Abstract
Background
Few studies have described the clinical and dermoscopic features of atypical Spitz tumors (AST).
Objective
To describe the clinical and dermoscopic features of a series of AST as compared to those of conventional Spitz nevi (SN).
Methods
Multicenter, retrospective, case-control study, analyzing the clinical and dermoscopic characteristics of 55 AST and 110 SN that were excised and diagnosed histopathologically.
Results
The majority of AST presented clinically as a plaque or nodule, dermoscopically typified by a multicomponent or nonspecific pattern. A proportion of lesions (16.4%) exhibited the typical non pigmented spitzoid pattern of dotted vessels and white lines under dermoscopy.
Nodularity, ulceration, linear vessels, polymorphic vessels, white lines, and blue/white veil were associated with AST by univariate analysis, but only nodularity and white lines remained significant after multivariate analysis. In contrast, a pigmented typical spitzoid pattern was a potent predictor of SN, associated with 6.5-fold increased probability.
Limitations
Differentiation from spitzoid melanoma and other non melanocytic lesions was not investigated.
Conclusion
Atypical Spitz tumors are polymorphic melanocytic proliferations with a nodular clinical appearance. Dermoscopically they demonstrate a multicomponent and nonspecific pattern. A typical non pigmented spitzoid pattern on dermoscopy (with dotted vessels and white lines) does not exclude AST.
Introduction
Atypical Spitz tumors (AST) are defined as melanocytic proliferations with intermediate histopathologic features between Spitz nevi (SN) and spitzoid melanoma, carrying uncertain malignant potential.1–9 The exact clinico-pathologic definition of AST is a matter of ongoing debate among dermatopathologists. Currently, some opinionleaders assert that only two diagnostic categories can be ascribed to spitzoid lesions, namely, SN and spitzoid melanoma.5 Others suggest that spitzoid lesions are a ‘morpho-biologic spectrum’ ranging from benign to clear-cut malignant lesions, with AST placed in the middle of this spectrum.6 Since the first description, by Smith and co-worker in 1989,2 several studies have investigated the histopathologic features of AST. There remains a lack of consensus, with diagnostic agreement among dermatopathologists consistently lower than for non-spitzoid melanocytic neoplasms.4 AST is a diagnostic definition increasingly used by pathologists, presenting the clinician with difficult patient management decisions.10–12 Despite numerous histopathologic studies, few have described the clinical and dermoscopic features of AST.8,10,12,13
We compared clinical and dermoscopic images of a series of AST with those of a control group of conventional SN in order to identify diagnostic features that might be used in the differential diagnosis between these two entities.
Material and Methods
We conducted a multicenter, case-control study analyzing clinical and dermoscopic characteristics of 55 AST and 110 SN that were excised and diagnosed histopathologically. Clinical and dermoscopic images of AST were collected from the databases of 7 pigmented lesions clinics in Italy (Reggio Emilia, Naples, Modena, Turin, Milan) and Spain (Barcelona, Tarragona). The inclusion criterion was the availability of a clinical and dermoscopic image of a histopathologically diagnosed AST. A control group of 110 consecutively selected SN was obtained from the database of the Skin Cancer Unit, ASMN-IRCCS, Reggio Emilia. The study period was 2003–2014. All lesions were examined by dermatopathologists specialized in the diagnosis of melanocytic skin tumors. Clinical images were acquired using a high resolution digital camera. Dermoscopic images were captured using polarized or non polarized dermoscopes (DermLite Foto, 3Gen LLC, Dana Point, CA).
Clinical information included the age and sex of each patient, and location of the tumor. For the AST group, additional information included sentinel lymph node biopsy (SLNB) status, performance of wide excision (with at least 1 cm clear margins) after initial biopsy, years of follow up, and outcome.
Clinical and dermoscopic images were evaluated in agreement by two observers (EM and GA) blinded with respect to the histopathologic diagnosis. When no agreement was achieved, a third evaluator (AL) was involved. Clinical evaluation included the assessment of palpability (macule, papule, plaque, nodule). The presence of ulceration and pigmentation were evaluated on dermoscopic images. Ulceration was scored as present or absent. Each lesion was defined as amelanotic (absence of pigmentation), hypomelanotic (pigmentation involving less than 50% of the lesion), or pigmented (pigmentation involving more than 50% of the lesion).14
Concerning dermoscopic characteristics, each lesion was assigned to a global pattern according to the following definitions: 1) Multicomponent pattern: combination of at least 2 different predominant features within a given lesion; 2) Typical pigmented spitzoid pattern: a pigmented lesion with regular starburst appearance (composed of streaks or large globules symmetrically distributed at the periphery); 3) Typical non-pigmented spitzoid pattern: a hypopigmented/amelanotic lesion with dotted vessels and/or reticular depigmentation;15 4) Globular pattern: brown globules involving most of the lesion; 5) Reticular pattern: pigment network occupying most of the lesion; 6) Homogeneous pattern: homogeneous pigmentation involving most of the lesion; 7) Nonspecific pattern: global pattern not ascribable to any of the above categories.
