We used five genetically distinct sub-populations of Australian barramundi (Lates calcarifer) to examine the extent of intraspecific variability in hypoxia tolerance at typical (26°C) and warm (36°C) temperatures. Critical oxygen tension ([O2]crit) was lower at 26°C than at 36°C, indicating greater hypoxia tolerance at the cooler temperature, but was not different between sub-populations.
Keywords: Barramundi, climate change, critical oxygen saturation hypoxia, Lates calcarifer, tropical
Abstract
Tropical coastal systems are particularly prone to periods of environmental hypoxia, which can result from organismal respiration as well as thermal stratification, and may be further exacerbated by anthropogenic disturbances. In this study, we used five genetically distinct sub-populations of Australian barramundi (Lates calcarifer) to examine the extent of intraspecific variability in hypoxia tolerance. Fish were maintained at two temperatures (26 or 36°C), representing the seasonal thermal range for this species across its tropical distribution in Australia. All fish maintained a constant oxygen consumption rate 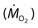 as air saturation of the water decreased from 100% down to a critical oxygen saturation ([O2]crit) of 15.44 ± 3.20 and 21.07 ± 3.92% (means ± SD) at 26 and 36°C, respectively. Mean [O2]crit, used as a performance measure of hypoxia tolerance, did not differ between sub-populations. No differences were found for resting
as air saturation of the water decreased from 100% down to a critical oxygen saturation ([O2]crit) of 15.44 ± 3.20 and 21.07 ± 3.92% (means ± SD) at 26 and 36°C, respectively. Mean [O2]crit, used as a performance measure of hypoxia tolerance, did not differ between sub-populations. No differences were found for resting 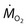 between sub-populations at 26°C, but modest differences were detected between two sub-populations at 36°C (3.36 ± 0.62 and 2.83 ± 0.27 mg O2 kg−1 min−1 for Gladstone and Broome sub-populations, respectively). Resting
between sub-populations at 26°C, but modest differences were detected between two sub-populations at 36°C (3.36 ± 0.62 and 2.83 ± 0.27 mg O2 kg−1 min−1 for Gladstone and Broome sub-populations, respectively). Resting 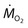 was lower for sub-populations at 26°C (1.46 ± 0.26 mg O2 kg−1 min−1) than at 36°C (3.10 ± 0.43 mg O2 kg−1 min−1), with a temperature coefficient (Q10) of 2.12 ± 0.30. We conclude that both hypoxia tolerance and resting
was lower for sub-populations at 26°C (1.46 ± 0.26 mg O2 kg−1 min−1) than at 36°C (3.10 ± 0.43 mg O2 kg−1 min−1), with a temperature coefficient (Q10) of 2.12 ± 0.30. We conclude that both hypoxia tolerance and resting 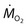 are conserved across the distribution of barramundi in Australia, which reflects the capacity of this species to cope in environments with large fluctuations in both temperature and dissolved oxygen.
are conserved across the distribution of barramundi in Australia, which reflects the capacity of this species to cope in environments with large fluctuations in both temperature and dissolved oxygen.
Introduction
The availability of dissolved oxygen (DO) in aquatic systems is critical in defining the abundance and distribution of biological communities (Pearson et al., 2003). In freshwater and estuarine systems, oxygen can fluctuate between normoxia and severe hypoxia due to respiration of organisms inhabiting such systems and thermal stratification (Pearson et al., 2003; Brauner and Val, 2005). In tropical regions, initial rains at the onset of the monsoon season can carry high loads of organic material into freshwater and estuarine systems, causing a rapid depletion of oxygen, which can be fatal for resident animal populations (Bishop, 1980; Townsend et al., 1992; Erskine et al., 2005). Furthermore, natural occurrences of hypoxia may be exacerbated by anthropogenic disturbances, such as agriculture and urbanization (Pollock et al., 2007).
Altered weather patterns resulting from climate change have the potential to exacerbate periods of environmental hypoxia. Higher water temperatures reduce the solubility of oxygen in water, while concurrently increasing the metabolic demand for oxygen in aquatic ectotherms (Weiss, 1970; Diaz and Breitburg, 2009). As such, the unprecedented rate of temperature rise predicted under current climate change scenarios may push many aquatic species close to, or above, their upper thermal thresholds (Tewksbury et al., 2008; Morrongiello et al., 2011; Huey et al., 2012). This may be particularly pronounced at very low latitudes near the equator, where species may be adapted to mean temperatures that may span only 1–2°C annually (Tewksbury et al., 2008). Thus, it is likely that the elevated temperatures predicted to occur in the future will progressively increase the prevalence and severity of hypoxia in aquatic systems, with flow-on effects to larger-scale processes, such as population growth, fitness and ecosystem dynamics (Pollock et al., 2007).
Fish can respond physiologically and behaviourally to fluctuating DO, although such responses are highly dependent on species and context. For example, fish that encounter hypoxic conditions may actively avoid areas of low DO (Herbert and Steffensen, 2005; Poulsen et al., 2011), increase gill ventilation volume (Jesse et al., 1967; Fernandes and Rantin, 1989), increase the number of red blood cells and haemoglobin concentration (Wells et al., 1989), and/or induce bradycardia (Randall and Smith, 1967). Exposure to acute or chronic hypoxia can have sub-lethal effects, such as altered behaviour and reduced growth and reproduction, which may be further exacerbated by increasing water temperatures (Vaquer-Sunyer and Duarte, 2008).
The physiological responses to hypoxia are regarded as being dependent on the frequency and severity of the hypoxic event (Farrell and Richards, 2009). Below a certain DO, most fish are unable to regulate their oxygen consumption rate 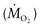 independently of ambient oxygen levels and consequently enter a state of oxygen conformity (Prosser and Brown, 1961; Fernandes et al., 1995; Schurmann and Steffensen, 1997). The level of DO at which this conformity occurs is referred to as the critical oxygen saturation ([O2]crit) and is commonly used as a measure of the hypoxia tolerance of a species (Prosser and Brown, 1961; Schurmann and Steffensen, 1997).
independently of ambient oxygen levels and consequently enter a state of oxygen conformity (Prosser and Brown, 1961; Fernandes et al., 1995; Schurmann and Steffensen, 1997). The level of DO at which this conformity occurs is referred to as the critical oxygen saturation ([O2]crit) and is commonly used as a measure of the hypoxia tolerance of a species (Prosser and Brown, 1961; Schurmann and Steffensen, 1997).
The prevalence of intraspecific local adaptation (or interdemic variation) may influence the capacity for sub-populations to respond to environmental changes (Grabowski et al., 2009; Tobler et al., 2011; Whitehead et al., 2011). It has previously been proposed that selection pressure for hypoxia tolerance may lead to variation among sub-populations for species with broad habitat ranges (Timmerman and Chapman, 2004). A number of studies to date have found evidence for local thermal adaptation in metabolic traits between sub-populations of species including killifish (Fundulus heteroclitus; Dhillon and Schulte, 2011) and Atlantic cod (Gadus morhua; Sylvestre et al., 2007; Grabowski et al., 2009). Local adaptation to hypoxia between sub-populations of fish species across broad spatial scales, however, is less well understood, and no study has investigated this for a higher-level predator.
