and number of people in howler monkeys inhabiting a forest fragment used for nature-based tourism. Am J Primatol 75: 1108–1116. [DOI] [PubMed] [Google Scholar]
- 2.Aldana AM, Beltrán M, Torres-Neira J, Stevenson PR. (2008) Habitat characterization and population density of brown spider monkeys (Ateles hybridus) in Magdalena Valley, Colombia. Neotrop Primates 15: 46–50. [Google Scholar]
- 3.Arroyo-Rodríguez V, Mandujano S. (2006) Forest fragmentation modifies habitat quality for Alouatta palliata. Int J Primatol 27: 1079–1096. [Google Scholar]
- 4.Baayen RH. (2010) Analyzing Linguistic Data: A Practical Introduction to Statistics. Cambridge University Press, Cambridge, UK. [Google Scholar]
- 5.Baayen RH. (2011) language R: data sets and functions with “Analyzing linguistic data: a practical introduction to statistics”, R package version 1.4. http://cran.r-project.org/package=languageR [Google Scholar]
- 6.Barja I, Silván G, Rosellinic S, Piñeiroc A, González-Gilb A, Camachob L, Illera JC. (2007) Stress physiological responses to tourist pressure in a wild population of European pine marten. J Steroid Biochem Mol Biol 104: 136–142. [DOI] [PubMed] [Google Scholar]
- 7.Bates D, Maechler M. (2010) lme4: Linear mixed-effects models using S4 classes. http://cran.r-project.org/package=lme4 [Google Scholar]
- 8.Bateson P, Bradshaw EL. (1997) Physiological effects of hunting red deer (Cervus elaphus). Proc Biol Sci 264: 1707–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behie AM, Pavelka MSM, Chapman CA. (2010) Sources of variation in fecal cortisol levels in howler monkeys in Belize. Am J Primatol 72: 600–606. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein I, Balcaen P, Dresdale L, Gouzoules H, Kavanagh M, Patterson T, Neyman-Warner P. (1976) Differential effects of forest degradation on primate populations. Primates 17: 401–411. [Google Scholar]
- 11.Bicca-Marques JC. (2003) How do howler monkeys cope with habitat fragmentation?. In Marsh LK, ed., Primates in Fragments: Ecology and Conservation: Kluwer Academic/Plenum Publishers, New York, pp 283–303. [Google Scholar]
- 12.Blumstein DT, Fernández-Juricic E, Zollner PA, Garity SC. (2005) Inter-specific variation in avian responses to human disturbance. J Appl Ecol 42: 943–953. [Google Scholar]
- 13.Boonstra R. (2005) Equipped for life: the adaptive role of the stress axis in male mammals. J Mammal 86: 236–247. [Google Scholar]
- 14.Boonstra R. (2013) Reality as the leading cause of stress: rethinking the impact of chronic stress in nature. Funct Ecol 27: 11–12. [Google Scholar]
- 15.Boubli J-P, Di Fiore A, Rylands AB, Mittermeier RA. (2008) Alouatta seniculus. In: IUCN 2013. IUCN Red List of Threatened Species. Version 2013.1. http://www.iucnredlist.org. (last accessed 25 July 2013). [Google Scholar]
- 16.Breazile JE. (1987) Physiologic basis and consequences of distress in animals. J Am Vet Med Assoc 191: 1212–1215. [PubMed] [Google Scholar]
- 17.Busch DS, Hayward LS. (2009) Stress in a conservation context: a discussion of glucocorticoid actions and how levels change with conservation-relevant variables. Biol Conserv 142: 2844–2853. [Google Scholar]
- 18.Cardillo M, Mace GM, Jones KE, Bielby J, Bininda-Emonds ORP, Sechrest W, Orme CDL, Purvis A. (2005) Multiple causes of high extinction risk in large mammal species. Science 309: 1239–1241. [DOI] [PubMed] [Google Scholar]
- 19.Cavigelli SA. (1999) Behavioural patterns associated with faecal cortisol levels in free-ranging female ring-tailed lemurs, Lemur catta. Anim Behav 57: 935–944. [DOI] [PubMed] [Google Scholar]