The presence of local dermoscopic features was also assessed, according to the definitions summarized in table 1. Lesions were scored for the presence of network, streaks, globules, regression, blue white veil, vascularity, homogeneous pigmentation, regression structures, and white lines.16 The latter feature included both inverse network and crystalline structures defined as shiny white parallel, orthogonal or disordered linear streaks or short lines that are only seen with polarized light dermoscopy.17–20 Because our study sample was composed of images taken either with polarized or with non polarized light dermatoscopes, we decided to consider inverse network and crystalline structures as one parameter called white lines (Table 1).
Table 1.
Definition of the observed dermoscopic features.
| Dermoscopic feature | Definition |
|---|---|
| Vessels type | Identification of a vascular component and classification into one of the following morphologic categories: linear, comma, hairpin, polymorphic, dotted, coiled, and arborizing. |
| Vessels distribution | Central, peripheral, diffuse, irregular. |
| Streaks | Brownish-black linear structures of variable thickness, not clearly combined with pigment network lines. Scored as regular (regularly distibuted at the periphery of the lesion), or irregular. |
| Homogeneous pigmentation | Areas of structureless color varying from black, brown, blue, pink, and red. |
| Superficial black network | Distinctive type of pigment network, visible on the suface of heavily pigmented lesions. |
| Brown globules | Regular: sharply circumscribed, usually round to oval, black to brown structures. Irregular: variously sized and colored globules irregularly distributed within the lesion. |
| White lines | White crossing lines in between the vascular structures or the pigmented globules and/or white parallel or orthogonal and disordered linear streaks or short lines. |
| Network | Regular: light- to dark-brown pigmented, regularly meshed and narrowly spaced network distributed more or less regularly throughout the lesion. Irregular: black, brown, or gray, irregularly meshed network, distributed more or less irregularly throughout the lesion and usually ending abruptly at the periphery. |
| Blue white veil | Confluent, gray-blue to whitish-blue, diffuse pigmentation. |
| Regression | Grey-blue areas, white areas, peppering. |
The definite histopathologic diagnosis of AST or Spitz nevus was used as the main outcome. Demographic, clinical and dermoscopic variables were included in the analysis. Colinearity was assessed via a correlation matrix, using Spearman’s Rho correlation coefficient. Variables that were statistically significantly correlated were included in the logistic regression analyses. Student’s t test and Non parametric Man-Whitney U-test were used to compare means, after normality explorations. Pearson’s Chi Square was used for non-parametric cross tabulation comparisons. Relative risks were calculated for dichotomous variables. Crude and adjusted odds ratios and corresponding 95 percent confidence intervals (95% CI) were calculated by univariate and conditional multivariate logistic regression, respectively. The Type I error probability associated with all tests in this study was set to 0.05. An alpha less than 0.10 is considered to signify a trend. All statistical calculations were made with the SPSS 17.0 (Statistical Package for Social Sciences, SPSS Inc., Chicago, Ill.).
Results
In all, 165 patients (65 males and 100 females) were included. Mean age was 28.4 ± 13.5 for females and 26.4 ± 31.9 years for males. Mean age was 20.8 ± 313.8 in the AST group, and 31.0 ± 312.3 in the SN group (Student’s t-test, p<0.0001).
Frequencies of the observed clinical and dermoscopic variables are reported in Tables 2 and 3. The majority of AST presented as nodular lesions (32/55, 58.2%), whereas SN were more frequently macules (47/110, 42.7%) or plaques (42/110, 38.2%). Approximately half of AST were pigmented (29/55, 52.7%) and half were hypo- or non-pigmented (26/55, 47.3%). The majority of SN were pigmented (78/110, 70.9%) (Table 2).
Table 2.
