This review examines physiological and morphological traits of native and invasive species occurring in environments characterized by low nutrient, water and light availability. Species invading low-resource environments possess traits associated with resource acquisition, resource conservation, or both acquisition and conservation.
Keywords: Invasion biology, leaf economics spectrum, resource acquisition, resource conservation, restoration ecology
Abstract
While invasive plant species primarily occur in disturbed, high-resource environments, many species have invaded ecosystems characterized by low nutrient, water, and light availability. Species adapted to low-resource systems often display traits associated with resource conservation, such as slow growth, high tissue longevity, and resource-use efficiency. This contrasts with our general understanding of invasive species physiology derived primarily from studies in high-resource environments. These studies suggest that invasive species succeed through high resource acquisition. This review examines physiological and morphological traits of native and invasive species in low-resource environments. Existing data support the idea that species invading low-resource environments possess traits associated with resource acquisition, resource conservation or both. Disturbance and climate change are affecting resource availability in many ecosystems, and understanding physiological differences between native and invasive species may suggest ways to restore invaded ecosystems.
Introduction
Low-resource environments are defined as those where plant productivity is severely limited by light, water, or soil nutrient availability, such as forest understories, deserts, and ancient landscapes. In many of these ecosystems, native plants have evolved mechanisms to tolerate stress and to facilitate the extraction of limiting resources. These adaptations have resulted in a high degree of species richness and functional diversity in many low-resource ecosystems (Dallman, 1998; Lambers et al., 2010; Olde Venterink, 2011). Native species appear to have a competitive advantage over invasive species in low-resource systems (Alpert et al., 2000; Daehler, 2003), and communities become more susceptible to invasion when resource availability is increased (Davis et al., 2000). While high-resource ecosystems tend to accumulate more exotic species than low-resource ecosystems (e.g. Huenneke et al., 1990; Gross et al., 2005; Stohlgren et al., 2008), many invasive species do occur in low-resource ecosystems. For example, several legumes have successfully invaded low-nitrogen soils in Hawaii, and many annual grasses and forbs dominate semi-arid grassland and shrub systems in California (Fig. 1).
Figure 1.

The legume Leucaena leucocephala invades young, low-nitrogen volcanic soils in Hawaii (top panel). Annual grasses and forbs, such as black mustard (Brassica nigra), aggressively invade semi-arid Mediterranean-climate ecosystems, such as southern California (bottom panel). Photo credit: Jennifer Funk.
It is difficult to identify a suite of general traits explaining invasiveness, because traits of invaders depend on characteristics of the invaded habitats (Pysek et al., 1995; Alpert et al., 2000; Daehler, 2003; Pysek and Richardson, 2007; Tecco et al., 2010). Specifically, the mechanisms allowing exotic species to invade low-resource ecosystems are likely to be very different from those allowing species to invade high-resource ecosystems. One way of thinking about invasion into low-resource environments is to focus on how plant species acquire and use resources. Competitive ability will be influenced by the ability of an individual to reduce the availability of a resource (e.g. resource acquisition, competitive effect, supply pre-emption), and by the ability to tolerate low resource availability (e.g. resource conservation, competitive response, concentration reduction; Tilman, 1982; Aarssen, 1983; Goldberg, 1990; Craine et al., 2005). While there is much debate about which competition mechanism predominates across environments, research conducted over the last three decades suggests that plants in high-resource ecosystems succeed through high rates of resource acquisition, while species adapted to low-resource ecosystems largely display traits associated with resource conservation (Chapin, 1980; Craine, 2009). However, the dichotomy between resource acquisition and conservation is not clear in some low-resource ecosystems, as species can effectively acquire (e.g. specialized roots, high root density) and conserve resources (e.g. high tissue longevity, nutrient resorption; e.g. Sack et al., 2003).
The trade-off between resource acquisition and conservation has been formalized in the leaf economics spectrum (LES), which shows that relationships exist among several key traits across a broad range of species and different climates (Reich et al., 1997; Wright et al., 2004). Plant species with low leaf mass per unit area (LMA), high rates of carbon assimilation, high leaf nitrogen (N) content, and short leaf lifespan occupy one end of the spectrum (fast return on investment), while plant species with high LMA, low rates of carbon assimilation, low leaf N content, and long leaf lifespans occupy the other (slow return on investment). With respect to invasion, several researchers have suggested that invasive species are positioned closer to the fast-return end of the LES (Leishman et al., 2007; Penuelas et al., 2010; Ordonez and Olff, 2012; but see Funk and Vitousek, 2007; Dawson et al., 2011). This ‘fast-return’ strategy seems at odds with an ability to tolerate low-resource conditions, as species adapted to low-resource systems often display slow growth, resource-use efficiency, high LMA, high tissue construction cost, and long-lived tissues (Chapin, 1980; Vitousek, 1982; Coley et al., 1985; Craine, 2009).
Do plant species invading low-resource ecosystems succeed through resource acquisition, resource conservation, or both? The theory of limiting similarity (MacArthur and Levins, 1967) predicts that invasive species will have different traits from native species and fill vacant niches (i.e. resource acquisition in the case of low-resource environments). In contrast, abiotic factors in low-resource environments are likely to constrain the range of possible traits (i.e. habitat filtering; Weiher et al., 1998), resulting in invasive species with similar resource conservation traits to native species. Ultimately, the specific strategy or traits of successful invaders will depend on the type and frequency of resource limitation, disturbance regimes, propagule pressure, and a number of other factors (Sher and Hyatt, 1999; Alpert et al., 2000; Theoharides and Dukes, 2007; Foxcroft et al., 2011). Resource levels in many historically low-resource ecosystems around the world are increasing due to changing disturbance regimes. In many cases, disturbance increases resource availability, with potentially large impacts on invasibility (Alpert et al., 2000; Fig. 2).
Figure 2.
Model for interactive effects of resource availability and disturbance on habitat invasibility. Disturbance often increases resource availability by removing competitors. Decreased frequency of disturbance (e.g. fire suppression) can prevent succession from being reset and favour strongly competitive invasive species. Adapted from Alpert et al. (2000).
In this review, I summarize our understanding of resource acquisition and use in native and invasive species occurring in low-resource ecosystems. I focus on soil nutrients, water, and light as limiting resources. Lastly, I discuss how we can use our understanding of resource acquisition and use in native and invasive species to restore native plant communities.
Soil nutrients
While plant growth can be limited by a number of macro- and micronutrients, the high mobility of N leads to N-limitation of plant growth in most ecosystems (Vitousek and Howarth, 1991). However, plant growth is often limited by phosphorus (P) availability in many tropical ecosystems with old, weathered soils. Additionally, plant species may be differentially limited by N and P in many systems. For example, plant growth in species with special adaptations for N (e.g. fixation) or P acquisition (e.g. cluster roots) may not be limited by the same nutrient as are neighbouring species (DiTommaso and Aarssen, 1989; Koerselman and Meuleman, 1996). Species also vary in their nutrient requirements. For example, grasses require lower amounts of P than forbs, possibly due to lower nucleic acid requirements associated with basal meristem leaf growth (Halsted and Lynch, 1996). Grasses with a C4 photosynthetic pathway can also operate at a lower N concentration due to higher photosynthetic nitrogen use efficiency (PNUE, i.e. carbon assimilation per unit of N; Sage and Pearcy, 1987).
The occurrence and degree of nutrient limitation in ecosystems is notoriously difficult to determine, because it depends on the process (e.g. plant growth) and time scale considered (Güsewell, 2004). Nutrient limitation is typically demonstrated when the addition of a nutrient increases plant growth (Vitousek and Howarth, 1991). As these types of experiments can be time consuming and labour intensive, element concentrations and ratios (e.g. N:P) of plant tissue have been used to demonstrate nutrient limitation in a variety of vegetation types. Across a diversity of ecosystems, N limitation is indicated by vegetation N:P ratios <10, P limitation is indicated by N:P ratios >20, and N and P can co-limit plant growth in between (Güsewell, 2004). Many researchers have also proposed specific N and P concentrations that characterize severely nutrient-limited soils. For example, N concentrations <13 mg g−1 and P concentrations <1 mg g−1 have been demonstrated to be limiting to plant growth (Wassen et al., 1995; Güsewell and Koerselman, 2002).
Many species can invade low-nutrient soils, and the best-studied examples are in ecosystems with young volcanic soils (e.g. Vitousek and Walker, 1989; Mack et al., 2001; Funk and Vitousek, 2007; Schoenfelder et al., 2010), grasslands (e.g. Drenovsky et al., 2012b; Han et al., 2012), and arid shrublands (James and Richards, 2006). However, very few invaders can invade severely nutrient-deficient soils. For example, there are very few invaders (e.g. Pinus) in Australia where soil P levels are below 200 p.p.m. Plants require a high activity of RNA to sustain rapid rates of protein synthesis (growth-rate hypothesis; Elser et al., 1996). Thus, invaders in P-limited systems should not be stereotypical fast-growing weeds. Historically, there have been fewer invasive species in saline- or serpentine-derived soils, which are characterized by low concentrations of macronutrients or high concentrations of salt or heavy metals (Lonsdale, 1999; Hoopes and Hall, 2002; Williamson and Harrison, 2002).
Efficiency of nutrient use
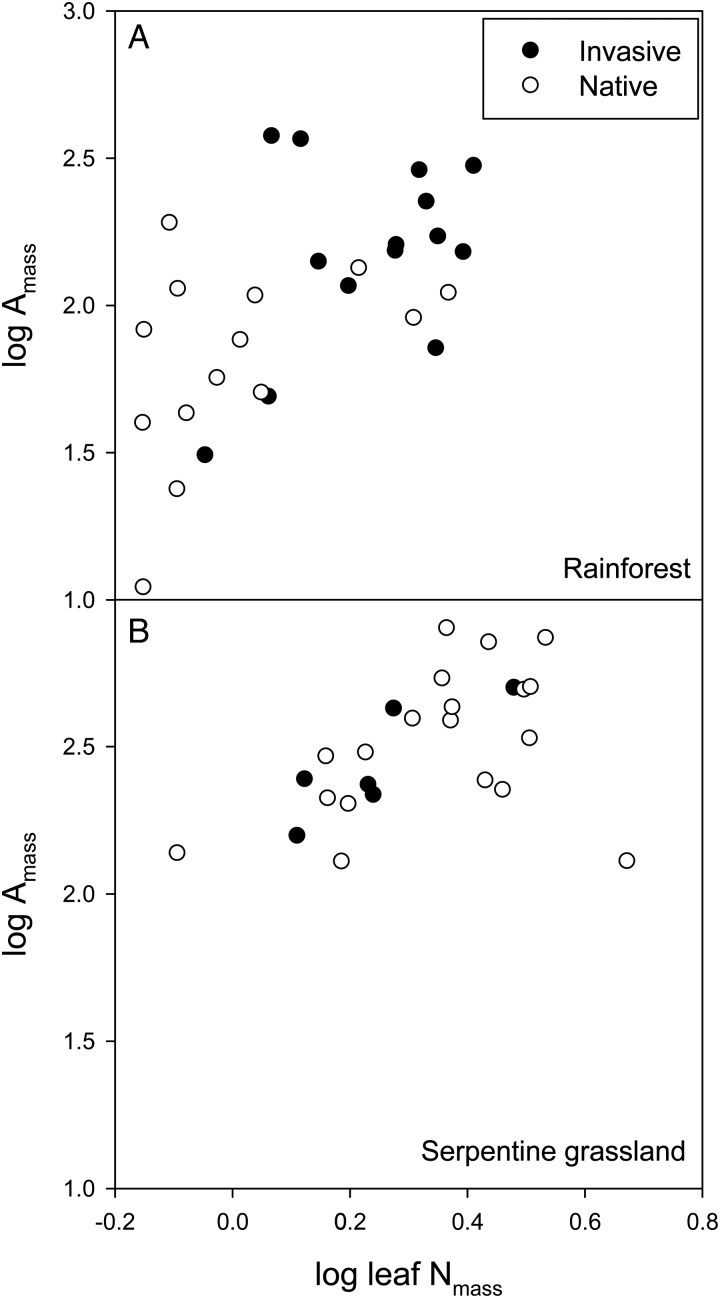
Across species, there is a positive correlation between leaf N and photosynthetic rate (Fig. 3; Field and Mooney, 1986). Researchers working across low- and high-nutrient environments have found that native species occupy the lower left corner of this relationship (slow return), while invasive species occupy the upper right (fast return; e.g. Leishman et al., 2007; Penuelas et al., 2010; Ordonez and Olff, 2012). However, this pattern has not been demonstrated in all communities examined. For example, this generalization holds for species occurring in N-limited Hawaiian rainforest (Fig. 3A), but the pattern does not hold for annual grasses and forbs occurring in serpentine soils in California (Fig. 3B). If the slope of the relationship between carbon assimilation and leaf N is similar for both native and invasive species, then physiological processes are similar; more leaf N leads to a corresponding increase in photosynthesis. However, if the two groups display different slopes, this means that PNUE is higher for one group, which implies that native and invasive species have different biochemical or morphological traits.
