We investigated reproductive aging in African elephants and found a relationship between females obtaining a high social status within their herd and a decline in ovarian steroid activity, which may be analogous to menopause. Understanding possible social constraints on reproductive fitness could enhance management of aging and growing elephant populations.
Keywords: Loxodonta africana, matriarch, non-invasive hormone monitoring, population management, post-partum duration, post-reproductive lifespan
Abstract
Free-ranging African elephants live in a fission–fusion society, at the centre of which is the matriarch. Matriarchs are generally older females that guide their families to resources and co-ordinate group defense. While much is known about elephant society, knowledge is generally lacking about how age affects the physiology of wild elephants. Investigation of the ovarian activity of free-ranging elephants could provide insight into the reproductive ageing process, with implications for population management. Faecal samples were collected from 46 individuals ranging in age from 14 to 60 years for a 2-year period, and progestagen metabolite analyses were used to examine relationships between social status, age, season, and ovarian activity in female elephants in Addo Elephant National Park, South Africa. Social status was the strongest predictor of faecal progestagen metabolite concentrations in non-pregnant elephants, with grand matriarchs (n = 6) having the lowest values compared with matriarchs (n = 21) and non-matriarch females (n = 19). Likewise, social status and age were the strongest predictors of faecal progestagen metabolite concentrations in pregnant elephants (n = 27). The number of years since a non-pregnant female gave birth to her last calf (post-partum duration) was longer for older females with a higher social status, as well as during the dry season. Our results indicate that social standing and age of elephants are related to reproductive function, and that older females exhibit reductions in ovarian capacity. These results expand our understanding of reproduction and fertility throughout an elephant's lifespan, and the factors that impact gonadal function in free-ranging females. Given that possible over-abundance of elephants in areas such as Addo Elephant National Park is fuelling the debate over how best to manage these populations, knowledge about the reproductive potential of high-ranking females can provide managers with biological data to identify the best candidates for controlling growth through translocation or contraception.
Introduction
In the wild, African elephants (Loxodonta africana) live in a fission–fusion society (Archie et al., 2006), and adult females and their offspring form the basis of the family unit (Douglas-Hamilton, 1972; Dublin, 1983; Archie et al., 2006). Females remain with the family group throughout their lives, whereas males leave their natal group at between 12 and 15 years of age (Poole, 1994). The largest, eldest female in the family is usually the matriarch (Douglas-Hamilton, 1972; Poole et al., 1989; Archie et al., 2006). However, age alone does not guarantee matriarchal status, because at the death of a matriarch the family can fission and may not always follow the next oldest female (B. A. Schulte, personal observation). When multiple, related family groups fuse into a kinship group, the eldest matriarch (which we term the ‘grand matriarch’) generally adopts the leadership role (Wittemyer et al., 2007c).
The importance of the matriarch is as a leader with crucial knowledge of natural resources and as a co-ordinator of group defense (Douglas-Hamilton, 1972; Dublin, 1983; Poole and Moss, 1989; Esposito, 2008; McComb et al., 2011). How she achieves this coordination is uncertain, but a matriarch seems to be aware of the location of family/kinship and non-family/kinship members, or the difference between relatives and non-relatives (Soltis et al., 2005a, b; Bates et al., 2008). The interactions of matriarchs and their families with related and unrelated groups may result in amiable fusion, tolerance, avoidance, or aggression (Esposito, 2008). Older matriarchs are more successful at distinguishing intruders (McComb et al., 2001) and the calls of male lions (McComb et al., 2011), and they facilitate family success in extreme conditions, such as severe poaching (Gobush et al., 2008). Age also affects a matriarch's rank among other matriarchs and the relative rank of her family; matriarchal rank contributes to the dominance status of non-matriarchal females in her kinship group in comparison with females from other kinship groups (Wittemyer and Getz, 2007c).
A positive relationship between social status and age has also been documented in a number of other species, yet knowledge is generally lacking about the biology of ageing and the pathological processes that sometimes accompany advanced age (Erwin et al., 2008). This lack of knowledge may hinder successful management, propagation, and conservation of endangered species, which depends upon a detailed understanding of reproduction and fertility throughout the lifespan (Erwin and Hof, 2008). In most species, gradual, age-related decreases in physiological functions occur in the majority of individuals and typically coincide with somatic and reproductive senescence (Kachel et al., 2011). Declines in fertility with age are a common feature of mammal life histories, particularly for long-lived species with long reproductive lifespans and inter-birth intervals (Bellino et al., 2003; Emery Thompson et al., 2007), such as chimpanzees (Emery Thompson et al., 2007), killer whales (McAuliffe et al., 2005; Johnstone et al., 2010), and free-ranging elephants (Laws et al., 1970; Smuts, 1975; Moss, 2001; Freeman et al., 2009; Robinson et al., 2012). Furthermore, African elephants in zoos exhibit a decline in ovarian activity that is associated with a high social rank and in some, but not all instances, a more advanced age (Freeman et al., 2010b). Hermes et al. (2004) referred to the decline in zoo elephant reproductive function as asymmetric or premature reproductive ageing. The rate of ageing and reproductive senescence is influenced by trade-offs in life-history and environmental variation, which can explain the differences in longevity observed within and between species (Nussey et al., 2008). It is not clear whether elephants exhibit these same trade-offs, or if age and social rank have similar suppressive effects on reproductive function.
Environmental factors also can impact reproductive success in free-ranging African elephants. Females can breed year round; however, conception and birth rates show strong seasonality relative to precipitation (Laws, 1970; Smuts, 1975; Dublin, 1983; Gough et al., 2006; Wittemyer et al., 2007a; Freeman et al., 2009; Foley et al., 2010). A few studies have investigated the impact of ecological factors on faecal progestagen metabolite (FPM) concentrations in free-ranging elephants as an indicator of reproductive activity (Whitehouse et al., 2000; Foley et al., 2001; Wittemyer et al., 2007b; Gobush et al., 2008). For example, poor vegetative quality during the dry season in Northern Kenya is correlated with lower FPM levels (Wittemyer et al., 2007b). Likewise, seasonal declines in FPM concentrations occur during the dry seasons in Tanzania, when water availability and food quality affect the body condition of the females (Foley et al., 2001). It is not known whether other populations of African elephants have similar seasonal variability in FPM concentrations, or how the ageing process impacts ovarian activity. Expanding our understanding of the reproductive physiology of free-ranging elephants in the presence of multiple environmental stressors would enhance our ability to evaluate and improve the efficacy of various conservation and management practices (Cooke et al., 2013).
The goal of the present study was to investigate relationships among female age, social status, and seasonal variability in precipitation with FPM concentrations in free-ranging elephants of Addo Elephant National Park (AENP). We hypothesized that low FPM concentrations would correlate inversely with female age, social status, post-partum duration, and seasonal precipitation. Understanding how elephant reproductive success is related to age and social status would contribute to the growing field of conservation physiology (Cooke et al., 2013) and broaden our understanding of the ageing process, potentially aiding the reproductive management of free-ranging and zoo elephants, and perhaps other long-lived species.
Materials and methods
Study animals
The study site was in the Eastern Cape of South Africa at Addo Elephant National Park, which consists of 13 500 ha of habitat that ranges from sub-tropical succulent thicket to open, grassy plains (Whitehouse and Hall-Martin, 2000). There were ∼415 elephants, comprising six matrilines, in AENP. Elephants were identified using ear tears and vein patterns, as well as other physical features (Fig. 1) that were compared with files consisting of photographs and descriptions of the individuals (Whitehouse and Hall-Martin, 2000; Whitehouse et al., 2001a). The date of birth was estimated to the month and year based upon photographic data from 1976 to the present (Whitehouse and Hall-Martin, 2000). For the younger females, the timing of the birth was known within a day or week of the event, based on observations (Whitehouse and Hall-Martin, 2000; Loizi et al., 2009). For females born before 1976, age was estimated using well-documented patterns of change in physical characteristics (e.g. shoulder height and body shape) relative to age in free-ranging elephants (Moss, 2001; Wittemyer et al., 2007a). Longitudinal studies of marked or recognizable individuals, such as those in AENP, are the most reliable sources for information about reproductive senescence in wild populations, because they allow researchers to separate within-individual ageing patterns from between-individual heterogeneity (Nussey et al., 2008).
Figure 1:

Non-matriarch and her calf. First sighting of a female African elephant in Addo Elephant National Park, South Africa with her newborn calf. She was identified as HAN by the distinctive notches in her ears and association with the rest of her family group (not shown).