Frequency of the evaluated clinical features in AST (atypical Spitz tumors) and SN (Spitz nevi).
| CLINICAL FEATURES | AST (%) | SN (%) | TOT (%) | |
|---|---|---|---|---|
|
| ||||
| SEX | M | 26 (47.3) | 39 (35.5) | 65 |
| F | 29 (52.7) | 71 (64.5) | 100 | |
|
| ||||
| LOCATION | trunk | 13 (23.6) | 21 (19.9) | 34 (20.6) |
| abdomen | 1 (1.8) | 5 (4.5) | 6 (3.6) | |
| lower limbs | 22 (40.0) | 52 (47.3) | 74 (44.8) | |
| upper limbs | 9 (16.4) | 22 (20.0) | 31 (18.8) | |
| acral | 5 (9.1) | 2 (1.8) | 7 (4.2) | |
| head/neck | 3 (5.5) | 5 (4.5) | 8 (4.8) | |
| buttocks | 1 (1.8) | 3 (2.7) | 4 (2.4) | |
| genital | 1 (1.8) | 0 | 1 (0.6) | |
|
| ||||
| PALPABILITY | macule | 9 (16.4) | 47 (42.7) | 56 (33.9) |
| papule | 3 (5.5) | 10 (9.1) | 13 (7.9) | |
| plaque | 11 (20.0) | 42 (38.2) | 53 (32.1) | |
| nodule | 32 (58.2) | 11 (10.9) | 43 (26.1) | |
|
| ||||
| PIGMENTATION | amelanotic | 14 (25.5) | 15 (13.6) | 29 (17.6) |
| hypomelanotic | 12 (21.8) | 17 (15.5) | 29 (17.6) | |
| pigmented | 29 (52.7) | 78 (70.9) | 107 (64.8) | |
|
| ||||
| ULCERATION | no | 42 (76.4) | 107 (97.3) | 149 (90.3) |
| Present | 13 (23.6) | 3 (2.7) | 16 (9.7) | |
Table 3.
Frequency of the observed dermoscopic features.
| Dermoscopic features | AST (N=55) (%) | SN (N=110) (%) | TOT (N=165) (%) | |
|---|---|---|---|---|
|
| ||||
| GLOBAL PATTERN | multicomponent | 24 (43.6) | 29 (26.4) | 53 (32.1) |
| typical spitzoid | 11 (20.0) | 58 (52.7) | 69 (41.8) | |
| unspecific | 11 (20.0) | 11 (10.0) | 22 (13.3) | |
| reticular | 3 (5.5) | 8 (7.3) | 11 (6.7) | |
| homogeneous | 6 (10.9) | 2 (1.8) | 8 (4.8) | |
| globular | 0 | 2 (1.8) | 2 (1.2) | |
|
| ||||
| VESSELS | absent | 21 (38.2) | 73 (66.4) | 94 (57.0) |
| linear | 5 (9.1) | 2 (1.8) | 7 (4.2) | |
| comma | 0 | 1 (0.9) | 1 (0.6) | |
| hairpin | 0 | 0 | 0 | |
| polymorphic | 14 (25.5) | 5 (4.5) | 19 (11.5) | |
| dotted | 13 (23.6) | 24 (21.8) | 37 (22.4) | |
| coiled | 1 (1.8) | 5 (4.5) | 6 (3.6) | |
| arborizing | 1 (1.8) | 0 | 1 (0.6) | |
|
| ||||
| VESSEL DISTRIBUTION | central | 7 (12.7) | 6 (5.5) | 13 (7.9) |
| peripheral | 7 (12.7) | 5 (4.5) | 12 (7.3) | |
| diffuse | 10 (18.2) | 20 (18.2) | 30 (18.2) | |
| irregular | 10 (18.2) | 7 (6.4) | 17 (10.3) | |
|
| ||||
| STREAKS | absent | 45 (81.8) | 60 (54.5) | 105 (63,6) |
| regular | 2 (3.6) | 34 (30.9) | 36 (21.8) | |
| irregular | 8 (14.5) | 15 (13.6) | 23 (13.9) | |
|
| ||||
| HOMOGENEOUS PIGMENTATION | absent | 27 (49.1) | 64 (58.2) | 91 (55.2) |
| regular | 12 (21.8) | 29 (26.4) | 41 (24.8) | |
| irregular | 16 (29.1) | 17 (15.5) | 33 (20.8) | |
|
| ||||
| SUPERFICIAL BLACK NETWORK | present | 0 | 11 (10.0) | 11 (6.7) |
|
| ||||
| BROWN GLOBULES | absent | 32 (58.2) | 68 (61.8) | 99 (60.0) |
| regular | 8 (14.5) | 20 (18.2) | 29 (17.5) | |
| irregular | 15 (27.3) | 22 (20.0) | 37 (22.4) | |
|
| ||||
| INVERSE NETWORK* | present | 15 (27.3) | 31 (28.2) | 46 (27.9) |
|
| ||||
| CRYSTALLINE STRUCTURES* | present | 20 (36.4) | 7 (6.4) | 27 (16.4) |
|
| ||||
| NETWORK | absent | 40 (72.7) | 88 (80.0) | 128 (77.6) |
| typical | 4 (7.3) | 9 (8.2) | 13 (7.9) | |
| atypical | 11 (20.0) | 13 (11.8) | 24 (14.5) | |
|
| ||||
| BLUE WHITE VEIL | present | 13 (23.6) | 13 (11.8) | 26 (15.8) |
|
| ||||
| REGRESSION | no | 45 (81.8) | 95 (86.4) | 25 (15.2) |
| present | 10 (18.2) | 15 (13.6) | ||
The features “inverse network” and “crystalline structures” were considered together in the statistical analysis, as a unique parameter named “white lines”. AST (atypical Spitz tumors) and SN (Spitz nevi).