- 20.Chapman CA, Saj TL, Snaith TV. (2007) Temporal dynamics of nutrition, parasitism, and stress in colobus monkeys: implications for population regulation and conservation. Am J Phys Anthropol 134: 240–250. [DOI] [PubMed] [Google Scholar]
- 21.Charbonnel N, Chaval Y, Berthier K, Deter J, Morand S, Palme R, Cosson JF. (2008) Stress and demographic decline: a potential effect mediated by impairment of reproduction and immune function in cyclic vole populations. Physiol Biochem Zool 81: 63–73. [DOI] [PubMed] [Google Scholar]
- 22.Cordeiro NJ, Howe HF. (2001) Low recruitment of trees dispersed by animals in African forest fragments. Conserv Biol 15: 1733–1741. [Google Scholar]
- 23.Cosson JF, Ringuet S, Claessens O, de Massary JC, Dalecky A, Villiers JF, Granjon L, Pons JM. (1999) Ecological changes in recent land-bridge islands in French Guiana, with emphasis on vertebrate communities. Biol Conserv 91: 213–222. [Google Scholar]
- 24.Creel S, Fox JE, Hardy A, Sands J, Garrott B, Peterson RO. (2002) Snowmobile activity and glucocorticoid stress responses in wolves and elk. Conserv Biol 16: 809–814. [Google Scholar]
- 25.Crockett CM. (1998) Conservation biology of the genus Alouatta. Int J Primatol 19: 549–578. [Google Scholar]
- 26.Cyr NE, Romero ML. (2007) Chronic stress in free-living European starlings reduces corticosterone concentrations and reproductive success. Gen Comp Endocrinol 151: 82–89. [DOI] [PubMed] [Google Scholar]
- 27.Di Fiore A, Campbell CJ. (2007) The atelines: variation in ecology, behavior, and social organization. In Campbell CJ, Fuentes A, MacKinnon KC, Panger M, Bearder SK, eds, Primates in Perspective. Oxford University Press, New York, pp 155–185. [Google Scholar]
- 28.Di Fiore A, Link A, Campbell CJ. (2010) The atelines: behavioral and socioecological diversity in a New World radiation. In Campbell CJ, Fuentes A, MacKinnon KC, Panger M, Beader SK, eds, Primates in Perspective, Ed 2 Oxford University Press, Oxford, pp 155–188. [Google Scholar]
- 29.Dunn JC, Cristóbal-Azkarate J, Veà JJ. (2010) Seasonal variations in the diet and feeding effort of two groups of howlers in different sized forest fragments. Int J Primatol 31: 887–903. [Google Scholar]
- 30.Dunn JC, Cristóbal-Azkarate J, Schulte-Herbrüggen B, Chavira R, Veà JJ. (2013) Travel time predicts fecal glucocorticoid levels in free-ranging howlers (Alouatta palliata). Int J Primatol 34: 246–259. [Google Scholar]
- 31.Ellenberg U, Mattern T, Seddon PJ, Luna Jorquera G. (2006) Physiological and reproductive consequences of human disturbance in Humboldt Penguins: the need for species-specific visitor management. Biol Conserv 133: 95–106. [Google Scholar]
- 32.Ellenberg U, Setiawan AN, Cree A, Houston DM, Seddon PJ. (2007) Elevated hormonal stress response and reduced reproductive output in Yellow-eyed Penguins exposed to unregulated tourism. Gen Comp Endocrinol 152: 54–63. [DOI] [PubMed] [Google Scholar]
- 33.Fahrig L. (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34: 487–515. [Google Scholar]
- 34.Field A. (2005) Discovering Statistics using SPSS. Sage Publications, London. [Google Scholar]
- 35.Foley JA, Defries R, Asner GP, Barford C, Bonan G, Carpenter SR, Chapin FS, Coe MT, Daily GC, Gibbs HK, et al. (2005) Global consequences of land use. Science 309: 570–574. [DOI] [PubMed] [Google Scholar]
- 36.Fowler GS. (1999) Behavioral and hormonal responses of Magellanic Penguins (Spheniscus magellanicus) to tourism and nest site visitation. Biol Conserv 90: 143–149. [Google Scholar]