Behavioural observations and faecal samples
The project was conducted from July 2007 to September 2009. In-depth analyses of AENP data since 1931 have established that there are six elephant matrilines in the park that form six kinship groups and two clans (Whitehouse and Harley, 2001). Additionally, 25 family groups, each with an identifiable matriarch, have been discovered through detailed field studies of the AENP population (from 1996 to 2009; Bagley, 2004; Gough and Kerley, 2006; Meyer, 2006; Esposito, 2008; Loizi et al., 2009; Merte et al., 2010). Given that the social rank of adult African elephants varies within and between matrilines (Wittemyer and Getz, 2007c), efforts were made to observe post-pubertal females (46 elephants; range, 14–60 years of age; Table 1) within each social category from all six of the AENP matrilines. The social status of each female within her matriline was assigned based upon the following criteria. Grand matriarchs (n = 6; age range, 39–60 years at the start of the study) were the head of their kinship group during fusion events. Matriarchs (n = 21; age range, 22–46 years) were the remaining heads of their respective family units during fission events. Non-matriarchs (n = 19; age range, 13–33 years) were the remaining females within the kinship group that did not assume a leadership role during fusion or fission events.
Table 1:
Description of the female African elephants studied in Addo Elephant National Park and faecal samples collected from each individual
| Elephant | Social status | Kinship group | Family groupa | Age range (years)b | Total faecal samples | Pregnant faecal samples |
|---|---|---|---|---|---|---|
| AND | Grand matriarch | A | AND | 51–53 | 16 | 0 |
| ALOc | Matriarch | A | ALO | 46–48 | 15 | 0 |
| LAGc | Matriarch | A | LAG | 44–46 | 14 | 0 |
| AMAc | Matriarch | A | AMA | 38–40 | 17 | 7 |
| ALLc | Matriarch | A | ALL | 35–37 | 19 | 5 |
| APPc | Matriarch | A | APP | 33–35 | 19 | 10 |
| AMBc | Matriarch | A | AMB | 30–32 | 13 | 0 |
| ANNc | Matriarch | A | ANN | 22–24 | 16 | 8 |
| ANGd | Non-matriarch | A | AND | 28–30 | 11 | 0 |
| ARR | Non-matriarch | A | LAG | 26–28 | 18 | 13 |
| ARA | Non-matriarch | A | ALL | 19–21 | 16 | 0 |
| AMO | Non-matriarch | A | AMA | 17–18 | 7 | 5 |
| TAN | Grand matriarch | B | TAN | 56–58 | 18 | 0 |
| CAT | Matriarch | B | CAT | 37–39 | 23 | 12 |
| BEVd | Matriarch | B | BEV | 37–39 | 16 | 12 |
| BLUc | Matriarch | B | BLU | 31–33 | 19 | 14 |
| BON | Matriarch | B | BON | 27–29 | 24 | 21 |
| BCH | Non-matriarch | B | BCH | 33–35 | 17 | 12 |
| BUB | Non-matriarch | B | BEV | 24–26 | 17 | 6 |
| BWI | Non-matriarch | B | CAT | 17–19 | 17 | 8 |
| BUL | Non-matriarch | B | CAT | 14–15 | 6 | 4 |
| HET | Grand matriarch | H | HET | 57–59 | 16 | 0 |
| HEId | Matriarch | H | HET | 35–37 | 14 | 0 |
| HILd | Non-matriarch | H | HET | 31–33 | 13 | 11 |
| HANd | Non-matriarch | H | HET | 26–28 | 14 | 10 |
| LLT | Grand matriarch | L | LLT | 39–41 | 7 | 5 |
| LAU | Matriarch | L | LAU | 35–37 | 5 | 0 |
| LUC | Non-matriarch | L | LAU | 22–24 | 5 | 4 |
| AFS | Grand matriarch | P | AFL | 58–60 | 12 | 0 |
| MARc | Matriarch | P | MAR | 44–46 | 13 | 0 |
| MEGc | Matriarch | P | MEG | 42–44 | 9 | 0 |
| PAUd | Matriarch | P | PAU | 38–40 | 13 | 0 |
| MAN | Matriarch | P | MAN | 35–36 | 7 | 0 |
| TIP | Matriarch | P | TIP | 34–36 | 14 | 6 |
| PHY | Matriarch | P | PHY | 26–28 | 21 | 0 |
| MOL | Matriarch | P | MAR | 26–28 | 18 | 0 |
| MUS | Non-matriarch | P | MEG | 24–26 | 12 | 10 |
| PIP | Non-matriarch | P | PAU | 22–24 | 12 | 4 |
| MIR | Non-matriarch | P | MEG | 18–20 | 17 | 0 |
| POP | Non-matriarch | P | PAU | 18–20 | 13 | 2 |
| MIL | Non-matriarch | P | MAR | 13–15 | 10 | 0 |
| REB | Grand matriarch | R | REB | 43–45 | 11 | 4 |
| ROZ | Matriarch | R | ROZ | 32–34 | 16 | 4 |
| RHOd | Non-matriarch | R | REB | 30–32 | 12 | 7 |
| RHI | Non-matriarch | R | ADD | 28–29 | 8 | 4 |
| RHE | Non-matriarch | R | RHO | 14–15 | 6 | 1 |
aFamily group was designated by the matriarch, or grand matriarch, of the group.
bAge range designates the age of the elephant over the course of faecal sample collections.
cSister of the grand matriarch.
dDaughter of the grand matriarch.
Addo Elephant National Park elephants have been monitored intensively since 1996 (Whitehouse and Hall-Martin, 2000) and are habituated to the presence of research and tourist vehicles, which made it easy to observe their behaviour and collect faecal samples from known individuals. During focal observations of the 46 females, reproductive events, including oestrous behaviours, mate guarding by males and copulations (Moss, 1983; Wittemyer et al., 2007b), and the development of teats were recorded. Pregnancy (n = 27 females; age range, 14–45 years) was determined according to these events and by back-dating parturition events. The date of conception was calculated by subtracting the average gestation period of 22 months (Laws, 1969; Wittemyer et al., 2007b) from the estimated date of birth. Based upon parturition events, we were able to confirm that two grand matriarchs (33.3%), 10 matriarchs (50.0%) and 15 non-matriarchs (78.9%) were pregnant during the course of this study. Faecal samples were collected when elephants were observed defaecating to ensure proper identification, and as close to monthly from each individual as possible. We analysed 636 faecal samples (13.83 ± 0.18 per individual; Table 1); 209 of these were from pregnant individuals (n = 27 elephants), while the remaining 427 were from non-pregnant animals (n = 46 elephants).
Our methods adhered to the Association for the Study of Animal Behaviour/Animal Behavior Society Guidelines for the Use of Animals in Research and have been approved by the Institutional Animal Care and Use Committee (IACUC) of George Mason University (#A3210-01). The fieldwork was performed with the permission (Permit # 2002-12-11 BSCH) and support of personnel in the South African National Parks.
Hormone analyses
A field method previously validated for elephants was used for extracting FPM (Freeman et al., 2010a, 2011). One millilitre aliquots of faecal extracts were placed into 12 mm × 75 mm polypropylene tubes (#2332 and #2305; Perfector Scientific), air-dried and heated to 72°C for 30 min before shipment to the Smithsonian Conservation Biology Institute for analysis. Dried faecal extracts were reconstituted with buffer (0.2 M NaH2PO4 and 0.2 M Na2HPO4 in 0.14 M NaCl) by vortexing the tubes for 1 h and then sonicating for 30 min. Reconstituted extracts were diluted and analysed using the enzyme immunoassay methods of Graham et al. (2001), with a monoclonal progesterone antibody (1:10 000 dilution CL425; C. Munro, University of California-Davis, CA, USA), horseradish peroxidase-conjugated label (1:40 000 dilution; C. Munro) and a phosphate–citrate buffer (#P4560; Sigma Aldrich, Inc.) substrate with tetramethylbenzidine (#T3405; Sigma Aldrich, Inc.). Assay sensitivity was 0.78 pg/well, and intra- and inter-assay coefficients of variation were <10%.