To help address this knowledge gap, we investigated [O2]crit and resting 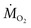 in five different sub-populations of Australian barramundi (Lates calcarifer) spanning ∼12° of latitude (Fig. 1). Experiments were performed at two temperatures chosen to represent typical (26°C) and warm summer conditions (36°C). The main aims of this study were as follows: (i) to investigate whether sub-populations differ in their [O2]crit and/or resting
in five different sub-populations of Australian barramundi (Lates calcarifer) spanning ∼12° of latitude (Fig. 1). Experiments were performed at two temperatures chosen to represent typical (26°C) and warm summer conditions (36°C). The main aims of this study were as follows: (i) to investigate whether sub-populations differ in their [O2]crit and/or resting 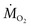 ; (ii) to quantify how different sub-populations respond to the combined challenges of temperature and hypoxia; and (iii) to determine whether hypoxia tolerance is related to latitudinal position. We expected barramundi sub-populations from lower latitudes to be more hypoxia tolerant than their higher latitude counterparts due to the inverse relationship between water temperature and oxygen solubility. We also expected barramundi (irrespective of sub-population) to exhibit higher metabolic rates at warmer temperatures, and consequently, to be less hypoxia tolerant due to the elevated metabolic demand for oxygen.
; (ii) to quantify how different sub-populations respond to the combined challenges of temperature and hypoxia; and (iii) to determine whether hypoxia tolerance is related to latitudinal position. We expected barramundi sub-populations from lower latitudes to be more hypoxia tolerant than their higher latitude counterparts due to the inverse relationship between water temperature and oxygen solubility. We also expected barramundi (irrespective of sub-population) to exhibit higher metabolic rates at warmer temperatures, and consequently, to be less hypoxia tolerant due to the elevated metabolic demand for oxygen.
Materials and methods
Experimental animals and holding conditions
Barramundi juveniles were obtained from five commercial hatcheries located at Broome (Broome Aquaculture Centre), Darwin (Darwin Aquaculture Centre), Karumba (Barramundi Discovery Centre), Townsville (Mainstream Aquaculture), and Gladstone (Gladstone Water Board Barramundi Hatchery; Fig. 1). All five hatcheries use broodstock sourced from local rivers that are separated by a minimum of 700 km (Fig. 1). Wild sub-populations of Australian barramundi differ genetically (Keenan, 1994; Doupé et al., 1999), and broodstock maintained at these locations have been identified as genetically distinct (Smith-Keune C, unpubublished data). Prior to experimental treatments, fish were grown on to a size of ∼200 g over ∼10 months in tanks containing fresh water connected to a recirculating system at the Marine and Aquaculture Research Facility Unit of James Cook University (Townsville, Queensland, Australia). All fish were fed ∼1% body weight per day using a commercial pelleted feed (Ridley Aquafeed, Narangba, Queensland, Australia), and maintained at 26 ± 1°C under a 12 h–12 h photoperiod. Dissolved oxygen was maintained at >75% saturation in the holding tanks, and water quality was monitored daily. Individual fish were weighed and measured prior to experiments and following respirometry. Mean (±SD) body mass and condition factor (K) of fish after respirometry were 194.16 ± 22.60 g and 1.17 ± 0.09, respectively. Condition factor was calculated according to the following formula: K = 100a × b−3, where a = weight (in grams) and b = total length (in centimetres; Froese, 2006).
Figure 1:
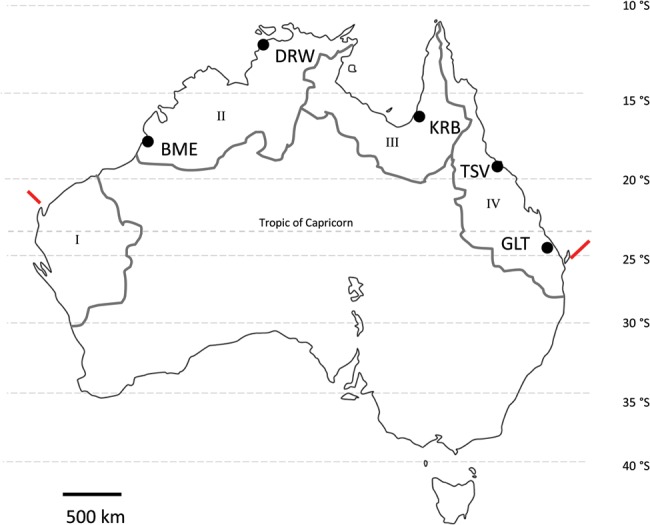
Map of Australia showing where individual sub-populations of barramundi were collected, as follows: BME, Broome; DRW, Darwin; GLT, Gladstone; KRB, Karumba; and TSV, Townsville. Major river drainage divisions of northern Australia have been redrawn from Hutchinson and Dowling (1991) and are indicated as follows: I, Indian Ocean; II, Kimberley and Arafura Sea; III, Gulf of Carpentaria; and IV, North East Coast. Red lines adjacent to the coast indicate the southerly limits of the species' distribution.
Experimental design
Temperature treatments were selected based on a review of atmospheric and sea-surface temperatures obtained from Australian government databases (AIMS, 2012; BOM, 2012), and from a comprehensive review of river temperatures from previously published data (Pusey et al., 1998; Stuart and Berghuis, 2002; Webster et al., 2005). Based on this information, the two temperature treatments were as follows: (i) a ‘typical’ temperature (26°C), which is representative of the annual mean across the species distribution in Australia and the temperature at which the fish had been held long term; and (ii) a ‘warm’ temperature (36°C), which is regarded to be representative of the upper limit that wild populations experience (Russell and Garrett, 1983), but still within the known tolerance limits for the species (Bermudes et al., 2010). Prior to experiments, fish were removed from their holding tanks and acclimated to one of two temperatures in a separate system where water temperature could be closely controlled. While fish in the 26°C treatment group did not undergo a temperature change relative to prior holding conditions, fish used in the 36°C treatment group were acclimated by increasing the temperature by 1°C per day and then held at 36°C for a minimum of 7 days prior to experimentation. All fish were fed as stated in the previous subsection, but food was withheld for 24 h prior to conducting respirometry to minimize the effect of specific dynamic action on oxygen consumption measurements.
Respirometry
All measurements were performed using intermittent flow-through respirometry, following best practices outlined in Clark et al. (2013). Respirometers (volume = 10.3 l) were fitted with a small Perspex window to allow fish to be observed during respirometry trials. Each respirometer was connected to two pumps; a single recirculating pump to keep water within the chamber mixed, and a flush pump to supply the chamber with aerated water between 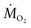 measurements. Respirometers and flush pumps were submerged in a shallow 1000 l tank, with vigorous aeration to provide both stable temperature conditions during experiments (26.2 ± 0.5 and 36.4 ± 0.2°C) and to supply the chambers with ∼100% saturated water during the flush cycle. Temperature-compensated oxygen concentration (in milligrams per litre) of the water within each chamber was continuously recorded (0.5 Hz) using oxygen-sensitive REDFLASH® dye on contactless spots (2 mm) adhered to the inside of each chamber and linked to a Firesting Optical Oxygen Meter (Pyro Science e. K, Aachen, Germany) via fibre-optic cables. Oxygen-sensing equipment was recalibrated daily using a one-point 100% saturation calibration and an electrical (factory-calibrated) zero. Following initial measurements of background respiration in the respirometers, individual fish that had been fasted for 24 h were placed in respirometers in the evening and allowed to acclimate to the respirometers for 16 h. During the acclimation period, the flush pumps attached to each respirometer were set to a 30 min–15 min on–off cycle, and
measurements. Respirometers and flush pumps were submerged in a shallow 1000 l tank, with vigorous aeration to provide both stable temperature conditions during experiments (26.2 ± 0.5 and 36.4 ± 0.2°C) and to supply the chambers with ∼100% saturated water during the flush cycle. Temperature-compensated oxygen concentration (in milligrams per litre) of the water within each chamber was continuously recorded (0.5 Hz) using oxygen-sensitive REDFLASH® dye on contactless spots (2 mm) adhered to the inside of each chamber and linked to a Firesting Optical Oxygen Meter (Pyro Science e. K, Aachen, Germany) via fibre-optic cables. Oxygen-sensing equipment was recalibrated daily using a one-point 100% saturation calibration and an electrical (factory-calibrated) zero. Following initial measurements of background respiration in the respirometers, individual fish that had been fasted for 24 h were placed in respirometers in the evening and allowed to acclimate to the respirometers for 16 h. During the acclimation period, the flush pumps attached to each respirometer were set to a 30 min–15 min on–off cycle, and 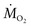 (in milligrams of O2 per kilogram per minute) was measured from the decline in oxygen in each respirometer during each 15 min off cycle. Resting
(in milligrams of O2 per kilogram per minute) was measured from the decline in oxygen in each respirometer during each 15 min off cycle. Resting 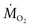 was calculated for each fish as the mean of the lowest three measurements recorded during the acclimation period.
was calculated for each fish as the mean of the lowest three measurements recorded during the acclimation period.
