Abstract
Objective
In this study we compared activity limitations, pain intensity, and global health in patients with rheumatoid arthritis (RA) in Sweden and the USA and aimed to determine whether nationality is associated with these outcomes.
Methods
We used longitudinal data from the ‘Swedish TIRA project’ (n = 149) and the University of California, San Francisco (UCSF) RA panel study (n = 85). Data were collected annually concerning use of medications [disease-modifying anti-rheumatic drugs (DMARDs), biologics, and corticosteroids], morning stiffness, number of swollen joints, and number of painful joints. Three self-reported outcome measures were examined: pain intensity measured on a 0–100 visual analogue scale (VAS), activity limitation according to the Health Assessment Questionnaire (HAQ), and global health. To analyse the data, the Student’s t-test, the χ2-test, and the generalized estimating equation (GEE) method were used.
Results
Nationality was significantly related to HAQ score and pain intensity, even after adjustment for covariates. The patients in the TIRA cohort reported a lower HAQ score and a higher pain intensity than the patients in the UCSF cohort. Nationality was not related to global health.
Conclusion
Patients with RA should be assessed with awareness of the psychosocial and cultural context because disability seems to be affected by nationality. Further knowledge to clarify how a multinational setting affects disability could improve the translation of interventions for patients with RA across nationalities.
Disability can be considered as one of the ‘side-effects’ of many chronic diseases such as rheumatoid arthritis (RA) (1). Disability can be quantified with physical measures. However, patient self-reported measures are both feasible and applicable (2). Several patient self-report instruments to assess RA were developed 30 years ago, and have been advocated as an infrastructure of daily rheumatology practice. The patient’s perspective has been highlighted by the Outcome Measures in Rheumatology (OMERACT) Committee (3).
Prevention of disability in RA is an important target. The prevalence of RA is about 0.5–0.7% in the developed world (4) and the disease is often associated with disability (1) and high direct and indirect costs (5). The course of disability is not directly related to the course of the disease activity and is instead associated with the initial level of disability itself (1), to personal factors as psychological distress (6), and to external factors such as nationality (7, 8). There are a limited number of studies comparing the outcome of RA between different nationalities. The large QUEST-RA (Quantitative Patient Questionnaires in Standard Monitoring of Patients with Rheumatoid Arthritis) study that included patients who received usual care in 25 countries (9) found disease activity and also the burden of RA to be greater in countries with low socio-economic status. The QUEST-RA study also indicated significant remission rates between countries and highlighted the importance of using the same assessment and standards in clinical care (10). Knowledge about differences in patient-reported disability between nationalities is limited. The objective of this study was to compare activity limitations, pain intensity, and global health in patients with RA in Sweden and the USA and to determine whether nationality is associated with these outcomes after adjustment for factors known to correlate with outcomes.
Methods
The ‘Swedish TIRA project’ (Swedish acronym for ‘early intervention in rheumatoid arthritis’) started in 1996 in cooperation with 10 rheumatology units where 320 patients with recent-onset RA (onset of joint swelling ≤12 months but ≥6 weeks) were recruited during a 27-month period (5). The 149 patients who remained in the TIRA project at the 8-year follow-up constitute the study group for the current study. Disease activity, disability, and health have been registered yearly at visits to the rheumatology units.
The University of California, San Francisco (UCSF) RA panel study started in 1982. A total of 822 patients were recruited from a random sample of rheumatologists in northern California. Data were collected by annual structured telephone interviews to assess disease variables such as joint counts and duration of morning stiffness, overall health status, and disability (11). There have been four additional enrolments from 1989 to 2004. In the present study all patients who were enrolled in 1999 or earlier were eligible for inclusion. Patients with disease onset before 1990 were excluded to create a sample of persons with recent-onset RA comparable to the TIRA sample, resulting in a sample of 85 patients.
Data collection
At baseline (1999), year of diagnosis, age, sex, nationality, and work status (working, not working, or retired) were obtained. In each cohort, the following variables were collected yearly (1999–2004): use of medications [disease-modifying anti-rheumatic drugs (DMARDs), biologics, and corticosteroids], morning stiffness (categorized as none, < 30 min, > 30 min, < 1 h, 1–2 h, 2–3 h, > 3 h), number of swollen joints (in the TIRA cohort 0–28 and in the UCSF cohort 0–17), and number of painful joints (in the TIRA cohort 0–28 and in the UCSF cohort 0–14).