Data analyses
Given that FPM concentrations were higher in faeces from pregnant than non-pregnant elephants (Freeman et al., 2011), samples were grouped for data analysis according to the reproductive state of the female at the time of sample collection. For non-pregnant elephants, the post-partum duration (PPD; i.e. the duration of the non-pregnant period) was determined by calculating the duration (in years) between the date of the sample collection and the parturition date of each elephant's previous calf (Wittemyer et al., 2007b). The inter-pregnancy interval (IPI) was calculated for pregnant elephants as the duration (in years) between the birth of the elephant's previous calf and the conception of the current fetus (Wittemyer et al., 2007b). As described previously, the month of gestation was determined by back-dating the average gestation period of 22 months (Laws, 1969; Wittemyer et al., 2007b) from the estimated date of birth. Given that newborns may not have been detected on the exact date of birth, our estimates for month of gestation may vary by ± 1–2 weeks. Thus, we used the trimester of gestation (first, 0–7 months; second, 8–14 months; and third, 15–22 months; Duer et al., 2002, 2007) to reflect the stages of pregnancy for model analyses. Total monthly precipitation values (in millimetres) for the AENP weather station were obtained from the South African Weather Service (Walmer, South Africa). Addo Elephant National Park is classified as semi-arid to arid and receives <455 mm precipitation per year on average (www.sanparks.org). Rainfall within AENP does occur throughout the year, but there are peaks in February–March and October–November. The AENP wet season was thus defined as October–March and the dry season as April–September.
Many of the explanatory variables were potentially correlated (e.g. the oldest elephants are most likely to be grand matriarchs). Thus, a linear mixed-effect (LME) model was employed to determine what factors contributed the most to FPM concentrations and PPD (Wittemyer et al., 2007b). Mixed-effects models can control for sources of between-individual heterogeneity, thus allowing for more accurate measurement of within-individual ageing patterns in longitudinally measured life-history traits (Nussey et al., 2008). Given that model data included repeated measures from individual elephants, female identity was incorporated as a random effect (Wittemyer et al., 2007b). The LME model assumes a Gaussian (or normal) distribution of the variables. Normality of the data was tested using the Kolmogorov–Smirnov test, and those variables that were not normally distributed were transformed (e.g. square root or Log10) prior to inclusion in the model. Pregnancy FPM concentrations were examined with respect to the fixed effects of age, social status, fetal sex, month of gestation, IPI, monthly precipitation, and wet/dry season. In comparison, FPM concentrations from non-pregnant elephants were evaluated based on age, social status, PPD, sex of her previous calf, monthly precipitation, and wet/dry season. Lastly, PPD in non-pregnant elephants was examined based on age, social status, sex of the previous calf, FPM concentrations, monthly precipitation, and wet/dry season; female identity was included as a random effect in the LME analyses of PPD. One elephant, MIR, was not included in the LME analyses because she had never given birth, and thus we could not calculate a PPD or provide a calf sex. Step-wise elimination of non-significant variables was conducted, and reduced models were compared with the full model using smaller values of Akaike's information criteria and Bayesian information criteria as a guide for model selection (Wittemyer et al., 2007a; Freeman et al., 2011).
Linear mixed-effects models were analysed using the free statistical package R (R Development Core Team, 2012) using the nlme package. When appropriate, post hoc analyses of the variables in the most parsimonious models were conducted using the HH package for Tukey's pair-wise comparisons. All other analyses were conducted using SigmaPlot (version 11.0 2008; Systat Software, Inc). For all analyses, P < 0.05 was considered significant, and all data were reported as means ± SEM, except for the LME tables, where the coefficient and standard error of the model were reported.
Results
The average FPM concentration for samples collected from female African elephants in AENP was 107.45 ± 5.15 ng/g faeces, with FPM concentrations from pregnant animals being higher (130.88 ± 5.69 ng/g faeces) than those from non-pregnant females (95.93 ± 2.56 ng/g faeces; Table 2). The average IPI for pregnant elephants in AENP was 1.88 ± 0.55 years (range, 0.08–4.67 years). There was little variability in IPI with respect to social status of the elephant within her family (Table 2). In contrast, non-pregnant grand matriarchs had the greatest PPD, followed by matriarchs, and then non-matriarchs (Table 2). The average PPD for all non-pregnant elephants was 2.65 ± 0.71 years (range, 0.08–27.43 years).
Table 2:
Physiological data collected from non-pregnant and pregnant free-ranging African elephants within Addo Elephant National Park, South Africa as they varied with respect to social rank of the individual within her family
| Non-pregnant elephants |
Pregnant elephants |
|||
|---|---|---|---|---|
| Social rank | FPM (ng/g faeces) | PPD (years) | FPM (ng/g faeces) | IPI (years) |
| Grand matriarch | 86.47 ± 5.01 (30.88–156.38) | 6.30 ± 2.46 (0.67–17.06) | 114.69 ± 27.08 (44.48–401.51) | 1.29 ± 0.54 (0.75–1.83) |
| Matriarch | 97.59 ± 3.21 (24.79–488.20) | 2.99 ± 1.27 (0.31–27.43) | 123.22 ± 6.82 (34.57–415.65) | 2.08 ± 0.59 (0.08–4.33) |
| Non-matriarch | 103.56 ± 5.81 (32.63–394.85) | 1.03 ± 0.26 (0.04–4.00) | 135.70 ± 4.36 (39.54–605.77) | 1.84 ± 0.32 (0.08–4.67) |
Data are presented as the mean value ± SEM and range (minimum–maximum) of faecal progestagen metabolite (FPM) concentrations, the number of years since non-pregnant females had their last calf (PPD), and the number of years between the conception of a pregnant female's current fetus and the birth of her previous calf (IPI).
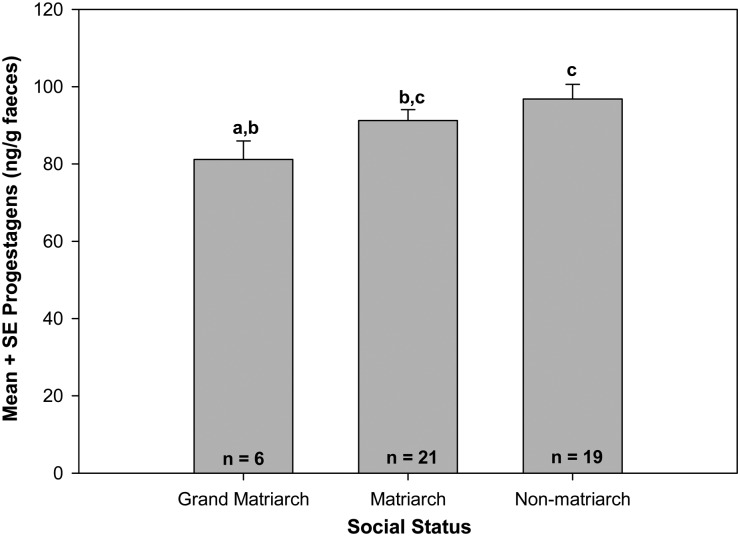
Several variables impacted FPM concentrations in elephants within AENP. Due to the relationship between age and social status, we ran the full model with an interaction of the two. However, based upon values of Akaike's information criteria and Bayesian information criteria, the most parsimonious model to predict the FPM concentrations in non-pregnant elephants was a reduced model with no interaction term (Table 3). The only significant variable was social status of the female (F2,41 = 3.30, P = 0.05). The FPM concentrations for non-pregnant non-matriarchs were significantly higher than for non-pregnant grand matriarchs (Tukey's test, P = 0.03); however, no differences in FPM concentrations were found between non-pregnant matriarchs and non-pregnant grand matriarchs (Tukey's test, P = 0.06) or non-pregnant non-matriarchs (Tukey's test, P = 0.57; Fig. 2). The age of the female, the interaction of age and social status, PPD, total monthly precipitation at the time of sample collection, the sex of her last calf, and whether the sample was collected in the wet or the dry season did not significantly contribute to FPM concentrations in non-pregnant AENP elephants (Table 3).
