Version Changes
Revised. Amendments from Version 1
Update of reference list to include a newly published paper [35] and also update of table 1 accordingly. Minor edits to the “Mitochondrial genome architecture and gene content” and “Mechanisms of mitochondrial gene expression“ sections to incorporate reference [35] and improve clarity. Minor corrections in figure 3, figure 4 legend and affiliation information.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Daniel Sloan, Colorado State University, Fort Collins, CO, USA
Thomas Becker, University of Freiburg, Freiburg, Germany
Abstract
Mitochondria are double membrane organelles of endosymbiotic origin, best known for constituting the centre of energetics of a eukaryotic cell. They contain their own mitochondrial genome, which as a consequence of gradual reduction during evolution typically contains less than two dozens of genes. In this review, we highlight the extremely diverse architecture of mitochondrial genomes and mechanisms of gene expression between the three sister groups constituting the phylum Euglenozoa - Euglenida, Diplonemea and Kinetoplastea. The earliest diverging euglenids possess a simplified mitochondrial genome and a conventional gene expression, whereas both are highly complex in the two other groups. The expression of their mitochondrial-encoded proteins requires extensive post-transcriptional modifications guided by complex protein machineries and multiple small RNA molecules. Moreover, the least studied diplonemids, which have been recently discovered as a highly abundant component of the world ocean plankton, possess one of the most complicated mitochondrial genome organisations known to date.
Keywords: mitochondria, euglenozoa, mitochondrial genome
Introduction
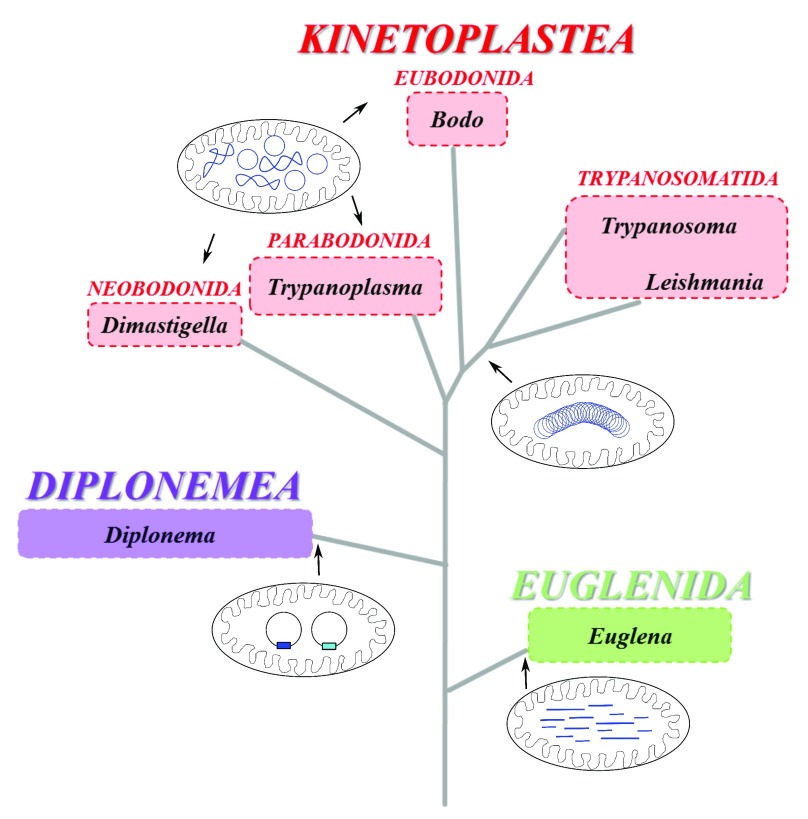
The phylum Euglenozoa, which is part of the supergroup Excavata that significantly diverged from other eukaryotic lineages, is composed of three geographically ubiquitous groups of flagellated protists: Euglenida, Diplonemea, and Kinetoplastea (the fourth group, Symbiontida, has no molecular data available and thus will not be discussed here) 1. The well-known representatives of these groups are Euglena for euglenids and Trypanosoma for kinetoplastids, whereas diplonemids are very poorly known. Although the Euglenozoa is a stable and highly supported group, mutual phylogenetic relationships among these three groups are not yet fully resolved, and euglenids likely constitute the earliest offshoot ( Figure 1) 1– 3.