Figure 3.
The relationship between mass-based photosynthetic rate (Amass) and leaf N content on a mass basis. Annual and perennial herbaceous and woody invasive species occupy the ‘high-return’ end of the spectrum in a rainforest in Hawaii (r = 0.59, P = 0.001; A); however, invasive grasses and forbs are similar to natives in a serpentine grassland in northern California (r = 0.47, P = 0.02; B). Data are from Funk and Vitousek (2007) and J. L. Funk (unpublished data).
The majority of studies examining nutrient-use efficiency in invasive species relative to co-occurring native species have found higher values in invasive species (Table 1). For example, Godoy et al. (2011) found higher PNUE in 20 Mediterranean invaders relative to natives in both low- and high-N conditions. Invasive lovegrass (Eragrostis curvula) had a higher PNUE relative to native grasses in low-nutrient soils in Australia (Firn et al., 2012). Likewise, when grown in low-P conditions, invasive members of the genus Pinus had higher PNUE than non-invasive members (Matzek, 2011). However, a handful of studies have found no differences in PNUE between native and invasive species (Table 1). For example, Schoenfelder et al. (2010) found that an invasive forb (Hypochaeris radicata) growing on nutrient-poor volcanic soils did not have higher PNUE relative to a confamilial native species. Instead, the researchers proposed that H. radicata invades this low-N system through superior N acquisition and by diluting tissue N in order to build more photosynthetic structures.
Table 1.
The number of studies that have observed trait differences between invasive and native or non-invasive exotic species in environments with (A) low soil nutrient availability, (B) low water availability and (C) low irradiance.
| Invasive > Native | No difference | Native > Invasive | References | |
|---|---|---|---|---|
| A. Low nutrient availability | ||||
| Resource conservation | ||||
| High NUE | 12 | 7 | 0 | 1-3,7-9,11,13,16,19, 20,27,28,31-33,42,44,49 |
| High LMA | 0 | 3 | 9 | 1-3,8,11,13,15,20,25,31,42,46 |
| High LLS | 0 | 1 | 1 | 15,35 |
| High resorption | 0 | 3 | 0 | 8,16,35 |
| Resource acquisition | ||||
| High R:S | 4 | 5 | 2 | 2,7,8,13,25,29,30,32,35,44,46 |
| High uptake per mass | 0 | 3 | 0 | 29,32,34 |
| Mycorrhizae | not enough data, but see 43 | |||
| Underutilized nutrient forms | not enough data | |||
| Specialized roots* | not enough data | |||
| B. Low water availability | ||||
| Resource conservation | ||||
| High WUE | 1 | 5 | 2 | 4,5,8,16,17,33,48,49 |
| High LMA | 0 | 3 | 5 | 2,5,8,15,17,45,48,49 |
| High LLS | not enough data, but see 15 | |||
| Water storage | not enough data | |||
| Specialized leaf morphology** | not enough data | |||
| Resource acquisition | ||||
| High R:S | 4 | 3 | 1 | 2,6,8,17,21,22,45,48 |
| Early phenology | 3 | 0 | 0 | 22,26,51 |
| Mycorrhizae | not enough data, but see 43 | |||
| Deep roots | not enough data | |||
| High SRL | not enough data | |||
| Fast tissue turnover | not enough data | |||
| C. Low irradiance | ||||
| Resource conservation | ||||
| High quantum yield | 3 | 7 | 1 | 13,14,16,18-20,24,38,39,41,47 |
| High LMA | 1 | 5 | 11 | 9,13-15,18-20,24,27,36, 38,41,45-47,50,52 |
| High LLS | 3 | 0 | 2 | 9,12,15,23,27 |
| High A/Rd | 4 | 3 | 1 | 9,20,24,27,33,36,38,41 |
| Resource acquisition | ||||
| Low R:S | 4 | 4 | 0 | 13,19,38,40,41,45,46,50 |
| High chlorophyll content | 2 | 2 | 1 | 9,14,18,39,40 |
| Low CC/High PEUE | 7 | 1 | 0 | 1,3,16,24,33,36,37,47 |
* Examples include nitrogen fixation and cluster roots; ** examples include low stomatal density, thick cuticle, trichomes. Abbreviations are: A/Rd, ratio of photosynthetic rate to dark respiration rate; CC, leaf construction cost; LMA, leaf mass per unit area; LLS, leaf lifespan; NUE, nutrient use efficiency; PEUE, photosynthetic energy use efficiency; R:S, root to shoot biomass ratio; SRL, specific root length; WUE, water use efficiency.
1Baruch and Goldstein 1999, 2Baruch and Jackson 2005, 3Baruch et al. 2000, 4Brock and Galen 2005, 5Cordell et al. 2002, 6DeFalco et al. 2003, 7Drenovsky et al. 2008, 8Drenovsky et al. 2012b, 9Durand and Goldstein 2001, 10Feng et al. 2007, 11Firn et al. 2012, 12Fridley 2012, 13Funk 2008, 14Funk and McDaniel 2010, 15Funk and Throop 2010, 16Funk and Vitousek 2007, 17Funk and Zachary 2010, 18Funk et al. 2013, 19Gleason and Ares 2004, 20Godoy et al. 2011, 21Grotkopp and Rejmanek 2007, 22Han et al. 2012, 23Harrington et al. 1989, 24Heberling and Fridley 2013, 25James and Drenovsky 2007, 26Kimball et al. 2011, 27Kloeppel and Abrams 1995, 28Laungani and Knops 2009, 29Leffler et al. 2011, 30Leishman and Thomson 2005, 31Leishman et al. 2010, 32Matzek 2011, 33McDowell 2002, 34Meisner et al. 2011, 35Morris et al. 2011, 36Nagel and Griffin 2004, 37Osunkoya et al. 2010a, 38Osunkoya et al. 2010b, 39Pammenter et al. 1986, 40Paquette et al. 2012, 41Pattison et al. 1998, 42Pavlik 1983, 43Pringle et al. 2009, 44Schoenfelder et al. 2010, 45Schumacher et al. 2008, 46Schumacher et al. 2009, 47Shen et al. 2011, 48Steers et al. 2011, 49Stratton and Goldstein 2001, 50van Kleunen et al. 2011, 51Wolkovich and Cleland 2011, 52Yamashita et al. 2000.
Few studies have examined the mechanisms of higher nutrient-use efficiency in invasive species. Plant species vary greatly in how they allocate N among photosynthetic and non-photosynthetic compounds in the leaf, and it is possible that invasive species with high PNUE allocate more N to photosynthetic compounds. Plants may allocate 5–32% of leaf N to ribulose-1,5-bisphosphate carboxylase oxygenase (photosynthetic) and 2–30% to cell walls (non-photosynthetic), with higher amounts of cell-wall protein occurring in longer-lived leaves (Evans, 1989; Warren and Adams, 2001; Onoda et al., 2004; Takashima et al., 2004; Harrison et al., 2009). Feng et al. (2009) found that, compared with native populations, invasive populations of Ageratina adenophora allocated more N to soluble protein at the expense of cell-wall protein, which increased PNUE. A study of five native and five invasive woody species from Hawaii also found that invasive species allocate less N to cell-wall protein than native species (Funk et al., 2013). While soluble protein content and PNUE did not differ between native and invasive species groups, invasive species allocated more N to amino acids, which may be used for rapid growth (Funk et al., 2013).
Leaf longevity and nutrient recycling may influence nutrient-use efficiency on longer time scales. Invasive species in low-nutrient systems tend to have lower LMA, but this does not seem to translate into shorter leaf lifespan (Table 1). While there are very few data on nutrient recycling, nutrient resorption appears to be similar among native and invasive species (Table 1). Similar levels of N or P resorption have been found between native and invasive grass and forb species from the Intermountain West of the USA (Drenovsky et al., 2012b), in invasive species of Acacia from Australia relative to co-occurring woody native species (reviewed by Morris et al., 2011), and in a structurally and taxonomically diverse group of native and invasive species occurring in low-nutrient soils in Hawaii (Funk and Vitousek, 2007).
Nutrient acquisition
Species occurring in N- and P-limited soils may possess morphological and physiological traits that facilitate N and P acquisition. Plants can maximize N uptake by increasing total root length, increasing specific root length, increasing root longevity, stimulating microbial decomposers via rhizodeposition, or allocating carbon to mycorrhizae. Few studies have surveyed root traits in native and invasive species and the existing data do not show clear differences between groups in root to shoot biomass ratio (R:S) or rates of nutrient uptake (Table 1). A high total root length appears to be more important in acquiring N than P (Olde Venterink and Güsewell, 2010). Instead, many native plants in P-limited soils have cluster roots and/or high phosphatase production in roots (Richardson et al., 2009; Olde Venterink, 2011). It is unclear whether invasive species in P-limited systems share these strategies, although several Lupinus species have cluster roots and invade low-P soils in Australia (Lambers et al., 2013).