The most frequent global dermoscopic pattern in AST was the multicomponent pattern, seen in 24/55 AST (43.6%), followed by nonspecific (11/55, 20.0%) and non-pigmented typical spitzoid (9/55, 16.4%), while a typical pigmented spitzoid pattern was found only in 2/55 AST (3.6%) (Table 3). In contrast, the typical pigmented spitzoid pattern was the most frequent global dermoscopic pattern of SN (40/110, 36.3%), followed by multicomponent (29/110, 26.4%) and non-pigmented typical spitzoid patterns (18/110, 16.4%).
According to the univariate logistic regression analysis, the most potent clinical predictors of AST were the nodular surface and the presence of ulceration, posing a 15-fold and 11-fold probability of AST, respectively. In contrast, the presence of pigmentation was associated with a 2.5-fold reduced probability of AST. The univariate analysis revealed several dermoscopic predictors of AST and SN. Specifically, the presence of linear or polymorphic vessels, blue white veil and white lines were independent predictors of AST, whereas the pigmented typical spitzoid pattern and the presence of regular streaks were suggestive of SN (table 4).
Table 4.
Univariate predictors of AST (Atypical Spitz Tumor) versus Spitz nevus (SN).
| Sig. | OR | 95% C.I. for EXP(B)
|
||
|---|---|---|---|---|
| Lower | Upper | |||
|
| ||||
| Palpability nodule | 0,000 | 15,192 | 5,651 | 40,838 |
|
| ||||
| Ulceration | 0,000 | 11,040 | 2,993 | 40,716 |
|
| ||||
| Global pattern typicalspitzoid | 0,001 | 0,229 | 0,099 | 0,532 |
|
| ||||
| Pigmentation pigmented | 0,033 | 0,398 | 0,171 | 0,926 |
| Vessels linear | 0,013 | 8,690 | 1,572 | 48,056 |
| Vessels polymorphic | 0,000 | 9,733 | 3,142 | 30,149 |
| Streaks regular | 0,001 | 0,078 | 0,018 | 0,344 |
| White lines | 0,032 | 2,066 | 1,064 | 4,013 |
| Bluewhiteveil | 0,054 | 2,310 | 0,987 | 5,402 |
After employing the multivariate conditional logistic regression model, which adjusted for all variables included in the model, nodularity and white lines remained significant predictors of AST, posing a 10-fold and 11-fold probability, respectively. In contrast, the presence of a pigmented typical spitzoid pattern was a potent predictor of SN, associated with 6.5-fold increased probability in the same multivariate model (table 5).
Table 5.
Multivariate Adjusted Predictors of AST (Atypical Spitz Tumor) versus Spitz Nevus. Odds Ratios (OR) mutually adjusted for all variables in the model.
| Sig. | OR | 95% C.I. for EXP(B) | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Palpability: nodule | 0,000 | 9,395 | 3,063 | 28,820 |
| Streaks regular | 0,020 | 0,154 | 0,032 | 0,744 |
Discussion
Our study provides novel insights into the clinical and dermoscopic morphology of AST, as compared to SN. According to our findings, AST develop either as pigmented nodular lesions with multicomponent or unspecific pattern dermoscopically, or as non-pigmented nodular lesions with a typical spitzoid pattern on dermoscopy. In contrast, detection of a pigmented typical spitzoid pattern (the so-called starburst pattern) is highly suggestive of SN.
Our observations might be valuable for improving the clinical recognition of AST and avoid its misinterpretation as SN. This is clinically relevant, since the management of these 2 entities differs significantly. AST are controversial tumors, because of their clinical and histopathologic morphologic overlap with spitzoid melanoma3–5. Accordingly, optimal management is wide local excision.
In contrast, SN is a benign tumor, similar to other benign melanocytic nevi. It is typified on dermoscopy by the so-called “starburst pattern”, consisting of symmetrically distributed peripheral streaks or pseudopods. A non-pigmented SN typically displays regularly distributed dotted vessels, with or without white lines intercrossing the red dots (reticular depigmentation or inverse network)10.