Table 3:
Linear mixed-effects model of factors that influence faecal progestagen metabolite concentrations in non-pregnant female African elephants in Addo Elephant National Park
| Full model | Coefficient ± SEM | d.f. | t-value | P-value | Akaike's information criteria Bayesian information criteria |
|---|---|---|---|---|---|
| Intercept | 61.34 ± 55.91 | 343 | 1.10 | 0.27 | 4035.82 4083.51 |
| Age | 0.53 ± 1.03 | 343 | 0.51 | 0.61 | |
| Square root of PPD (years) | −0.75 ± 2.29 | 343 | −0.33 | 0.74 | |
| Log10 precipitation (mm) | −6.02 ± 5.87 | 343 | −1.03 | 0.31 | |
| Season (dry) | |||||
| Wet season | −5.55 ± 5.01 | 343 | −1.11 | 0.27 | |
| Sex of last calf (female) | |||||
| Last calf male | 3.27 ± 4.24 | 40 | 0.77 | 0.45 | |
| Social status (grand matriarch) | |||||
| Matriarch status | −28.68 ± 57.15 | 40 | 0.50 | 0.62 | |
| Non-matriarch status | −38.96 ± 57.89 | 40 | 0.67 | 0.51 | |
| Age × social status (grand matriarch) | |||||
| Age × matriarch status | −0.25 ± 1.08 | 343 | −0.23 | 0.82 | |
| Age × non-matriarch status | −0.37 ± 1.23 | 343 | −0.30 | 0.77 | |
| Reduced model | Coefficient ± SEM | d.f. | t-value | P-value | Akaike's information criteria Bayesian information criteria |
| Intercept | 83.25 ± 4.98 | 348 | 16.73 | <0.001 | |
| Season (dry) | |||||
| Wet season | −7.45 ± 4.58 | 348 | −1.63 | 0.10 | |
| Social status (grand matriarch) | 4007.85 | ||||
| Matriarch status | 10.29 ± 5.55 | 41 | 1.85 | 0.07 | 4031.63 |
| Non-matriarch status | 15.04 ± 6.11 | 41 | 2.46 | 0.02 |
Figure 2:
Faecal progestagens and pregnancy. Relationship between mean faecal progestagen metabolite concentrations and social rank for non-pregnant elephants in Addo Elephant National Park, South Africa. Superscripts designate significant differences (P < 0.05, Tukey's test).
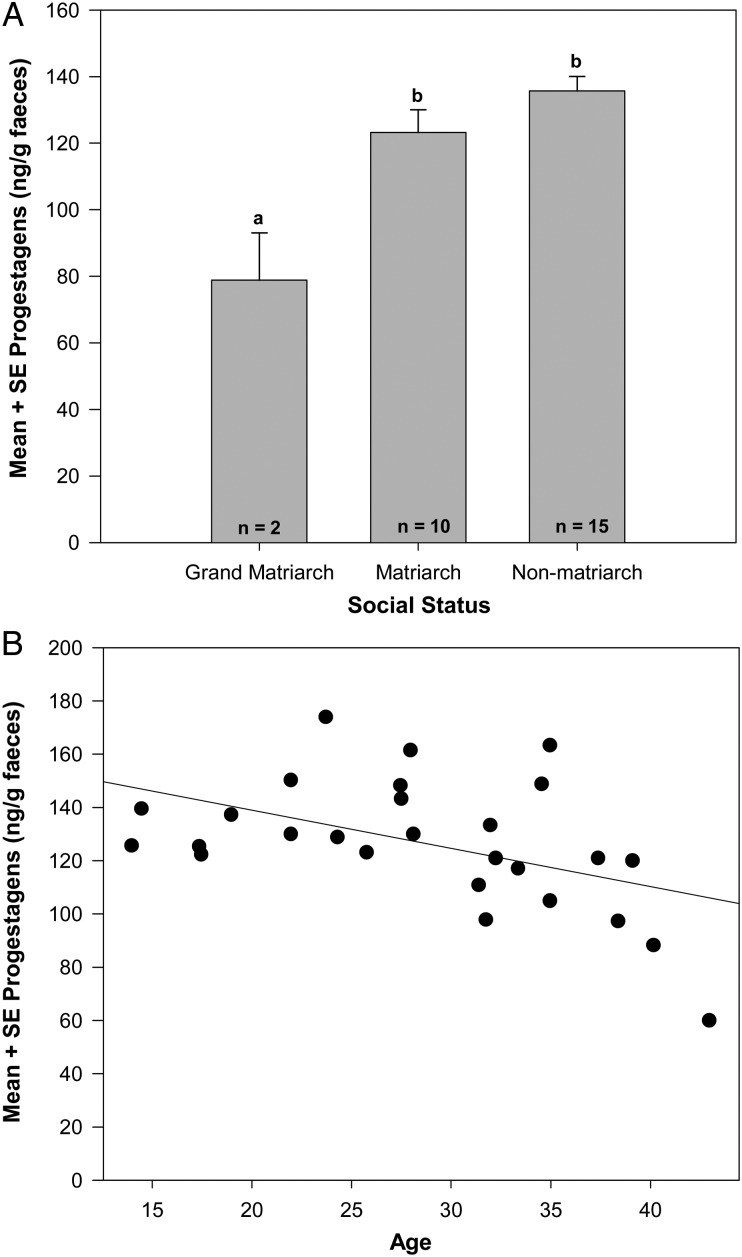
In order to determine what factors contributed to FPM concentrations in pregnant elephants, we included the interaction of age and social status again. With these models, the interaction of age and social status impacted the FPM concentrations in pregnant elephants (Table 4), in both the full and the reduced models. Additionally, the sex of the fetus was significant in the reduced model, but had only a borderline relationship in the full model (Table 4). Based upon Akaike's information criteria and Bayesian information criteria, the full model was more parsimonious than the reduced model. Older, pregnant grand matriarchs had lower FPM concentrations than pregnant matriarchs (Tukey's test, P < 0.05) and pregnant non-matriarchs (Tukey's test, P < 0.05; Fig. 3A); no differences were found between older, pregnant matriarchs and pregnant non-matriarchs (Tukey's test, P = 0.41). While neither age (F1,173 = 0.29, P = 0.57) nor social status (F1,24 = 0.72, P = 0.50) alone contributed to FPM concentrations in pregnant AENP elephants, the interaction of age and social status was significant (F2,173 = 5.21, P < 0.01; Fig. 3B). None of the other variables (e.g. trimester, IPI, precipitation, season) contributed (P > 0.05) to FPM concentrations in pregnant AENP elephants (Table 4).
Table 4:
Linear mixed-effects model of factors that influence faecal progestagen metabolite concentrations in pregnant female African elephants in Addo Elephant National Park
| Full model | Coefficient ± SEM | d.f. | t-value | P-value | Akaike's information criteria Bayesian information criteria |
|---|---|---|---|---|---|
| Intercept | 1852.02 ± 799.43 | 173 | 2.32 | 0.02 | 2359.64 2405.61 |
| Age | −41.90 ± 19.37 | 173 | −2.16 | 0.03 | |
| Trimester (first) | |||||
| Second | 25.31 ± 15.07 | 173 | 1.68 | 0.10 | |
| Third | 4.05 ± 15.00 | 173 | 0. 27 | 0.78 | |
| IPI (years) | 4.59 ± 5.47 | 173 | 0.84 | 0.40 | |
| Log10 precipitation (mm) | −4.82 ± 15.90 | 173 | −0.30 | 0.76 | |
| Sex of fetus (female) | |||||
| Male fetus | −27.58 ± 15.15 | 173 | −1.82 | 0.07 | |
| Social status (grand matriarch) | |||||
| Matriarch status | −1625.96 ± 804.96 | 24 | −2.02 | 0.05 | |
| Non-matriarch status | −1816.64 ± 800.05 | 24 | −2.27 | 0.03 | |
| Season (dry) | |||||
| Wet season | 7.46 ± 13.68 | 173 | 0.55 | 0.59 | |
| Age × social status (grand matriarch) | |||||
| Age × matriarch status | 39.01 ± 19.46 | 173 | 2.00 | 0.05 | |
| Age × non-matriarch status | 45.52 ± 19.24 | 173 | 2.37 | 0.02 | |
| Reduced model | Coefficient ± SEM | d.f. | t-value | P-value | Akaike's information criteria Bayesian information criteria |
| Intercept | 1605.07 ± 753.00 | 178 | 2.13 | 0.03 | 2387.55 |
| Age | −35.60 ± 18.17 | 178 | −1.96 | 0.05 | 2417.33 |
| Sex of the fetus (female) | |||||
| Male | −1834.35 ± 791.50 | 178 | −2.33 | 0.02 | |
| Social status (grand matriarch) | |||||
| Matriarch status | −1383.57 ± 761.95 | 24 | −1.82 | 0.08 | |
| Non-matriarch status | −1564.44 ± 753.95 | 24 | −2.08 | 0.04 | |
| Age × social status (grand matriarch) | |||||
| Age × matriarch status | −33.27 ± 18.42 | 178 | 1.81 | 0.07 | |
| Age × non-matriarch status | −39.79 ± 18.18 | 178 | 2.19 | 0.03 |
Figure 3:
Faecal progestagens, social rank and age. Relationships between mean faecal progestagen metabolite concentrations and social rank (A) or age (B) of pregnant elephants in Addo Elephant National Park, South Africa. Superscripts designate significant differences (P < 0.05, Tukey's test).