Native species in P- and N-limited soils frequently form associations with mycorrhizal fungi, which help plants to sequester P and N and may also protect them from soil pathogens and drought stress (Auge, 2001; Willis et al., 2013). A review of the limited data on mycorrhizal dynamics in native and invasive plant species suggested that many invasive plants do not associate with mycorrhizae, are facultatively mycorrhizal, or can partner with various types (arbuscular mycorrhizae vs. ectomycorrhizae) and species of fungi (Pringle et al., 2009). Patterns appear to vary by region. An analysis of the California flora concluded that fewer invasive species than native species form mycorrhizal associations, while the pattern was reversed in Great Britain (Pringle et al., 2009). There are examples of obligate mycorrhizal invasive species that use novel species of mycorrhizal fungi in the introduced habitat to outcompete native species (e.g. Centaurea maculosa; Marler et al., 1999). Conversely, there are examples where novel mycorrhizal symbionts inhibit the growth of invasive species (e.g. Plantago lanceolata; Bever, 2002). There are also examples where an invasive species negatively affects neighbouring native species by disrupting mycorrhizal associations (e.g. Vogelsang and Bever, 2009; Meinhardt and Gehring, 2012). Understanding how native and invasive species associate with mycorrhizae is critical in nutrient-poor soils, and more data are needed to understand taxonomic and geographical patterns among species.
Many successful invaders in N-limited systems have symbiotic associations with N-fixing bacteria. For example, Myrica faya, Leucaena leucocephala, and other nitrogen-fixing species have invaded young, N-limited volcanic soils in Hawaii, filling an empty niche, because no native nitrogen-fixing species occur during primary succession on these soils (Fig. 1; Vitousek and Walker, 1989). Another example is Australian Acacia spp. that invade low-nutrient coastal dunes in Portugal (Rodríguez-Echeverría et al., 2009) and low-nutrient fynbos in South Africa (e.g. Witkowski, 1991; Yelenik et al., 2004). While nitrogen fixation may facilitate the invasion of these species into low-N ecosystems, nitrogen-fixing species may possess other traits that increase access to below-ground resources. For example, invasive Australian acacias allocate more biomass below ground (higher root mass ratio and root depth) compared with co-occurring native species, allowing them greater access to water and nutrients (Witkowski, 1991; Morris et al., 2011). There is also evidence that some invasive species may nodulate more readily and fix greater amounts of N than co-occurring N-fixing species (Rodríguez-Echeverría et al., 2009), although it is not known whether greater nodulation arises through a plant's ability to form associations with multiple bacterial partners (e.g. greater symbiotic promiscuity) and nodulate with low bacterial population sizes, or through differences in the bacteria themselves. Bacteria genera and strains vary in growth rate and the efficiency of N-fixation (e.g. Simms et al., 2006). For example, invasive Australian acacias mainly associate with slow-growing Bradyrhizobium, but have been found occasionally to associate with fast-growing Rhizobium (Rodríguez-Echeverría et al., 2011). Very little is known about how native and invasive species associate with different strains of N-fixing bacteria, and this is an interesting area for future research.
Lastly, invasive species may access forms of nutrients that neighbouring species are not using, including amino acids (Lipson and Näsholm, 2001). Very few studies have examined how native and invasive species compete for HN4+, NO3−, and organic N. Aanderud and Bledsoe (2009) concluded that invasive grass species used HN4+, the dominant form of N, forcing subordinate native species to use NO3− and amino acids. Leffler et al. (2011) found that an invasive annual grass (Bromus tectorum) had a high mass-specific absorption rate and a high rate of whole-plant N uptake, implying that this species could access NO3− that other species (including one exotic invasive and three native species) could not use. The potential for invasive species to use different forms of N is another exciting area for research, although organic N uptake may be restricted to cold, wet environments with low rates of N mineralization (Craine, 2009).
Water availability
Arid environments (e.g. deserts, tundra, xeric shrubland) are characterized by < 250 mm of annual precipitation, while semi-arid environments (e.g. grassland, savanna, Mediterranean shrubland, seasonally dry tropical forests) receive 250–500 mm of annual precipitation (Holdridge, 1967). The degree to which water and N co-limit plant growth in arid systems has been investigated by several researchers (DeFalco et al., 2003; James and Richards, 2005, 2006; Barker et al., 2006), and results suggest that water availability most strongly limits plant growth in normal precipitation years, while N availability limits plant growth in wet years. Plants cope with water limitation by integrating biochemical, physiological, and morphological processes across multiple levels of organization (i.e. cell, organ, plant). As I discuss below, there is evidence that some invasive species possess drought-tolerant traits, while others do not.
Efficiency of water use
Many leaf-level traits, including thick cuticles and trichomes, function to reduce the amount of water lost from leaves (Sandquist and Ehleringer, 1998). Additionally, plants in arid environments tend to have high LMA. Across taxonomically diverse plant species, high LMA leads to lower leaf-level carbon assimilation rates, representing one of the key trade-offs of the LES (Reich et al., 1997; Wright et al., 2004). However, high LMA in arid systems has been linked to larger amounts of mesophyll tissue (which contains the photosynthetic machinery) rather than higher amounts of structural tissue (Wright and Westoby, 2002). Thus, plants can increase their water-use efficiency (WUE) by investing more resources in photosynthetic enzymes and pigments to draw down intercellular CO2 concentrations and reduce transpiration loss (Westoby et al., 2002). Given that photosynthetic enzymes require N, plants adapted to arid regions generally have high leaf N content, presumably to increase WUE (Wright and Westoby, 2002). Thus, LMA and photosynthetic rate are not necessarily negatively correlated in arid and semi-arid systems (e.g. Steers et al., 2011).
The existing data suggest that LMA is lower in invasive species than in native species occurring in arid environments (Table 1). For example, in the Mojave desert, two annual exotic grasses (Bromus madritensis and Schismus barbatus) and one annual exotic forb (Erodium cicutarium) produce many thin (low LMA) leaves (Steers et al., 2011). Thin leaves generally have low quantities of structural carbohydrates, which results in low energetic or construction cost of the leaf (Griffin, 1994). Low construction cost is often associated with higher plant growth rates (Nagel and Griffin, 2004), because resources are available to produce more photosynthetic tissue, which maximizes plant-level carbon assimilation. However, while cheap structures may provide an initial growth advantage, more leaf area leads to higher plant-level transpiration rates, and this may render these exotic species more prone to water stress in low-precipitation years. Nevertheless, many annual species (particularly invasive annuals) may employ this strategy, where the production of cheap structures facilitates a rapid response to unpredictable precipitation events (Angert et al., 2007; Huxman et al., 2008). This mechanism may explain why exotic species can spread in wet years and remain in the seed bank during dry years (e.g. Pennisetum setaceum in Hawaii; Cordell et al., 2002).
Cavaleri and Sack (2010) conducted a meta-analysis of 40 studies examining water use in native and invasive plants worldwide and found similar values for leaf-level WUE expressed on an instantaneous basis (photosynthetic rate/transpiration rate) and integrated over leaf lifespan (δ13C). This same pattern emerges when the analysis is restricted to arid and semi-arid systems (Table 1). Cavaleri and Sack (2010) also found that invasive species had lower pre-dawn water potential (ψpd) than native species, particularly in regions with low mean annual precipitation. They suggest that invasive species may favour drier microsites within habitats, deplete soil moisture levels more than natives, or have higher nocturnal transpiration (e.g. Dawson, 1993).
Water acquisition and drought tolerance
Root depth, root to shoot biomass ratio (R:S), and mycorrhizal associations strongly influence water uptake. The influence of rooting depth on plant performance depends on the magnitude and frequency of precipitation; more frequent large precipitation events increase the productivity of deep-rooted shrubs, while more frequent small events increase the productivity of shallow-rooted species (Weltzin and McPherson, 1997; Gebauer and Ehleringer, 2000; Loik, 2007). I am not aware of any study that has quantified root depth for native and invasive species in an arid system. Several studies have demonstrated enhanced water acquisition in species invading arid and semi-arid systems through higher R:S (Table 1). For example, a study of 12 phylogenetically controlled pairs of native and invasive woody species in California found that invasive species allocated more biomass to roots, which may help them tolerate summer drought (Grotkopp and Rejmanek, 2007). Additionally, in a study of annual species from the Mojave Desert, DeFalco et al. (2003) found that Bromus madritensis uses more water, takes up water at a faster rate, and draws down soil water content to a lower level than neighbouring native forb and grass species due to greater biomass allocation below ground and greater root surface area. These same traits also confer an advantage in N acquisition; B. madritensis had a higher N content and N uptake rates in some treatment conditions (DeFalco et al., 2003). Given that species adapted to arid and semi-arid environments must maintain a high N status to achieve high WUE (Wright and Westoby, 2002), the ability to take up N during precipitation events may strongly impact plant N status and, consequently, plant fitness.
Understanding species responses to short-term changes in water availability is important because global climate models project intensified intra-annual variation in precipitation in many arid environments, resulting in larger precipitation events with longer intervening dry periods (Diffenbaugh et al., 2005; Knapp et al., 2008). Han et al. (2012) found a higher R:S in invasive species relative to native species in variable irrigation conditions, suggesting that invasive species may demonstrate enhanced physiological plasticity to changing environments. In contrast, a study of native and invasive shrubs in southern California found no clear differences in how water-stressed individuals of these groups responded to precipitation events (Funk and Zachary, 2010). One native (Salvia mellifera) and one invasive species (Ricinus communis) displayed rapid photosynthetic recovery following drought, but this was attributable to enhanced leaf-level function (WUE) rather than new root growth.
Much of the work on water acquisition in native and invasive species has focused on root traits rather than differences in water conductance through the xylem. Across taxonomic groups, there appears to be a trade-off between water conductance and vulnerability to cavitation (Hacke et al., 2001; Preston and Ackerly, 2003). While reinforcement of water-conducting vessels and tracheids prevents xylem cavitation at low water potential, transport efficiency is reduced by increased wall thickness in reinforced cells. Woody species adapted to arid and semi-arid environments may reduce water conduction in order to prevent cavitation, and it is unknown whether woody species invading these environments are similar to native species in this way. Caplan and Yeakley (2010) found that an invasive blackberry (Rubus armeniacus) maintained higher stomatal conductance and lower hydraulic resistance throughout the year relative to two native congeners. Greater rates of water transport were probably driven by access to deeper water sources and shoot water storage, although species differences in stomata anatomy and xylem embolism were not examined.
Phenology
Plants growing in arid and semi-arid systems display a broad range of phenological patterns that limit the severity of water stress (Williams et al., 1997; Sandquist and Ehleringer, 1998). Annual species are common in arid systems, because this strategy enables them to complete a short life-cycle during the favourable wet season. Many perennial species are drought deciduous, which allows them to be dormant during the hot, dry summer months (Nilsen and Muller, 1981; Comstock and Ehleringer, 1986). However, drought deciduousness is more economically feasible in nutrient-rich environments, where the costly loss of nutrients in shed leaves does not adversely affect plant fitness (reviewed by Morris et al., 2011).
Plant species may cope with fluctuations in the timing and magnitude of water availability by altering their phenology. An analysis of several US plant databases found that exotic species generally develop leaves earlier in the year than natives, which may allow them to pre-empt resources by being active earlier (Table 1; Wolkovich and Cleland, 2011). For example, African lovegrass (Eragrostis curvula), which occupies nutrient- and water-depleted sites in Australia, germinates and grows faster than functionally similar native grass species (Han et al., 2012), potentially enhancing its competitive ability. Phenological patterns also correlate with plant function. In the Sonoran Desert, Kimball et al. (2011) found that annual species with high WUE, including the invasive forb Erodium cicutarium, germinate earlier in the growing season and reproduce for a longer time period. Species with low WUE germinate later, following several rainfall events, but the plants experience higher risk of mortality associated with warmer temperatures later in the growing season.
