Abstract
Proinsulin folding within the endoplasmic reticulum (ER) remains incompletely understood, but it is clear that in mutant INS gene–induced diabetes of youth (MIDY), progression of the (three) native disulfide bonds of proinsulin becomes derailed, causing insulin deficiency, β-cell ER stress, and onset of diabetes. Herein, we have undertaken a molecular dissection of proinsulin disulfide bond formation, using bioengineered proinsulins that can form only two (or even only one) of the native proinsulin disulfide bonds. In the absence of preexisting proinsulin disulfide pairing, Cys(B19)-Cys(A20) (a major determinant of ER stress response activation and proinsulin stability) preferentially initiates B-A chain disulfide bond formation, whereas Cys(B7)-Cys(A7) can initiate only under oxidizing conditions beyond that existing within the ER of β-cells. Interestingly, formation of these two “interchain” disulfide bonds demonstrates cooperativity, and together, they are sufficient to confer intracellular transport competence to proinsulin. The three most common proinsulin disulfide mispairings in the ER appear to involve Cys(A11)-Cys(A20), Cys(A7)-Cys(A20), and Cys(B19)-Cys(A11), each disrupting the critical Cys(B19)-Cys(A20) pairing. MIDY mutations inhibit Cys(B19)-Cys(A20) formation, but treatment to force oxidation of this disulfide bond improves folding and results in a small but detectable increase of proinsulin export. These data suggest possible therapeutic avenues to ameliorate ER stress and diabetes.
Introduction
Diabetes onset and progression have been ascribed to both hyperglycemia-mediated and dyslipidemia-mediated β-cell toxicity (1,2). Both stimuli initially cause β-cells to adjust their secretory capacity, including changes in the endoplasmic reticulum (ER) that are adapted to limit accumulation of misfolded proinsulin, the major β-cell secretory protein product (3–5). Despite this regulation, there are numerous pathological circumstances in which proinsulin misfolding outstrips the genetically programmed adaptive capacity of the ER (6); this basic realization has been recognized for more than a decade (7).
The clearest model of β-cell failure triggered by proinsulin misfolding currently comes from a cluster of autosomal dominant INS gene mutations that produce the syndrome we call MIDY (mutant INS gene–induced diabetes of youth) (8). To the best of our knowledge, all MIDY mutations perturb the native disulfide pairing of proinsulin (9,10). The process of proinsulin disulfide bond formation in vivo is complicated by the rapid time scale (<1 min) for proinsulin translation/translocation into the ER lumen (11), the timing of preproinsulin signal peptide cleavage (12), the regulation of the ER lumenal redox environment (13,14), and activity of ER oxidoreductases (15). It is recognized that all three disulfide bonds of insulin, the two “interchain” disulfide bonds called Cys(B7)-Cys(A7) and Cys(B19)-Cys(A20) as well as the intra-A chain disulfide Cys(A6)-Cys(A11)—all of which form within proinsulin—must be intact for optimal insulin bioactivity on the insulin receptor (16). Nevertheless, few studies have actually examined the pathway(s) of proinsulin folding and misfolding within the β-cell ER. What is known is that in vitro refolding of proteins of the insulin/IGF superfamily proceeds stepwise via one- and two-disulfide intermediates, in which a molten globule containing the B19-A20 disulfide bond is critical (17–19). Protein disulfide isomerase (PDI) enhances this in vitro folding progression of proinsulin (20,21). However, within β-cells, overexpression of PDI causes proinsulin accumulation within the ER (22) and knockdown of PDI expression actually enhances ER export of properly folded proinsulin (23). These findings highlight the importance of examining proinsulin folding in a cellular context.
In this report, we probe proinsulin folding in the ER of pancreatic β-cells, clearly demonstrating both preferential initiation of the Cys(B19)-Cys(A20) disulfide bond and cooperativity in formation of the two interchain disulfides required for the intracellular transport of proinsulin. We have identified cysteine interlopers that can lure uncommitted Cys(B19) or Cys(A20) into infidelity of disulfide pairing, which is pathognomonic of proinsulin misfolding. Moreover, native proinsulin disulfide bond formation appears linked to the oxidative environment of the ER lumen, which controls the dwell time of free cysteine thiols that may participate in proinsulin disulfide mispairing, which can lead to ER stress and diabetes (24).
Research Design and Methods
Materials
Bis-Tris 4–12% polyacrylamide (NuPAGE) gels were from Life Technologies; protease inhibitor was from Roche. Mouse anti-tubulin and diamide were from Sigma-Aldrich. Guinea pig anti-insulin was from Millipore. Rabbit anti-myc (RMYC-45A) and chicken anti-myc (CMYC-45A) were from Immunology Consultants Laboratories. Horseradish peroxidase–conjugated antibodies were from Jackson ImmunoResearch. Enhanced chemiluminescence Western blotting substrate was from Millipore. Trans35S label and pure 35S-methionine were from Perkin Elmer. ProSieve 50 was from Lonza.
Plasmids
Human myc-tagged preproinsulin cDNA in pTARGET has previously been described (9,25). Cysteine missense mutations C(A7)Y, C(A6)S, C(A11)S, C(A20)S, C(B7)S, and B(19)S were introduced as a single, double (“lose mutants”), or quadruplet (“keep mutants”) in the human myc-tagged preproinsulin plasmid (QuikChange site-directed mutagenesis kit, Agilent). Two methionines were introduced into the human myc-tagged preproinsulin C-peptide using the following primers: 5′-CGCCGGGAGGCAGAGGACATGCAGGTGATGCAGGTGGAGCTGGGCGGG-3′ and 5′-CCCGCCCAGCTCCACCTGCATCACCTGCATGTCCTCTGCCTCCCGGCG-3′.
Cell Culture and Transfection
INS1 β-cells were cultured in RPMI medium supplemented with 10% FBS, 10 mmol/L HEPES, 1 mmol/L sodium pyruvate, penicillin streptomycin, and 50 μmol/L 2-mercaptoethanol (26). Human embryonic kidney 293T (293T) cells were cultured in DMEM with 10% FBS, penicillin (100 units/mL), and streptomycin (100 μg/mL). INS1 or 293T cells seeded in 12-well plates were transfected with Lipofectamine 2000 and lysed 48 h later by boiling in SDS-gel sample buffer.
SDS-PAGE and Western Blotting
Proteins in islet lysates or media were resolved by Bis-Tris 4–12% polyacrylamide gels, electrotransferred to nitrocellulose, and probed with primary anti-insulin, anti-myc, or anti–α-tubulin primary antibodies and appropriate peroxidase-conjugated secondary antibodies. Western blots were quantified using ImageJ software.
Human Proinsulin Secretion
At 30 h after transfection of 293T cells, the bathing medium was changed to fresh medium, which was then collected for 18 h before analysis.
Metabolic Labeling and Immunoprecipitation
At 48 h posttransfection, cells were incubated in media lacking cysteine/methionine for 30 min and then pulse labeled with 35S-Met (specific activity >1,000 Ci per mmol) or 35S-Cys/Met. At appropriate chase times, cells were washed once with PBS containing 20 mmol/L N-ethyl maleimide (NEM) and then lysed in radioimmunoprecipitation assay buffer (25 mmol/L Tris, pH 7.5; 1% Triton X-100; 0.2% deoxycholic acid; 0.1% SDS; 10 mmol/L EDTA; and 100 mmol/L NaCl) plus 2 mmol/L NEM and a protease inhibitor cocktail. Cell lysates, normalized to trichloroacetic acid precipitable counts, were precleared with zysorbin and immunoprecipitated with anti-myc or anti-insulin antibodies and protein A agarose overnight at 4°C. Immunoprecipitates were analyzed by nonreducing and reducing Tris–tricine–urea–SDS-PAGE, with phosphorimaging. Bands were quantified using ImageJ software.