Spitzoid melanoma can also display some of these features (peripheral streaks, dotted vessels or inverse network), but usually lacks the characteristic symmetry of SN. Accordingly, lesions not overall characterized by one of the 2 typical spitzoid patterns should be excised, even in the presence of local spitzoid features.
Our results demonstrate that the majority of AST do not display a typical spitzoid, but a multicomponent or unspecific pattern. Consequently, the use of dermoscopy might prompt clinicians to excise these tumors, facilitating their appropriate management.
Diagnostic difficulty with spitzoid tumors is not only associated with dermoscopically asymmetric lesions, but also with those displaying a perfectly symmetric typical spitzoid pattern. Although the vast majority of these are SN, some will turn out to be melanomas and, others AST. In our study, 16.4% of AST were dermoscopically characterized by a typical non-pigmented spitzoid pattern, namely dotted vessels and white lines. In contrast, the pigmented typical spitzoid (starburst) pattern was a potent predictor of SN. This information, added to the previously existing knowledge on the morphologic diversity of spitzoid lesions, might further enhance their optimal management.
To overcome the problem of accurately interpreting a spitzoid-looking lesion, some clinicians are prone to remove all of them, while others follow a more conservative approach, especially in children.21–24 As shown by a recent study, the possibility of a perfectly symmetric spitzoid-looking lesion to be a melanoma significantly increases with age, being exceedingly low until the age of 20 years and rising to 50% after the age of 50 years25. Conservative management of Spitz nevi in children is increasing, as highlighted by a survey recently published.26 In this study, respondents included 175 pediatric dermatologists from the United States and around the world, with about 80% utilizing dermoscopy. In children with a clinical diagnosis of Spitz nevus, the option of clinical follow-up was chosen by 49.3% of respondents in the context of small, stable, non-pigmented lesions and by 29.7% in the context of pigmented lesions with a typical starburst pattern on dermoscopy.26 Thus, pigmented lesions, even if exhibiting a typical starburst pattern, seemed to be of most concern to the majority of respondents.
Our results highlight that the presence of a starburst pattern on dermoscopy almost invariably corresponds to SN. Monitoring such a lesion seems a safe management strategy. In contrast, surgical excision might represent the optimal choice for hypopigmented and amelanotic nodules dermoscopically displaying dotted vessels and white lines, since these findings characterize almost 20% of AST.
One limitation of our study is that we included only melanocytic lesions (SN and AST) in the analysis. We did not investigate either the differential diagnosis with spitzoid melanoma, or non melanocytic lesions, such as angioma and pyogenic granuloma, which can simulate AST both clinically and dermoscopically.8,10,12,27,28 Further studies are needed to evaluate this differential diagnosis. An additional limitation can be identified in the selection of the study sample, with multicenter collection of AST, whereas SN were selected in a single center. This was due to the relative rarity of AST compared to SN.
In conclusion, AST are polymorphic melanocytic tumors typically appearing as nodular lesions and dermoscopically typified by a multicomponent or unspecific pattern. Amelanotic and hypomelanotic nodules with a typical spitzoid pattern under dermoscopy cannot be safely classified as SN. Elucidation of the clinical and dermoscopic features of histopathologically equivocal spitzoid lesions may help the clinician select lesions that merit excision irrespective of patient age.
Figure 1.
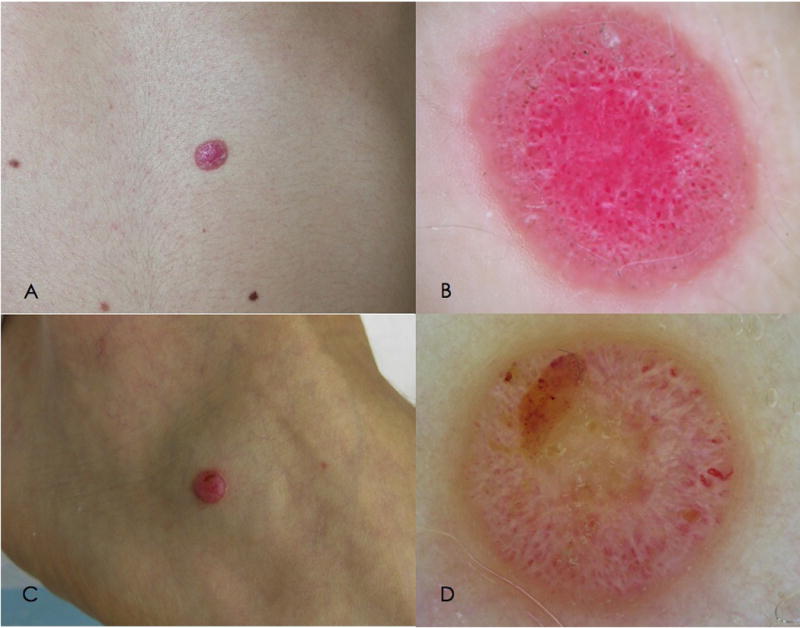
Clinical and dermoscopic features of amelanotic AST (atypical Spitz tumor). A. Clinical image of an amelanotic nodular lesion, arising on the abdomen of a 25 year-old woman. B. On dermoscopy, dotted vessels and white lines are visible over a pinkish background.