Following the chamber acclimation period and resting 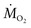 measurements, each flush pump was turned off, and fish were permitted to deplete the oxygen within their respective respirometers down to 5% air saturation. The
measurements, each flush pump was turned off, and fish were permitted to deplete the oxygen within their respective respirometers down to 5% air saturation. The 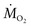 was calculated for each consecutive 5 min period during the decline in oxygen (∼1.5–4 h depending on temperature). Upon reaching 5% air saturation, each flush pump was turned on to restore oxygen levels to 100% saturation. Preliminary trials conducted using similar-sized fish indicated that depletion to 5% saturation was sufficient to calculate [O2]crit but still above the oxygen levels that induce loss of equilibrium. The following two values were identified from each [O2]crit experiment: (i) pre-hypoxia,
was calculated for each consecutive 5 min period during the decline in oxygen (∼1.5–4 h depending on temperature). Upon reaching 5% air saturation, each flush pump was turned on to restore oxygen levels to 100% saturation. Preliminary trials conducted using similar-sized fish indicated that depletion to 5% saturation was sufficient to calculate [O2]crit but still above the oxygen levels that induce loss of equilibrium. The following two values were identified from each [O2]crit experiment: (i) pre-hypoxia, 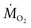 , which was calculated from the mean of the lowest three 5 min measurements recorded between 100 and 75% saturation after flush pumps had been turned off; and (ii) [O2]crit, which was determined using previously established methods (Corkum and Gamperl, 2009; Nilsson et al., 2010). Briefly, [O2]crit was determined by fitting two linear regression lines to the measurements (one line based on the calculated pre-hypoxia,
, which was calculated from the mean of the lowest three 5 min measurements recorded between 100 and 75% saturation after flush pumps had been turned off; and (ii) [O2]crit, which was determined using previously established methods (Corkum and Gamperl, 2009; Nilsson et al., 2010). Briefly, [O2]crit was determined by fitting two linear regression lines to the measurements (one line based on the calculated pre-hypoxia, 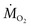 and another line based on the decrease in
and another line based on the decrease in 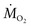 observed during the later stages of the [O2]crit test), and calculating the intersection point of the two lines (Ott et al., 1980; Nilsson et al., 2004). After the completion of each trial, additional background respiration measurements were obtained for each chamber in the absence of fish. Any change in background respiration between the start and end of experiments was assumed to be linear when correcting fish
observed during the later stages of the [O2]crit test), and calculating the intersection point of the two lines (Ott et al., 1980; Nilsson et al., 2004). After the completion of each trial, additional background respiration measurements were obtained for each chamber in the absence of fish. Any change in background respiration between the start and end of experiments was assumed to be linear when correcting fish 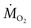 .
.
Data analyses and statistics
All oxygen consumption rate measurements (in milligrams of O2 per kilogram per minute) were calculated using commercial software (LabChart v. 7; ADInstruments, Sydney, NSW, Australia) from the slope of the decline in oxygen concentration according to the formula:
where slopea and slopeb are the declines in oxygen (in milligrams per litre per second) measured in the presence and absence of fish within the chamber, respectively, and Vc and Mb are the volumes (in litres) of the chamber and fish, respectively.
Resting, 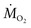 pre-hypoxia,
pre-hypoxia, 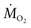 and [O2]crit measurements were obtained for 114 barramundi from the five geographically distinct sub-populations, with individual fish considered as an individual replicate for population and temperature treatments. Individual replicates where [O2]crit was not calculated (n = 7) were excluded from further analyses. The temperature coefficient (Q10) for resting
and [O2]crit measurements were obtained for 114 barramundi from the five geographically distinct sub-populations, with individual fish considered as an individual replicate for population and temperature treatments. Individual replicates where [O2]crit was not calculated (n = 7) were excluded from further analyses. The temperature coefficient (Q10) for resting 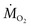 for each population over the 10°C temperature increment was calculated using the formula:
for each population over the 10°C temperature increment was calculated using the formula:
where R is resting, 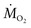 T is temperature, 1 represents values at 26°C, and 2 represents values at 36°C.
T is temperature, 1 represents values at 26°C, and 2 represents values at 36°C.
Statistical analyses were performed using SPSS v. 20 (IBM, Chicago, IL, USA). A general linear model was used to assess the effect of the two main factors (sub-population and temperature) on resting, 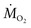 pre-hypoxia
pre-hypoxia 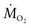 and [O2]crit. These analyses were followed by one-way ANOVA with Student–Newman–Keuls post hoc tests for [O2]crit and the non-parametric Kruskal–Wallis test with Dunn's post hoc comparison (where the assumption of normality was not met) for resting
and [O2]crit. These analyses were followed by one-way ANOVA with Student–Newman–Keuls post hoc tests for [O2]crit and the non-parametric Kruskal–Wallis test with Dunn's post hoc comparison (where the assumption of normality was not met) for resting 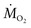 and pre-hypoxia
and pre-hypoxia 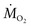 to assess sub-population differences at each temperature (Zar, 2010). Homogeneity of variance and normality were assessed using Levene's test and normal quantile–quantile (Q–Q) plot, respectively. Data are presented as means ± SD, and results were considered statistically significant at P < 0.05.
to assess sub-population differences at each temperature (Zar, 2010). Homogeneity of variance and normality were assessed using Levene's test and normal quantile–quantile (Q–Q) plot, respectively. Data are presented as means ± SD, and results were considered statistically significant at P < 0.05.
Results
Irrespective of population, the resting 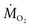 of fish acclimated to 26°C was significantly lower than the resting
of fish acclimated to 26°C was significantly lower than the resting 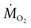 of fish acclimated to 36°C (1.47 ± 0.24 vs. 3.10 ± 0.43 mg O2 kg−1 min−1 for all sub-populations combined; F1, 110 = 563.57, P < 0.001; Fig. 2). There was a significant interaction between sub-population and temperature for resting
of fish acclimated to 36°C (1.47 ± 0.24 vs. 3.10 ± 0.43 mg O2 kg−1 min−1 for all sub-populations combined; F1, 110 = 563.57, P < 0.001; Fig. 2). There was a significant interaction between sub-population and temperature for resting 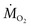 (P = 0.025). No differences were found for resting
(P = 0.025). No differences were found for resting 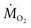 between sub-populations at 26°C (H5 = 1.446, P = 0.84). At 36°C, resting
between sub-populations at 26°C (H5 = 1.446, P = 0.84). At 36°C, resting 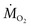 differed from that at 26°C (H5 = 11.25, P = 0.0239; Fig. 2), although Dunn's post hoc comparison did not identify differences between individual sub-populations. Pre-hypoxia
differed from that at 26°C (H5 = 11.25, P = 0.0239; Fig. 2), although Dunn's post hoc comparison did not identify differences between individual sub-populations. Pre-hypoxia 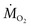 was higher for the Townsville sub-population than for the Darwin sub-population at 26°C (H5 = 11.90, P = 0.018), and was higher for the Gladstone sub-population than for the Broome or Karumba sub-populations at 36°C (H5 = 17.02, P = 0.002). The average Q10 for resting
was higher for the Townsville sub-population than for the Darwin sub-population at 26°C (H5 = 11.90, P = 0.018), and was higher for the Gladstone sub-population than for the Broome or Karumba sub-populations at 36°C (H5 = 17.02, P = 0.002). The average Q10 for resting 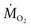 and pre-hypoxia
and pre-hypoxia 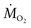 across all populations was 2.12 ± 0.30 and 2.12 ± 0.36, respectively.
across all populations was 2.12 ± 0.30 and 2.12 ± 0.36, respectively.