Outcome
Three self-reported outcome measures were examined: pain intensity measured on a 0–100 visual analogue scale (VAS); activity limitation according to the Health Assessment Questionnaire (HAQ); and global health. The slightly modified Swedish version of the HAQ was used in the TIRA cohort (12). Global health was reported on a VAS in the TIRA cohort, and on an ordinal scale in the UCSF cohort. Based on the distribution, global health was categorized into poor/fair and excellent/good in both cohorts.
Statistical analysis
Differences between the Swedish and the UCSF cohorts were analysed using the Student’s t-test and χ2. Independent associations between variables were investigated by the generalized estimating equation (GEE) method, which is a regression method that allows the investigation of longitudinal data while adjusting for within-patient correlation across multiple observations. The GEE method was used to determine the importance of nationality for the outcome variables and also to create multivariate regression models separately for the UCSF cohort and the TIRA cohort with the chosen outcomes as dependent variables.
Results
The 149 patients in the Swedish TIRA cohort had a mean (SD) disease duration of 2.4 (0.7) years at baseline in 1999. The 85 patients from the UCSF cohort had a mean (SD) disease duration of 2.6 (4.3) years. Sixty-eight per cent of the patients in the TIRA cohort were women and the corresponding number in the UCSF cohort was 86% (p = 0.001) (Table 1).
Table 1.
Demographics, disease characteristics, and disability in the TIRA project (n = 149) and the UCSF RA panel study (n = 85) at baseline (1999) and after 5 years (2004).
| 1999
|
2004
|
|||||
|---|---|---|---|---|---|---|
| TIRA | UCSF | p-value | TIRA | UCSF | p-value | |
| Years since diagnosis, mean (SD) | 2.4 (0.7) | 2.6 (4.3) | NS | – | – | |
| Age (years), mean (SD) | 56 (14) | 53 (16) | NS | – | – | |
| Sex (% females) | 68 | 86 | 0.001 | – | – | |
| Work status (%) | NS | |||||
| Working | 42 | 57 | – | – | ||
| Unable | 24 | 14 | – | – | ||
| Retired | 34 | 29 | – | – | ||
| Medication (% using) | ||||||
| DMARDs | 78 | 84 | NS | 66 | 75 | NS |
| Biologics | 2 | 11 | 0.01 | 14 | 38 | <0.0001 |
| Steroids | 41 | 40 | NS | 41 | 23 | 0.022 |
| Morning stiffness (%) | 0.03 | NS | ||||
| None | 15 | 17 | 22 | 23 | ||
| < 30 min | 20 | 40 | 18 | 35 | ||
| > 30 min and < 1 h | 22 | 15 | 27 | 20 | ||
| 1–2 h | 20 | 14 | 20 | 12 | ||
| 2–3 h | 13 | 9 | 9 | 5 | ||
| > 3 h | 10 | 5 | 4 | 5 | ||
| HAQ, mean (SD) | 0.6 (0.6) | 0.8 (0.7) | 0.04 | 0.7 (0.6) | 0.8 (0.7) | NS |
| Pain, mean (SD) | 37 (25) | 25 (25) | 0.001 | 40 (26) | 27 (28) | 0.003 |
| Global health (%) | NS | 0.004 | ||||
| Excellent or good | 53 | 61 | 51 | 73 | ||
| Fair or poor | 47 | 39 | 49 | 27 | ||
At baseline, a higher proportion of patients from the USA received biological agents. At the follow-up in 2004, the patients from the USA were more likely to receive biological agents but less likely to receive steroids than the Swedish patients. The patients in the UCSF cohort reported a higher number of painful joints but a shorter period of morning stiffness than the TIRA cohort.
The patients in the TIRA cohort reported higher pain intensity but better global health than the patients in the UCSF cohort. Activity limitations measured by the HAQ were higher in the UCSF cohort at baseline. During the study period, the mean HAQ score varied between 0.64 and 0.74 in the TIRA cohort and between 0.74 and 0.85 in the UCSF cohort. At the last follow-up in 2004, there was no statistical difference in HAQ score between the two cohorts (Figure 1).
Figure 1.