With respect to phenology, evergreen species may be more constrained in their ability to respond morphologically to precipitation events than deciduous perennials or annual species (Grime et al., 1986). However, retaining long-lived leaves may provide evergreen species with an advantage over those that must produce new leaves following a precipitation event. For example, Eragrostis lehmanniana, an invasive grass in the southwest USA, can up-regulate photosynthesis quickly following summer precipitation events, while a co-occurring native bunchgrass, Heteropogon contortus, lags behind as it grows new leaves (Ignace et al., 2007). In contrast, species with short-lived or inexpensive tissues can track the limiting resource over time and invest in tissues that are more appropriate for the new environment. While this has primarily been demonstrated for variation in light availability (Ackerly and Bazzaz, 1995), there is some evidence of a stress-tracking ability in response to drought as well. Two exotic species (Ricinus communis and Nicotiana glauca) in a semi-arid coastal sage scrub community in southern California responded to drought-induced high-light stress with new growth and large decreases in the function of existing leaves (photosynthetic rate, light harvesting), suggesting that these species respond to stress by turnover of existing tissue rather than acclimatization of existing tissue (Funk and Zachary, 2010).
Light availability
Plant species occurring in low-light environments demonstrate a trade-off between shade tolerance and growth rate (Bazzaz, 1979; Valladares and Niinemets, 2008). Shade-intolerant species grow rapidly in order to reach higher light levels at the top of the canopy and display high photosynthetic rates, early reproduction, and short lifespan. Some shade-intolerant exotic species can take advantage of disturbances that create high-light gaps. The exotic tree species Ailanthus altissima is shade intolerant (Martin et al., 2010) but succeeds in low-light forests because it requires only a small gap to initiate rapid growth to reach the canopy (Knapp and Canham, 2000). Higher leaf area ratios and low R:S, characteristics of many species invading forests (Table 1; Pattison et al., 1998; Standish et al., 2001; Reinhart et al., 2006; Schumacher et al., 2009; Paquette et al., 2012), suggest that these species are able to take advantage of high-light conditions and grow rapidly in response to natural or human-induced canopy gaps. For example, Leicht and Silander (2006) found that an invasive liana (Celastrus orbiculatus) grew taller than a congeneric native (Celastrus scandens), which allows it to forage more efficiently for canopy gaps. Once established, some shade-intolerant species change the structure of the forest, promoting high-light conditions that favour exotic species. For example, understory species in forests can create an environment suitable for them by suppressing recruitment of native canopy species (Standish et al., 2001; Funk and McDaniel, 2010). However, most species invading forests are shade tolerant (Martin et al., 2009), and therefore shade tolerance is the focus of the following section.
Shade tolerance
Researchers have characterized the traits associated with shade tolerance, although the focus has been on leaf traits as opposed to shoot and root traits (Mooney, 1972; Bjorkman, 1981; Chazdon, 1988; Givnish, 1988). Species adapted to low-light environments possess a suite of physiological traits to maximize light capture, such as high quantum yield (carbon assimilated per photon absorbed), high chlorophyll content, low respiratory rates, low light compensation points, and allocation of nitrogen to proteins associated with light-harvesting functions at the expense of carbon-assimilation functions (Bjorkman, 1981; Evans and Poorter, 2001; Givnish et al., 2004; Craine, 2009; but see Walters and Reich, 2000; Janse-ten Klooster et al., 2007; Wyka et al., 2007). Shade-tolerant species typically possess leaves with large amounts of structural tissue, which helps protect them against physical stresses and herbivory (Lusk and Warton, 2007). This increases LMA, leaf longevity and the lifetime carbon assimilation of the leaf (Reich et al., 1997; Westoby et al., 2002), which is advantageous in low-light habitats on long time scales.
There have been few publications on understory invaders despite their presence, and they may have been overlooked historically because they do not spread quickly or possess traits more commonly ascribed to fast-growing exotic species (Martin et al., 2009). While some studies in low-light environments have found higher photosynthetic rate and quantum yield in invasive species relative to native species, the majority of studies found no significant differences in these traits (Table 1). Some invasive species may achieve high rates of carbon assimilation at low irradiance by allocating more resources to the light-harvesting components of photosynthesis, such as chlorophyll (Table 1; Durand and Goldstein, 2001). However, increasing photosynthetic capacity can be costly. High metabolic activity leads to higher respiratory costs resulting from higher rates of protein turnover and maintenance of solute gradients required for phloem loading (Lambers et al., 2008); thus, leaves with high photosynthetic rates typically have higher light compensation points associated with higher respiratory costs (e.g. Givnish et al., 2004). However, several studies of photosynthetic function in native and invasive species found that invasive species in low-light conditions achieved high photosynthetic rates at a low respiratory cost (Table 1). As the mechanism for this pattern was not examined in these studies, there is a need for additional research in this area.
Invasive species in low-light environments have lower LMA and leaf construction costs relative to native species (Table 1). When coupled with higher photosynthetic rates, lower construction costs can increase photosynthetic energy-use efficiency (PEUE; carbon assimilated per unit of energy invested in leaf construction). Building cheaper leaves allows a plant to produce more photosynthetic structures for the same energy cost, which maximizes whole-plant carbon gain. However, there is a trade-off between low construction cost and leaf lifespan, in that cheaper leaves often have lower leaf lifespan. Long leaf lifespan is a characteristic of shade-tolerant species, because this allows a plant to assimilate carbon over a longer time period for the same initial investment in leaf construction. Several surveys of native and exotic species in forests have found earlier bud break and longer leaf lifespans in exotic species (Harrington et al., 1989; Kloeppel and Abrams, 1995; Fridley, 2012; but see Durand and Goldstein, 2001; Funk and Throop, 2010), which seems at odds with the general pattern of lower leaf construction cost in invasive species. Nevertheless, the effect on PEUE is the same, in that longer leaf lifespan will increase PEUE as more carbon is assimilated per resource invested over time.
Measures of resource-use efficiency integrated over a leaf's lifespan suggest very different scenarios for the success of invasive species compared with the instantaneous measures (PNUE, WUE, and quantum yield) that I have presented thus far. Instantaneous measures of resource-use efficiency may reflect performance on short time scales, while measures integrated over leaf lifespan may more accurately reflect performance on longer time scales (e.g. multiple seasons; Funk and Vitousek, 2007). The appropriate measure of resource-use efficiency will be context dependent, and this may partly explain discrepancies across studies, such as the finding that some invasive species in low-light environments display lower leaf construction costs, while others maximize leaf lifespan.
Shade tolerance and rapid growth: the best of both worlds
Several studies have found that invasive species do not adhere to the growth rate–shade tolerance trade-off (see Table 2 of Catford et al., 2012). Norway maple (Acer platanoides), one of the most common forest invaders in the northeastern USA and in riparian and montane forests in the northern Rocky Mountains in the USA, is a well-studied species that displays both high survivorship in low-light conditions (2% full sun) and high growth rates in high-light conditions (80% full sun; Martin et al., 2010). The departure of A. platanoides from the growth rate–shade tolerance trade-off probably results from a combination of plant- and leaf-level traits. Acer platanoides has a lower R:S than co-occurring native species in high-light conditions (Paquette et al., 2012) and in deeply shaded forests (Reinhart et al., 2006). Allocation of biomass to photosynthesizing tissues can result in higher plant-level assimilation and growth, which is advantageous when plants are competing primarily for light. At the leaf level, A. platanoides has high rates of photosynthesis and high LMA compared with the native congener Acer saccharum, but the denser leaves result from more chloroplasts in the palisade and mesophyll cells rather than increased structural tissue (reviewed by Kloeppel and Abrams, 1995). Despite the increased allocation to photosynthetic tissue, the two Acer species did not differ in respiratory costs (Kloeppel and Abrams, 1995).
Other invasive species display both shade-tolerance traits and rapid growth. Durand and Goldstein (2001) found that an invasive tree fern (Sphaeropteris cooperi) had higher chlorophyll content (shade tolerance) and larger annual height growth compared with native tree ferns in Hawaii. Pammenter et al. (1986) compared an invasive and native Agrostis species on a light-limited sub-Antarctic island and found that the invasive species had higher light-use efficiency over a wide temperature range. Additionally, the invasive Agrostis had thinner leaves and allocated relatively more carbon to photosynthetic tissue, presumably resulting in higher plant-level assimilation and growth. As discussed above, this strategy is shared by several exotic annual species in arid environments. Lastly, when compared with native species of varying successional status, Yamashita et al. (2000) found that an exotic tree species (Bischofia javanica) responded faster physiologically (increased photosynthesis of existing leaves) and morphologically (new leaf formation) to increased light levels simulating a canopy gap. Furthermore, following a transition from shade to sun, B. javanica decreased leaf chlorophyll content and increased PNUE, suggesting that this species reallocates N from light-harvesting to carboxylation components of photosynthesis.
Implications for restoration and conservation
Given that many low-resource environments have high species and functional diversity, it is essential to understand invasion dynamics in these systems in order to conserve and restore native biodiversity. While invasive species outperform native species in many communities, native species generally have an advantage or hold their own in low-resource environments (Daehler, 2003), which means that opportunities exist for control and restoration. Restoration techniques are diverse and range from methods that target specific invaders to those that manipulate community-level processes, such as disturbance, seed dispersal, and resource availability.
When native and invasive species differ in the timing or magnitude of resource acquisition or use, reinstating natural disturbance regimes or lowering resource availability may facilitate the restoration of native plant species (Fig. 4). For example, many studies have shown that adding carbon to soil can lower plant-available N and, consequently, reduce the abundance of invasive species (e.g. Blumenthal et al., 2003; Corbin and D'Antonio, 2004; Cherwin et al., 2009; Steers et al., 2011; but see James et al., 2011). Additionally, eliminating disturbance that creates canopy gaps in forests can exclude shade-intolerant invasive species (Funk and McDaniel, 2010; Emer and Fonseca, 2011). Community-level manipulations may be particularly effective if native and invasive species differ in the timing of resource use. Marushia et al. (2010) found that early season application of herbicide reduced exotic cover without affecting cover of native desert annuals. This method was effective because, as discussed above, many exotic species in desert systems display a rapid phenology and germinate before native species (Wolkovich and Cleland, 2011).
Figure 4.
Traits associated with resource acquisition and use may suggest restoration strategies for invaded plant communities. Restoration approaches are separated into two categories, namely those that directly target invasive species and those that seek to alter a community-level process. 1When native and invasive species differ in the timing of germination or reproduction, practitioners can apply herbicide, mow, or graze during periods when invasive species are active or flowering. 2Original disturbance regimes should be restored when altered disturbance facilitates invasion, such as where canopy gaps increase light availability or fire reduces competition. 3Resource availability should be reduced when invasive species have higher resource requirements than native species. Examples include lowering soil nutrient availability by adding carbon to the soil, establishing canopy trees to reduce light, and tarping to reduce vertical or horizontal water flow. 4If native and invasive species are using resources in similar ways, but populations of native species are dispersal limited, practitioners can introduce native plants or seeds to overcome this barrier.