Confocal Imaging
Cells plated on chamber slides (Nunc) were transfected, fixed in 3.7% formaldehyde (in PBS, pH 7.4) for 20 min at room temperature, washed with PBS, permeabilized with 0.4% Triton X-100 in TBS, blocked with TBS containing 3% BSA and 0.2% Triton X-100, and stained overnight at 4°C with primary antibodies: guinea pig anti-insulin and chicken anti-myc diluted in TBS containing 3% BSA and 0.2% Tween 20. Thereafter, slides were rinsed and incubated with secondary antibodies conjugated to Alexa-488 or -568, mounted with Prolong Gold with DAPI, and imaged by epifluorescence in an Olympus FV500 confocal microscope with a ×60 oil objective.
BiP Promoter–Driven Luciferase Assay
BiP luciferase was performed as previously described (9). Briefly, INS1 cells were cotransfected with BiP-firefly-luciferase reporter plasmid (27) and human proinsulin constructs at a DNA ratio of 1:10. At 48 h posttransfection, cell extracts were prepared for the firefly luciferase reporter assay, normalized to proinsulin expression.
Statistical Analysis
Results are presented as means ± SEM, calculated using GraphPad Prism software, version 6.0. Statistical differences were determined using unpaired Mann-Whitney t test or ANOVA.
Results
Minimum Requirements for Oxidative Folding and ER Exit of Proinsulin
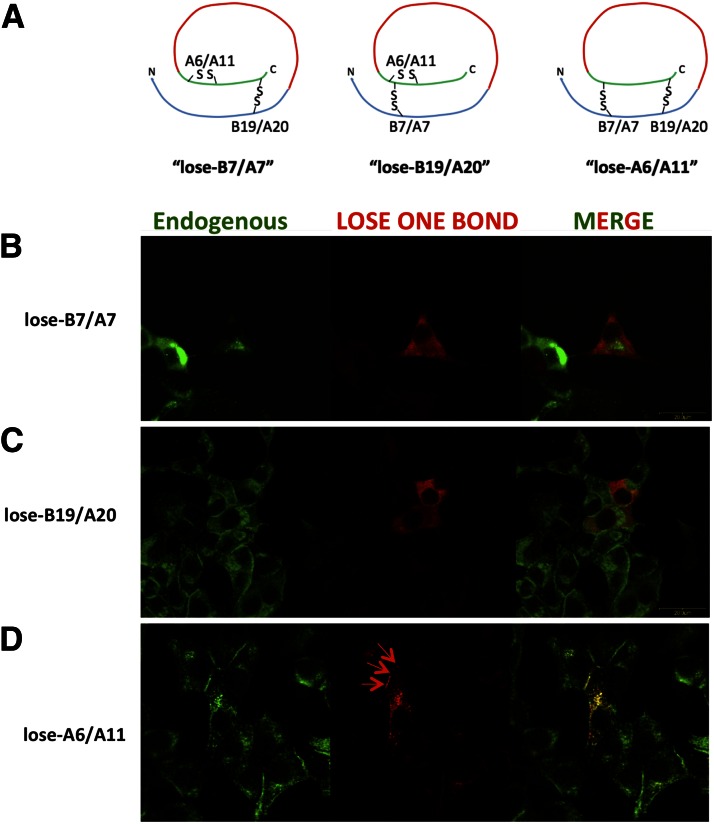
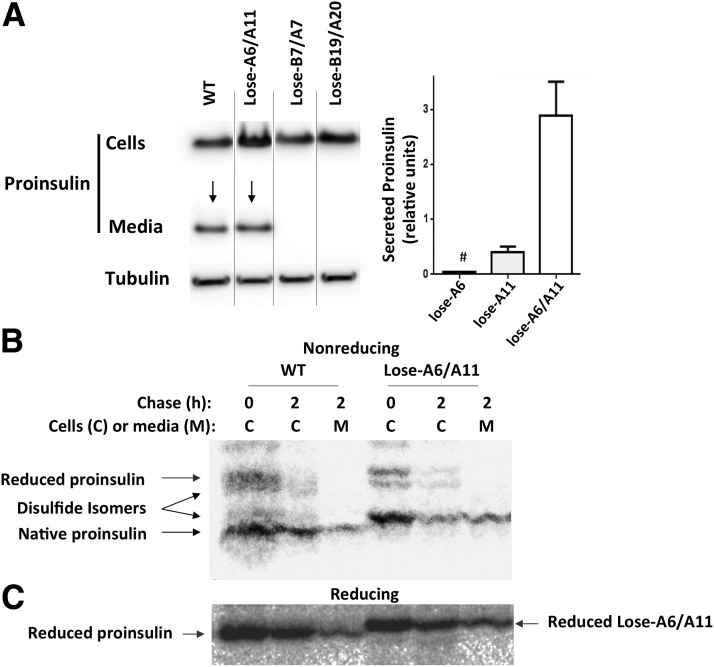
It has been suggested that of proinsulin’s three disulfide bonds, the intra-A chain (A6-A11) pairing contributes least to three-dimensional structure and is not essential for quality control of proinsulin exit from the ER (16,28–30). To examine this directly, we generated three proinsulin missense mutants, each replacing precisely two Cys residues that normally partner in a native proinsulin disulfide bond (Fig. 1A). When expressed in transiently transfected INS1 pancreatic β-cells, proinsulin constructs that lose-B7/A7 or lose-B19/A20 exhibited an ER-like intracellular distribution that poorly colocalized with endogenous insulin (Fig. 1B and C). By contrast, lose-A6/A11 exhibited a punctate staining in juxtanuclear and subplasmalemmal regions that colocalized with insulin secretory granules (Fig. 1D). Further, in transiently transfected 293T cells, whereas lose-B7/A7 and lose-B19/A20 were not secreted, lose-A6/A11 was recovered in the culture medium at high levels (Fig., 2A left). These data confirm that the Cys(A6)-Cys(A11) disulfide bond is not essential for proinsulin exit from the ER; yet, this seems surprising because diabetes in the Ins2-Munich proinsulin-C(A6)S heterozygous mouse (which begins at 1 month of age) has been specifically attributed to loss of the Cys(A6)-Cys(A11) disulfide bond (31). We therefore examined proinsulins with single Cys missense mutations (to Ser) at either A6 or A11 positions. Interestingly, while proinsulin-C(A6)S export was blocked, secretion of proinsulin-C(A11)S was consistently detectable (Fig. 2A, right) and the lose-A6/A11 double mutant was secreted at much higher levels (Fig. 2A–C). The results imply that diabetes development in the Ins2-Munich mouse is not due to loss of the A6-A11 disulfide bond but is caused by catastrophic proinsulin misfolding linked to the presence of the remaining unpaired Cys(A11) residue.
Figure 1.
Expression of proinsulin “lose one bond” mutants in pancreatic β-cells. A: Schematic of the “lose mutants”; all constructs were myc tagged within the C-peptide (shown in red). B–D: Indirect immunofluorescence localization of lose mutants in INS1 cells (anti-myc [in red]) compared with that observed for anti-insulin that detects primarily the endogenous Ins1 and Ins2 gene products (in green)—a merged image is shown at right. The red arrows indicate the subplasmalemmal distribution of insulin secretory granules. Scale bar = 20 µm.