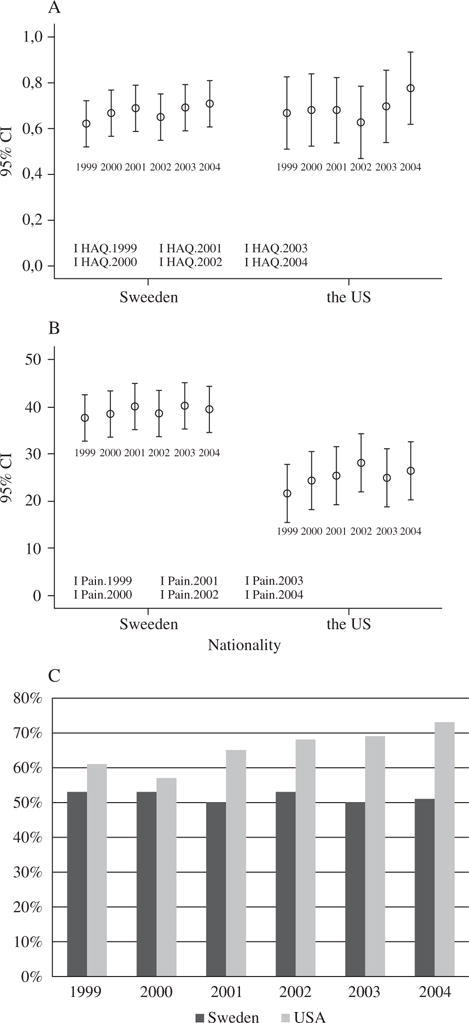
Mean values and 95% confidence intervals (CIs) for (A) the HAQ score and (B) pain intensity, and (C) the percentage with reported excellent/good health in the TIRA and the UCSF cohort from 1999 to 2004.
According to the multivariate GEE model with the total study group (Table 2), nationality was significantly related to HAQ score and pain intensity when controlling for the covariates, with the patients in the TIRA cohort reporting a lower HAQ score (0.28 on average) and a higher pain intensity (9.5 on average) than the patients in the UCSF cohort. However, nationality was not related to global health.
Table 2.
Relationship between nationality and the dependent variables HAQ score, pain intensity, and global health.
| HAQ score, mean (SD)
|
Pain intensity
|
Global health
|
||||||
|---|---|---|---|---|---|---|---|---|
| Sweden Mean (SD) | USA Mean (SD) | GEE coefficient (95% CI) | Sweden Mean (SD) | USA Mean (SD) | GEE coefficient (95% CI) | Sweden Median (IQR) | USA Median (IQR) | GEE coefficient (95%CI) |
| 0.68 (0.58) | 0.80 (0.65) | −0.28*** (−0.44 to −0.13)a |
39 (26) | 28 (26) | 9.5*** (4.9–14.0)b |
1 (1) | 1 (1) | 0.06 (−0.07 to 0.18)c |
Association between HAQ and nationality, adjusting for time, years since diagnosis, age*, sex***, work status at baseline***, use of DMARDs, biologics, and steroids***, morning stiffness*, global health***, and pain intensity***.
Association between pain intensity and nationality, adjusting for time**, years since diagnosis, age, sex, work status at baseline, use of DMARDs, biologics, and steroids, morning stiffness**, global health***, and HAQ***.
Association between rating of global health and nationality, adjusting for time, years since diagnosis, age, sex, work status at baseline, use of DMARDs, biologics*, and steroids, morning stiffness**, pain intensity***, and HAQ***.
p < 0.05,
p < 0.01,
p < 0.001.
When separate GEE models were calculated for the TIRA and the UCSF cohorts, sex, pain intensity, and global health were associated with HAQ score in both cohorts. Additionally, work status was associated with HAQ score in the TIRA cohort and with use of steroids in the UCSF cohort.
In both cohorts, morning stiffness, global health, and HAQ score were identified as variables related to pain intensity. Of note, an improved HAQ score and improved health were associated with decreased pain intensity to a greater degree in the TIRA cohort than in the UCSF cohort. The reverse can be seen in morning stiffness, which had a stronger association with pain intensity in the UCSF cohort than in the Swedish. Global health was related to pain intensity and HAQ score in both cohorts and also by the use of biologics in the TIRA cohort, where not using biologics was associated with worse health.
Discussion
The main result of this analysis was that nationality was significantly related to HAQ score and pain intensity, even after adjustment for variables known to affect these outcomes. We found that the HAQ score was lower by 0.28 points and the pain intensity higher by 9.5 points in the TIRA cohort than in the UCSF cohort. The minimally important difference for the HAQ to be considered somewhat/much better has been set to −0.20 (13), which indicates that the Swedish patients have a clinically meaningful lower level of reported activity limitations than US patients. In the time period used in the study (1999–2004), there was a substantial increase in the use of DMARDs, which went together with the improved mean HAQ score (14) seen in both cohorts. Global health was, unlike pain intensity and activity limitations, not affected by nationality in the present study, perhaps because of the dichotomization of the variable. Self-reported measures as pain, quality of life, and health are otherwise often closely linked (15), as was also seen in our GEE analyses.