Similar to the models predicting FPM concentrations, the interaction of age and social rank was included in the exploration of factors that contributed to PPD in non-pregnant elephants (Table 5). Based upon Akaike's information criteria and Bayesian information criteria, we selected the reduced model where age, social status, and season contributed to PPD. The PPD increased with the age of the female (F1,359 = 1056.11, P < 0.001; Fig. 4A) and was longer during the dry season in comparison to the wet season (F1,359 = 5.78, P = 0.02; Fig. 4C). Additionally, PPD was significantly longer (F2,42 = 24.67, P < 0.001; Fig. 4B) in non-pregnant, grand matriarchs than matriarchs (Tukey's test, P < 0.001) and non-matriarchs (Tukey's test, P < 0.001); PPD was also longer in matriarchs than in non-matriarchs (Tukey's test, P < 0.001). None of the other variables, FPM concentration of each sample, sex of the previous calf, and the total monthly precipitation at the time of sample collection, was related to PPD (Table 5).
Table 5:
Linear mixed-effects models of factors that influence the number of post-partum years exhibited by non-pregnant female African elephants in Addo Elephant National Park
| Full model | Coefficient ± SEM | d.f. | t-value | P-value | Akaike's information criteria Bayesian information criteria |
|---|---|---|---|---|---|
| Intercept | −42.88 ± 3.40 | 357 | −12.62 | <0.001 | 561.95 |
| Age | 0.96 ± 0.05 | 357 | 20.09 | <0.001 | 609.79 |
| FPM (ng/g faeces) | <0.01 ± <0.01 | 357 | 0.04 | 0.97 | |
| Log10 precipitation (mm) | −0.04 ± 0.04 | 357 | −0.95 | 0.34 | |
| Season (dry) | |||||
| Wet season | −0.05 ± 0.04 | 357 | −1.35 | 0.17 | |
| Sex of last calf (female) | |||||
| Male calf | −0.89 ± 1.61 | 41 | −0.57 | 0.58 | |
| Social status (grand matriarch) | |||||
| Matriarch status | 17.94 ± 3.71 | 41 | 4.83 | <0.001 | |
| Non-matriarch status | 28.92 ± 3.73 | 41 | 7.75 | <0.001 | |
| Age × social status (grand matriarch) | |||||
| Age × matriarch status | −0.18 ± 0.06 | 357 | −3.01 | <0.01 | |
| Age × non-matriarch status | −0.31 ± 0.07 | 357 | −4.46 | <0.001 | |
| Reduced model | Coefficient ± SEM | d.f. | t-value | P-value | Akaike's information criteria Bayesian information criteria |
| Intercept | −43.60 ± 3.27 | 359 | −13.32 | <0.001 | |
| Age | 0.96 ± 0.05 | 359 | 20.36 | <0.001 | |
| Season (dry) | |||||
| Wet season | −0.06 ± 0.03 | 359 | −1.86 | 0.06 | |
| Social status (grand matriarch) | 541.30 | ||||
| Matriarch status | 18.13 ± 3.69 | 42 | 4.91 | <0.001 | 577.25 |
| Non-matriarch status | 29.17 ± 3.71 | 42 | 7.87 | <0.001 | |
| Age × social status (grand matriarch) | |||||
| Age × matriarch status | −0.18 ± 0.06 | 359 | −3.06 | <0.01 | |
| Age × non-matriarch status | −0.32 ± 0.07 | 359 | −4.51 | <0.001 |
Figure 4:
Post-partum duration in non-pregnant elephants. Relationship between post-partum duration and age (A), social rank (B), or wet/dry season (C) for African elephants in Addo Elephant National Park, South Africa. Superscripts designate significant differences (P < 0.05, Tukey's test).
Discussion
Linear mixed-effects models indicated that social status was the most significant predictor of FPM concentrations in non-pregnant elephants within AENP. Likewise, both age and social status influenced FPM concentrations in pregnant AENP elephants. The negative relationships within each model demonstrated that grand matriarchs have lower FPM concentrations than matriarchs and non-matriarchs and that older pregnant females have lower FPM concentrations than younger pregnant elephants. For PPD, positive relationships were also demonstrated between age and social status, with non-pregnant grand matriarchs and older females having the longest intervals since the birth of their last calf. In addition, season was related to PPD; a shorter PPD was found during the wet than the dry season, because elephants were more likely to give birth in the wet season (59% of births in the present study). As our results demonstrate, because age and social status were strongly related, it is difficult to tease apart the impact of these two factors on reproductive function. Nevertheless, this is the first study to provide evidence that ovarian activity declines in older African elephants as they attain grand matriarch status.
Reproduction in female mammals is dynamic and can be affected by the interplay of multiple biotic and abiotic factors (Atsalis et al., 2008). Females that survive in the wild to an advanced age are typically reproductively robust (Walker et al., 2008; Finch et al., 2010) and may exhibit behavioural traits that enhance their health and survivability (Walker and Herndon, 2008). In wild populations, mortality rates can be high, whether caused by predation, disease, or other factors. As a result, most females fail to reach an advanced age, which precludes the observation of high rates of reproductive senescence in wild populations (Atsalis and Margulis, 2008). Thus, a continuation of reproductive function, even at a diminished level, and survival beyond the natural fertile period is a rare event that deserves notice (Erwin and Hof, 2008). Ovarian or endocrine data on reproductive ageing have been studied in only a few mammalian species (Finch and Holmes, 2010), but all of the studies demonstrate the importance of ovarian cycling and steroidogenesis in the maintenance of female reproduction (Ottinger, 2010).
Like humans, most female mammals display a decline in fertility with advanced age (Bellino and Wise, 2003; McAuliffe and Whitehead, 2005; Kachel et al., 2011). Although female African elephants in the wild have been known to reproduce into their fifties (Laws et al., 1970; Smuts, 1975; Freeman et al., 2009; Robinson et al., 2012), a lack of detectable corpora lutea in most females over 50 years of age in Kruger National Park, South Africa indicated that they had inactive ovaries and were no longer reproductively viable (Freeman et al., 2009). By 50 years of age, those females were most likely to be the matriarchs of their family or grand matriarchs of their kinship group.
As predicted, an inverse relationship between age, social status, and ovarian function (FPM) was found among the adult African elephants in AENP. The relatively low FPM concentrations and long PPD observed among the older AENP elephants, and longitudinal FPM profiles that remained at baseline in an earlier study (Freeman et al., 2011), indicate that the four grand matriarchs over the age of 50 years and one matriarch (aged 44 years at study onset) may have entered reproductive senescence. Additionally, only three of the 12 elephants in this study that were ≥ 40 years of age were pregnant; two were younger grand matriarchs (39 and 43 years) and one a matriarch (38 years). Due to possible lost pregnancies (e.g. miscarriages) and females that gave birth after the study ended, the percentage of older pregnant females (25%) in AENP was probably under-estimated. However, it is likely that many of the AENP females over the age of 45 years were not pregnant and did not have functional corpora lutea, similar to the Kruger population. There is likely to be considerable individual variability in ovarian responses to ageing. Variable numbers of oocytes endowed to AENP females at birth (Finch and Holmes, 2010) could explain why some older matriarchs and grand matriarchs were still giving birth, while others had lower FPM concentrations and a longer PPD. It was somewhat unexpected that age was related to FPM concentrations only in pregnant elephants, but the results nevertheless corroborate those found among free-ranging females in Kenya (Wittemyer et al., 2007b). That study did not relate FPM concentrations to social status; additional studies are needed to determine whether similar relationships exist for other elephant populations. The decrease in FPM taken together with the longer PPD with age and elevated social status among elephants provide physiological evidence of age-related reproductive senescence (Wittemyer et al., 2007b) or menopause in elephants, as has long been suspected (Laws, 1970). A logical next step would be to examine how FPM concentrations and longer PPD in older, higher ranking elephants might be related to calf birth weights and survival, and/or milk yield.
Behavioural mechanisms may promote older female elephants reaching reproductive senescence (Laws, 1969, 1970). Senescence is an adaptive trade-off between continued reproduction and assisting kin (McAuliffe and Whitehead, 2005). In large, long-lived, highly social animals, such as elephants, cessation of reproduction before death may be selected for when the energy devoted to the care and survival of offspring can increase inclusive fitness (Hamilton, 1964). The demonstrated benefits that the social knowledge of older matriarchs impart on the family unit (McComb et al., 2011) and high rates of co-operative behaviours among related females (Douglas-Hamilton, 1972; Wittemyer et al., 2005; McComb et al., 2011) suggest that early reproductive senescence may be selected for in elephants. Additionally, older elephants are more likely to have close relatives in their social group than young females, which increases the benefits of ceasing reproduction to assist close kin (Johnstone and Cant, 2010). In turn, the higher reproductive rates of kin impart fitness benefits that promote cessation of reproduction in the matriarch or grand matriarch. These social dynamics may be why reproductive success (Laws et al., 1970; Smuts, 1975; Moss, 2001; Freeman et al., 2009) and FPM concentrations of wild African elephants decline when females reach advanced age (>45–50 years) and matriarchal social status (e.g. become grand matriarchs).