Figure 2.
Secretion of proinsulin “lose mutants” from pancreatic β-cells. A: 293T cells were transfected with myc-tagged proinsulin bearing wild-type B and A chains (WT), lose-A6/A11, lose-B7/A7, lose-B19/A20, or single C(A6)S or C(A11)S. At 30 h posttransfection, cell culture media were collected overnight, and cells were lysed. Left: Secretion and cellular content of myc-tagged proinsulin were analyzed by Western blotting using anti-myc (for proinsulin) and anti-tubulin antibodies. Right: Quantitation of secretion as measured by Western blotting (from 8 individual samples in 3 independent experiments. Additional proinsulin secretion results, with the identical conclusion, were obtained as measured by rat insulin radioimmunoassay [not shown]). B and C: 293T cells transfected with myc-tagged proinsulin bearing wild-type B and A chains or lose-A6/A11 were pulse labeled with 35S-labeled amino acids for 30 min and chased as indicated. The media were collected and cells lysed; both samples were immunoprecipitated with anti-insulin and then analyzed by nonreducing (B) and reducing (C) Tris–tricine–urea–SDS-PAGE and autoradiography. In this gel system, lose-A6/A11 migrates more slowly than wild type even under reducing conditions, but this does not confuse identification of disulfide isomers that clearly migrate much more slowly under nonreducing conditions.
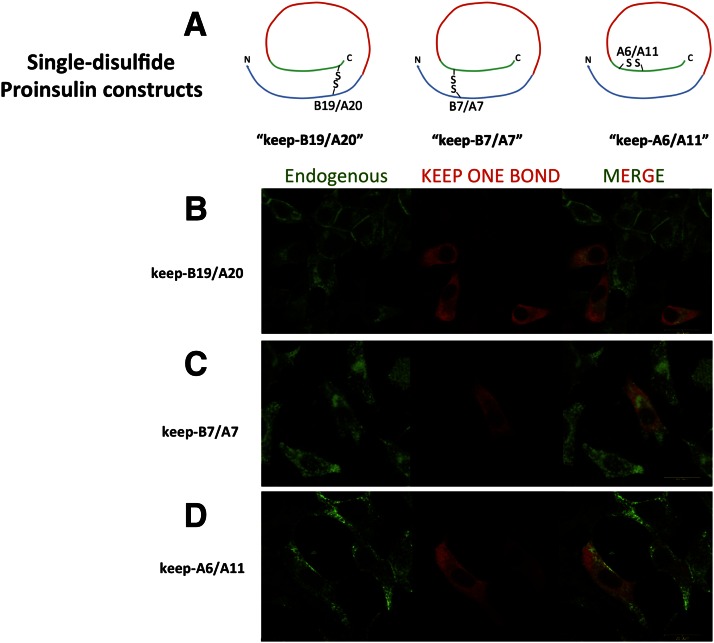
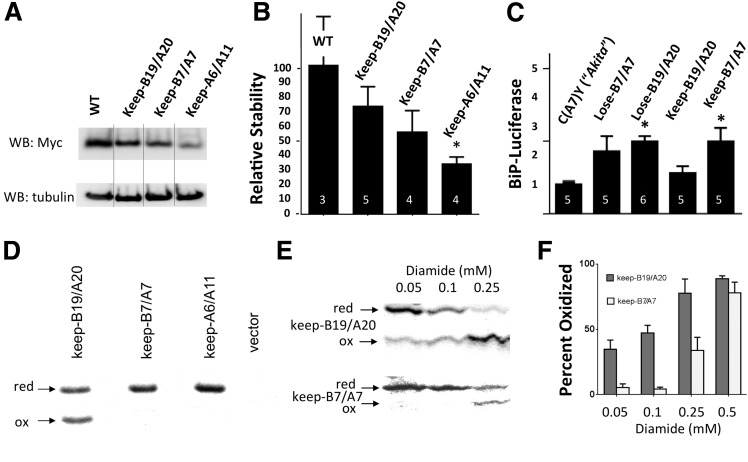
Unlike the Cys(A6)-Cys(A11) disulfide bond, anterograde transport of proinsulin requires that both interchain disulfide bonds must be intact (32); i.e., absence of either exposes structural information that causes proinsulin to be retained within the ER (29). With this in mind, we went on to prepare three distinct proinsulin missense mutants, each potentially capable of making only a single native disulfide bond (Fig. 3A), with the understanding that none of these mutants can be exported. Importantly, each of these “keep one bond” mutants was synthesized at a comparable rate that was 80–95% that of wild-type proinsulin (Supplementary Fig. 1), and as expected, each was entrapped within the ER in INS1 β-cells (Fig. 3B–D), where they colocalized with calnexin (not shown). When the steady-state levels of these proteins were examined by Western blotting, keep-A6/A11 exhibited a major loss of proinsulin stability in the ER due to its complete absence of interchain disulfide bonds, whereas keep-B7/A7 and keep-B19/A20 had levels of stability that were intermediate between keep-A6/A11 and wild-type proinsulin (Fig. 4A and B). Misfolding of MIDY proinsulin mutants results in activation of an ER stress response that can be measured using a BiP promoter–luciferase reporter assay (9). We found that when compared with Akita proinsulin as a positive control (9), proinsulin constructs lacking cysteines B19/A20 exhibited a significant further increase of ER stress response in INS1 cells, whereas keep-B19/A20 (despite encoding only one potential disulfide bond) provided the least additional ER stress response activation (Fig. 4C).
Figure 3.
Expression of proinsulin “keep one bond” mutants in pancreatic β-cells. A: Schematic of the “keep mutants”; all constructs were myc tagged within the C-peptide (shown in red). B–D: Immunofluorescence localization of keep mutants in INS1 cells (anti-myc [red]) compared with that observed for anti-insulin that detects primarily the endogenous Ins1 and Ins2 gene products (in green)—a merged image is shown at right. Scale bar = 20 μm.
Figure 4.
Oxidation and stability of proinsulin keep mutants in pancreatic β-cells. A and B: INS1 cells were transiently transfected with myc-tagged proinsulin bearing wild-type B and A chains (WT), keep-B19/A20, keep-B7/A7, or keep-A6/A11. At 48 h, cells were lysed and analyzed by reducing SDS-PAGE and Western blotting (WB) with anti-myc and anti-tubulin (A), with bands quantified by ImageJ and normalized to the synthesis of newly made proinsulin (Supplementary Fig. 1) (B) (the numbers in white reflect independent replicates); data represent the mean ± SEM (*P < 0.05 relative to wild type). C: INS1 cells were transiently cotransfected with a BiP promoter–firefly luciferase plasmid and plasmid encoding myc-tagged proinsulin C(A7)Y (Akita), lose mutants, or keep mutants at a ratio of 1:10, respectively. At 48 h posttransfection, cells were lysed and firefly luciferase was measured per cellular content of myc-tagged proinsulin. The numbers in white reflect independent replicates; data represent the mean ± SEM (*P < 0.05 relative to Akita proinsulin). D and E: INS1 cells were transiently transfected with keep-B19/A20, keep-B7/A7, or keep-A6/A11. At 48 h, cells were pulse labeled with 35S-Met for 15 min (D) and treated with diamide for 2 min at the concentrations shown (E). The cells were washed with ice-cold PBS containing 20 mmol/L NEM and lysed in the presence of 2 mmol/L NEM. Cell lysates were immunoprecipitated with anti-myc antibodies, and newly synthesized proinsulin was analyzed by nonreducing Tris–tricine–urea–SDS-PAGE and phosphorimaging. The positions of both reduced (red) and oxidized (ox) forms of newly synthesized keep-B19/A20 and keep-B7/A7 are indicated. F: Oxidized and reduced proinsulin expressions from three independent experiments similar to that shown in E were quantified by densitometry.