C. Clinical image of an amelanotic nodule arising on the extremity of a 19 year-old boy. B. On dermoscopy, the lesion shows a nonspecific pattern, with polymorphic vessels, ulceration and white lines.
Figure 2.
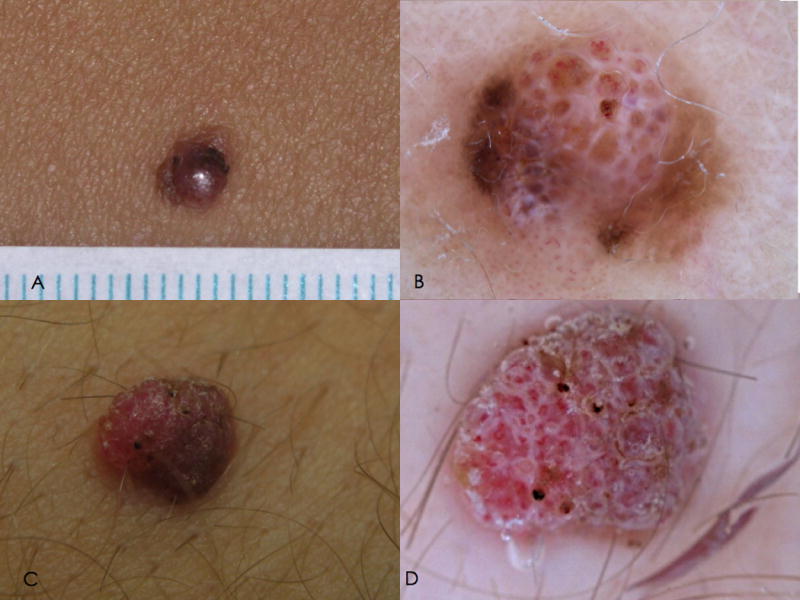
Clinical and dermoscopic features of hypomelanotic AST (atypical Spitz tumor). A. Clinical appearance of a hypomelanotic nodule arising on the lower limb of a 34 year-old woman. B. On dermoscopy the lesion exhibits a multicomponent pattern, with homogeneous brown pigmentation, white lines and polymorphic vessels.
C. Clinical appearance of a hypopigmented nodule arising on the leg of a 22 year-old boy. D. On dermoscopy, nonspecific pattern is detected, with polymorphic vessels, brownish pigmentation, and ulceration.
Figure 3.
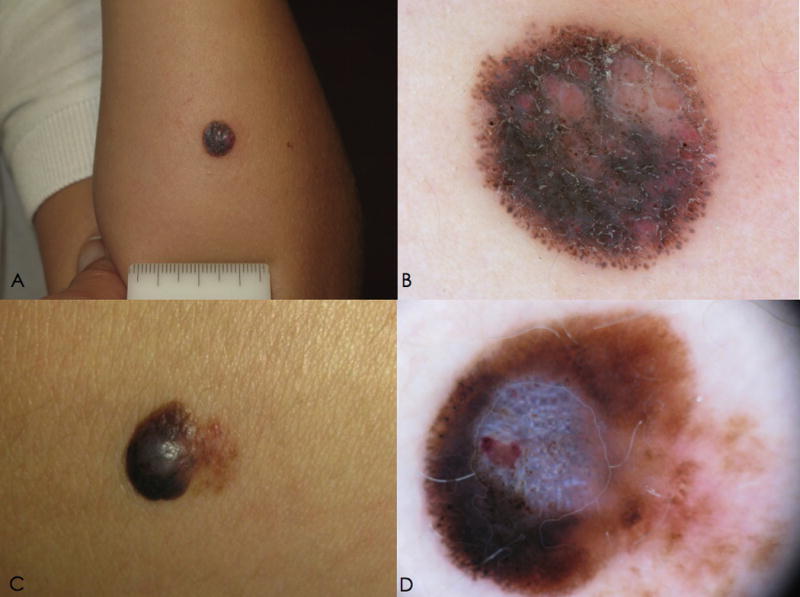
Clinical and dermoscopic features of pigmented AST (atypical Spitz tumor).A. Clinical image of a nodule arising on the forearm of a 3 year-old girl. B. On dermoscopy, a multicomponent pattern is detected, with irregular globules, and irregular homogeneous brown and pink pigmentation.