Figure 2:
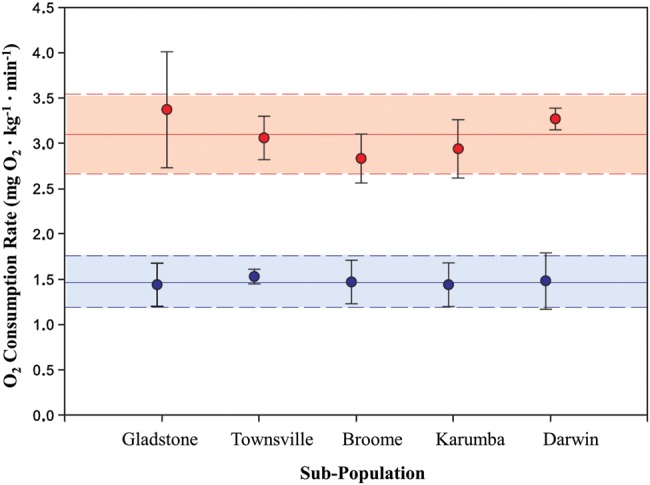
Resting oxygen consumption rates (in milligrams of O2 per kilogram per minute; means ± SD) for five barramundi sub-populations at 26°C (blue; n = 48) and 36°C (red; n = 59). Oxygen consumption rates were calculated from the average slope of the decline in chamber [O2] across a 15 min period during the ‘closed’ cycle of intermittent flow-through respirometry, and measured every 45 min. The continous and dashed horizontal lines represent the mean and standard deviation for all populations at each temperature.
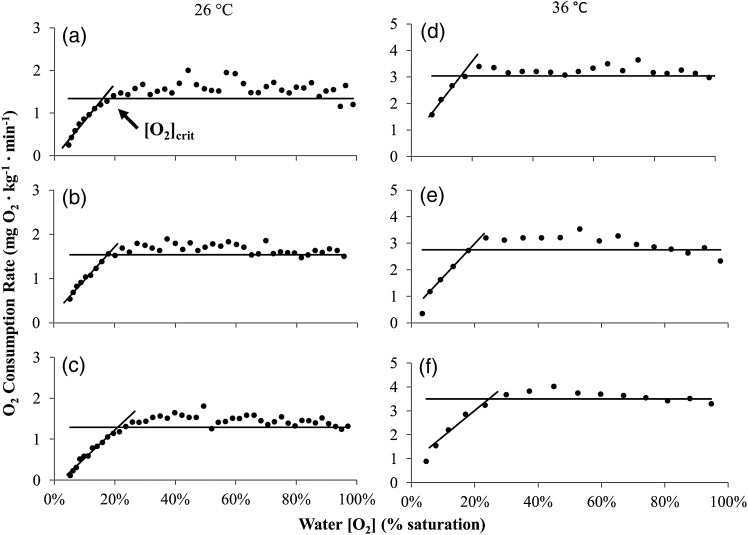
Barramundi exhibited a common and clear trend in response to decreasing oxygen, with fish maintaining a relatively constant 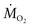 above the [O2]crit, followed by a steep decline (Fig. 3). There was no significant interaction between population and temperature for [O2]crit (P = 0.486). Temperature had a significant effect on mean [O2]crit across all populations, with lower [O2]crit at 26 than at 36°C (F1,114 = 69.97; P < 0.001; Fig. 4). No significant differences were observed for mean [O2]crit between four of the five sub-populations tested at both 26 and 36°C (Fig. 4). The exception was that mean [O2]crit was highest for fish from Darwin at both 26 and 36°C (18.91 ± 2.97 and 23.84 ± 3.49%, respectively). Critical oxygen saturation measurements varied between individuals, with minimal and maximal values spanning 8.63–23.02% saturation at 26°C (mean = 15.44 ± 3.20% saturation) and 13.47–31.17% saturation at 36°C (mean = 21.07 ± 3.92% saturation). Fish displayed signs of distress, such as erratic movements and pale body colour, below [O2]crit; however, all fish recovered fully upon returning to 100% saturated conditions.
above the [O2]crit, followed by a steep decline (Fig. 3). There was no significant interaction between population and temperature for [O2]crit (P = 0.486). Temperature had a significant effect on mean [O2]crit across all populations, with lower [O2]crit at 26 than at 36°C (F1,114 = 69.97; P < 0.001; Fig. 4). No significant differences were observed for mean [O2]crit between four of the five sub-populations tested at both 26 and 36°C (Fig. 4). The exception was that mean [O2]crit was highest for fish from Darwin at both 26 and 36°C (18.91 ± 2.97 and 23.84 ± 3.49%, respectively). Critical oxygen saturation measurements varied between individuals, with minimal and maximal values spanning 8.63–23.02% saturation at 26°C (mean = 15.44 ± 3.20% saturation) and 13.47–31.17% saturation at 36°C (mean = 21.07 ± 3.92% saturation). Fish displayed signs of distress, such as erratic movements and pale body colour, below [O2]crit; however, all fish recovered fully upon returning to 100% saturated conditions.
Figure 3:
Representative graphs displaying oxygen consumption rate 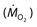 as water oxygen concentration (percentage saturation) decreases for six individuals from three barramundi populations at 26 and 36°C. Populations are indicated by each panel as follows: Gladstone (a and d), Broome (b and e) and Darwin (c and f). The
as water oxygen concentration (percentage saturation) decreases for six individuals from three barramundi populations at 26 and 36°C. Populations are indicated by each panel as follows: Gladstone (a and d), Broome (b and e) and Darwin (c and f). The 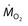 was calculated from the average slope of the decline in chamber [O2] for every 5 min period. The critical oxygen saturation ([O2]crit), indicated by the arrow in the top left panel, was calculated from the intersection between two linear regressions; one for pre-hypoxia
was calculated from the average slope of the decline in chamber [O2] for every 5 min period. The critical oxygen saturation ([O2]crit), indicated by the arrow in the top left panel, was calculated from the intersection between two linear regressions; one for pre-hypoxia 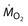 , and a second for the steep decline in
, and a second for the steep decline in 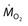 during the later stages of the [O2]crit test. Fish took ∼4 h to deplete oxygen within the chambers at 26°C and ∼1.5 h at 36°C. The difference in the vertical scale for fish at 26 and 36°C reflects the increased
during the later stages of the [O2]crit test. Fish took ∼4 h to deplete oxygen within the chambers at 26°C and ∼1.5 h at 36°C. The difference in the vertical scale for fish at 26 and 36°C reflects the increased 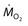 of barramundi at the higher temperature.
of barramundi at the higher temperature.