Using the keep mutants, we asked whether formation of native disulfide bonds occurs as a stochastic process or whether there is a preferred step initiating proinsulin oxidative folding in the ER. Notably, de novo formation of an initial proinsulin Cys(B7)-Cys(A7) disulfide in the ER of INS1 cells was below detectable levels, whereas keep-B19/A20 readily demonstrated its ability to oxidize in the absence of any preexisting disulfide bond (Fig. 4D). Does this mean that it is not possible for proinsulin to initiate folding via Cys(B7)-Cys(A7) disulfide pairing? To explore the contribution of the ER oxidative environment to Cys(B7)-Cys(A7) and Cys(B19)-Cys(A20) disulfide bonding, immediately after synthesis of proinsulin keep mutants we examined the effect of a very brief exposure of cells to varying doses of diamide, which consumes reduced glutathione and oxidizes the ER lumen (33,34). Treatment with 0.25 mmol/L diamide not only resulted in most proinsulin molecules being able to initiate interchain disulfide bond formation via Cys(B19)-Cys(A20) (Fig. 4E, upper panel) but also, remarkably, now enabled a fraction of proinsulin to initiate oxidative folding via Cys(B7)-Cys(A7) (Fig. 4E, lower panel). Indeed, INS1 cells briefly exposed to 0.5 mmol/L diamide were able to promote oxidation of nearly the entire population of either keep-B19/A20 or keep-B7/A7 molecules (Fig. 4F). Although diamide treatment clearly overdrives thiol oxidation (35), the data in Fig. 4, taken together, support that Cys(B19)-Cys(A20) initiates proinsulin interchain disulfide bond formation and also suggest that control of the ER intralumenal oxidative environment can regulate the efficiency of proinsulin oxidative maturation in pancreatic β-cells. Indeed, in INS1 cells in the absence of diamide treatment, there was a range between experiments in the fraction of proinsulin molecules that formed an initial Cys(B19)-Cys(A20) disulfide (never being a majority, on average representing approximately one-third of molecules).
Cooperativity in Oxidative Folding of Proinsulin
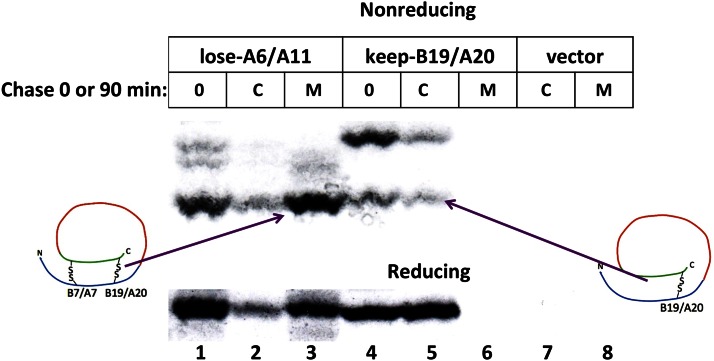
The fact that only a minority of newly synthesized proinsulin in INS1 cells can initiate interchain disulfide bonding via Cys(B19)-Cys(A20) (Fig. 5, lane 4—and further progression was not detected as a function of time after synthesis—see lane 5) and virtually none can initiate via Cys(B7)-Cys(A7) (Fig. 4D and E) makes it all the more impressive that lose-A6/A11, bearing only four cysteines, exhibits efficient cellular export (Fig. 2A) that depends upon both interchain disulfide bonds being intact. Consistently, we found that the majority of lose-A6/A11 molecules became fully oxidized immediately after synthesis (Fig. 5, lane 1) and the oxidized species was the primary secreted form (Fig. 2B and Fig. 5, lane 3). This remarkably synergistic enhancement of interchain disulfide bond formation indicates cooperativity in the oxidative folding of proinsulin in the ER of pancreatic β-cells.
Figure 5.
Cooperativity in proinsulin interchain disulfide pairing. INS1 cells transfected with lose-A6/A11 or keep-B20/A20 (or empty vector) were pulse labeled with 35S-labeled amino acids for 15 min and either lysed without chase (0) or chased for 90 min. After washing of cells in ice-cold PBS containing 20 mmol/L NEM, cells were lysed in the presence of 2 mmol/L NEM. After immunoprecipitation with anti-myc, cell lysates (C) and media (M) were analyzed by nonreducing (upper) or reducing (lower) Tris–tricine–urea–SDS-PAGE and autoradiography.
Disulfide Promiscuity Linked to Proinsulin Misfolding
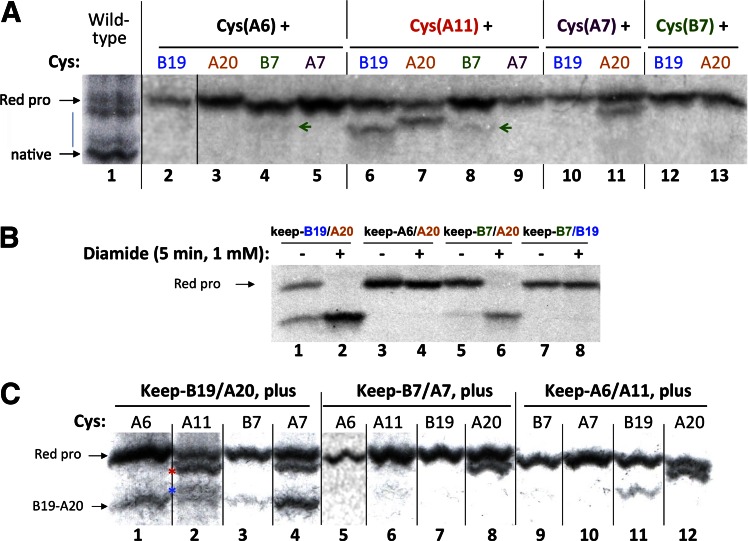
The foregoing results suggested that proper proinsulin folding in β-cells requires an initiating Cys(B19)-Cys(A20) interchain linkage with cooperative formation of the Cys(B7)-Cys(A7) disulfide bond. These findings led us to pursue the fidelity of intramolecular cysteine pairings, i.e., formation of improper interactions that may compete with native proinsulin disulfide bond formation in the ER of pancreatic β-cells. For this, we began with a series of simple two-cysteine proinsulin constructs, each with the potential to form 1 of 12 possible improper disulfide pairings, and analyzed the newly synthesized proinsulins by nonreducing Tris–tricine–urea–SDS-PAGE. We immediately noted that an unpaired Cys(A6) was relatively resistant to intramolecular disulfide mispairing, although very tiny quantities of newly synthesized Cys(B7)-Cys(A6) could be detected (Fig. 6A, lane 4, green arrow). Remarkably, even when partnered with the highly reactive Cys(A20) (Fig. 6A, lane 3), a disulfide mispairing with Cys(A6) could not be forced despite treatment of cells with 1.0 mmol/L diamide (Fig. 6B, lane 4). Thus, diabetogenic proinsulin misfolding is much more likely to arise from an unpaired Cys(A11) residue than from an unpaired Cys(A6), consistent with the phenotype of the Munich diabetic mouse that expresses the Ins2-proinsulin-Ser(A6) mutant (31). Indeed, separate from the native disulfide pairing of Cys(A11), it was apparent that Cys(A11) could substantively mispair with three alternative intramolecular partners: Cys(B19), Cys(A20), and, more weakly, Cys(B7) (Fig. 6A, lanes 6–8). In addition to inappropriate pairing involving Cys(A11), Cys(A20) could also engage in an inappropriate partnership with Cys(A7) (Fig. 6A, lanes 7 and 11). Other minor disulfide mispairings such as Cys(A20) with Cys(B7) (Fig. 6A, lane 13) only became obvious in an ER environment that was hyperoxidized by diamide treatment (Fig. 6B, lane 6, and data not shown). Most importantly, each of the three most abundantly observed proinsulin disulfide mispairings [Cys(A11)-Cys(A20), Cys(A7)-Cys(A20), and Cys(B19)-Cys(A11)] competes with formation of the critical initiating interchain disulfide bond Cys(B19)-Cys(A20).