C. Clinical appearance of a pigmented nodule arising on the lower limb of a 15 year-old girl. D. On dermoscopy, a multicomponent pattern is visible, with irregular streaks, homogeneous brown pigmentation, blue-white veil and crystalline structures.
Acknowledgments
Funding/Support: This study was supported in part by the Italian Ministry of Health (RF-2010-2316524). Research at the Melanoma Unit in Hospital Clinic is partially founded by Grants 03/0019, 05/0302, 06/0265, 09/1393 and 12/00840 from Fondo de Investigaciones Sanitarias, Spain; by the CIBER de Enfermedades Raras of the Instituto de Salud Carlos III, Spain; by the AGAUR 2009 SGR 1337 of the Catalan Government, Spain; by the European Commission under the 6th Framework Programme, Contract nº: LSHC-CT-2006-018702 (GenoMEL); and by the National Cancer Institute (NCI) of the US National Institute of Health (NIH) (CA83115).
Footnotes
Role of the Sponsors: The sponsors had no role in the design and conduct of the study; in the collection, analysis and interpretation of data; or in the preparation, review, or approval of the manuscript.
References
- 1.Kernen JA, Ackerman LV. Spindle cell nevi and epithelioid cell nevi (so-called juvenile melanomas) in children and adults: a clinicopathologic study of 27 cases. Cancer. 1960;13:612–625. doi: 10.1002/1097-0142(196005/06)13:3<612::aid-cncr2820130324>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 2.Smith KJ, Barrett TL, Skelton HG, 3rd, et al. Spindle cell and epithelioid cell nevi with atypia and metastasis (malignant Spitz nevus) Am J Surg Pathol. 1989;13:931–939. doi: 10.1097/00000478-198911000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Barnhill RL, Flotte TJ, Fleischli M, et al. Cutaneous melanoma and atypical Spitz tumors in children. Cancer. 1995;76:1833–1845. doi: 10.1002/1097-0142(19951115)76:10<1833::aid-cncr2820761024>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 4.Barnhill RL, Argenyi ZB, From L, et al. Atypical Spitz nevi/tumor: lack of consensus for diagnosis, discrimination from melanoma, and prediction of outcome. Hum Pathol. 1999;30:513–520. doi: 10.1016/s0046-8177(99)90193-4. [DOI] [PubMed] [Google Scholar]
- 5.Mones JM, Ackerman AB. “Atypical”tSpitz’s nevus, “Malignant”aSpitz’s nevus, and “Metastasizing”eSpitz’s nevus: a critique in historical perspective of three concepts flawed fatally. Am J Dermatopathol. 2004;26:310–333. doi: 10.1097/00000372-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Casso EM, Grin-Jorgensen CM, Grant-Kels JM. Spitz nevi. J Am Acad Dermatol. 1992;27:901–913. doi: 10.1016/0190-9622(92)70286-o. [DOI] [PubMed] [Google Scholar]
- 7.Wiesner T, He J, Yelensky R, Esteve-Puig R, et al. Kinase fusions are frequent in Spitz tumors and spitzoid melanomas. Nat Commun. 2014;5:3116. doi: 10.1038/ncomms4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrara G, Zalaudek I, Savarese I, et al. Pediatric atypical spitzoid neoplasms. A review with emphasis on ‘red’(‘Spitz’) tumors and ‘blue’l(‘Blitz’) tumors. Dermatology. 2010;220:306–10. doi: 10.1159/000300093. [DOI] [PubMed] [Google Scholar]
- 9.Ludgate MW, Fullen DR, Lee J, et al. The atypical Spitz tumor of uncertain biologic potential: a series of 67 patients from a single institution. Cancer. 2009;115:631. doi: 10.1002/cncr.24047. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara G, Gianotti R, Cavicchini S, et al. Spitz nevus, Spitz tumor, and Spitzoid melanoma: a comprehensive clinicopathologic overview. Dermatol Clin. 2013;31:589–98. viii. doi: 10.1016/j.det.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Lallas A, Kyrgidis A, Ferrara G, et al. Atypical Spitz tumours and sentinel lymph node biopsy: a systematic review. Lancet Oncol. 2014;15:e178. doi: 10.1016/S1470-2045(13)70608-9. [DOI] [PubMed] [Google Scholar]