Community-level restoration approaches will be most effective when native and invasive species differ in the timing and magnitude of resource use (Emer and Fonseca, 2011; Steers et al., 2011). As highlighted above, many invasive species have similar or higher resource-use efficiency compared with neighbouring native species. In these cases, lowering resource availability will not suppress the growth of invasive species. When confronted with resource-use-efficient invasive species, the best restoration options may be manual control of invasive species, planting or reseeding functionally similar native species, controlled burns, or herbicide treatment (Funk et al., 2008; Fig. 4). A better understanding of physiological and morphological traits can help in the identification of possible restoration strategies in a given community (Funk et al., 2008; Drenovsky et al., 2012a).
Conclusions
Invasion is a community-level process, and the traits of invasive species depend on many factors, including the traits of native species, as well as propagule pressure, and the type and frequency of disturbance and resource limitation. While there is significant variation in results from studies of invasive species conducted in low-resource systems, it is possible to make a few generalizations. With respect to resource conservation, invasive species appear to use nutrients more efficiently than natives in low-nutrient soils. However, invasive and native species are similarly efficient at using water and light in arid and light-limited systems, respectively. With respect to resource acquisition, invasive species tend to have higher R:S in arid systems and lower R:S in light-limited systems, relative to co-occurring native species. Additionally, invasive species have lower leaf construction costs and higher PEUE in light-limited systems. Earlier phenology in arid systems may also help invasive species to outcompete native species for resources.
There are several gaps in our understanding of how species invade low-resource systems. In low-nutrient systems, we need more information on how native and invasive species associate with different strains/species of N-fixing bacteria and mycorrhizae. Additionally, we have a limited understanding of how native and invasive species differ in the timing and form of nutrient use, as well as the capacity for recycling nutrients. Much of the research in arid and semi-arid ecosystems has focused on morphological traits (such as biomass allocation and LMA) and a few physiological traits (e.g. WUE), and we know very little about water relations in native and invasive species in these environments. The few studies that have examined physiology in species invading forest systems have focused on a few species (e.g. A. platanoides, A. altissima). More studies are needed to determine whether invasive species adhere to the shade tolerance–growth rate trade-off and to determine the physiological mechanisms underlying any deviations. For example, studies at the cellular level are needed to understand how some invasive species can increase carbon gain without a corresponding increase in respiratory costs.
Propagule pressure, trait plasticity, and the type of species comparison confound our understanding of invasion in low-resource systems. Firstly, invasion in low-resource ecosystems may be influenced by seed and vegetative dispersal. For example, in low-N fields, annual grasses can dominate, even though they are weak competitors relative to native perennial grasses, because native species are dispersal limited (Seabloom et al., 2003). Secondly, plant species may benefit from physiological or morphological plasticity in low-resource environments, where resources can vary temporally or spatially (e.g. Poorter and Lambers, 1986; Davis et al., 2000; Valladares et al., 2000; Balaguer et al., 2001; Valladares et al., 2002; Funk, 2008). Several studies suggest that invasive species can be more plastic than native species in specific environmental conditions (for review see Davidson et al., 2011; Palacio-López and Gianoli, 2011). Thus, caution should be used when interpreting trait data across environmental gradients, because species may differ in the plasticity of traits and, importantly, trait plasticity may not necessarily result in increased fitness or translate into increased abundance (Funk, 2008; Osunkoya et al., 2010b; Godoy et al., 2011; van Kleunen et al., 2011; Dawson et al., 2012; Firn et al., 2012; Matzek, 2012). Lastly, most of the studies that examined invasive species in low-resource environments have compared invasive species with co-occurring native species (but see Leishman and Thomson, 2005; Feng et al., 2007; Matzek, 2011; Shen et al., 2011; van Kleunen et al., 2011). Comparisons of invasive and non-invasive exotic species answer different questions from conventional comparisons of native and invasive species (e.g. why some exotic species become invasive, while others do not; van Kleunen et al., 2010), and our understanding of invasion in low-resource ecosystems could benefit from these types of comparisons.
Many low-resource environments are experiencing radical changes as N deposition and land-use legacies increase nutrient availability in low-N and low-P systems, climate change alters the frequency and magnitude of precipitation in arid and semi-arid systems, and deforestation alters light availability. The effects of global change factors and their interactions on invasive species are still largely unresolved, and more research is needed on this important topic. Understanding the physiological mechanisms by which native and invasive species respond to current and future resource availability will help restoration efforts. Specifically, leaf- and plant-level traits can suggest ways to manipulate community-level properties to restore invaded ecosystems.
Acknowledgements
I gratefully acknowledge Virginia Matzek, Lawren Sack, Rachel Standish, Mark van Kleunen, and one anonymous reviewer who provided thoughtful comments on the manuscript. This work was supported by the National Science Foundation (grant OISE-1132994) and a Jasper Ridge Restoration Fellowship from Stanford University.
References
- 1.Aanderud ZT, Bledsoe CS. (2009) Preferences for 15N-ammonium, 15N-nitrate, and 15N-glycine differ among dominant exotic and subordinate native grasses from a California oak woodland. Environ Exp Bot 65: 205–209. [Google Scholar]
- 2.Aarssen LW. (1983) Ecological combining ability and competitive combining ability in plants: toward a general evolutionary theory of coexistence in systems of competition. Am Nat 122: 707–731. [Google Scholar]
- 3.Ackerly DD, Bazzaz FA. (1995) Leaf dynamics, self-shading and carbon gain in seedlings of a tropical pioneer tree. Oecologia 101: 289–298. [DOI] [PubMed] [Google Scholar]
- 4.Alpert P, Bone E, Holzapfel C. (2000) Invasiveness, invasibility and the role of environmental stress in the spread of non-native plants. Perspect Plant Ecol Evol Syst 3: 52–66. [Google Scholar]
- 5.Angert AL, Huxman TE, Barron-Gafford GA, Gerst KL, Venable DL. (2007) Linking growth strategies to long-term population dynamics in a guild of desert annuals. J Ecol 95: 321–331. [Google Scholar]
- 6.Auge RM. (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11: 3–42. [Google Scholar]
- 7.Balaguer L, Martinez-Ferri E, Valladares F, Perez-Corona ME, Baquedano FJ, Castillo FJ, Manrique E. (2001) Population divergence in the plasticity of the response to Quercus coccifera to the light environment. Funct Ecol 15: 124–135. [Google Scholar]
- 8.Barker DH, Vanier C, Naumburg E, Charlet TN, Nielsen KM, Newingham BA, Smith SD. (2006) Enhanced monsoon precipitation and nitrogen deposition affect leaf traits and photosynthesis differently in spring and summer in the desert shrub Larrea tridentata. New Phytol 169: 799–808. [DOI] [PubMed] [Google Scholar]
- 9.Baruch Z, Goldstein G. (1999) Leaf construction cost, nutrient concentration, and net CO2 assimilation of native and invasive species in Hawaii. Oecologia 121: 183–192. [DOI] [PubMed] [Google Scholar]
- 10.Baruch Z, Pattison RR, Goldstein G. (2000) Responses to light and water availability of four invasive Melastomataceae in the Hawaiian islands. Int J Plant Sci 161: 107–118. [DOI] [PubMed] [Google Scholar]
- 11.Baruch Z, Jackson RB. (2005) Responses of tropical native and invader C4 grasses to water stress, clipping and increased atmospheric CO2 concentration. Oecologia 145: 522–532. [DOI] [PubMed] [Google Scholar]
- 12.Bazzaz FA. (1979) The physiological ecology of plant succession. Annu Rev Ecol Syst 10: 351–371. [Google Scholar]
- 13.Bever JD. (2002) Negative feedback within a mutualism: host-specific growth of mycorrhizal fungi reduces plant benefit. Proc Biol Sci 269: 2595–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bjorkman O. (1981) Responses to different quantum flux densities. In Lange OL, Nobel PS, Osmond CB, Ziegler H, eds, Encyclopedia of Plant Physiology, Vol 12A Springer-Verlag, Heidelberg, pp 57–107. [Google Scholar]
- 15.Blumenthal DM, Jordan NR, Russelle MP. (2003) Soil carbon addition controls weeds and facilitates prairie restoration. Ecol Appl 13: 605–615. [Google Scholar]
- 16.Brock MT, Galen C. (2005) Drought tolerance in the alpine dandelion, Taraxacum ceratophorum (Asteraceae), its exotic congener T. officinale, and interspecific hybrids under natural and experimental conditions. Am J Bot 92: 1311–1321. [DOI] [PubMed] [Google Scholar]
- 17.Caplan JS, Yeakley JA. (2010) Water relations advantages for invasive Rubus armeniacus over two native ruderal congeners. Plant Ecol 210: 169–179. [Google Scholar]
- 18.Catford JA, Daehler CC, Murphy HT, Sheppard AW, Hardesty BD, Westcott DA, Rejmanek M, Bellingham PJ, Pergl J, Horvitz CC, Hulme PE. (2012) The intermediate disturbance hypothesis and plant invasions: implications for species richness and management. Perspect Ecol Evol Syst 14: 231–241. [Google Scholar]
- 19.Cavaleri MA, Sack L. (2010) Comparative water use of native and invasive plants at multiple scales: a global meta-analysis. Ecology 91: 2705–2715. [DOI] [PubMed] [Google Scholar]
- 20.Chapin FS., III (1980) The mineral nutrition of wild plants. Annu Rev Ecol Syst 11: 233–260. [Google Scholar]
- 21.Chazdon RL. (1988) Sunflecks and their importance to forest understorey plants. Adv Ecol Res 18: 1–63. [Google Scholar]
- 22.Cherwin KL, Seastedt TR, Suding KN. (2009) Effects of nutrient manipulations and grass removal on cover, species composition, and invasibility of a novel grassland in Colorado. Restor Ecol 17: 818–826. [Google Scholar]