Figure 6.
Potential infidelity of proinsulin disulfide bond formation. A: INS1 cells were transiently transfected with proinsulin mutants bearing only two Cys residues that can potentially form only nonnative disulfide partnerships. At 24 h after transfection, cells were pulse labeled with 35S-amino acids for 10 min, lysed as in Fig. 5, and immunoprecipitated with anti-insulin followed by nonreducing Tris–tricine–urea–SDS-PAGE and phosphorimaging. Lane 1 (separated by black line) is a wild-type proinsulin control analyzed separately. Lane 2 (separated by black line) is from the same gel but was underloaded and was intentionally overexposed to facilitate comparison. Small green arrows denote minor misfolded species formed by Cys(B7), as described in the text. B: 293T were transiently transfected to express keep-B19/A20 or selected novel keep mutants bearing only two Cys residues that can potentially form only nonnative disulfide partnerships. After pulse labeling for 10 min as in A, cells were either untreated (–) or treated with 1 mmol/L diamide (+) for 5 min, lysed, and immunoprecipitated and analyzed as in A. C: INS1 cells were transiently transfected with myc-tagged keep mutants, each bearing one additional interloper cysteine. At 48 h after transfection, cells were labeled with 35S-amino acids for 15 min, lysed as in Fig. 5, immunoprecipitated with anti-myc, and resolved by nonreducing Tris–tricine–urea–SDS-PAGE and phosphorimaging.
To directly demonstrate the potential for such competition in the ER of pancreatic β-cells, we transfected INS1 cells and proceeded to examine the oxidation of keep-B19/A20 in the presence of an unpaired interloper Cys residue (Supplementary Fig. 2). By itself, keep-B19/A20 appears as only two possible bands by nonreducing Tris–tricine–urea–SDS-PAGE: a faster migrating proinsulin with disulfide intact or a slower migrating proinsulin with disulfide absent (Fig. 6B, lanes 1–2). However, in the presence of a third cysteine, specifically, unpaired Cys(A11) such as occurs in the Munich proinsulin C(A6)S, major new bands appeared that (based on mispairing standards shown in Fig. 6A) correspond to Cys(A11)-Cys(A20) and Cys(A11)-Cys(B19) mispairs (Fig. 6C, asterisks, lane 2). Additionally, an unpaired Cys(A7) formed a readily detectable Cys(A7)-Cys(A20) mispairing (Fig. 6A, lane 11, and Fig. 6C, lane 4, respectively). Thus, particularly Cys(A11) and also Cys(A7) are potential interlopers that can interfere with proinsulin initiating its critical Cys(B19)-Cys(A20) interchain disulfide bond in the ER of pancreatic β-cells. Reciprocally, an unpaired Cys(A20) is a potential interloper in the Cys(B7)-Cys(A7) pairing (Fig. 6C, lane 8) as well as interfering with the intrachain Cys(A6)-Cys(A11) pairing (lane 12). By contrast, proinsulin Cys(A6) appears to be quite nonreactive in intramolecular disulfide mispairing, and Cys(B7) is also somewhat limited in intramolecular mispairing, although such mispairing can be forced with diamide treatment (Fig. 6B, lane 6).
Significance for MIDY
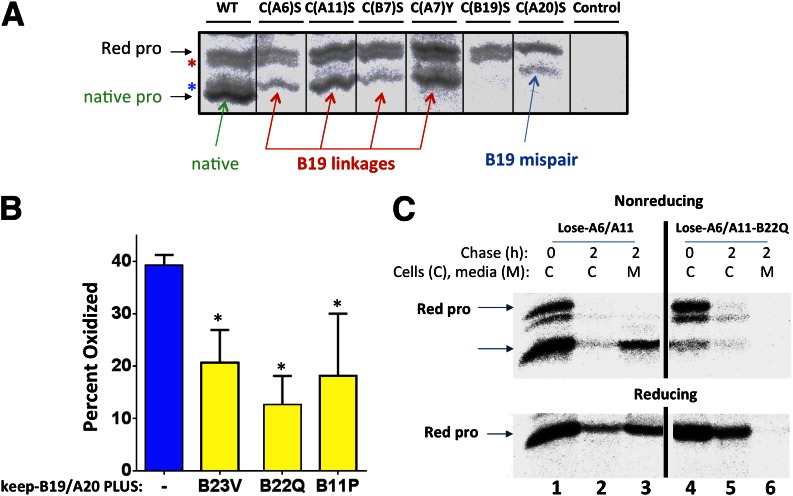
Proinsulin missense mutations reported to cause the syndrome of MIDY include those replacing individual Cys(B7) or Cys(B19), Cys(A6), Cys(A7), or Cys(A20) (36), i.e., all except Cys(A11). On the basis of our growing understanding from the preceding analysis, we examined each individual proinsulin Cys missense mutant for the presence of intramolecular disulfide mispairing. From nonreduced samples of each of the first five mutants, we noted the presence of a novel mispaired proinsulin disulfide isomer that migrated just beneath fully reduced proinsulin position (Fig. 7A, upper asterisk). On the basis of band migration (Fig. 6), we could infer that each mutant formed at least one mispaired disulfide isomer involving Cys(A20). Indeed, this particular band (Fig. 7A, upper asterisk) could not be detected in a proinsulin C(A20)S mutant. Most importantly, a Cys(A20)-containing mispaired disulfide isomer was also apparent in newly synthesized proinsulin with wild-type B and A chains (Fig. 7A, first lane). Moreover, each of the first four proinsulin mutants also appeared capable of initiating an interchain interaction either via the native Cys(B19)-Cys(A20) linkage or via Cys(B19) mispairing (Fig. 7A, lower asterisk). This band was notably absent in a proinsulin-C(B19)S mutant (Fig. 7A). Once again, such a disulfide isomer was present even for proinsulin with wild-type B and A chains (Fig. 7A, first lane). Altogether, these data indicate that the two most common improper intramolecular disulfide pairings involve Cys(A20) and Cys(B19)—these are fractionally minor in wild-type proinsulin but become fractionally major in MIDY mutants.
Figure 7.