A high reported pain intensity among the Swedish patients is in agreement with Albers et al (16) in a European comparison. Previous reports have shown that pain intensity can be associated with culture (6) and psychological distress in RA in both Europe and the USA (17). According to a study of patients with osteoarthritis, cultural differences in pain and function were explained by body mass index (BMI) and depressive symptoms, and cultural differences in function may also be largely influenced by pain (15). In the present study, we do not have information about depressive symptoms, BMI, or other contextual factors in the two cohorts and we therefore do not have the possibility to compare baseline values in the two cohorts. Some patients in the UCSF cohort have a longer disease duration, which may explain some of the differences in pain intensity because of improved pain management or medical treatments. In general, women report more pain than men, although this is not so obvious in chronic inflammatory diseases such as RA (18). In an earlier study in the TIRA cohort we did not find any significant sex differences in pain intensity (19). This is further reinforced in the present study, where gender was not significantly associated with pain intensity in the GEE analysis. This shows that the pain in RA is complex and influenced by many factors. Because pain has been identified as a priority symptom for the treatment of both European and American patients (17), a comprehensive examination of patients’ perspectives regarding pain is important for pain management in clinical care.
Differences in the way the data were collected (by telephone interview in the USA and at visits to the clinic in Sweden) could also lead to differences in pain ratings. This may also be a problem in other variables. According to Barton et al (20), joint count performed by a trained assessor, as in the TIRA cohort, and self-reported swollen joints, as in the UCSF cohort, show a low level of correlation. Furthermore, in the TIRA cohort, the slightly modified Swedish version of the HAQ was used. A few questions in the Swedish version of the HAQ are slightly modified to take in to account the sociocultural differences between the USA and Sweden (12) and the scoring of an auxiliary device or of another person’s help is slightly different (12). According to the developers of the Swedish version, these small modifications should not be of major importance in the result (12) and therefore do not explain the differences in the present study. Finally, unmeasured covariates, such as depression and BMI, as mentioned earlier, as well as education, type of work, income, grip force, or the 28-joint Disease Activity Score (DAS28), may explain differences between the cohorts. The results from the study need to be further examined by a more defined study design to test in what way culture or nationality affect the disability in RA. With a larger sample it would be interesting to analyse whether the nationality affects self-reported disability differently in men and women with RA.
In conclusion, disability in RA seems to be affected by nationality, so assessing patients with awareness of the psychosocial context is of importance. Additional research to elucidate how disability is affected in a multinational setting could improve the translation of interventions for patients with RA across nationalities.
Acknowledgments
We thank the interviewers and the co-workers for data collection in the two cohorts, and the data manager, Stephanie Rush. The study was supported by a grant from the Swedish Rheumatism Association and the Swedish Council for Working Life and Social Research.
References
- 1.Pincus T, Callahan LF. The ‘side effects’ of rheumatoid arthritis: joint destruction, disability and early mortality. Br J Rheumatol. 1993;32(Suppl 1):28–37. [PubMed] [Google Scholar]
- 2.El Miedany Y, El Gaafary M, Youssef SS, Palmer D. Incorporating patient reported outcome measures in clinical practice: development and validation of a questionnaire for inflammatory arthritis. Clin Exp Rheumatol. 2010;28:734–44. [PubMed] [Google Scholar]
- 3.Tugwell P, Boers M. Developing consensus on preliminary core efficacy endpoints for rheumatoid arthritis clinical trials. OMERACT Committee. J Rheumatol. 1993;20:555–6. [PubMed] [Google Scholar]