- 12.Moscarella E, Piccolo V, Argenziano G, et al. Problematic lesions in children. Dermatol Clin. 2013 Oct;31(4):535–47. vii. doi: 10.1016/j.det.2013.06.003. Epub 2013 Jul 10. [DOI] [PubMed] [Google Scholar]
- 13.Urso C. A new perspective for Spitz tumors? Am J Dermatopathol. 2005;27:364–365. doi: 10.1097/01.dad.0000144261.92614.42. [DOI] [PubMed] [Google Scholar]
- 14.Menzies SW, Kreusch J, Byth K, et al. Dermoscopic evaluation of amelanotic and hypomelanotic melanoma. Arch Dermatol. 2008 Sep;144(9):1120–7. doi: 10.1001/archderm.144.9.1120. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara G, Argenziano G, Soyer HP, et al. The spectrum of Spitz nevi: a clinicopathologic study of 83 cases. Arch Dermatol. 2005;141:1381–1387. doi: 10.1001/archderm.141.11.1381. [DOI] [PubMed] [Google Scholar]
- 16.Argenziano G, Soyer HP, Chimenti S, et al. Dermoscopy of pigmented skin lesions: results of a consensus meeting via the Internet. J Am Acad Dermatol. 2003 May;48(5):679–93. doi: 10.1067/mjd.2003.281. [DOI] [PubMed] [Google Scholar]
- 17.Shitara D, Ishioka P, Alonso-Pinedo Y, et al. Shiny white streaks: a sign of malignancy at dermoscopy of pigmented skin lesions. Acta Derm Venereol. 2014 Mar;94(2):132–7. doi: 10.2340/00015555-1683. [DOI] [PubMed] [Google Scholar]
- 18.Botella-Estrada R, Requena C, Traves V, et al. Chrysalis and negative pigment network in Spitz nevi. Am J Dermatopathol. 2012 Apr;34(2):188–91. doi: 10.1097/DAD.0b013e3182222ac1. [DOI] [PubMed] [Google Scholar]
- 19.Marghoob AA, Cowell L, Kopf AW, et al. Observation of chrysalis structures with polarized dermoscopy. Arch Dermatol. 2009 May;145(5):618. doi: 10.1001/archdermatol.2009.28. [DOI] [PubMed] [Google Scholar]
- 20.Pizzichetta MA, Canzonieri V, Soyer PH, et al. Negative pigment network and shiny white streaks: a dermoscopic-pathological correlation study. Am J Dermatopathol. 2014 May;36(5):433–8. doi: 10.1097/DAD.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 21.Broganelli P, Titli S, Lallas A, et al. Spitz/Reed nevi: proposal of management recommendations by the Dermoscopy Study Group of the Italian Society of Dermatology (SIDeMaST) G Ital Dermatol Venereol. 2014 Oct;149(5):601–6. [PubMed] [Google Scholar]
- 22.Argenziano G, Agozzino M, Bonifazi E, et al. Natural evolution of Spitz nevi. Dermatology. 2011;222(3):256–60. doi: 10.1159/000326109. [DOI] [PubMed] [Google Scholar]
- 23.Nino M, Brunetti B, Delfino S, et al. Spitz nevus: follow-up study of 8 cases of childhood starburst type and proposal for management. Dermatology. 2009;218(1):48–51. doi: 10.1159/000161120. [DOI] [PubMed] [Google Scholar]
- 24.Brunetti B, Nino M, Sammarco E, et al. Spitz naevus: a proposal for management. J Eur Acad Dermatol Venereol. 2005 May;19(3):391–3. doi: 10.1111/j.1468-3083.2004.01137.x. [DOI] [PubMed] [Google Scholar]
- 25.Lallas A, Moscarella E, Longo C, et al. Likelihood of finding melanoma when removing a Spitzoid-looking lesion in patients aged 12 years or older. J Am Acad Dermatol. 2015 Jan;72(1):47–53. doi: 10.1016/j.jaad.2014.09.037. [DOI] [PubMed] [Google Scholar]
- 26.Tlougan BE, Orlow SJ, Schaffer JV. Spitz nevi: beliefs, behaviors, and experiences of pediatric dermatologists. JAMA Dermatol. 2013 Mar;149(3):283–91. doi: 10.1001/jamadermatol.2013.1124. [DOI] [PubMed] [Google Scholar]
- 27.Spitz S. Melanoma of childhood. Am J Pathol. 1948;24:591–609. [PMC free article] [PubMed] [Google Scholar]
- 28.Moscarella E, Zalaudek I, Cerroni L, et al. Excised melanocytic lesions in children and adolescents - a 10-year survey. Br J Dermatol. 2012;167:368–373. doi: 10.1111/j.1365-2133.2012.10952.x. [DOI] [PubMed] [Google Scholar]