Figure 1. Schematic phylogenetic tree of representative genera of Euglenozoa depicting the organization of their mitochondrial genomes.
The scheme is based on Adl et al. 1 (2012). Different organization of their mitochondrial genomes (in blue) is shown for the three major lineages: Kinetoplastea (in red), Diplonemea (in purple), and Euglenida (in green). Whereas Euglenida possess an array of linear mitochondrial DNA molecules of variable length, Diplonemea and Kinetoplastida contain in their organelle circular DNA molecules in different arrangements. In Diplonemea, circular molecules of two sizes are non-catenated and supercoiled. The kinetoplast DNA of Eubodonida, Parabodonida, and Neobodonida is composed of numerous free, non-catenated relaxed or supercoiled DNA circles, whereas in Trypanosomatida it is constituted of thousands of relaxed circles, mutually interlocked into a single giant network composed of interlocked maxicircles and minicircles that together with proteins are packaged into a single compact disk.
Euglenozoans have several common morphological features, such as subpellicular microtubules and a single flagellum or two heterodynamic flagella, protruding from an anterior pocket. Their lifestyles vary greatly, ranging from the free-living photosynthetic euglenids to intra- and extracellular parasites of plants, insects, and mammals, including humans. We do not yet know the predominant lifestyle of diplonemids, a group that recently came into the spotlight thanks to the Tara Oceans expedition, which revealed their global presence and extreme abundance in the world ocean. Indeed, diplonemids may comprise the sixth most abundant and third most species-rich group of marine eukaryotes 4, 5. The euglenids and diplonemids display different modes of nutrition; the former are characterized by photoautotrophy, whereas the latter are likely phagotrophs, osmotrophs, or parasites or a combination of these. The predominantly parasitic kinetoplastids make use of the carbon sources provided by their hosts 6, 7.
A hallmark feature of euglenozoans is a single large mitochondrion, frequently reticulated and displaying cristae with a discoid structure 1, 8. Like all mitochondria of aerobic protists, this organelle contains mitochondrial DNA (mtDNA) 7. Although as vestigial as mtDNAs of other eukaryotes, this organellar genome evolved in euglenozoan protists into a stunning variety of structures and organizations, as described in more detail below. With the advent of affordable high-throughput sequencing, thousands of mt genomes are being assembled and annotated. However, their selection remains strongly skewed toward the metazoans, which mostly harbor standard, highly reduced, and streamlined mt genomes 9. Yet the majority of extant eukaryotic diversity is constituted by protists 10, of which only a very small fraction has their mtDNA characterized. Still, the available mt genomes of protists show a range of bizarre gene arrangements, modes of organization, and complex post-transcriptional maturations 11, 12. Hence, it does not come as a surprise that some authors consider further sequencing of mt genomes of metazoans as superfluous and non-informative but that at the same time they call for focusing efforts onto the organellar genomes of hitherto-neglected protist groups 12.
Mitochondrial genome architecture and gene content
Standard mt genomes are usually represented by a circular or linear DNA molecule encoding an average of fewer than two dozen genes ranging from 2 to 66 proteins in Chromera velia and Andalucia godoyi, respectively 13, 14. Although euglenozoans harbor a low number of genes in their mt genomes, they developed an extremely variable genome architecture. In euglenids and diplonemids, mtDNA seems to be evenly distributed throughout the lumen of the organelle 8, 15, whereas in kinetoplastids, the picture is more complex ( Figure 2). In the obligatory parasitic trypanosomatids mtDNA is invariably compacted into a single disk-shaped structure of concatenated DNA termed the kinetoplast DNA (kDNA), the free-living or commensalic bodonids have their kDNA distributed either evenly or in foci in the mt lumen 16.