Matriarchs and grand matriarchs also benefit the family unit by sharing information about the location of historical food sources (Gobush et al., 2008). Gobush et al. (2008) studied the impact of the loss of old matriarchs (similar in age to grand matriarchs in the present study) on the remaining family members within a heavily poached elephant population of Mikumi National Park, Tanzania. Adult females without an older matriarch had lower reproductive output and higher stress levels. These studies (Gobush et al., 2008; McComb et al., 2011) reinforce the importance of elephant matriarchs and the benefits they can impart to their families. If cultural transmission of knowledge plays a role in the evolution of a post-reproductive lifespan, it may be through more subtle means than increasing the survival of offspring and grand offspring (Ward et al., 2009). More research is required to determine how reproductive senescence is attributed to homologous physiological patterns in ovarian decline (Finch and Holmes, 2010), or the evolutionary need for kin selection or the transfer of inter-generational knowledge.
In our study, there was an environmental effect on reproductive activity. Specifically, the post-partum duration in AENP elephants was shorter during the wet season, when vegetative quality is generally higher. Enhanced vegetative quality during periods of higher rainfall can positively influence birth rates of elephants (Gough and Kerley, 2006; Foley and Faust, 2010). Addo Elephant National Par elephants have higher birth rates during wet than dry years (Gough and Kerley, 2006). Although elephants can give birth year round, most (33 of 80 births) during the course of our study occurred during AENP rainfall peaks in October–November and February–March. Giving birth in the wet season ensures that females are in optimal body condition as lactational demands increase (Laws and Parker, 1968). Given that elephants typically nurse a calf until the next one is born, most juveniles are weaned during the wet season when vegetative quality can compensate for the calories no longer gained from milk. Poor nutritional quality during the dry season causes a decline in body condition, which may negatively impact the success of implantations and early pregnancies (Laws and Parker, 1968; Foley et al., 2001) as well as contributing to lower FPM concentrations for both pregnant (Foley et al., 2001; Wittemyer et al., 2007b) and non-pregnant elephants (Wittemyer et al., 2007b). Females that do not cycle because of a lack of available browse and poor body condition during the dry season are unlikely to conceive. Such natural regulation of oestrous cycle activity may help to regulate the timing of conceptions and births. Reproductive seasonality has been documented for populations of elephants in Kenya, South Africa, Tanzania, Uganda, and Zambia (Laws and Parker, 1968; Laws, 1969; Smuts, 1975; Poole, 1989; Stuart-Hill et al., 1993; Foley et al., 2001; Wittemyer et al., 2007a; Freeman et al., 2009; Foley and Faust, 2010).
We expected to find seasonal variability in FPM concentrations, similar to the studies in Kenya (Wittemyer et al., 2007b) and Tanzania (Foley et al., 2001); however, no such relationship was found between precipitation or wet/dry season and FPM concentrations in pregnant or non-pregnant elephants in AENP. Similar to these two populations (Wittemyer et al., 2007a; Foley and Faust, 2010), elephant birth rates in AENP are positively correlated with rainfall in the year of conception (Gough and Kerley, 2006). Unlike those populations, AENP elephants have access to drought-resistant vegetation (Stuart-Hill and Aucamp, 1993), artificial water sources, and rainfall year round (Gough and Kerley, 2006), and show very little variation in body condition throughout the year (J. M. Meyer, personal observation). The consistent condition of the elephants year round may explain why no differences in FPM concentrations with respect to precipitation or season were found in the AENP population.
The access to water and the consistent body condition of the elephants in AENP may also contribute to their relatively high population growth rate (5.8 ± 3.1%; Gough and Kerley, 2006). The average IPI found in the present study (1.88 ± 0.55 months) would produce a similar inter-calving interval (3.3 ± 0.8 years) to that reported previously for AENP (Gough and Kerley, 2006), assuming a 22 month gestation (Laws, 1969; Wittemyer et al., 2007b). One third of the pregnant females within our sample AENP population (n = 9) conceived within a year of giving birth; four of those females lost their calf and appear to have re-cycled and conceived shortly thereafter. In spite of the rapidly expanding population and a density that has exceeded recommendations for 50 years (Kerley et al., 2006), the elephant population in AENP has yet to experience any density-dependent regulation (Gough and Kerley, 2006).
Conclusions
Our study advances knowledge about reproductive physiology in free-ranging elephants by providing evidence of a relationship between older females obtaining the highest social status within their family and declines in FPM concentrations and increases in PPD. Reproductive senescence contributes to a post-reproductive lifespan for elephant matriarchs and grand matriarchs, when they may provide survival benefits to their offspring and extended family members because of the knowledge they impart. However, a post-reproductive lifespan will only evolve if the indirect fitness benefits that accrue outweigh additional attempts at direct fitness output once females reach advanced age (>45 years) and high social rank. Owing to the high density of elephants within AENP (Kerley and Landman, 2006), there may be further selective pressures on the oldest females to stop adding more individuals to the population. The present study provides further evidence of a decline in reproductive success with advanced age in elephants. More research on other populations with larger numbers of matriarchs and grand matriarchs, and lower population densities, is needed to determine whether selective pressures have led to the evolution of menopause in female elephants, as suggested by Laws (1969).
Knowledge about reproductive physiology of high-ranking females can provide managers with biological data to identify the best candidates for policy decisions when population growth needs to be regulated. Elephant over-population is a growing problem in some areas of Africa (Owen-Smith et al., 2006), and the density of elephants in AENP has exceeded recommended limits, by up to 8-fold, for 50 years (Kerley and Landman, 2006). Although the AENP elephants have a high population growth rate, which is coupled with low juvenile and adult mortality, these demographic factors are not density dependent in the AENP population (Gough and Kerley, 2006). The influence of the AENP elephants on the succulent thicket vegetation is well documented (Lombard et al., 2001; Kerley and Landman, 2006; Landman et al., 2008). It is predicted that the elephant population in AENP will continue to grow and reproduce at a high rate until the vegetative resources are irreversibly depleted (Gough and Kerley, 2006). Although fluctuating elephant populations can be beneficial to biodiversity (Whyte et al., 1999), it is not known whether uncontrolled growth will irreversibly harm it (Dickson and Adams, 2009) or be detrimental to ecosystem functioning in the long term (Landman et al., 2012). Furthermore, competition with elephants for dwindling resources in AENP appears to be impacting the health (Aronoff JT, Santymire RM, Freeman EW, Meyer J, Gillespie TR, unpublished), foraging opportunities and diet (Landman and Kerley, 2013; Landman et al., 2013), and activity patterns (Tambling CJ, Meyer J, Minnie L, Freeman EW, Santymire RM, Addendorf J, Kerley GIH, unpublished) of the critically endangered black rhinoceros (Diceros bicornis bicornis). Thus, for the sake of other animal and plant species, growth of the AENP elephant population may need to be controlled before it naturally reaches carrying capacity (Whyte, 2001; Gough and Kerley, 2006).
There is much debate about the best tools to manage growing elephant populations (Owen-Smith et al., 2006) and whether scientific data prove that they need to be regulated at all (Dickson and Adams, 2009). Historically, elephant populations in South Africa were controlled through culling, and in 2008 South Africa voted to resume this practice (Dickson and Adams, 2009). Contraception is another possible means to control elephant populations, and its use has been tested repeatedly in South Africa (Stetter et al., 2006; Kerley et al., 2007; Fayrer-Hosken et al., 1999; Druce et al., 2011, 2013). Translocations, reintroductions, and the creation of mega-transfrontier parks (van Aarde et al., 2006, 2007) are also proposed as management tools. Regardless of the methods selected, physiological data (Cooke et al., 2013), such as those gained through non-invasive endocrine monitoring, can provide critical information to population managers. For instance, monitoring of hormone patterns can demonstrate the efficacy of culling and contraceptives (Wasser et al., 1996; Foley et al., 2001) on reproductive function, and identify the best candidates for translocation (Freeman et al., 2011; Cooke et al., 2013). Additionally, measures of both reproductive (e.g. FPM) and stress hormones (e.g. glucocorticoids) can be used to assess the impact of human disturbance (e.g. poaching and habitat fragmentation) and environmental change on overall reproductive health and animal welfare, with implications for conservation management.