Figure 4:
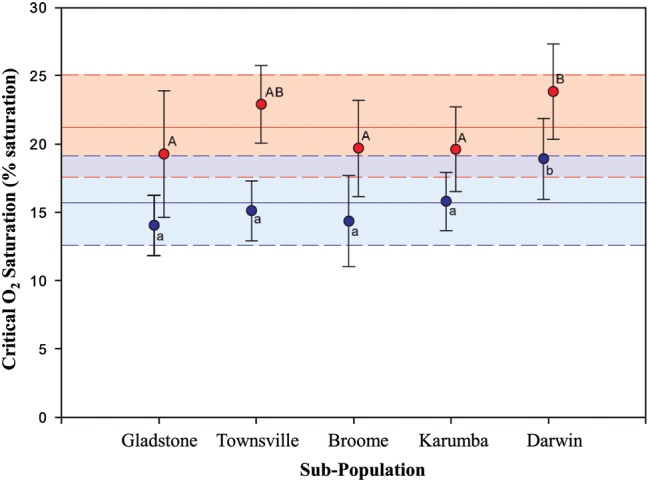
Mean critical oxygen saturation (percentage saturation; mean ± SD) for five sub-populations of barramundi at 26°C (blue; n = 50) and 36°C (red; n = 63; populations given in Fig. 1). The continuous and dashed horizontal lines represent mean and standard deviation, respectively, of critical oxygen saturation for all populations at each temperature. Letters indicate significant differences (P < 0.05; a < b and A < B).
Discussion
Species inhabiting thermally stable environments (e.g. equatorial or polar) are thought to be physiologically constrained within a narrow range of environmental thresholds, suggesting that they may be particularly susceptible to increasing temperatures and increasing variability in mean temperatures (Tewksbury et al., 2008). Due to the inverse relationship between temperature and oxygen solubility in water (Diaz and Breitburg, 2009), we expected barramundi from lower latitudes to be more hypoxia tolerant than their higher-latitude counterparts. Contrary to expectations, Darwin fish (low latitude) displayed a slightly higher average [O2]crit (less hypoxia tolerant) than fish from the other four populations tested at both control and warm temperatures. However, on the whole, we were unable to find consistent and conclusive evidence for local adaptation in hypoxia tolerance between Australian barramundi sub-populations separated by large longitudinal and latitudinal distances.
Interspecific variation in hypoxia tolerance, as derived from [O2]crit measurements, exists for a number of temperate and tropical fish species at temperatures that are routine or typical (Table 1). Species such as Nile tilapia (Oreochromis niloticus) can regulate 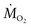 down to 11 ± 3% saturation, whereas other species, such as Atlantic salmon (Salmo salar), can regulate
down to 11 ± 3% saturation, whereas other species, such as Atlantic salmon (Salmo salar), can regulate 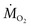 to only 36 ± 3% saturation (Table 1). Schofield and Chapman (2000) reported a [O2]crit of 15% saturation for juvenile Nile perch (Lates niloticus) at 20°C, and also noted the tendency of Nile perch to congregate at the surface layer of water during periods of aquatic hypoxia in an attempt to enhance oxygen uptake from the more aerated surface waters (commonly referred to as aquatic surface respiration). It has been suggested, however, that Nile perch are inefficient at using this technique to obtain oxygen (Schofield and Chapman, 2000). Anecdotal reports exist of farmed barramundi aggregating at the surface during periods of low DO; however, the capacity for barramundi to use aquatic surface respiration remains unclear. No attempt was made to measure aquatic surface respiration in this study; however, future experiments using respirometers with an air pocket at the surface would help to elucidate the potential for aquatic surface respiration in barramundi, and therefore their capacity to exist for extended periods in hypoxic waters below [O2]crit.
to only 36 ± 3% saturation (Table 1). Schofield and Chapman (2000) reported a [O2]crit of 15% saturation for juvenile Nile perch (Lates niloticus) at 20°C, and also noted the tendency of Nile perch to congregate at the surface layer of water during periods of aquatic hypoxia in an attempt to enhance oxygen uptake from the more aerated surface waters (commonly referred to as aquatic surface respiration). It has been suggested, however, that Nile perch are inefficient at using this technique to obtain oxygen (Schofield and Chapman, 2000). Anecdotal reports exist of farmed barramundi aggregating at the surface during periods of low DO; however, the capacity for barramundi to use aquatic surface respiration remains unclear. No attempt was made to measure aquatic surface respiration in this study; however, future experiments using respirometers with an air pocket at the surface would help to elucidate the potential for aquatic surface respiration in barramundi, and therefore their capacity to exist for extended periods in hypoxic waters below [O2]crit.
Table 1:
Critical oxygen tension ([O2]crit; expressed as percentage saturation) measurements for a range of temperate and tropical fish species
| Species | [O2]crit (% saturation) | Temperature (°C) | Fish mass (g) | Authors |
|---|---|---|---|---|
| Salmo salar | 36 ± 3 | 18 | 151 ± 41 | Barnes et al. (2011)a |
| 45 ± 4 | 22 | |||
| Lates niloticus | 15 ± 2 | 20 | 4–28 | Schofield and Chapman (2000)b |
| Prochilodus scrofa | 14 | 25 | 244 ± 76 | Fernandes et al. (1995)b |
| 28 | 35 | 295 ± 49 | ||
| Anguilla anguilla | 16 | 25 | (yellow phase) | Cruz-Neto and Steffensen (1997)b |
| Oreochromis niloticus | 11 ± 3 | 25 | 301 ± 46 | Fernandes and Rantin (1989)b |
| 19 ± 0 | 35 | |||
| Lates calcarifer | 15 ± 3 | 26 | 191 ± 23 | Present study |
| 21 ± 4 | 36 |
aValues of [O2]crit were converted to percentage saturation from milligrams per litre (100% saturation = 9.4 mg l−1 at 18°C and 8.7 mg l−1 at 22°C).
bValues of [O2]crit were converted to percentage saturation from millimetres of mercury (100% saturation = 159 mmHg).
Barramundi are featured in mass-mortality events in northern Australia (Townsend et al., 1992), and previous reports indicate that barramundi succumb rapidly to hypoxia in severe conditions. Pearson et al. (2003) reported a ‘lethal DO concentration’ of ∼15% saturation for barramundi living in temperatures ranging from 28 to 30°C; however, the details of the fish and experimental design were unclear, making the results difficult to interpret in the context of the present study. In contrast, Wu (1990) reported no mortality for 150–250 g barramundi held in saltwater after 8 h of exposure to ∼15% saturation at 25°C, but exposure to ∼8% saturation resulted in 50% mortality after 6.5 h. Butler et al. (2007) reported an ‘acute asphyxiation concentration’ of 4% saturation for 200 g barramundi in freshwater at 28°C. Fish were visibly distressed in severe acute hypoxia in the present study, but there was only one mortality during the [O2]crit tests, despite the fact that each individual fish was exposed to DO of ∼4–5% saturation in order to obtain precise [O2]crit measurements.
As well as measuring ‘acute asphyxiation’, Butler et al. (2007) investigated the relationship between gill ventilation volume and frequency in response to declining DO for barramundi at 28°C. Butler et al. (2007) demonstrated that this species increases ventilation rates down to ∼15–20% saturation, followed by a steep decline in ventilation rate as DO continues to decline. The maximal ventilation rate observed by Butler et al. (2007) corresponds closely to our observed [O2]crit measurements. This suggests that increasingly elevated ventilatory requirements may be needed to maintain a state of oxygen regulation by the fish in acute hypoxic conditions. Furthermore, a sharp decline in both ventilation rate and 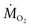 may be inevitable once DO drops below [O2]crit.
may be inevitable once DO drops below [O2]crit.