- 23.Coley P, Bryant J, Chapin FI. (1985) Resource availability and plant antiherbivore defense. Science 230: 895–899. [DOI] [PubMed] [Google Scholar]
- 24.Comstock JP, Ehleringer JR. (1986) Canopy dynamics and carbon gain in response to soil water availability in Encelia frutescens Gray, a drought-deciduous shrub. Oecologia 68: 271–278. [DOI] [PubMed] [Google Scholar]
- 25.Corbin JD, D'Antonio CM. (2004) Can carbon addition increase competitiveness of native grasses? A case study from California. Restor Ecol 12: 36–43. [Google Scholar]
- 26.Cordell S, Cabin RJ, Hadway LJ. (2002) Physiological ecology of native and alien dry forest shrubs in Hawaii. Biol Invasions 4: 387–396. [Google Scholar]
- 27.Craine JM, Fargione J, Sugita S. (2005) Supply pre-emption, not concentration reduction, is the mechanism of competition for nutrients. New Phytol 166: 933–940. [DOI] [PubMed] [Google Scholar]
- 28.Craine JM. (2009) Resource Strategies of Wild Plants. Princeton University Press, Princeton. [Google Scholar]
- 29.Daehler CC. (2003) Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Annu Rev Ecol Evol Syst 34: 183–211. [Google Scholar]
- 30.Dallman PR. (1998) Plant Life in the World's Mediterranean Climates: California, Chile, South Africa, Australia, and the Mediterranean Basin. University of California Press, Berkeley. [Google Scholar]
- 31.Davidson AM, Jennions M, Nicotra AB. (2011) Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol Lett 14: 419–431. [DOI] [PubMed] [Google Scholar]
- 32.Davis MA, Grime JP, Thompson K. (2000) Fluctuating resources in plant communities: a general theory of invasibility. J Ecol 88: 528–534. [Google Scholar]
- 33.Dawson TE. (1993) Hydraulic lift and water use by plants: implications for water balance, performance and plant-plant interactions. Oecologia 95: 565–574. [DOI] [PubMed] [Google Scholar]
- 34.Dawson W, Burslem DFRP, Hulme PE. (2011) The comparative importance of species traits and introduction characteristics in tropical plant invasions. Divers Distrib 17: 1111–1121. [Google Scholar]
- 35.Dawson W, Rohr RP, van Kleunen M, Fischer M. (2012) Alien plant species with a wider global distribution are better able to capitalize on increased resource availability. New Phytol 194: 859–867. [DOI] [PubMed] [Google Scholar]
- 36.DeFalco LA, Bryla DR, Smith-Longozo V, Nowak RS. (2003) Are Mojave Desert annual species equal? Resource acquisition and allocation for the invasive grass Bromus madritensis subsp. rubens (Poaceae) and two native species. Am J Bot 90: 1045–1053. [DOI] [PubMed] [Google Scholar]
- 37.Diffenbaugh NS, Pal JS, Trapp RJ, Giorgi F. (2005) Fine-scale processes regulate the response of extreme events to global climate change. Proc Natl Acad Sci USA 102: 15774–15778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DiTommaso A, Aarssen LW. (1989) Resource manipulations in natural vegetation: a review. Vegetatio 84: 9–29. [Google Scholar]
- 39.Drenovsky RE, Martin CE, Falasco MR, James JJ. (2008) Variation in resource acquisition and utilization traits between native and invasive perennial forbs. Am J Bot 95: 681–687. [DOI] [PubMed] [Google Scholar]
- 40.Drenovsky RE, Grewell BJ, D'Antonio CM, Funk JL, James JJ, Molinari N, Parker IM, Richards CL. (2012a) A functional trait perspective on plant invasion. Ann Bot 110: 141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drenovsky RE, Khasanova A, James JJ. (2012b) Trait convergence and plasticity among native and invasive species in resource-poor environments. Am J Bot 99: 629–639. [DOI] [PubMed] [Google Scholar]
- 42.Durand LZ, Goldstein G. (2001) Photosynthesis, photoinhibition, and nitrogen use efficiency in native and invasive tree ferns in Hawaii. Oecologia 126: 345–354. [DOI] [PubMed] [Google Scholar]
- 43.Elser JJ, Dobberfuhl DR, MacKay NA, Schampel JH. (1996) Organism size, life history, and N:P stoichiometry: toward a unified view of cellular and ecosystem processes. Bioscience 46: 674–684. [Google Scholar]
- 44.Emer C, Fonseca CR. (2011) Araucaria forest conservation: mechanisms providing resistance to invasion by exotic timber trees. Biol Invasions 13: 189–202. [Google Scholar]
- 45.Evans JR. (1989) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78: 9–19. [DOI] [PubMed] [Google Scholar]
- 46.Evans JR, Poorter H. (2001) Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ 24: 755–767. [Google Scholar]
- 47.Feng Y, Wang J, Sang W. (2007) Biomass allocation, morphology and photosynthesis of invasive and noninvasive exotic species grown at four irradiance levels. Acta Oecologica 31: 40–47. [Google Scholar]
- 48.Feng YL, Lei YB, Wang RF, Callaway RM, Valiente-Banuet A, Inderjit, Li YP, Zheng YL. (2009) Evolutionary tradeoffs for nitrogen allocation to photosynthesis versus cell walls in an invasive plant. Proc Natl Acad Sci USA 106: 1853–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Field CB, Mooney HA. (1986) The photosynthesis-nitrogen relationship in wild plants. In Givnish TJ, ed, On the Economy of Plant Form and Function. Cambridge University Press, Cambridge, pp 25–55. [Google Scholar]
- 50.Firn J, Prober SM, Buckley YM. (2012) Plastic traits of an exotic grass contribute to its abundance but are not always favorable. PLOS One 7: e35870 doi:35810.31371/journal.pone.0035870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foxcroft LC, Pickett STA, Cadenasso ML. (2011) Expanding the conceptual frameworks of plant invasion ecology. Perspect Plant Ecol Evol Syst 13: 89–100. [Google Scholar]
- 52.Fridley JD. (2012) Extended leaf phenology and the autumn niche in deciduous forest invasions. Nature 485: 359–362. [DOI] [PubMed] [Google Scholar]
- 53.Funk JL, Vitousek PM. (2007) Resource use efficiency and plant invasion in low-resource systems. Nature 446: 1079–1081. [DOI] [PubMed] [Google Scholar]
- 54.Funk JL. (2008) Differences in plasticity between invasive and native plants from a low resource environment. J Ecol 96: 1162–1174. [Google Scholar]
- 55.Funk JL, Cleland EE, Suding KN, Zavaleta ES. (2008) Restoration through re-assembly: plant traits and invasion resistance. Trends Ecol Evol 23: 695–703. [DOI] [PubMed] [Google Scholar]
- 56.Funk JL, McDaniel S. (2010) Altering light availability to restore invaded forest: the predictive role of plant traits. Restor Ecol 18: 865–872. [Google Scholar]
- 57.Funk JL, Throop HL. (2010) Enemy release and plant invasion: patterns of defensive traits and leaf damage in Hawaii. Oecologia 162: 815–823. [DOI] [PubMed] [Google Scholar]
- 58.Funk JL, Zachary VA. (2010) Physiological responses to short-term water and light stress in native and invasive plant species in southern California. Biol Invasions 12: 1685–1694. [Google Scholar]
- 59.Funk JL, Glenwinkel LA, Sack L. (2013) Differential allocation to photosynthetic and non-photosynthetic nitrogen fractions among native and invasive species. PLOS One 8: e64502 doi:10.1371/journal.pone.0064502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gebauer RLE, Ehleringer JR. (2000) Water and nitrogen uptake patterns following moisture pulses in a cold desert community. Ecology 81: 1415–1424. [Google Scholar]
- 61.Givnish TJ. (1988) Adaptation to sun and shade: a whole-plant perspective. Aust J Plant Physiol 15: 63–92. [Google Scholar]
- 62.Givnish TJ, Montgomery RA, Goldstein G. (2004) Adaptive radiation of photosynthetic physiology in the Hawaiian lobeliads: light regimes, static light responses, and whole-plant compensation points. Am J Bot 91: 228–246. [DOI] [PubMed] [Google Scholar]
- 63.Gleason SM, Ares A. (2004) Photosynthesis, carbohydrate storage and survival of a native and an introduced species in relation to light and defoliation. Tree Physiol 24: 1087–1097. [DOI] [PubMed] [Google Scholar]
- 64.Godoy O, Valladares F, Castro-Dıez P. (2011) Multispecies comparison reveals that invasive and native plants differ in their traits but not in their plasticity. Funct Ecol 25: 1248–1259. [Google Scholar]
- 65.Goldberg DE. (1990) Components of resource competition in plant communities. In Grace JB, Tilman D, eds, Perspectives on Plant Competition. Academic Press, San Diego, p 2749. [Google Scholar]
- 66.Griffin KL. (1994) Calorimetric estimates of construction cost and their use in ecological studies. Funct Ecol 8: 551–562. [Google Scholar]
- 67.Grime JP, Crick JC, Rincon JE. (1986) The ecological significance of plasticity. In Jennings DH, Trewavas AJ, eds, Plasticity in Plants. The Company of Biologists Limited, Cambridge, pp 5–29. [PubMed] [Google Scholar]
- 68.Gross KL, Mittelbach GG, Reynolds HL. (2005) Grassland invasibility and diversity: responses to nutrients, seed input, and disturbance. Ecology 86: 476–486. [Google Scholar]
- 69.Grotkopp E, Rejmanek M. (2007) High seedling relative growth rate and specific leaf area are traits of invasive species: phylogenetically independent contrasts of woody angiosperms. Am J Bot 94: 526–532. [DOI] [PubMed] [Google Scholar]
- 70.Güsewell S, Koerselman W. (2002) Variation in nitrogen and phosphorus concentrations of wetland plants. Perspect Ecol Evol Syst 5: 37–61. [Google Scholar]
- 71.Güsewell S. (2004) N:P ratios in terrestrial plants: variation and functional signficance. New Phytol 164: 243–266. [DOI] [PubMed] [Google Scholar]
- 72.Hae UG, Sperry JS, Pockman WT, Davis SD, McCulloh KA. (2001) Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 126: 457–461. [DOI] [PubMed] [Google Scholar]
- 73.Halsted M, Lynch J. (1996) Phosphorus responses of C3 and C4 species. J Exp Bot 47: 497–505. [Google Scholar]
- 74.Han Y, Buckley YM, Firn J. (2012) An invasive grass shows colonization advantages over native grasses under conditions of low resource availability. Plant Ecol 213: 1117–1130. [Google Scholar]
- 75.Harrington RA, Brown BJ, Reich PB. (1989) Ecophysiology of exotic and native shrubs in Southern Wisconsin. I. Relationship of leaf characteristics, resource availability, and phenology to seasonal patterns of carbon gain. Oecologia 80: 356–367. [DOI] [PubMed] [Google Scholar]
- 76.Harrison MT, Edwards EJ, Farquhar GD, Nicotra AB, Evans JR. (2009) Nitrogen in cell walls of sclerophyllous leaves accounts for little of the variation in photosynthetic nitrogen-use efficiency. Plant Cell Environ 32: 259–270. [DOI] [PubMed] [Google Scholar]
- 77.Heberling JM, Fridley JD. (2013) Resource-use strategies of native and invasive plants in Eastern North American forests. New Phytol 200: 523–533. [DOI] [PubMed] [Google Scholar]
- 78.Holdridge LR. (1967) Life Zone Ecology. Tropical Science Center, San Jose, Costa Rica. [Google Scholar]
- 79.Hoopes MF, Hall LM. (2002) Edaphic factors and competition affect pattern formation and invasion in a California grassland. Ecol Appl 12: 24–39. [Google Scholar]