With our growing understanding of the relationships among age, social status, and progestagen concentrations, the endocrine status of females should be monitored prior to selecting them for any population-control programmes. Due to the high costs and controversial nature of most policies for regulating elephant populations (Dickson and Adams, 2009), knowledge of the reproductive status of individuals in the population would enhance the efficacy of these management decisions. For instance, discovering that a female is no longer reproductively viable would eliminate her as a candidate for contraception. In particular, grand matriarchs should be excluded from consideration for culling, contraception, and/or translocation without assessing their reproductive status first, because of the important role they play in elephant society and the likelihood that these females are no longer cycling.
Acknowledgements
We wish to thank Laura Broederdorf, Michelle Schroeder, and Remy Schoenemann for their assistance in the field and laboratory. Additionally, we appreciate the continued support of our work by South African National Parks, including but not limited to John Adendorff and Angela Gaylard. This work was supported by the Smithsonian Women's Committee, Walcott Endowment and Scholarly Studies Program, as well as the International Elephant Foundation.
References
- 1.Archie EA, Morrison TA, Foley CAH, Moss CJ, Alberts SC. (2006) Dominance rank relationships among wild female African elephants, Loxodonta africana. Anim Behav 71: 117–127. [Google Scholar]
- 2.Atsalis S, Margulis S. (2008) Primate reproductive aging: from lemurs to humans. In Atsalis S, Margulis SW, Hof PR, eds, Primate Reproductive Aging: Cross-Taxon Perspectives on Reproduction, Vol 36 Karger, Basil, pp 186–194. [DOI] [PubMed] [Google Scholar]
- 3.Bagley KR. (2004) Chemosensory behavior and development of African male elephants (Loxodonta africana). MS thesis Georgia Southern University, Statesboro, GA, USA. [Google Scholar]
- 4.Bates LA, Poole JH, Byrne RW. (2008) Elephant cognition. Curr Biol 18: 544–546. [DOI] [PubMed] [Google Scholar]
- 5.Bellino FL, Wise PM. (2003) Nonhuman primate models of menopause workshop. Biol Reprod 68: 10–18. [DOI] [PubMed] [Google Scholar]
- 6.Cooke SJ, Sack L, Franklin CE, Farrell AP, Beardall J, Wikelski M, Chown SL. (2013) What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conserv Physiol 1: doi:10.1093/conphys/cot1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dickson P, Adams WM. (2009) Science and uncertainty in South Africa's elephant culling debate. Envir & Plan C: Govt & Pol 27: 110–123. [Google Scholar]
- 8.Douglas-Hamilton I. (1972) On the ecology and behaviour of the African elephant. Doctorate of Philosophy thesis, University of Oxford, UK. [Google Scholar]
- 9.Druce HC, Mackey RL, Slotow R. (2011) How immunocontraception can contribute to elephant management in small, enclosed reserves: Munyawana population as a case study. PLoS One 6: e27952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Druce HC, Mackey RL, Pretorius K, Slotow R. (2013) The intermediate-term effects of PZP immunocontraception: behavioural monitoring of the treated elephant females and associated family groups. Anim Conserv 16: 180–187. [Google Scholar]
- 11.Dublin HT. (1983) Cooperation and reproductive competition among female African elephants. In SK Wagner, ed, Social Behavior of Female Vertebrates. Academic Press, New York, pp 291–313. [Google Scholar]
- 12.Duer C, Carden M, Schmitt D, Tomasi T. (2002) Utility of maternal serum total testosterone analysis for fetal gender determination in Asian elephants (Elephas maximus). Anim Reprod Sci 69: 47–52. [DOI] [PubMed] [Google Scholar]
- 13.Duer C, Carden M, Tomasi T. (2007) Detection of fetal gender differences in maternal serum progesterone concentrations of Asian elephants (Elephas maximus). Anim Reprod Sci 97: 278–283. [DOI] [PubMed] [Google Scholar]
- 14.Emery Thompson M, Jones JH, Pusey AE, Brewer-Marsden S, Goodall J, Marsden D, Matsuzawa T, Nishida T, Reynolds V, Sugiyama Y, et al. (2007) Aging and fertility patterns in wild chimpanzees provide insights into the evolution of menopause. Curr Biol 17: 2150–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erwin JM, Hof PR. (2008) Menopause and reproductive senescence in comparative context. In Atsalis S, Margulis SW, Hof PR, eds, Primate Reproductive Aging: Cross-Taxon Perspectives on Reproduction, Vol 36 Karger, Basil, pp 4–16. [DOI] [PubMed] [Google Scholar]
- 16.Esposito RMM. (2008) The effect of matriarchs on group interactions, kinship fitness, and differences in chemosensory behavior of African elephants (Loxodonta africana). MS thesis, Georgia Southern University, Statesboro, GA, USA. [Google Scholar]
- 17.Fayrer-Hosken RA, Bertschinger HJ, Kirkpatrick JF, Grobler D, Lamberski N, Honneyman G, Ulrich T. (1999) Contraceptive potential of the porcine zona pellucida vaccine in the African elephant (Loxodonta africana). Theriogenology 52: 835–846. [DOI] [PubMed] [Google Scholar]
- 18.Finch CE, Holmes DJ. (2010) Ovarian aging in developmental and evolutionary contexts. Ann NY Acad Sci 1204: 82–94. [DOI] [PubMed] [Google Scholar]
- 19.Foley CAH, Papageorge S, Wasser SK. (2001) Noninvasive stress and reproductive measures of social and ecological pressures in free-ranging African elephants. Conserv Biol 15: 1134–1142. [Google Scholar]
- 20.Foley CAH, Faust LJ. (2010) Rapid population growth in an elephant Loxodonta africana population recovering from poaching in Tarangire National Park, Tanzania. Oryx 44: 205–212. [Google Scholar]
- 21.Freeman EW, Whyte I, Brown JL. (2009) Reproductive evaluation of elephants culled in Kruger National Park, South Africa between 1976 and 1995. Afr J Ecol 47: 192–201. [Google Scholar]
- 22.Freeman EW, Abbondanza FN, Meyer J, Schulte BA, Brown JL. (2010a) A simplified method for monitoring progestagens in African elephants under field conditions. Meth Ecol Evol 1: 86–91. [Google Scholar]
- 23.Freeman EW, Schulte BA, Brown JL. (2010b) Investigating the impact of rank and ovarian activity on the social behavior of captive female African elephants. Zoo Biol 29: 154–167. [DOI] [PubMed] [Google Scholar]
- 24.Freeman EW, Meyer JM, Putman S, Schulte BA, Brown JL. (2011) Using a simplified field progestagen method to assess ovarian activity in female African elephants. Biol Conserv 144: 2105–2111. [Google Scholar]
- 25.Gobush KS, Mutayoba BM, Wasser SK. (2008) Long-term impacts of poaching and relatedness, stress physiology, and reproductive output of adult female African elephants. Conserv Biol 22: 1590–1599. [DOI] [PubMed] [Google Scholar]
- 26.Gough KF, Kerley GIH. (2006) Demography and population dynamics in the elephants Loxodonta africana of Addo Elephant National Park, South Africa: is there evidence of density dependent regulation? Oryx 40: 434–441. [Google Scholar]
- 27.Graham L, Schwarzenberger F, Mostl E, Galama W, Savage A. (2001) A versatile enzyme immunoassay for the determination of progestogens in feces and serum. Zoo Biol 20: 227–236. [Google Scholar]
- 28.Hamilton WD. (1964) The genetic evolution of social behavior. I. J Theor Biol 7: 1–16. [DOI] [PubMed] [Google Scholar]
- 29.Hermes R, Hildebrandt TB, Göritz F. (2004) Reproductive problems directly attributable to long-term captivity–asymmetric reproductive aging. Anim Reprod Sci 82–83: 49–69. [DOI] [PubMed] [Google Scholar]
- 30.Johnstone RA, Cant MA. (2010) The evolution of menopause in cetaceans and humans: the role of demography. Proc Biol Sci 277: 3765–3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kachel AF, Premo LS, Hublin J-J. (2011) Grandmothering and natural selection. Proc Biol Sci 278: 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerley GIH, Landman M. (2006) The impacts of elephants on biodiversity in the Eastern Cape Subtropical Thickets. S Afr J Sci 102: 395–402. [Google Scholar]