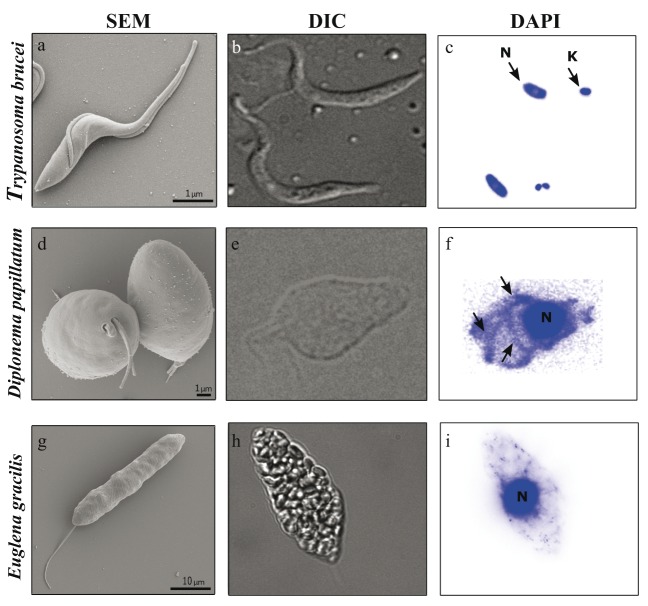
Figure 2. Morphology of representatives of Kinetoplastea ( Trypanosoma brucei), Diplonemea ( Diplonema papillatum), and Euglenida ( Euglena gracilis).
Scanning electron microscopy (SEM) ( a, d, g) and differential interference contrast (DIC) ( b, e, h) reveal cell morphology, whereas 4′,6-diamidino-2-phenylindole (DAPI) staining provides information about the amount and distribution of mitochondrial DNA ( c, f, i). ( c) Trypanosoma with distinct nucleus (N) and kinetoplast (K). ( f) In Diplonema, arrows point to large amounts of mitochondrial DNA meandering through the cell. Scale bars = 1 μm ( a, d) and 10 μm ( g).
The best-studied kDNA is that of the human pathogen and model organism Trypanosoma brucei (for current reviews, see 17– 19). It is composed of thousands of DNA circles mutually interlocked into a single network ( Figure 2) that is densely packed into a disk-shaped structure located close to the basal body of the flagellum. The kDNA network of T. brucei and related flagellates is formed of dozens of maxicircles, each about 20 kb long, and of approximately 5,000 uniformly sized (~1.0 kb) minicircles 20. The maxicircle is composed of a single conserved region, which contains all protein-coding and rRNA genes, and a shorter variable region of species-specific size and sequence, which probably plays a role in replication 21– 23. The conserved region carries 18 protein-coding genes, mostly subunits of respiratory complexes (complex I: nad1, nad2, nad3, nad4, nad5, nad7, nad8, and nad9; complex III: cob; complex IV: cox1, cox2, and cox3; complex V: atp6), one ribosomal protein ( rps12), small and large mito-rRNA genes ( 12S and 9S), and four open reading frames of unknown function ( MURF2, MURF5, cr3, and cr4) ( Figure 3) 24, 25. Each minicircle codes for three to five small guide RNA (gRNA) genes, accounting for a total coding capacity of the kDNA of approximately 1,200 different gRNAs within approximately 250 distinct minicircle classes 26. The gRNAs provide information for post-transcriptional RNA editing in the form of multiple insertions and deletions of uridine residues into the maxicircle-derived mRNAs (for recent reviews, see 19, 27, 28). The kDNA does not encode tRNA genes, and hence all tRNA molecules must be imported from the cytosol 29, 30.