- 37.Fox J, Weisberg S. (2011) An R Companion to Applied Regression. Sage Publications, London. [Google Scholar]
- 38.French SS, Denardo DF, Greives TJ, Strand CR, Demas GE. (2010) Human disturbance alters endocrine and immune responses in the Galapagos marine iguana (Amblyrhynchus cristatus). Horm Behav 58: 792–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ganswindt A, Palme R, Heistermann M, Borragan S, Hodges JK. (2003) Non-invasive assessment of adrenocortical function in the male African elephant (Loxodonta africana) and its relation to musth. Gen Comp Endocrinol 134: 156–166. [DOI] [PubMed] [Google Scholar]
- 40.Ganzhorn JU. (1995) Low-level forest disturbance effects on primary production, leaf chemistry, and lemur populations. Ecology 76: 2084–2096. [Google Scholar]
- 41.Gobush KS, Mutayoba BM, Wasser SK. (2008) Long-term impacts of poaching on relatedness, stress physiology, and reproductive output of adult female African elephants. Conserv Biol 22: 1590–1599. [DOI] [PubMed] [Google Scholar]
- 42.Gómez-Posada C, Roncancio N, Hincapie P, Betancourt A. (2010) Densidad y composición de grupos en tres poblaciones de mono aullador rojo (Alouatta seniculus) en Valle y Cauca, Colombia. Boletín Científico del Centro de Museos, Universidad de Caldas 14: 79–91. [Google Scholar]
- 43.González-Solís J, Guix JC, Mateos E, Llorens L. (2001) Population density of primates in a large fragment of the Brazilian Atlantic rainforest. Biodivers Conserv 10: 1267–1282. [Google Scholar]
- 44.Goymann W. (2012) On the use of non-invasive hormone research in uncontrolled, natural environments: the problem with sex, diet, metabolic rate and the individual. Methods Ecol Evol 3: 757–765. [Google Scholar]
- 45.Hannah L, Carr JL, Lankerani A. (1995) Human disturbance and natural habitat: a biome level analysis of a global data set. Biodivers Conserv 4: 128–155. [Google Scholar]
- 46.Harcourt AH, Coppeto SA, Parks SA. (2002) Rarity, specialization and extinction in primates. J Biogeogr 29: 445–456. [Google Scholar]
- 47.Heistermann M, Ademmer C, Kaumanns W. (2004) Ovarian cycle and effect of social changes on adrenal and ovarian function in Pygathrix nemaeus. Int J Primatol 25: 689–708. [Google Scholar]
- 48.Henle K, Davie KF, Kleyer M, Margules C, Settele J. (2004) Predictors of species sensitivity to fragmentation. Biodivers Conserv 13: 207–251. [Google Scholar]
- 49.Hinam HL, St Clair CC. (2008) High levels of habitat loss and fragmentation limit reproductive success by reducing home range size and provisioning rates of Northern Saw-whet Owls. Biol Conserv 141: 524–535. [Google Scholar]
- 50.Homan RN, Regosin JV, Rodrigues DM, Reed JM, Windmiller BS, Romero LM. (2003) Impacts of varying habitat quality on the physiological stress of spotted salamanders (Ambystoma maculatum). Anim Conserv 6: 11–18. [Google Scholar]
- 51.Hunt KE, Wasser SK. (2003) Effect of long-term preservation methods on fecal glucocorticoid concentrations of grizzly bear and African elephant. Physiol Biochem Zool 76: 918–928. [DOI] [PubMed] [Google Scholar]
- 52.Irwin MT. (2008) Diademed sifaka (Propithecus diadema) ranging and habitat use in continuous and fragmented forest: higher density but lower viability in fragments? Biotropica 40: 231–240. [Google Scholar]
- 53.IUCN (2012) The IUCN Red List of Threatened Species. Version 2012.2. http://www.iucnredlist.org (last accessed 5 December 2012). [Google Scholar]