There is varying evidence in the literature for local adaptation of performance traits in sub-populations of Australian barramundi. Cairns (latitude 16°S) and Burrum River (26°S) barramundi have been found to exhibit no differences in specific growth rate, critical thermal minima, or critical thermal maxima between populations (Rodgers and Bloomfield, 1993; Burke, 1994; Keenan, 2000). Recent research, however, has documented differences in performance traits at high temperatures for Australian barramundi from lower (northern) latitudes compared with those from higher (southern) latitudes through the measurement of critical swimming speed (Edmunds et al., 2010) and time to loss of swimming equilibrium when challenged with acute water heating (Newton et al., 2010). Edmunds et al. (2012) found elevated transcript abundance of the glycolytic enzyme lactate dehydrogenase-B (ldh-b) in southern (Gladstone) sub-populations of Australian barramundi compared with northern (Darwin) sub-populations, and suggested that southern populations may be adapted to cooler temperatures. Differences were observed for pre-hypoxia 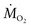 in this study, but such differences were not consistent with resting
in this study, but such differences were not consistent with resting 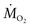 , nor was there conclusive evidence for a latitudinal trend. The results from our study indicate that both resting
, nor was there conclusive evidence for a latitudinal trend. The results from our study indicate that both resting 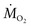 and hypoxia tolerance are conserved across sub-populations of Australian barramundi.
and hypoxia tolerance are conserved across sub-populations of Australian barramundi.
Higher temperatures consistently result in elevated 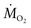 for teleost fish, irrespective of species or life history, reflecting increased metabolic requirements at higher temperatures (Fry and Hart, 1948; Brett and Groves, 1979; Clark et al., 2011). Barramundi elicit a 2-fold increase in resting
for teleost fish, irrespective of species or life history, reflecting increased metabolic requirements at higher temperatures (Fry and Hart, 1948; Brett and Groves, 1979; Clark et al., 2011). Barramundi elicit a 2-fold increase in resting 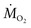 (Q10 = 2.12) over a 10°C increase in temperature. Higher
(Q10 = 2.12) over a 10°C increase in temperature. Higher 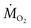 at warmer temperatures might be expected to induce a decrease in hypoxia tolerance in all fish species due to the increased oxygen demands of the fish. Results from previous studies on species such as Nile tilapia (Fernandes and Rantin, 1989; Mamun et al., 2013), rainbow trout (Oncorhynchus mykiss; Ott et al., 1980), and common carp (Cyprinus carpio; Ott et al., 1980) indicate that [O2]crit appears to be less temperature sensitive than what is typically found for resting
at warmer temperatures might be expected to induce a decrease in hypoxia tolerance in all fish species due to the increased oxygen demands of the fish. Results from previous studies on species such as Nile tilapia (Fernandes and Rantin, 1989; Mamun et al., 2013), rainbow trout (Oncorhynchus mykiss; Ott et al., 1980), and common carp (Cyprinus carpio; Ott et al., 1980) indicate that [O2]crit appears to be less temperature sensitive than what is typically found for resting 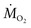 ; however, this is not consistent across all teleosts. Species such as Atlantic salmon (Barnes et al., 2011), Doederlein's cardinal fish (Ostorhinchus doederleini; Nilsson et al., 2010), and Atlantic cod (Schurmann and Steffensen, 1997) elicit a large increase in [O2]crit with increasing temperature, particularly towards upper thermal tolerance limits. Our results indicate that [O2]crit displays only a small increase for barramundi from typical (26°C) to high temperatures (36°C), demonstrating the resilient nature of this species to the synergistic effects of temperature and environmental hypoxia.
; however, this is not consistent across all teleosts. Species such as Atlantic salmon (Barnes et al., 2011), Doederlein's cardinal fish (Ostorhinchus doederleini; Nilsson et al., 2010), and Atlantic cod (Schurmann and Steffensen, 1997) elicit a large increase in [O2]crit with increasing temperature, particularly towards upper thermal tolerance limits. Our results indicate that [O2]crit displays only a small increase for barramundi from typical (26°C) to high temperatures (36°C), demonstrating the resilient nature of this species to the synergistic effects of temperature and environmental hypoxia.
Aside from temperature, a number of other environmental parameters can impact the hypoxia tolerance of fish. The ability to adjust to hypoxic conditions through a lowering of [O2]crit following pre-exposure has been reported for the epaulette shark (Hemiscyllium ocellatum; Routley et al., 2002) and goldfish (Carassius auratus; Fu et al., 2011), although Cook et al. (2011) found no differences in [O2]crit between naïve and hypoxia-conditioned silver sea bream (Pagrus auratus). Henriksson et al. (2008) demonstrated that alterations in salinity can increase the [O2]crit by up to 30% in prickly sculpin (Cottus asper) acclimated to freshwater, compared with fish adapted to seawater; however, the same trend was not observed in the closely related Pacific staghorn sculpin (Leptocottus armatus). Barramundi inhabit environments that are prone to acute and chronic hypoxia, and such environments also experience broad fluctuations in salinity and temperature. Currently, there is no understanding of the effects of exposure to repetitive (short-term) or chronic (long-term) hypoxia on the physiology, and consequently, performance of barramundi. However, this topic warrants future research to elucidate more fully the capacity of fishes to tolerate extreme and variable environments.
Elevated temperatures due to climate change are predicted to increase the frequency and severity of hypoxic events through lower O2 solubility, increased animal respiration rates, and enhanced stratification (Justic et al., 2001; Diaz and Breitburg, 2009). Such conditions have the potential to increase habitat availability at higher latitudes, while leading to the deterioration of habitats at lower latitudes (Graham and Harrod, 2009; Gardiner et al., 2010). Nutrient inputs to aquatic coastal systems from human waste and agriculture, combined with habitat degradation through increased land use, have the potential to exacerbate further the effects of environmental hypoxia on physiological systems. The present study has shown, for the first time, that a tropical euryhaline fish, barramundi, does not display obvious local adaptation in resting 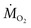 and hypoxia tolerance. This strongly suggests that all sub-populations of this species in northern Australia can cope equally well in environments with large fluctuations in both temperature and dissolved oxygen.
and hypoxia tolerance. This strongly suggests that all sub-populations of this species in northern Australia can cope equally well in environments with large fluctuations in both temperature and dissolved oxygen.
Acknowledgements
This project was supported by funding from the National Climate Change Adaptation Research Facility (NCCARF). G.M.C. was supported by an Australian Postgraduate Award and funding from the AIMS@JCU Collaborative Research Program. The Australian Institute of Marine Science supported T.D.C. and much of the experimental equipment used in this study. The authors wish to thank Professor Dean Jerry for his contribution to this project. All procedures used in this research were approved by the Animal Ethics Committee of James Cook University, Approval A1652.