- 80.Huenneke LF, Hamburg SP, Koide R, Mooney HA, Vitousek PM. (1990) Effects of soil resources on plant invasion and community structure in California serpentine grassland. Ecology 71: 478–491. [Google Scholar]
- 81.Huxman TE, Barron-Gafford G, Gerst KL, Angert AL, Tyler AP, Venable DL. (2008) Photosynthetic resource-use efficiency and demographic variability in desert winter annual plants. Ecology 89: 1554–1563. [DOI] [PubMed] [Google Scholar]
- 82.Ignace DD, Huxman TE, Weltzin JF, Williams DG. (2007) Leaf gas exchange and water status responses of a native and non-native grass to precipitation across contrasting soil surfaces in the Sonoran Desert. Oecologia 152: 401–413. [DOI] [PubMed] [Google Scholar]
- 83.James JJ, Richards JH. (2005) Plant nitrogen capture from pulses: effects of pulse size, growth rate, and other soil resources. Oecologia 145: 113–122. [DOI] [PubMed] [Google Scholar]
- 84.James JJ, Richards JH. (2006) Plant nitrogen capture in pulse-driven systems: interactions between root responses and soil processes. J Ecol 94: 765–777. [Google Scholar]
- 85.James JJ, Drenovsky RE. (2007) A basis for relative growth rate differences between native and invasive forb seedlings. Rangeland Ecol Manage 60: 395–400. [Google Scholar]
- 86.James JJ, Drenovsky RE, Monaco TA, Rinella MJ. (2011) Managing soil nitrogen to restore annual grass-infested plant communities: effective strategy or incomplete framework? Ecol Appl 21: 490–502. [DOI] [PubMed] [Google Scholar]
- 87.Janse-ten Klooster SH, Thomas EJP, Sterck FJ. (2007) Explaining interspecific differences in sapling growth and shade tolerance in temperate forests. J Ecol 95: 1250–1260. [Google Scholar]
- 88.Kimball S, Angert AL, Huxman TE, Venable DL. (2011) Differences in the timing of germination and reproduction relate to growth physiology and population dynamics of Sonoran Desert winter annuals. Am J Bot 98: 1773–1781. [DOI] [PubMed] [Google Scholar]
- 89.Kloeppel BD, Abrams MD. (1995) Ecophysiological attributes of the native Acer saccharum and the exotic Acer platanoides in urban oak forests in Pennsylvania, USA. Tree Physiol 15: 739–746. [DOI] [PubMed] [Google Scholar]
- 90.Knapp AK, Beier C, Briske DD, Classen AT, Luo Y, Reichstein M, Smith MD, Smith SE, Bell JE, Fay PA, et al. (2008) Consequences of more extreme precipitation regimes for terrestrial ecosystems. Bioscience 58: 811–821. [Google Scholar]
- 91.Knapp LB, Canham CD. (2000) Invasion of an old-growth forest in New York by Ailanthus altissima: sapling growth and recruitment in canopy gaps. J Torrey Bot Soc 127: 307–315. [Google Scholar]
- 92.Koerselman W, Meuleman AFM. (1996) The vegetation N:P ratio: a new tool to detect the nature of nutrient limitation. J Appl Ecol 33: 1441–1450. [Google Scholar]
- 93.Lambers H, Chapin FS, III, Pons TL. (2008) Plant Physiological Ecology, Ed 2 Springer, New York. [Google Scholar]
- 94.Lambers H, Brundrett MC, Raven JA, Hopper SD. (2010) Plant mineral nutrition in ancient landscapes: high plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant Soil 334: 11–31. [Google Scholar]
- 95.Lambers H, Clements JC, Nelson MN. (2013) How a phosphorus-acquisition strategy based on carboxylate exudation powers the success and agronomic potential of lupines (Lupinus, Fabaceae). Am J Bot 100: 263–288. [DOI] [PubMed] [Google Scholar]
- 96.Laungani R, Knops JMH. (2009) Species-driven changes in nitrogen cycling can provide a mechanism for plant invasions. Proc Natl Acad Sci USA 106: 12400–12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leffler AJ, Monaco TA, James JJ. (2011) Nitrogen acquisition by annual and perennial grass seedlings: testing the roles of performance and plasticity to explain plant invasion. Plant Ecol 212: 1601–1611. [Google Scholar]
- 98.Leicht SA, Silander JA. (2006) Differential responses of invasive Celastrus orbiculatus (Celastraceae) and native C. scandens to changes in light quality. Am J Bot 93: 972–977. [DOI] [PubMed] [Google Scholar]
- 99.Leishman MR, Thomson VP. (2005) Experimental evidence for the effects of additional water, nutrients and physical disturbance on invasive plants in low fertility Hawkesbury Sandstone soils, Sydney, Australia. J Ecol 93: 38–49. [Google Scholar]
- 100.Leishman MR, Haslehurst T, Ares A, Baruch Z. (2007) Leaf trait relationships of native and invasive plants: community- and global-scale comparisons. New Phytol 176: 635–643. [DOI] [PubMed] [Google Scholar]
- 101.Leishman MR, Thomson VP, Cooke J. (2010) Native and exotic invasive plants have fundamentally similar carbon capture strategies. J Ecol 98: 28–42. [Google Scholar]
- 102.Lipson D, Näsholm T. (2001) The unexpected versatility of plants: organic nitrogen use and availability in terrestrial ecosystems. Oecologia 128: 305–316. [DOI] [PubMed] [Google Scholar]
- 103.Loik ME. (2007) Sensitivity of water relations and photosynthesis to summer precipitation pulses for Artemisia tridentata and Purshia tridentata. Plant Ecol 191: 95–108. [Google Scholar]
- 104.Lonsdale WM. (1999) Global patterns of plant invasions and the concept of invasibility. Ecology 80: 1522–1536. [Google Scholar]
- 105.Lusk CH, Warton DI. (2007) Global meta-analysis shows that relationships of leaf mass per area with species shade tolerance depend on leaf habit and ontogeny. New Phytol 176: 764–774. [DOI] [PubMed] [Google Scholar]
- 106.MacArthur RH, Levins R. (1967) The limiting similarity, convergence and divergence of coexisting species. Am Nat 101: 377–385. [Google Scholar]
- 107.McDowell SCL. (2002) Photosynthetic characteristics of invasive and noninvasive species of Rubus (Rosaceae). Am J Bot 89: 1431–1438. [DOI] [PubMed] [Google Scholar]
- 108.Mack MC, D'Antonio CM, Ley RE. (2001) Alteration of ecosystem nitrogen dynamics by exotic plants: a case study of C4 grasses in Hawaii. Ecol Appl 11: 1323–1335. [Google Scholar]
- 109.Marler MJ, Zabinski CA, Callaway RM. (1999) Mycorrhizae indirectly enhance competitive effects of an invasive forb on a native bunchgrass. Ecology 80: 1180–1186. [Google Scholar]
- 110.Martin PH, Canham CD, Marks PL. (2009) Why forests appear resistant to exotic plant invasions: intentional introductions, stand dynamics, and the role of shade tolerance. Front Ecol Environ 7: 142–149. [Google Scholar]
- 111.Martin PH, Canham CD, Kobe RK. (2010) Divergence from the growth-survival trade-off and extreme high growth rates drive patterns of exotic tree invasions in closed-canopy forests. J Ecol 98: 778–789. [Google Scholar]
- 112.Marushia RG, Cadotte MW, Holt JS. (2010) Phenology as a basis for management of exotic annual plants in desert invasions. J Appl Ecol 47: 1290–1299. [Google Scholar]
- 113.Matzek V. (2011) Superior performance and nutrient-use efficiency of invasive plants over non-invasive congeners in a resource-limited environment. Biol Invasions 13: 3005–3014. [Google Scholar]
- 114.Matzek V. (2012) Trait values, not trait plasticity, best explain invasive species' performance in a changing environment. PLoS One 7: e48821 doi:48810.41371/journal.pone.0048821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Meinhardt KA, Gehring CA. (2012) Disrupting mycorrhizal mutualisms: a potential mechanism by which exotic tamarisk outcompetes native cottonwoods. Ecol Appl 22: 532–549. [DOI] [PubMed] [Google Scholar]
- 116.Meisner A, de Boer W, Verhoeven KJF, Boschker HTS, van der Putten WH. (2011) Comparison of nutrient acquisition in exotic plant species and congeneric natives. J Ecol 99: 1308–1315. [Google Scholar]
- 117.Mooney HA. (1972) The carbon balance of plants. Annu Rev Ecol Syst 3: 315–346. [Google Scholar]
- 118.Morris TL, Esler KJ, Barger NN, Jacobs SM, Cramer MD. (2011) Ecophysiological traits associated with the competitive ability of invasive Australian acacias. Divers Distrib 17: 898–910. [Google Scholar]
- 119.Nagel JM, Griffin KL. (2004) Can gas-exchange characteristics help explain the invasive success of Lythrum salicaria? Biol Invasions 6: 101–111. [Google Scholar]
- 120.Nilsen ET, Muller WH. (1981) Phenology of the drought-deciduous shrub Lotus scoparius: climatic controls and adaptive significance. Ecol Monogr 51: 323–342. [Google Scholar]
- 121.Olde Venterink H, Güsewell S. (2010) Competitive interactions between two meadow grasses under nitrogen and phosphorus limitation. Funct Ecol 24: 877–886. [Google Scholar]
- 122.Olde Venterink H. (2011) Does phosphorus limitation promote species-rich plant communities? Plant Soil 345: 1–9. [Google Scholar]
- 123.Onoda Y, Hikosaka K, Hirose T. (2004) Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Funct Ecol 18: 419–425. [Google Scholar]
- 124.Ordonez A, Olff H. (2012) Do alien plant species profit more from high resource supply than natives? A trait-based analysis. Glob Ecol Biogeogr 22: 648–658. [Google Scholar]
- 125.Osunkoya OO, Bayliss D, Panetta FD, Vivian-Smith G. (2010a) Leaf trait co-ordination in relation to construction cost, carbon gain and resource-use efficiency in exotic invasive and native woody vine species. Ann Bot 106: 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Osunkoya OO, Bayliss D, Panetta FD, Vivian-Smith G. (2010b) Variation in ecophysiology and carbon economy of invasive and native woody vines of riparian zones in south-eastern Queensland. Austral Ecology 35: 636–649. [Google Scholar]
- 127.Palacio-López K, Gianoli E. (2011) Invasive plants do not display greater phenotypic plasticity than their native or non-invasive counterparts: a meta-analysis. Oikos 120: 1393–1401. [Google Scholar]
- 128.Pammenter NW, Drennan PM, Smith VR. (1986) Physiological and anatomical aspects of photosynthesis of two Agrostis species at a sub-antarctic island. New Phytol 102: 143–160. [DOI] [PubMed] [Google Scholar]
- 129.Paquette A, Fontaine B, Berninger F, Dubois K, Lechowicz MJ, Messier C, Posada JM, Valladares F, Brisson J. (2012) Norway maple displays greater seasonal growth and phenotypic plasticity to light than native sugar maple. Tree Physiol 32: 1339–1347. [DOI] [PubMed] [Google Scholar]
- 130.Pattison RR, Goldstein G, Ares A. (1998) Growth, biomass allocation and photosynthesis of invasive and native Hawaiian rainforest species. Oecologia 117: 449–459. [DOI] [PubMed] [Google Scholar]
- 131.Pavlik BM. (1983) Nutrient and productivity relations of the dune grass Ammoophila arenaria and Elymus mollis. Oecologia 57: 227–232. [DOI] [PubMed] [Google Scholar]
- 132.Penuelas J, Sardans J, Llusia J, Owen SM, Carnicer J, Giambelluca TW, Rezende EL, Waite M, Niinemets U. (2010) Faster return on ‘leaf economics’ and different biogeochemical niche in invasive compared with native plant species. Glob Change Biol 16: 2171–2185. [Google Scholar]