- 54.Jaimez NA, Bribiescas RG, Aronsen GP, Anestis SA, Watts DP. (2012) Urinary cortisol levels of gray-cheeked mangabeys are higher in disturbed compared to undisturbed forest areas in Kibale National Park, Uganda. Anim Conserv 15: 242–247. [Google Scholar]
- 55.Janin A, Léna J-P, Joly P. (2011) Beyond occurrence: body condition and stress hormone as integrative indicators of habitat availability and fragmentation in the common toad. Biol Conserv 144: 1008–1016. [Google Scholar]
- 56.Johns AD. (1988) Effects of “selective” timber extraction on rain forest structure and composition and some consequences for frugivores and folivores. Biotropica 20: 31–37. [Google Scholar]
- 57.Johns AD, Skorupa JP. (1987) Responses of rain-forest primates to habitat disturbance: a review. Int J Primatol 8: 157–191. [Google Scholar]
- 58.Julliot C, Sabatier D. (1993) Diet of the red howler monkey (Alouatta seniculus) in French Guiana. Int J Primatol 14: 527–550. [Google Scholar]
- 59.Keay JM, Singh J, Gaunt MC, Kaur T. (2006) Fecal glucocorticoids and their metabolites as indicators of stress in various mammalian species: a literature review. J Zoo Wildl Med 37: 234–244. [DOI] [PubMed] [Google Scholar]
- 60.Keselman HJ, Algina J, Kowalchuk RK. (2001) The analysis of repeated measures designs: a review. Br J Math Stat Psychol 54: 1–20. [DOI] [PubMed] [Google Scholar]
- 61.Klein LL, Klein DJ. (1977) Feeding behaviour of the Colombian spider monkey, Ateles belzebuth. In Clutton-Brock TH, ed., Primate Ecology: Studies of Feeding and Ranging Behavior in Lemurs, Monkeys, and Apes. Academic Press, London, pp 153–181. [Google Scholar]
- 62.Landys MM, Ramenofsky M, Wingfield JC. (2006) Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen Comp Endocrinol 148: 132–149. [DOI] [PubMed] [Google Scholar]
- 63.Link A, de Luna AG, Alfonso F, Giraldo-Beltran P, Ramirez F. (2010) Initial effects of fragmentation on the density of three neotropical primate species in two lowland forests of Colombia. Endang Species Res 13: 41–50. [Google Scholar]
- 64.Lopez GO, Terborgh J, Ceballos N. (2005) Food selection by a hyperdense population of red howler monkeys (Alouatta seniculus). J Trop Ecol 21: 445–450. [Google Scholar]
- 65.Lucas JR, Freeberg TM, Egbert J, Schwabl H. (2006) Fecal corticosterone, body mass, and caching rates of Carolina chickadees (Poecile carolinensis) from disturbed and undisturbed sites. Horm Behav 49: 634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martínez-Mota R, Valdespino C, Sánchez-Ramos MA, Serio-Silva JC. (2007) Effects of forest fragmentation on the physiological stress response of black howler monkeys. Anim Conserv 10: 374–379. [Google Scholar]
- 67.Menon S, Poirier FE. (1996) Lion-tailed macaques (Macaca silenus) in a disturbed forest fragment: activity patterns and time budget. Int J Primatol 17: 969–985. [Google Scholar]
- 68.Michalski F, Peres CA. (2005) Anthropogenic determinants of primate and carnivore local extinctions in a fragmented forest landscape of southern Amazonia. Biol Conserv 124: 383–396. [Google Scholar]
- 69.Millspaugh JJ, Washburn BE. (2004) Use of fecal glucocorticoid metabolite measures in conservation biology research: considerations for application and interpretation. Gen Comp Endocrinol 138: 189–99. [DOI] [PubMed] [Google Scholar]
- 70.Milton K. (1980) The Foraging Strategy of Howler Monkeys: A Study of Primate Economics. Columbia University Press, New York. [Google Scholar]