Effects of MIDY mutants on proinsulin disulfide bond formation. A: INS1 cells were transfected to express myc-tagged proinsulin wild-type (WT) or missense mutants as indicated. At 42 h posttransfection, the cells were pulse labeled with 35S-amino acids for 15 min, lysed as in Fig. 5, immunoprecipitated with anti-myc, and analyzed by nonreducing Tris–tricine–urea–SDS-PAGE and phosphorimaging. Control, untransfected cells. B: 293T cells were transiently transfected with keep-B19/A20 (–) or that bearing an additional MIDY mutation as indicated. At 48 h, cells were labeled with 35S-amino acids for 15 min, lysed as in Fig. 5, immunoprecipitated with anti-myc, and analyzed by nonreducing SDS-PAGE and phosphorimaging, and the oxidized and reduced proinsulin bands were quantified by scanning densitometry (n = 3) (*P < 0.05 compared with keep-B19/A20). C: 293T cells transfected with lose-A6/A11 or lose-A6/A11 bearing the B22Q MIDY mutation were pulse labeled with 35S-amino acids for 30 min and chased for the times indicated. After washing of cells in ice-cold PBS containing 20 mmol/L NEM, cells were lysed in the presence of 2 mmol/L NEM and immunoprecipitated with anti-insulin. Both cell lysates (C) and media (M) were analyzed by nonreducing (upper) and reducing (lower) Tris–tricine–urea–SDS-PAGE and autoradiography.
Because MIDY mutants are not limited to cysteine missense mutations, it was important to determine the extent to which non-Cys proinsulin mutants may disrupt the critical Cys(B19)-Cys(A20) interchain disulfide bond. Using the assay described in Fig. 4, we determined that MIDY mutations G(B23)V, R(B22)Q, and L(B11)P each decreased the efficiency of Cys(B19)-Cys(A20) disulfide pairing by at least 50% (Fig. 7B). Because of both cooperativity in interchain disulfide bonding (Fig. 5) and the propensity for Cys(A20) and Cys(B19) to form disulfide mispairs (Fig. 6A and C), MIDY mutants that inhibit Cys(B19)-Cys(A20) disulfide bond formation are expected to amplify their effects on proinsulin misfolding. To test this, we introduced the R(B22)Q MIDY substitution into the lose-A6/A11 backbone. As noted above, the majority of lose-A6/A11 molecules showed rapid native-like proinsulin oxidation with subsequent secretion to the medium (Fig. 7C). By contrast, the majority of lose-A6/A11 molecules bearing the R(B22)Q MIDY substitution were unable to acquire native-like proinsulin oxidation [with increased relative abundance of fully reduced proinsulin and a presumptive Cys(A20)-containing disulfide isomer] and were completely blocked in proinsulin secretion (Fig. 7C and Supplementary Fig. 3). These data provide insight into the catastrophic proinsulin misfolding, insulin deficiency, ER stress, and diabetes seen in the β-cells of MIDY patients.
Disulfide Intervention for MIDY
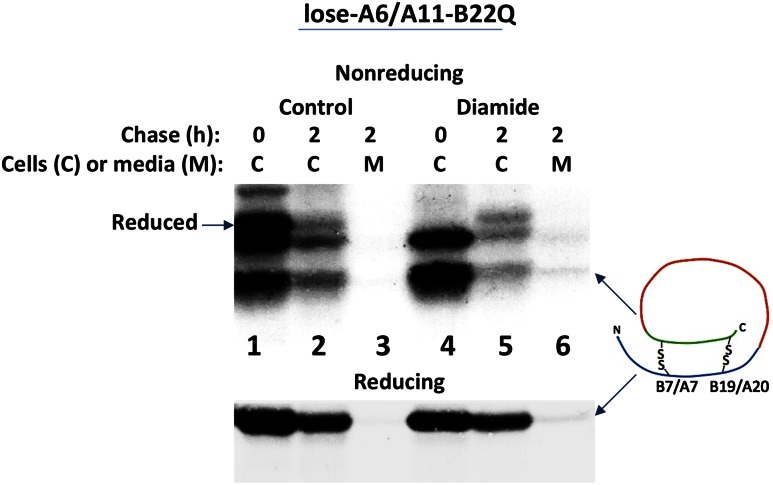
MIDY creates a toxic gain-of-function mutation from misfolded proinsulin because MIDY mutants block insulin production from the wild-type INS allele, which occurs prior to any decrease in β-cell mass (36). Increased expression of ER oxidoreductin-1 (Ero1), which helps to catalyze disulfide bond formation in the ER (37), has been found to promote significant escape/rescue of the wild-type proinsulin that is coexpressed with MIDY mutants in the ER (12). Curiously, we also discovered that increased Ero1 expression could directly rescue ER export of a very small fraction of some of the MIDY mutants themselves (14), and this effect has not been adequately explained. To test whether an improvement of proinsulin oxidative folding might account for a small degree of secretory rescue achievable with MIDY mutants, we briefly exposed cells to diamide (1 mmol/L), followed immediately thereafter by diamide washout. Without diamide, most lose-A6/A11 bearing the R(B22)Q substitution failed to achieve proper interchain disulfide oxidation, and this was accompanied by blockade of proinsulin secretion (Figs. 7C and 8, lanes 1–3). Upon diamide treatment, an increased fraction of proinsulin acquired interchain disulfide oxidation (Fig. 8, lane 4) and a very small fraction of the oxidized MIDY mutant could now be detected in the secretion (Fig. 8, lane 6). The experiment was conducted in 293T cells that express no wild-type proinsulin, strongly suggesting that although the mutation is still present, disulfide bond formation allowed a small fraction of MIDY mutant molecules to directly pass ER quality control and become secreted.
Figure 8.
Partial rescue of the native proinsulin disulfide bond formation that is perturbed in MIDY. 293T cells transfected with lose-A6/A11 bearing the B22Q MIDY mutation were pulse labeled with 35S-amino acids for 30 min and either untreated (control) or treated with 1 mmol/L diamide for 5 min (diamide) and chased for the times indicated. After washing of cells in ice-cold PBS containing 20 mmol/L NEM, cells were lysed in the presence of 2 mmol/L NEM and immunoprecipitated with anti-insulin. Both cell lysates (C) and media (M) were analyzed by nonreducing (upper) and reducing (lower) Tris–tricine–urea–SDS-PAGE and autoradiography.
Discussion
Combating proinsulin misfolding in the β-cell ER is a potentially achievable goal that might help to limit onset and progression of diabetes. In recent years, reports have surfaced regarding pharmacological approaches to correcting misfolding of a subfraction of proteins in the secretory pathway, including mutant enzymes, receptors, transporters, and ion channels (38), such as the cystic fibrosis transmembrane regulator (39), ATP-binding cassette transporters (40), gonadotropin-releasing hormone receptor (41), superoxide dismutase 1 (42), lysosomal enzymes like β-glucosidase (43), and others. Pharmacotherapies are beginning to be reported that can ameliorate ER stress in pancreatic β-cells as well (44–47); however, no pharmacotherapies that specifically help correct proinsulin misfolding in the ER have yet been reported. In part, this may be attributed to our still-limited understanding of proinsulin misfolding in the ER context.
We have been greatly influenced by the work of Weiss (19), Feng and colleagues (48), and others who have studied in vitro refolding of single-chain insulins and proinsulin and suggested that the Cys(B19)-Cys(A20) disulfide bond is kinetically preferred (i.e., it forms first) although the one-disulfide intermediate is generally not recovered because once this interchain bond is initiated, the remaining disulfides form rapidly thereafter. We believe that cooperativity in proinsulin disulfide bond formation is already implied by in vitro studies showing that oxidative folding of the remaining cysteines is significantly slowed in a proinsulin lacking Cys(B7) and Cys(A7) (49). To explore this directly in pancreatic β-cells, we expressed potential single-disulfide proinsulins (keep mutants [Fig. 3]) and potential two-disulfide proinsulins (lose mutants [Fig. 1]). Indeed, our results clearly establish that in the ER of β-cells, newly synthesized proinsulin prefers to initiate interchain covalent interaction via the Cys(B19)-Cys(A20) disulfide bond (Fig. 4D–F), and this shows cooperativity with the second, Cys(B7)-Cys(A7) interchain disulfide, which together satisfy ER quality-control requirements for proinsulin export (Figs. 1D, 2B, 5, and 7C). This cooperativity may be as simple as the possibility that the entropic barrier for formation of the B7-A7 disulfide is lowered because the polypeptide becomes more constrained after formation of the initiating B19-A20 disulfide. This can produce a sequentiality to the steps of proinsulin folding; however, in the more complex scenario of newly synthesized proinsulin initially bearing six unpaired Cys residues, there is considerable room for things to go wrong.