References
- 1.AIMS (2012) AIMS Data Centre, Townsville. http://maps.aims.gov.au/index.html?intro=false&z=4&ll=142.91883,-17.51872&l0=aims_aims:AIMS%20-%20Temperature%20Loggers,ea_World_NE2-coast-cities-reefs_Baselayer (last accessed 19 August 2012). [Google Scholar]
- 2.Barnes R, King H, Carter CG. (2011) Hypoxia tolerance and oxygen regulation in Atlantic salmon, Salmo salar from a Tasmanian population. Aquaculture 318: 397–401. [Google Scholar]
- 3.Bermudes M, Glencross B, Austen K, Hawkins W. (2010) The effects of temperature and size on the growth, energy budget and waste outputs of barramundi (Lates calcarifer). Aquaculture 306: 160–166. [Google Scholar]
- 4.Bishop KA. (1980) Fish kills in relation to physical and chemical changes in Magela Creek (East Alligator River system, Northern Territory) at the beginning of the tropical wet season. Australian Zoologist 20: 485–500. [Google Scholar]
- 5.BOM (2012) Bureau of Meteorology – Commonwealth of Australia, Canberra. http://www.bom.gov.au/ (last accessed 19 August 2012). [Google Scholar]
- 6.Brauner CJ, Val AL. (2005) Oxygen transfer. In Val AL, Almeida Val VMFD, Randall DJ, eds, Fish Physiology, Vol 21 Academic Press, New York, pp 277–306. [Google Scholar]
- 7.Brett JR, Groves TDD. (1979) Physiological energetics. In Hoar WS, Randall DJ, Brett JR, eds, Fish Physiology, Ed, Vol. 8 Academic Press, New York, pp 279–352. [Google Scholar]
- 8.Burke JB. (1994) Temperature and salinity requirements in the early life-history of two catadromous fishes, Barramundi, Lates calcarifer (Bloch), and Australia Bass, Macquaria novemaculeata (Steindachner) in Eastern Australia. Masters thesis University of New South Wales, Sydney. [Google Scholar]
- 9.Butler B, Burrows D, Pearson RG. (2007) Testing the Hypoxia Tolerance of Tropical Freshwater Fishes, Ed, Vol ACTFR Report No. 07/31 Australian Centre for Tropical Freshwater Research, James Cook University, Townsville. [Google Scholar]
- 10.Clark TD, Jeffries KM, Hinch SG, Farrell AP. (2011) Exceptional aerobic scope and cardiovascular performance of pink salmon (Oncorhynchus gorbuscha) may underlie resilience in a warming climate. J Exp Biol 214: 3074–3081. [DOI] [PubMed] [Google Scholar]
- 11.Clark TD, Sandblom E, Jutfelt F. (2013) Aerobic scope measurements of fishes in an era of climate change: respirometry, relevance and recommendations. J Exp Biol 216: 2771–2782. [DOI] [PubMed] [Google Scholar]
- 12.Cook DG, Iftikar FI, Baker DW, Hickey AJR, Herbert NA. (2013) Low O2 acclimation shifts the hypoxia avoidance behaviour of snapper (Pagrus auratus) with only subtle changes in aerobic and anaerobic function. J Exp Biol 216: 369–378. [DOI] [PubMed] [Google Scholar]
- 13.Corkum CP, Gamperl AK. (2009) Does the ability to metabolically downregulate alter the hypoxia tolerance of fishes?: a comparative study using cunner (T. adspersus) and Greenland cod (G. ogac). J Exp Zool A Ecol Genet Physiol 311: 231–239. [DOI] [PubMed] [Google Scholar]
- 14.Cruz-Neto AP, Steffensen JF. (1997) The effects of acute hypoxia and hypercapnia on oxygen consumption of the freshwater European eel. J Fish Biol 50: 759–769. [Google Scholar]
- 15.Dhillon RS, Schulte PM. (2011) Intraspecific variation in the thermal plasticity of mitochondria in killifish. J Exp Biol 214: 3639–3648. [DOI] [PubMed] [Google Scholar]
- 16.Diaz RJ, Breitburg DL. (2009) Chapter 1, The hypoxic environment. In Richards JG, Farrell AP, Brauner CJ, eds, Fish Physiology, Vol 27 Academic Press, New York, pp 1–23. [Google Scholar]
- 17.Doupé RG, Horwitz P, Lymbery AJ. (1999) Mitochondrial genealogy of Western Australian barramundi: applications of inbreeding coefficients and coalescent analysis for separating temporal population processes. J Fish Biol 54: 1197–1209. [Google Scholar]
- 18.Edmunds RC, van Herwerden L, Fulton CJ. (2010) Population-specific locomotor phenotypes are displayed by barramundi, Lates calcarifer, in response to thermal stress. Can J Fish Aquat Sci 67: 1068–1074. [Google Scholar]
- 19.Edmunds RC, Smith-Keune C, Van Herwerden L, Fulton CJ, Jerry DR. (2012) Exposing local adaptation: synergistic stressors elicit population-specific lactate dehydrogenase-B (ldh-b) expression profiles in Australian barramundi, Lates calcarifer. Aquat Sci 74: 171–178. [Google Scholar]
- 20.Erskine WD, Saynor MJ, Erskine L, Evans KG, Moliere DR. (2005) A preliminary typology of Australian tropical rivers and implications for fish community ecology. Mar Freshw Res 56: 253–267. [Google Scholar]
- 21.Farrell AP, Richards JG. (2009) Chapter 11, Defining hypoxia: an integrative synthesis of the responses of fish to hypoxia. In JG Richards, AP Farrell, CJ Brauner, eds, Fish Physiology, Vol 27 Academic Press, NewYork, pp 487–503. [Google Scholar]
- 22.Fernandes MN, Rantin FT. (1989) Respiratory responses of Oreochromis niloticus (Pisces, Cichlidae) to environmental hypoxia under different thermal conditions. J Fish Biol 35: 509–519. [Google Scholar]
- 23.Fernandes MN, Barrionuevo WR, Rantin FT. (1995) Effects of thermal stress on respiratory responses to hypoxia of a South American Prochilodontid fish, Prochilodus scrofa. J Fish Biol 46: 123–133. [Google Scholar]
- 24.Froese R. (2006) Cube law, condition factor and weight–length relationships: history, meta-analysis and recommendations. J Appl Ichthyol 22: 241–253. [Google Scholar]
- 25.Fry FEJ, Hart JS. (1948) The relation of temperature to oxygen consumption in the goldfish. Biol Bull 94: 66–77. [PubMed] [Google Scholar]
- 26.Fu S-J, Brauner CJ, Cao Z-D, Richards JG, Peng J-L, Dhillon R, Wang Y-X. (2011) The effect of acclimation to hypoxia and sustained exercise on subsequent hypoxia tolerance and swimming performance in goldfish (Carassius auratus). J Exp Biol 214: 2080–2088. [DOI] [PubMed] [Google Scholar]
- 27.Gardiner NM, Munday PL, Nilsson GE. (2010) Counter-gradient variation in respiratory performance of coral reef fishes at elevated temperatures. PLoS One 5: e13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grabowski T, Young S, Libungan L, Steinarsson A, Marteinsdóttir G. (2009) Evidence of phenotypic plasticity and local adaption in metabolic rates between components of the Icelandic cod (Gadus morhua; L.) stock. Environ Biol Fish 86: 361–370. [Google Scholar]
- 29.Graham CT, Harrod C. (2009) Implications of climate change for the fishes of the British Isles. J Fish Biol 74: 1143–1205. [DOI] [PubMed] [Google Scholar]
- 30.Henriksson P, Mandic M, Richards Jeffrey G. (2008) The osmorespiratory compromise in sculpins: impaired gas exchange is associated with freshwater tolerance. Physiol Biochem Zool 81: 310–319. [DOI] [PubMed] [Google Scholar]
- 31.Herbert NA, Steffensen JF. (2005) The response of Atlantic cod, Gadus morhua, to progressive hypoxia: fish swimming speed and physiological stress. Mar Biol 147: 1403–1412. [Google Scholar]
- 32.Huey RB, Kearney MR, Krockenberger A, Holtum JAM, Jess M, Williams SE. (2012) Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Philos Trans R Soc Lond B Biol Sci 367: 1665–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hutchinson MF, Dowling TI. (1991) A continental hydrological assessment of a new grid-based digital elevation model of Australia. Hydrol Process 5: 45–58. [Google Scholar]