- 71.Mittermeier RA, Rylands AB, Taylor LA, Chiozza F, Williamson EA, Wallis J, Schwitzer C. (2012) Primates in Peril: The World's 25 Most Endangered Primates 2012–2014. IUCN/SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), and Bristol Conservation and Science Foundation, Bristol, UK. [Google Scholar]
- 72.Monclús R, Rödel HG, Von Holst D, De Miguel J. (2005) Behavioural and physiological responses of naïve European rabbits to predator odour. Anim Behav 70: 753–761. [Google Scholar]
- 73.Muehlenbein MP, Ancrenaz M, Sakong R, Ambu L, Prall S, Fuller G, Raghanti MA. (2012) Ape conservation physiology: fecal glucocorticoid responses in wild Pongo pygmaeus morio following human visitation. PLoS One 7: e33357 doi:10.1371/journal.pone.0033357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muller MN, Wrangham RW. (2004) Dominance, cortisol and stress in wild chimpanzees (Pan troglodytes schweinfurthii). Behav Ecol Sociobiol 55: 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Müllner A, Linsenmair EK, Wikelski M. (2004) Exposure to ecotourism reduces survival and affects stress response in hoatzin chicks (Opisthocomus hoazin). Biol Conserv 118: 549–558. [Google Scholar]
- 76.Munshi-South J, Tchignoumba L, Brown J, Abbondanza N, Maldonado JE, Henderson A, Alonso A. (2008) Physiological indicators of stress in African forest elephants (Loxodonta africana cyclotis) in relation to petroleum operations in Gabon, Central Africa. Divers Distrib 14: 995–1003. [Google Scholar]
- 77.Neville MK. (1972) The population structure of red howler monkeys (Alouatta seniculus) in Trinidad and Venezuela. Folia Primatol 17: 56–86. [DOI] [PubMed] [Google Scholar]
- 78.Ostro LET, Silver SC, Koontz FW, Horwich RH, Brockett R. (2001) Shifts in social structure of black howler (Alouatta pigra) groups associated with natural and experimental variation in population density. Int J Primatol 22: 733–748. [Google Scholar]
- 79.Peres CA. (2001) Synergistic effects of subsistence hunting and habitat fragmentation on Amazonian forest vertebrates. Conserv Biol 15: 1490–1505. [Google Scholar]
- 80.Pickering AD, Pottinger TG, Sumpter JP, Carragher JF, Le Bail PY. (1991) Effects of acute and chronic stress on the levels of circulating growth hormone in the rainbow trout, Oncorhynchus mykiss. Gen Comp Endocrinol 83: 86–93. [DOI] [PubMed] [Google Scholar]
- 81.Piñeiro A, Barja I, Silván G, Illera JC. (2012) Effects of tourist pressure and reproduction on physiological stress response in wildcats: management implications for species conservation. Wildl Res 39: 532–539. [Google Scholar]
- 82.Purvis A, Gittleman JL, Cowlishaw G, Mace GM. (2000) Predicting extinction risk in declining species. Proc Biol Sci 267: 1947–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Putz FE, Leigh EGJ, Wright SJ. (1990) Solitary confinement in Panama. Garden 2: 18–23. [Google Scholar]
- 84.R Development Core Team (2012) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org [Google Scholar]
- 85.Rangel-Negrín A, Alfaro JL, Valdez RA, Romano MC, Serio-Silva JC. (2009) Stress in Yucatan spider monkeys: effects of environmental conditions on fecal cortisol levels in wild and captive populations. Anim Conserv 12: 496–502. [Google Scholar]
- 86.Rimbach R, Heymann EW, Link A, Heistermann M. (2013) Validation of an enzyme immunoassay for assessing adrenocortical activity and characterization of factors that affect fecal glucocorticoid metabolite levels in two New World primates. Gen Comp Endocrinol 191: 13–23. [DOI] [PubMed] [Google Scholar]
- 87.Rumiz DI. (1990) Alouatta caraya: population density and demography. Am J Primatol 21: 279–294. [DOI] [PubMed] [Google Scholar]