For example, whereas the intrachain Cys(A6)-Cys(A11) disulfide bond is entirely unnecessary for proinsulin export from the ER (Fig. 2), the presence of an unpaired Cys(A11) poses a direct threat to the native proinsulin folding pathway by attacking either unpaired Cys(A20) or Cys(B19) or even Cys(B7) (Fig. 6A and C), completely blocking proinsulin secretion (Fig. 2A, right). This not only provides an initial explanation for the severe neonatal diabetes of the Munich mouse (31) but also may explain why Cys(A11) itself is the only Cys site that has not been reported as a MIDY mutation—because an unpaired Cys(A6) is relatively innocuous. Cys(A6) accessibility is presumably limited such that it does not effectively combine with any cysteine other than its native partner (Fig. 6A)—even when the ER environment is hyperoxidized (Fig. 6B). Thus, it emerges that formation of the Cys(A6)-Cys(A11) disulfide bond (which is most difficult to study) is critical insofar as Cys(A6) must sequester Cys(A11) before Cys(A11) engages, as an interloper, other cysteine partners to damage the fidelity of their critical interchain disulfide bonds.
We believe these realizations provide considerable new insight into the pathogenesis of MIDY. Using keep-B19/A20 as a template, we establish directly that several different MIDY mutants impair Cys(B19)-Cys(A20) disulfide bond formation (Fig. 7B). This failure essentially propagates proinsulin folding failure by not making the Cys(B7)-Cys(A7) pairing as well (Fig. 4D). However, we remain intrigued with the idea that the reluctant Cys(B7)-Cys(A7) pairing can be encouraged under conditions of enhanced oxidation in the ER (Fig. 4E). Thus, when providing the β-cell ER an exposure to hyperoxidizing conditions, the distribution of newly synthesized MIDY mutant can be shifted toward an increased fraction of proper intramolecular disulfide pairing (Fig. 8)—which will make this mutant less likely to engage in intermolecular disulfide attack (9)—and can even rescue export of a tiny fraction of the mutant protein itself (Fig. 8). These findings may explain recent results establishing that overexpression of Ero1 (a natural oxidant of the ER) can promote escape of wild-type proinsulin that is coexpressed alongside a MIDY mutant proinsulin, thereby increasing insulin production (12), as well as rescuing a tiny fraction of the MIDY mutant molecules (14). Conversely, loss of Ero1 activity is accompanied by a marked worsening of diabetes in Akita mice, leading to islet destruction (13). These findings, together with our current results, suggest that selective enhancement of oxidative folding in pancreatic β-cells could represent a potential therapeutic approach to improve insulin production in patients with MIDY.
Finally, we note that wild-type proinsulin also exhibits a predisposition to misfold (Figs. 2B, 6A, and 7A). Studies are now needed to determine whether enhanced ER oxidation may limit proinsulin misfolding and ER stress that has been suggested to occur in type 2 diabetes.
Supplementary Material
Article Information
Acknowledgments. The authors acknowledge Bill and Dee Brehm for helping to advance diabetes research and helping to establish the Brehm Center for Diabetes Research at the University of Michigan.
Funding. This work was funded primarily by National Institutes of Health (NIH), by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R01-DK-48280 (to P.A.), by NIDDK R01-DK-088856, by research grants from the National Natural Science Foundation of China (81070629 and 81370895) (to M.L.), and by the University of Michigan Protein Folding Diseases Initiative (to B.T., M.L., and P.A.). The authors also acknowledge assistance from the University of Michigan Diabetes Research Center, funded by the NIH, NIDDK (P60-DK-20572), to support the Administrative, Molecular Biology, and the Morphology & Image Analysis Cores.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. L.H. and A.S. researched data. L.H., N.M., B.T., M.L., and P.A. contributed to discussion. L.H., M.L., and P.A. wrote the manuscript. L.H., N.M., B.T., M.L., and P.A. reviewed and edited the manuscript. P.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db15-1345/-/DC1.
References
- 1.Eizirik DL, Cnop M. ER stress in pancreatic beta cells: the thin red line between adaptation and failure. Sci Signal 2010;3:pe7. [DOI] [PubMed] [Google Scholar]
- 2.Bensellam M, Laybutt DR, Jonas JC. The molecular mechanisms of pancreatic β-cell glucotoxicity: recent findings and future research directions. Mol Cell Endocrinol 2012;364:1–27 [DOI] [PubMed] [Google Scholar]
- 3.Scheuner D, Vander Mierde D, Song B, et al. Control of mRNA translation preserves endoplasmic reticulum function in beta cells and maintains glucose homeostasis. Nat Med 2005;11:757–764 [DOI] [PubMed] [Google Scholar]
- 4.Volchuk A, Ron D. The endoplasmic reticulum stress response in the pancreatic β-cell. Diabetes Obes Metab 2010;12(Suppl. 2):48–57 [DOI] [PubMed] [Google Scholar]
- 5.Rabhi N, Salas E, Froguel P, Annicotte JS. Role of the unfolded protein response in β cell compensation and failure during diabetes. J Diabetes Res 2014;2014:795171 [DOI] [PMC free article] [PubMed]
- 6.Sun J, Cui J, He Q, Chen Z, Arvan P, Liu M. Proinsulin misfolding and endoplasmic reticulum stress during the development and progression of diabetes. Mol Aspects Med 2015;42:105–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev 2006;86:1133–1149 [DOI] [PubMed] [Google Scholar]
- 8.Liu M, Hodish I, Haataja L, et al. Proinsulin misfolding and diabetes: mutant INS gene-induced diabetes of youth. Trends Endocrinol Metab 2010;21:652–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu M, Haataja L, Wright J, et al. Mutant INS-gene induced diabetes of youth: proinsulin cysteine residues impose dominant-negative inhibition on wild-type proinsulin transport. PLoS One 2010;5:e13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss MA. Diabetes mellitus due to the toxic misfolding of proinsulin variants. FEBS Lett 2013;587:1942–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang XF, Arvan P. Intracellular transport of proinsulin in pancreatic beta-cells. Structural maturation probed by disulfide accessibility. J Biol Chem 1995;270:20417–20423 [DOI] [PubMed] [Google Scholar]
- 12.Liu M, Lara-Lemus R, Shan SO, et al. Impaired cleavage of preproinsulin signal peptide linked to autosomal-dominant diabetes. Diabetes 2012;61:828–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zito E, Chin KT, Blais J, Harding HP, Ron D. ERO1-beta, a pancreas-specific disulfide oxidase, promotes insulin biogenesis and glucose homeostasis. J Cell Biol 2010;188:821–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright J, Birk J, Haataja L, et al. Endoplasmic reticulum oxidoreductin-1α (Ero1α) improves folding and secretion of mutant proinsulin and limits mutant proinsulin-induced endoplasmic reticulum stress. J Biol Chem 2013;288:31010–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajpal G, Arvan P. Disulfide bond formation. In Handbook of Biologically Active Peptides. 2nd ed. Kastin A, Ed. San Diego, CA, Elsevier/Academic Press, 2013, p. 1721–1729 [Google Scholar]