- 33.Landman M, Kerley GIH, Schoeman DS. (2008) Relevance of elephant herbivory as a threat to important plants in the Addo Elephant National Park, South Africa. J Zool 274: 51–58. [Google Scholar]
- 34.Landman M, Schoeman DS, Hall-Martin AJ, Kerley GIH. (2012) Understanding long-term variations in an elephant piosphere effect to manage impacts. PLoS One 7: e45334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landman M, Kerley GIH. (2013) Elephant both increase and decrease availability of browse resources for black rhinoceros. Biotropica doi:10.1111/btp.12066. [Google Scholar]
- 36.Landman M, Schoeman DS, Kerley GIH. (2013) Shift in black rhinoceros diet in the presence of elephant: evidence for competition? PLoS One 8: e69771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laws RM, Parker ISC. (1968) Recent studies on elephant populations in East Africa. Symp Zool Soc Lond 21: 319–359. [Google Scholar]
- 38.Laws RM. (1969) Aspects of reproduction in the African elephant, Loxodonta africana. J Reprod Fert 6: 193–217. [Google Scholar]
- 39.Laws RM. (1970) Biology of African elephants. Science Prog 58: 251–262. [PubMed] [Google Scholar]
- 40.Laws RM, Parker ISC, Johnstone RCB. (1970) Elephants and habitats in North Bunyoro, Uganda. E Afr Wildl J 8: 163–180. [Google Scholar]
- 41.Loizi H, Goodwin TE, Rasmussen LEL, Whitehouse AM, Schulte BA. (2009) Sexual dimorphism in the performance of chemosensory investigatory behaviours by African elephants (Loxodonta africana). Behaviour 146: 373–392. [Google Scholar]
- 42.Lombard AT, Johnson CF, Cowling RM, Pressey RL. (2001) Protecting plants from elephants: botanical reserve scenarios within the Addo Elephant National Park, South Africa. Biol Conserv 102: 191–203. [Google Scholar]
- 43.McAuliffe K, Whitehead H. (2005) Eusociality, menopause and information in matrilineal whales. Trends Ecol Evol 20: 650–650. [DOI] [PubMed] [Google Scholar]
- 44.McComb K, Moss C, Durant SM, Baker L, Sayialel S. (2001) Matriarchs as repositories of social knowledge in African elephants. Science 292: 491–494. [DOI] [PubMed] [Google Scholar]
- 45.McComb K, Shannon G, Durant SM, Sayialel K, Slotow R, Poole J, Moss C. (2011) Leadership in elephants: the adaptive value of age. Proc Biol Sci 278: 3270–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merte CE, Goodwin TE, Schulte BA. (2010) Male and female developmental differences in chemosensory investigations by African elephants (Loxodonta africana) approaching waterholes. Behav Ecol Sociobiol 64: 401–408. [Google Scholar]
- 47.Meyer J. (2006) Sexually dimorphic social development and female intrasexual chemical signaling of African elephants (Loxodonta africana). MS thesis. Georgia Southern University, Statesboro, GA, USA. [Google Scholar]
- 48.Moss CJ. (1983) Oestrous behavior and female choice in the African elephant. Behaviour 86: 167–196. [Google Scholar]
- 49.Moss CJ. (2001) The demography of an African elephant (Loxodonta africana) population in Amboseli, Kenya. J Zool 255: 145–156. [Google Scholar]
- 50.Nussey DH, Coulson T, Festa-Bianchet M, Gaillard JM. (2008) Measuring senescence in wild animal populations: towards a longitudinal approach. Func Ecol 22: 393–406. [Google Scholar]
- 51.Ottinger MA. (2010) Mechanisms of reproductive aging: conserved mechanisms and environmental factors. Ann NY Acad Sci 1204: 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Owen-Smith N, Kerley GIH, Page B, Slotow R, van Aarde RJ. (2006) A scientific perspective on the management of elephants in the Kruger National Park and elsewhere. S Afr J Sci 102: 389–394. [Google Scholar]
- 53.Poole JH. (1989) Mate guarding, reproductive success and female choice in African elephants. Anim Behav 27: 842–849. [Google Scholar]
- 54.Poole JH, Moss CJ. (1989) Elephant mate searching: group dynamics and vocal and olfactory communication. Symp Zool Soc Lond 61: 111–125. [Google Scholar]
- 55.Poole JH. (1994) Sex differences in the behaviour of African elephants. In Short RV, Balaban E, eds, The Differences Between the Sexes. Cambridge University Press, Cambridge, UK, pp 331–346. [Google Scholar]
- 56.Robinson MR, Mar KU, Lummaa V. (2012) Senescence and age-specific trade-offs between reproduction and survival in female Asian elephants. Ecol Lett 15: 260–266. [DOI] [PubMed] [Google Scholar]
- 57.Smuts GL. (1975) Reproduction and population characteristics of elephants in Kruger National Park. J Sth Afr Wildl Mgmt Assoc 5: 1–10. [Google Scholar]
- 58.Soltis J, Leong K, Savage A. (2005a) African elephant vocal communication I: antiphonal calling behaviour among affiliated females. Anim Behav 70: 579–588. [Google Scholar]
- 59.Soltis J, Leong K, Savage A. (2005b) African elephant vocal communication II: rumble variation reflects the individual identity and emotional state of callers. Anim Behav 70: 589–599. [Google Scholar]
- 60.Stetter M, Henderickson D, Zuba J, Stetter K, Grobler D, van Altena J, Small L-A. (2006) Laproscopic vasectomy as a potential population control method in free ranging African elephants (Loxodonta africana). In D Olson ed., International Elephant Conservation and Research Symposium, Copenhagen, The International Elephant Foundation, Azle, TX, pp 37. [Google Scholar]
- 61.Stuart-Hill G, Aucamp A. (1993) Carrying capacity of the succulent valley bushveld of the Eastern Cape. Afr J Range Forage Sci 10: 1–10. [Google Scholar]
- 62.van Aarde RJ, Jackson TP. (2007) Megaparks for metapopulations: addressing the causes of locally high elephant numbers in southern Africa. Biol Conserv 134: 289–297. [Google Scholar]
- 63.van Aarde RJ, Jackson TP, Ferreira SM. (2006) Conservation science and elephants management in southern Africa. S Afr J Sci 102: 385–389. [Google Scholar]
- 64.Walker ML, Herndon JG. (2008) Menopause in nonhuman primates. Biol Reprod 79: 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ward EJ, Parsons K, Holmes EE, Balcomb KC, 3rd, Ford JK. (2009) The role of menopause and reproductive senescence in a long-lived social mammal. Front Zool 6, 4. doi:10.1186/1742-9994-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wasser SK, Papageorge S, Foley C, Brown JL. (1996) Excretory fate of estradiol and progesterone in the African elephant (Loxodonta africana) and patterns of fecal steroid concentrations throughout the estrous cycle. Gen Comp Endocrinol 102: 255–262. [DOI] [PubMed] [Google Scholar]
- 67.Whitehouse AM, Hall-Martin AJ. (2000) Elephants in Addo Elephant National Park, South Africa: reconstruction of the population's history. Oryx 34: 46–55. [Google Scholar]
- 68.Whitehouse AM, Harley EH. (2001) Post-bottleneck genetic diversity of elephant populations in South Africa, revealed using microsatellite analysis. Mol Ecol 10: 2139–2149. [DOI] [PubMed] [Google Scholar]
- 69.Whitehouse AM, Hall-Martin AJ, Knight MH. (2001) A comparison of methods used to count the elephant population of the Addo Elephant National Park, South Africa. Afr J Ecol 39: 140–145. [Google Scholar]
- 70.Whyte IJ, Biggs HC, Gaylard A, Braack LEO. (1999) A new policy for the management of the Kruger National Park's elephant population. Koedoe 42: 111–132. [Google Scholar]
- 71.Whyte IJ. (2001) Conservation management of the Kruger National Park elephant population. PhD thesis, University of Pretoria, Pretoria, South Africa. [Google Scholar]
- 72.Wittemyer G, Douglas-Hamilton I, Getz WM. (2005) The socioecology of elephants: analysis of the processes creating multitiered social structures. Anim Behav 69: 1357–1371. [Google Scholar]
- 73.Wittemyer G, Barner Rasmussen H, Douglas-Hamilton I. (2007a) Breeding phenology in relation to NDVI variability in free-ranging African elephant. Ecography 30: 42–50. [Google Scholar]
- 74.Wittemyer G, Ganswindt A, Hodges K. (2007b) The impact of ecological variability on the reproductive endocrinology of wild female African elephants. Horm Behav 51: 346–354. [DOI] [PubMed] [Google Scholar]
- 75.Wittemyer G, Getz WM. (2007c) Hierarchical dominance structure and social organization in African elephants, Loxodonta africana. Anim Behav 73: 671–681. [Google Scholar]