- 88.Sapolsky RM, Romero ML, Munck AU. (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21: 55–89. [DOI] [PubMed] [Google Scholar]
- 89.Selye H. (1956) The Stress of Life. McGraw-Hill, New York. [Google Scholar]
- 90.Setchell JM, Smith T, Wickings EJ, Knapp LA. (2010) Stress, social behaviour, and secondary sexual traits in a male primate. Horm Behav 58: 720–728. [DOI] [PubMed] [Google Scholar]
- 91.Shutt K, Setchell JM, Heistermann M. (2012) Non-invasive monitoring of physiological stress in the Western lowland gorilla (Gorilla gorilla gorilla): validation of a fecal glucocorticoid assay and methods for practical application in the field. Gen Comp Endocrinol 179: 167–177. [DOI] [PubMed] [Google Scholar]
- 92.Strasser EH, Heath JA. (2013) Reproductive failure of a human-tolerant species, the American kestrel, is associated with stress and human disturbance. J Appl Ecol 50: 912–919. [Google Scholar]
- 93.Stratakis CA, Chrousos GP. (1995) Neuroendocrinology and pathophysiology of the stress system. Ann N Y Acad Sci 77: 1–18. [DOI] [PubMed] [Google Scholar]
- 94.Sumner J, Moritz C, Shine R. (1999) Shrinking forest shrinks skink: morphological change in response to rainforest fragmentation in the prickly forest skink (Gnypetoscincus queenslandiae). Biol Conserv 91: 159–167. [Google Scholar]
- 95.Symington MM. (1988) Food competition and foraging party size in the black spider monkey (Ateles paniscus chamek). Behaviour 105: 117–134. [Google Scholar]
- 96.Tecot SR. (2008) Seasonality and predictability: the hormonal and behavioral responses of the red-bellied lemur, Eulemur rubriventer, in southeastern Madagascar. PhD thesis. University of Texas at Austin. [Google Scholar]
- 97.Thiel D, Palme R, Jenni L, Jenni-Eiermann S. (2011) Winter tourism increases stress hormone levels in the Capercaillie Tetrao urogallus. Ibis 153: 122–133. [Google Scholar]
- 98.Umapathy G, Hussain S, Shivaji S. (2011) Impact of habitat fragmentation on the demography of lion-tailed macaque (Macaca silenus) populations in the rainforest of Anamalai Hills, Western Ghats, India. Int J Primatol 32: 889–900. [Google Scholar]
- 99.Urbani B, Morales AL, Link A, Stevenson PR. (2008) Ateles hybridus. In: IUCN 2013. IUCN Red List of Threatened Species. Version 2013.1. http://www.iucnredlist.org. (last accessed 25 July 2013). [Google Scholar]
- 100.von der Ohe CG, Wasser SK, Hunt KE, Servheen C. (2004) Factors associated with fecal glucocorticoids in Alaskan brown bears (Ursus arctos horribilis). Physiol Biochem Zool 77: 313–320. [DOI] [PubMed] [Google Scholar]
- 101.Wasser SK, Bevis K, King G, Hanson E. (1997) Noninvasive physiological measures of disturbance in the Northern Spotted Owl. Conserv Biol 11: 1019–1022. [Google Scholar]
- 102.Wasser SK, Davenport B, Ramage ER, Hunt KE, Parker M, Clarke C, Stenhouse G. (2004) Scat detection dogs in wildlife research and management: application to grizzly and black bears in the Yellowhead Ecosystem, Alberta, Canada. Can J Zool 82: 475–492. [Google Scholar]
- 103.Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn S, Ramenofsky M, Richardson RD. (1998) Ecological bases of hormone-behavior interactions: the “emergency life history stage”. Integr Comp Biol 38: 191–206. [Google Scholar]
- 104.Zwijacz-Kozica T, Selva N, Barja I, Silván G, Martínez-Fernández L, Illera JC, Jodłowski M. (2012) Concentration of fecal cortisol metabolites in chamois in relation to tourist pressure in Tatra National Park (South Poland). Acta Theriol 58: 215–222. [Google Scholar]