- 16.Chang SG, Choi KD, Jang SH, Shin HC. Role of disulfide bonds in the structure and activity of human insulin. Mol Cells 2003;16:323–330 [PubMed] [Google Scholar]
- 17.Jia XY, Guo ZY, Wang Y, Xu Y, Duan SS, Feng YM. Peptide models of four possible insulin folding intermediates with two disulfides. Protein Sci 2003;12:2412–2419 [DOI] [PMC free article] [PubMed]
- 18.Qiao ZS, Min CY, Hua QX, Weiss MA, Feng YM. In vitro refolding of human proinsulin. Kinetic intermediates, putative disulfide-forming pathway folding initiation site, and potential role of C-peptide in folding process. J Biol Chem 2003;278:17800–17809 [DOI] [PubMed] [Google Scholar]
- 19.Hua QX, Mayer JP, Jia W, Zhang J, Weiss MA. The folding nucleus of the insulin superfamily: a flexible peptide model foreshadows the native state. J Biol Chem 2006;281:28131–28142 [DOI] [PubMed] [Google Scholar]
- 20.Winter J, Klappa P, Freedman RB, Lilie H, Rudolph R. Catalytic activity and chaperone function of human protein-disulfide isomerase are required for the efficient refolding of proinsulin. J Biol Chem 2002;277:310–317 [DOI] [PubMed] [Google Scholar]
- 21.Winter J, Gleiter S, Klappa P, Lilie H. Protein disulfide isomerase isomerizes non-native disulfide bonds in human proinsulin independent of its peptide-binding activity. Protein Sci 2011;20:588–596 [DOI] [PMC free article] [PubMed]
- 22.Zhang L, Lai E, Teodoro T, Volchuk A. GRP78, but not protein-disulfide isomerase, partially reverses hyperglycemia-induced inhibition of insulin synthesis and secretion in pancreatic {beta}-cells. J Biol Chem 2009;284:5289–5298 [DOI] [PubMed] [Google Scholar]
- 23.Rajpal G, Schuiki I, Liu M, Volchuk A, Arvan P. Action of protein disulfide isomerase on proinsulin exit from endoplasmic reticulum of pancreatic β-cells. J Biol Chem 2012;287:43–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oyadomari S, Koizumi A, Takeda K, et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest 2002;109:525–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haataja L, Snapp E, Wright J, et al. Proinsulin intermolecular interactions during secretory trafficking in pancreatic β cells. J Biol Chem 2013;288:1896–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology 1992;130:167–178 [DOI] [PubMed] [Google Scholar]
- 27.Tirasophon W, Welihinda AA, Kaufman RJ. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev 1998;12:1812–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hua QX, Nakagawa SH, Jia W, et al. Hierarchical protein folding: asymmetric unfolding of an insulin analogue lacking the A7-B7 interchain disulfide bridge. Biochemistry 2001;40:12299–12311 [DOI] [PubMed] [Google Scholar]
- 29.Liu M, Ramos-Castañeda J, Arvan P. Role of the connecting peptide in insulin biosynthesis. J Biol Chem 2003;278:14798–14805 [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Gong H, Sun Y, et al. Dissecting the role of disulfide bonds on the amyloid formation of insulin. Biochem Biophys Res Commun 2012;423:373–378 [DOI] [PubMed] [Google Scholar]
- 31.Herbach N, Rathkolb B, Kemter E, et al. Dominant-negative effects of a novel mutated Ins2 allele causes early-onset diabetes and severe beta-cell loss in Munich Ins2C95S mutant mice. Diabetes 2007;56:1268–1276 [DOI] [PubMed] [Google Scholar]
- 32.Guo ZY, Feng YM. Effects of cysteine to serine substitutions in the two inter-chain disulfide bonds of insulin. Biol Chem 2001;382:443–448 [DOI] [PubMed] [Google Scholar]
- 33.Frand AR, Kaiser CA. The ERO1 gene of yeast is required for oxidation of protein dithiols in the endoplasmic reticulum. Mol Cell 1998;1:161–170 [DOI] [PubMed] [Google Scholar]
- 34.Tortorella D, Story CM, Huppa JB, et al. Dislocation of type I membrane proteins from the ER to the cytosol is sensitive to changes in redox potential. J Cell Biol 1998;142:365–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watson WH, Pohl J, Montfort WR, et al. Redox potential of human thioredoxin 1 and identification of a second dithiol/disulfide motif. J Biol Chem 2003;278:33408–33415 [DOI] [PubMed] [Google Scholar]
- 36.Liu M, Sun J, Cui J, et al. INS-gene mutations: from genetics and beta cell biology to clinical disease. Mol Aspects Med 2015;42:3–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Araki K, Iemura S, Kamiya Y, et al. Ero1-α and PDIs constitute a hierarchical electron transfer network of endoplasmic reticulum oxidoreductases. J Cell Biol 2013;202:861–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leidenheimer NJ, Ryder KG. Pharmacological chaperoning: a primer on mechanism and pharmacology. Pharmacol Res 2014;83:10–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okiyoneda T, Veit G, Dekkers JF, et al. Mechanism-based corrector combination restores ΔF508-CFTR folding and function. Nat Chem Biol 2013;9:444–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudashevskaya EL, Stockner T, Trauner M, Freissmuth M, Chiba P. Pharmacological correction of misfolding of ABC proteins. Drug Discov Today Technol 2014;12:e87–e94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janovick JA, Stewart MD, Jacob D, et al. Restoration of testis function in hypogonadotropic hypogonadal mice harboring a misfolded GnRHR mutant by pharmacoperone drug therapy. Proc Natl Acad Sci USA 2013;110:21030–21035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Das I, Krzyzosiak A, Schneider K, et al. Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit. Science 2015;348:239–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lieberman RL, Wustman BA, Huertas P, et al. Structure of acid beta-glucosidase with pharmacological chaperone provides insight into Gaucher disease. Nat Chem Biol 2007;3:101–107 [DOI] [PubMed] [Google Scholar]
- 44.Chan JY, Luzuriaga J, Bensellam M, Biden TJ, Laybutt DR. Failure of the adaptive unfolded protein response in islets of obese mice is linked with abnormalities in β-cell gene expression and progression to diabetes. Diabetes 2013;62:1557–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cadavez L, Montane J, Alcarraz-Vizán G, et al. Chaperones ameliorate beta cell dysfunction associated with human islet amyloid polypeptide overexpression. PLoS One 2014;9:e101797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shang L, Hua H, Foo K, et al. β-Cell dysfunction due to increased ER stress in a stem cell model of Wolfram syndrome. Diabetes 2014;63:923–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kozuka C, Sunagawa S, Ueda R, et al. γ-Oryzanol protects pancreatic β-cells against endoplasmic reticulum stress in male mice. Endocrinology 2015;156:1242–1250 [DOI] [PubMed] [Google Scholar]
- 48.Yan H, Guo ZY, Gong XW, Xi D, Feng YM A peptide model of insulin folding intermediate with one disulfide. Protein Sci 2003;12:768–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y, Tang JG. Influence of A7-B7 disulfide bond deletion on the refolding and structure of proinsulin. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 2003;35:122–126 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.