- 133.Poorter H, Lambers H. (1986) Growth and competitive ability of a highly plastic and marginally plastic genotype of Plantago major in a fluctuating environment. Physiol Plant 67: 217–222. [Google Scholar]
- 134.Preston KA, Ackerly DD. (2003) Hydraulic architecture and the evolution of shoot allometry in contrasting climates. Am J Bot 90: 1502–1512. [DOI] [PubMed] [Google Scholar]
- 135.Pringle A, Bever JD, Gardes M, Parrent JL, Rillig MC, Klironomos JN. (2009) Mycorrhizal symbioses and plant invasions. Annu Rev Ecol Syst 40: 699–715. [Google Scholar]
- 136.Pysek P, Prach D, Smilauer P. (1995) Relating invasion success to plant traits: an analysis of the Czech alien flora. In Pysek P, Prach D, Rejmanek M, Wade M, eds, Plant Invasions: General Aspects and Special Problems. SPB Academic Publishing, Amsterdam, pp 39–66. [Google Scholar]
- 137.Pysek P, Richardson DM. (2007) Traits associated with invasiveness in alien plants: where do we stand? In Nentwig W, ed, Biological Invasions, Ecological Studies, Vol 193 Springer-Verlag, Berlin, pp 97–125. [Google Scholar]
- 138.Reich PB, Walters MB, Ellsworth DS. (1997) From tropics to tundra: global convergence in plant functioning. Proc Natl Acad Sci USA 94: 13730–13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reinhart KO, Gurnee J, Tirado R, Callaway RM. (2006) Invasion through quantitative effects: intense shade drives native decline and invasive success. Ecol Appl 16: 1821–1831. [DOI] [PubMed] [Google Scholar]
- 140.Richardson AE, Barea JM, McNeill AM, Prigent-Combaret C. (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321: 305–339. [Google Scholar]
- 141.Rodríguez-Echeverría S, Crisostomo JA, Nabais C, Freitas H. (2009) Belowground mutualists and the invasive ability of Acacia longifolia in coastal dunes of Portugal. Biol Invasions 11: 651–661. [Google Scholar]
- 142.Rodríguez-Echeverría S, Le Roux JJ, Crisostomo JA, Ndlovu J. (2011) Jack-of-all-trades and master of many? How does associated rhizobial diversity influence the colonization success of Australian Acacia species? Divers Distrib 17: 946–957. [Google Scholar]
- 143.Sack L, Grubb PJ, Marañón T. (2003) The functional morphology of juvenile plants tolerant of strong summer drought in shaded forest understories in southern Spain. Plant Ecol 168: 139–163. [Google Scholar]
- 144.Sage RG, Pearcy RW. (1987) The nitrogen use efficiency of C3 and C4 plants II. Leaf nitrogen effects on the gas exchange characteristics of Chenopodium album (L.) and Amaranthus retroflexus (L.). Plant Physiol 84: 959–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sandquist DR, Ehleringer JR. (1998) Intraspecific variation of drought adaptation in brittlebush: leaf pubescence and timing of leaf loss vary with rainfall. Oecologia 113: 162–169. [DOI] [PubMed] [Google Scholar]
- 146.Schoenfelder AC, Bishop JG, Martinson HM, Fagan WF. (2010) Resource use efficiency and community effects of invasive Hypochaeris radicata (Asteraceae) during primary succession. Am J Bot 97: 1772–1779. [DOI] [PubMed] [Google Scholar]
- 147.Schumacher E, Kueffer C, Tobler M, Gmür V, Edwards PJ, Dietz H. (2008) Influence of drought and shade on seedling growth of native and invasive trees in the Seychelles. Biotropica 40: 543–549. [Google Scholar]
- 148.Schumacher E, Kueffer C, Edwards PJ, Dietz H. (2009) Influence of light and nutrient conditions on seedling growth of native and invasive trees in the Seychelles. Biol Invasions 11: 1941–1954. [Google Scholar]
- 149.Seabloom EW, Harpole WS, Reichman OJ, Tilman D. (2003) Invasion, competitive dominance, and resource use by exotic and native California grassland species. Proc Natl Acad Sci USA 100: 13384–13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shen XY, Peng SL, Chen BM, Pang JX, Chen LY, Xu HM, Hou YP. (2011) Do higher resource capture ability and utilization efficiency facilitate the successful invasion of native plants? Biol Invasions 13: 869–881. [Google Scholar]
- 151.Sher AA, Hyatt LA. (1999) The disturbed resource-flux invasion matrix: a new framework for patterns of plant invasion. Biol Invasions 1: 107–114. [Google Scholar]
- 152.Simms EL, Taylor DL, Povich J, Shefferson RP, Sachs JL, Urbina M, Tausczik Y. (2006) An empirical test of partner choice mechanisms in a wild legume–rhizobium interaction. Proc Biol Sci 273: 77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Standish RJ, Robertson AW, Williams PA. (2001) The impact of an invasive weed Tradescantia fluminensis on native forest regeneration. J Appl Ecol 38: 1253–1263. [Google Scholar]
- 154.Steers RJ, Funk JL, Allen EB. (2011) Can resource-use traits predict native vs. exotic plant success in carbon amended soils? Ecol Appl 21: 1211–1224. [DOI] [PubMed] [Google Scholar]
- 155.Stohlgren TJ, Barnett DT, Jarnevich CS, Flather C, Kartesz JT. (2008) The myth of plant species saturation. Ecol Lett 11: 313–326. [DOI] [PubMed] [Google Scholar]
- 156.Stratton LC, Goldstein G. (2001) Carbon uptake, growth and resource-use efficiency in one invasive and six native Hawaiian dry forest tree species. Tree Physiol 21: 1327–1334. [DOI] [PubMed] [Google Scholar]
- 157.Takashima T, Hikosaka K, Hirose T. (2004) Photosynthesis or persistence: nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant Cell Environ 27: 1047–1054. [Google Scholar]
- 158.Tecco PA, Diaz S, Cabido M, Urcelay C. (2010) Functional traits of alien plants across contrasting climatic and land-use regimes: do aliens join the locals or try harder than them? J Ecol 98: 17–27. [Google Scholar]
- 159.Theoharides KA, Dukes JS. (2007) Plant invasion across space and time: factors affecting nonindigenous species success during four stages of invasion. New Phytol 176: 256–273. [DOI] [PubMed] [Google Scholar]
- 160.Tilman D. (1982) Resource Competition and Community Structure. Princeton University Press, Princeton. [PubMed] [Google Scholar]
- 161.Valladares F, Wright SJ, Lasso E, Kitajima K, Pearcy RW. (2000) Plastic phenotypic response to light of 16 congeneric shrubs from a Panamanian rainforest. Ecology 81: 1925–1936. [Google Scholar]
- 162.Valladares F, Chico JM, Aranda I, Balaguer L, Dizengremel P, Manrique E, Dreyer E. (2002) The greater seedling high-light tolerance of Quercus robur over Fagus sylvatica is linked to a greater physiological plasticity. Trees 16: 395–403. [Google Scholar]
- 163.Valladares F, Niinemets U. (2008) Shade tolerance, a key plant feature of complex nature and consequences. Annu Rev Ecol Syst 39: 237–257. [Google Scholar]
- 164.van Kleunen M, Dawson W, Schlaepfer DR, Jeschke JM, Fischer M. (2010) Are invaders different? A conceptual framework of comparative approaches for assessing determinants of invasiveness. Ecol Lett 13: 947–958. [DOI] [PubMed] [Google Scholar]
- 165.van Kleunen M, Schlaepfer DR, Glaettli M, Fischer M. (2011) Preadapted for invasiveness: do species traits or their plastic response to shading differ between invasive and non-invasive plant species in their native range? J Biogeogr 38: 1294–1304. [Google Scholar]
- 166.Vitousek P. (1982) Nutrient cycling and nutrient use efficiency. Am Nat 119: 553–572. [Google Scholar]
- 167.Vitousek PM, Walker LR. (1989) Biological invasion by Myrica faya in Hawaii: plant demography, nitrogen fixation, ecosystem effects. Ecol Monogr 59: 247–265. [Google Scholar]
- 168.Vitousek PM, Howarth RW. (1991) Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13: 87–115. [Google Scholar]
- 169.Vogelsang KM, Bever JD. (2009) Mycorrhizal densities decline in association with nonnative plants and contribute to plant invasion. Ecology 90: 399–407. [DOI] [PubMed] [Google Scholar]
- 170.Walters MB, Reich PB. (2000) Trade-offs in low-light CO2 exchange: a component of variation in shade tolerance among cold temperate tree seedlings. Funct Ecol 14: 155–165. [Google Scholar]
- 171.Warren CR, Adams MA. (2001) Distribution of N, Rubisco and photosynthesis in Pinus pinaster and acclimation to light. Plant Cell Environ 24: 597–609. [Google Scholar]
- 172.Wassen MJ, Olde Venterink HGM, de Swart EOAM. (1995) Nutrient concentrations in mire vegetation as a measure of nutrient limitation in mire ecosystems. J Veg Sci 6: 5–16. [Google Scholar]
- 173.Weiher E, Clarke GDP, Keddy PA. (1998) Assembly rules, morphological dispersion, and the coexistence of plant species. Oikos 81: 309–322. [Google Scholar]
- 174.Weltzin JF, McPherson GR. (1997) Spatial and temporal soil moisture resource partitioning by trees and grasses in a temperate savanna, Arizona, USA. Oecologia 112: 156–164. [DOI] [PubMed] [Google Scholar]
- 175.Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ. (2002) Plant ecological strategies: some leading dimensions of variation between species. Annu Rev Ecol Syst 33: 125–159. [Google Scholar]
- 176.Williams RJ, Myers BA, Muller WJ, Duff GA, Eamus D. (1997) Leaf phenology of woody species in a North Australian tropical savanna. Ecology 78: 2542–2558. [Google Scholar]
- 177.Williamson J, Harrison S. (2002) Biotic and abiotic limits to the spread of exotic revegetation species. Ecol Appl 12: 40–51. [Google Scholar]
- 178.Willis A, Rodrigues BF, Harris PJC. (2013) The ecology of arbuscular mycorrhizal fungi. Crit Rev Plant Sci 32: 1–20. [Google Scholar]
- 179.Witkowski ETF. (1991) Growth and competition between seedlings of Protea repens (L.) L. and the alien invasive, Acacia saligna (Labill.) Wendl. in relation to nutrient availability. Funct Ecol 5: 101–110. [Google Scholar]
- 180.Wolkovich EM, Cleland EE. (2011) The phenology of plant invasions: a community ecology perspective. Front Ecol Environ 9: 287–294. [Google Scholar]
- 181.Wright IJ, Westoby M. (2002) Leaves at low versus high rainfall: coordination of structure, lifespan and physiology. New Phytol 155: 403–416. [DOI] [PubMed] [Google Scholar]
- 182.Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JH, Diemer M, et al. (2004) The worldwide leaf economics spectrum. Nature 428: 821–827. [DOI] [PubMed] [Google Scholar]
- 183.Wyka T, Robakowski P, Zytkowiak R. (2007) Acclimation of leaves to contrasting irradiance in juvenile trees differing in shade tolerance. Tree Physiol 27: 1293–1306. [DOI] [PubMed] [Google Scholar]
- 184.Yamashita N, Ishida A, Kushima H, Tanaka N. (2000) Acclimation to sudden increase in light favoring an invasive over native trees in subtropical islands, Japan. Oecologia 125: 412–419. [DOI] [PubMed] [Google Scholar]
- 185.Yelenik SG, Stock WD, Richardson DM. (2004) Ecosystem level impacts of invasive Acacia saligna in the South African fynbos. Restor Ecol 12: 44–51. [Google Scholar]