- 34.Jesse MJ, Shub C, Fishman AP. (1967) Lung and gill ventilation of the African lung fish. Respir Physiol 3: 267–287. [DOI] [PubMed] [Google Scholar]
- 35.Justic D, Rabalais NN, Turner RE. (2001) Implications of global climate change for coastal and estuarine hypoxia: hypothesis, observations and models for the Northern Gulf of Mexico. Ed, Proceedings of the 6th International Symposium on Fish Physiology, Athens, Georgia. [Google Scholar]
- 36.Keenan C. (1994) Recent evolution of population structure in Australian barramundi, Lates calcarifer (Bloch): an example of isolation by distance in one dimension. Mar Freshw Res 45: 1123–1148. [Google Scholar]
- 37.Keenan CP. (2000) Should we allow human-induced migration of the Indo–West Pacific fish, barramundi Lates calcarifer (Bloch) within Australia? Aquac Res 31: 121–131. [Google Scholar]
- 38.Mamun S, Focken U, Becker K. (2013) A respirometer system to measure critical and recovery oxygen tensions of fish under simulated diurnal fluctuations in dissolved oxygen. Aquac Int 21: 31–44. [Google Scholar]
- 39.Morrongiello JR, Beatty SJ, Bennett JC, Crook DA, Ikedife DNEN, Kennard MJ, Kerezsy A, Lintermans M, McNeil DG, Pusey BJ, et al. (2011) Climate change and its implications for Australia's freshwater fish. Mar Freshw Res 62: 1082–1098. [Google Scholar]
- 40.Newton JR, Smith-Keune C, Jerry DR. (2010) Thermal tolerance varies in tropical and sub-tropical populations of barramundi (Lates calcarifer) consistent with local adaptation. Aquaculture 308 Supplement 1: S128–S132. [Google Scholar]
- 41.Nilsson GE, Hobbs J-P, Munday PL, Östlund-Nilsson S. (2004) Coward or braveheart: extreme habitat fidelity through hypoxia tolerance in a coral-dwelling goby. J Exp Biol 207: 33–39. [DOI] [PubMed] [Google Scholar]
- 42.Nilsson GE, Östlund-Nilsson S, Munday PL. (2010) Effects of elevated temperature on coral reef fishes: loss of hypoxia tolerance and inability to acclimate. Comp Biochem Physiol A Mol Integr Physiol 156: 389–393. [DOI] [PubMed] [Google Scholar]
- 43.Ott ME, Heisler N, Ultsch GR. (1980) A re-evaluation of the relationship between temperature and the critical oxygen tension in freshwater fishes. Comp Biochem Physiol A Physiol 67: 337–340. [Google Scholar]
- 44.Pearson RG, Crossland MR, Butler B, Manwaring S. (2003) Effects of Cane-field Drainage on the Ecology of Tropical Waterways, Ed, Vol Report No. 03/04-1,2,3 to Sugar R&D Corporation James Cook University, Townsville. [Google Scholar]
- 45.Pollock MS, Clarke LMJ, Dubé MG. (2007) The effects of hypoxia on fishes: from ecological relevance to physiological effects. Environ Rev 15: 1–14. [Google Scholar]
- 46.Poulsen SB, Jensen LF, Nielsen KS, Malte H, Aarestrup K, Svendsen JC. (2011) Behaviour of rainbow trout Oncorhynchus mykiss presented with a choice of normoxia and stepwise progressive hypoxia. J Fish Biol 79: 969–979. [DOI] [PubMed] [Google Scholar]
- 47.Prosser CL, Brown FA. (1961) Comparative Animal Physiology. Saunders, Philadelphia. [Google Scholar]
- 48.Pusey B, Arthington A, Read M. (1998) Freshwater fishes of the Burdekin River, Australia: biogeography, history and spatial variation in community structure. Environ Biol Fish 53: 303–318. [Google Scholar]
- 49.Randall DJ, Smith JC. (1967) The regulation of cardiac activity in fish in a hypoxic environment. Physiol Zool 40: 104–113. [Google Scholar]
- 50.Rodgers LJ, Bloomfield JP. (1993) Comparison of growth, condition and mortality between stocks of barramundi Lates calcarifer: 1. Cairns and Burrum River strains. In Barlow CG, Rimmer MA, eds, Larval and Juvenile Culture of Barramundi Lates calcarifer. Fishing Industry Research and Development Council, Canberra, pp 9.1–9. [Google Scholar]
- 51.Routley MH, Nilsson GE, Renshaw GMC. (2002) Exposure to hypoxia primes the respiratory and metabolic responses of the epaulette shark to progressive hypoxia. Comp Biochem Physiol A Mol Integr Physiol 131: 313–321. [DOI] [PubMed] [Google Scholar]
- 52.Russell D, Garrett R. (1983) Use by juvenile barramundi, Lates calcarifer (Bloch), and other fishes of temporary supralittoral habitats in a tropical estuary in Northern Australia. Mar Freshw Res 34: 805–811. [Google Scholar]
- 53.Schofield PJ, Chapman LJ. (2000) Hypoxia tolerance of introduced Nile perch: implications for survival of indigenous fishes in the Lake Victoria basin. Afr Zool 35: 35–42. [Google Scholar]
- 54.Schurmann H, Steffensen JF. (1997) Effects of temperature, hypoxia and activity on the metabolism of juvenile Atlantic cod. J Fish Biol 50: 1166–1180. [Google Scholar]
- 55.Stuart IG, Berghuis AP. (2002) Upstream passage of fish through a vertical-slot fishway in an Australian subtropical river. Fish Manag Ecol 9: 111–122. [Google Scholar]
- 56.Sylvestre E-L, Lapointe D, Dutil J-D, Guderley H. (2007) Thermal sensitivity of metabolic rates and swimming performance in two latitudinally separated populations of cod, Gadus morhua L. J Comp Physiol B 177: 447–460. [DOI] [PubMed] [Google Scholar]
- 57.Tewksbury JJ, Huey RB, Deutsch CA. (2008) Putting the heat on tropical animals. Science 320: 1296–1297. [DOI] [PubMed] [Google Scholar]
- 58.Timmerman CM, Chapman LJ. (2004) Hypoxia and interdemic variation in Poecilia latipinna. J Fish Biol 65: 635–650. [Google Scholar]
- 59.Tobler M, Palacios M, Chapman LJ, Mitrofanov I, Bierbach D, Plath M, Arias-Rodriguez L, Garcí de Leóna FJ, Mateos M. (2011) Evolution in extreme environments: replicated phenotypic differentiation in livebearing fish inhabiting sulfidic springs. Evolution 65: 2213–2228. [DOI] [PubMed] [Google Scholar]
- 60.Townsend SA, Boland KT, Wrigley TJ. (1992) Factors contributing to a fish kill in the Australian wet/dry tropics. Water Res 26: 1039–1044. [Google Scholar]
- 61.Vaquer-Sunyer R, Duarte CM. (2008) Thresholds of hypoxia for marine biodiversity. Proc Natl Acad Sci USA 105: 15452–15457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Webster IT, Rea N, Padovan AV, Dostine P, Townsend SA, Cook S. (2005) An analysis of primary production in the Daly River, a relatively unimpacted tropical river in northern Australia. Mar Freshw Res 56: 303–316. [Google Scholar]
- 63.Weiss RF. (1970) The solubility of nitrogen, oxygen and argon in water and seawater. Deep Sea Res Oceanogr Abstr 17: 721–735. [Google Scholar]
- 64.Wells RMG, Grigg GC, Beard LA, Summers G. (1989) Hypoxic responses in a fish from a stable environment: blood oxygen transport in the Antarctic fish Pagothenia Borchgrevinki. J Exp Biol 141: 97–111. [Google Scholar]
- 65.Whitehead A, Galvez F, Zhang S, Williams LM, Oleksiak MF. (2011) Functional genomics of physiological plasticity and local adaptation in killifish. J Hered 102: 499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu RSS. (1990) Environmental Tolerance of some Marine Fish: Applications in Mariculture Management. US Environmental and Protection Agency, Guangzhou. [Google Scholar]
- 67.Zar JH. (2010) Biostatistical Analysis, Ed 5 Pearson Prentice-Hall, Upper Saddle River. [Google Scholar]