- 4.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hallert E, Husberg M, Skogh T. Costs and course of disease and function in early rheumatoid arthritis: a 3-year follow-up (the Swedish TIRA project) Rheumatology (Oxford) 2006;45:325–31. doi: 10.1093/rheumatology/kei157. [DOI] [PubMed] [Google Scholar]
- 6.Bai M, Tomenson B, Creed F, Mantis D, Tsifetaki N, Voulgari PV, et al. The role of psychological distress and personality variables in the disablement process in rheumatoid arthritis. Scand J Rheumatol. 2009;38:419–30. doi: 10.3109/03009740903015135. [DOI] [PubMed] [Google Scholar]
- 7.Chung CP, Sokka T, Arbogast PG, Pincus T. Work disability in early rheumatoid arthritis: higher rates but better clinical status in Finland compared with the US. Ann Rheum Dis. 2006;65:1653–7. doi: 10.1136/ard.2005.048439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sokka T, Toloza S, Cutolo M, Kautiainen H, Makinen H, Gogus F, et al. Women, men, and rheumatoid arthritis: analyses of disease activity, disease characteristics, and treatments in the QUEST-RA study. Arthritis Res Ther. 2009;11:R7. doi: 10.1186/ar2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sokka T, Kautiainen H, Pincus T, Toloza S, da Rocha Castelar Pinheiro G, Lazovskis J, et al. Disparities in rheumatoid arthritis disease activity according to gross domestic product in 25 countries in the QUEST-RA database. Ann Rheum Dis. 2009;68:1666–72. doi: 10.1136/ard.2009.109983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sokka T, Hetland ML, Makinen H, Kautiainen H, Hørslev-Petersen K, Luukkainen RK, et al. Remission and rheumatoid arthritis: data on patients receiving usual care in twenty-four countries. Arthritis Rheum. 2008;58:2642–51. doi: 10.1002/art.23794. [DOI] [PubMed] [Google Scholar]
- 11.Yelin EH, Criswell LA, Feigenbaum PG. Health care utilization and outcomes among persons with rheumatoid arthritis in fee-for-service and prepaid group practice settings. J Am Med Assoc. 1996;276:1048–53. [PubMed] [Google Scholar]
- 12.Ekdahl C, Eberhardt K, Andersson I, Svensson B. Assessing disability in patients with rheumatoid arthritis. Use of a Swedish version of the Stanford Health Assessment Questionnaire. Scand J Rheumatol. 1988;17:263–71. doi: 10.3109/03009748809098795. [DOI] [PubMed] [Google Scholar]
- 13.Pope JE, Khanna D, Norrie D, Ouimet JM. The minimally important difference for the Health Assessment Questionnaire in rheumatoid arthritis clinical practice is smaller than in randomized controlled trials. J Rheumatol. 2009;36:254–9. doi: 10.3899/jrheum.080479. [DOI] [PubMed] [Google Scholar]
- 14.Soderlin MK, Lindroth Y, Turesson C, Jacobsson LT. A more active treatment has profound effects on the health status of rheumatoid arthritis (RA) patients: results from a population-based RA register in Malmö, Sweden, 1997–2005. Scand J Rheumatol. 2010;39:206–11. doi: 10.3109/03009740903313621. [DOI] [PubMed] [Google Scholar]
- 15.Kosinski M, Zhao SZ, Dedhiya S, Osterhaus JT, Ware JE., Jr Determining minimally important changes in generic and disease-specific health-related quality of life questionnaires in clinical trials of rheumatoid arthritis. Arthritis Rheum. 2000;43:1478–87. doi: 10.1002/1529-0131(200007)43:7<1478::AID-ANR10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 16.Albers JM, Paimela L, Kurki P, Eberhardt KB, Emery P, van ‘t Hof MA, et al. Treatment strategy, disease activity, and outcome in four cohorts of patients with early rheumatoid arthritis. Ann Rheum Dis. 2001;60:453–8. doi: 10.1136/ard.60.5.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor P, Manger B, Alvaro-Gracia J, Johnstone R, Gomez-Reino J, Eberhardt E, et al. Patient perceptions concerning pain management in the treatment of rheumatoid arthritis. J Int Med Res. 2010;38:1213–24. doi: 10.1177/147323001003800402. [DOI] [PubMed] [Google Scholar]
- 18.Cairns BE, Gazerani P. Sex-related differences in pain. Maturitas. 2009;63:292–6. doi: 10.1016/j.maturitas.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Bjork M, Gerdle B, Thyberg I, Peolsson M. Multivariate relationships between pain intensity and other aspects of health in rheumatoid arthritis –cross sectional and five year longitudinal analyses (the Swedish TIRA project) Disabil Rehabil. 2008;30:1429–38. doi: 10.1080/09638280701623356. [DOI] [PubMed] [Google Scholar]
- 20.Barton JL, Criswell LA, Kaiser R, Chen YH, Schillinger D. Systematic review and metaanalysis of patient self-report versus trained assessor joint counts in rheumatoid arthritis. J Rheumatol. 2009;36:2635–41. doi: 10.3899/jrheum.090569. [DOI] [PMC free article] [PubMed] [Google Scholar]