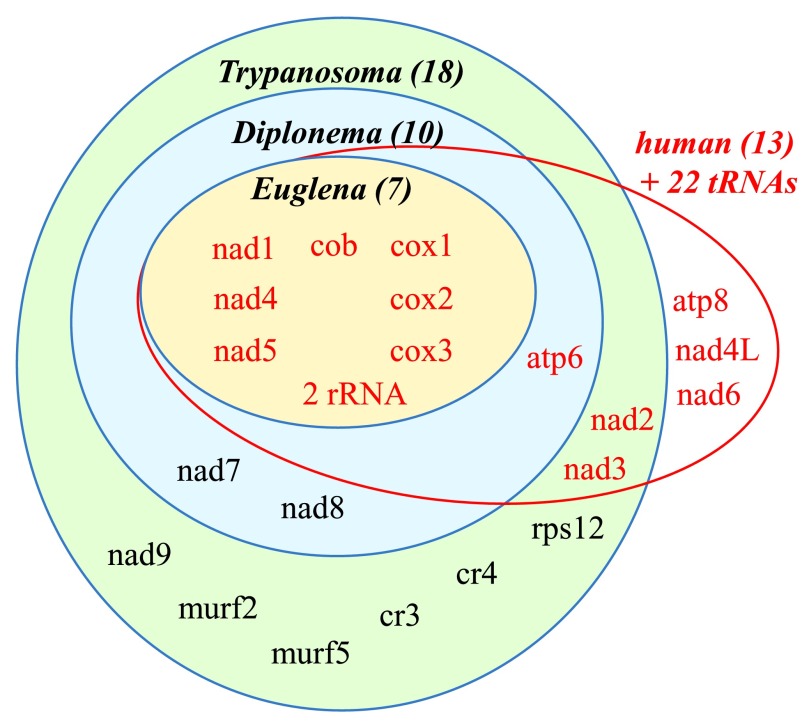
Figure 3. Schematic representation of the gene content of mitochondrial genomes of representative euglenozoans and human.
The number of protein-coding genes is shown in parentheses next to selected euglenozoan flagellates. The number of protein-coding genes found in Euglena (orange oval), Diplonema (blue oval), and Trypanosoma is depicted (green oval). In comparison, human mitochondrial genome encodes 13 proteins (in red). All euglenozoans most likely contain small subunit and large subunit mito-rRNAs that in Diplonema and Euglena are likely to be highly divergent.
The mtDNA of the diplonemid Diplonema papillatum is composed of numerous free DNA circles ( Figure 1) with a total size of about 600 kb 31. The circles fall into two classes that are distinguished by their size (6 or 7 kb) and by the sequence of their non-coding regions that makes up about 95% of the circle. Each circle also carries a single cassette composed of a piece of a gene (gene module) that is flanked on both sides by a unique sequence on average 50 bp in length 31– 34. Although the genes are uniquely fragmented, the gene content of Diplonema is rather standard and similar to that of kDNA of the kinetoplastid flagellates, as it specifies subunits of respiratory complexes (complex I: nad1, 4, 5, 7, and 8; complex III: cob; complex IV: cox1-3; complex V: atp6) and large subunit (LSU) mito-rRNA 31. The small subunit (SSU) mito-rRNA has been identified only very recently because its sequence is extremely diverged, which has made its identification challenging 34, 35. So far, all identified mt-encoded genes of Diplonema are fragmented, hence must be uniquely trans-spliced into fully translatable mRNAs by a hitherto-unknown mechanism.
The mtDNA of Euglena, the model euglenid, is surprisingly simple when compared with the highly complex genomes of its sister groups. Indeed, the genome is streamlined in terms of both its architecture and gene content. The mtDNA of Euglena is represented by a pool of recombination-prone short linear molecules containing repeats, pieces of non-functional gene fragments, and full-length gene copies, which ensure the production of functional proteins. The set of only three protein-coding genes ( cox1-3) 36, 37 was recently complemented by four intact genes encoding additional subunits of the respiratory chain ( nad1, 4, and 5 and cob) 38. The genes encoding subunits of complex V and ribosomal proteins are missing. The SSU and LSU of mito-rRNA are likely split into two fragments each (SSU-R, SSU-L and LSU-R, LSU-L). However, only the SSU-R fragment has been identified to date and this is most likely due to the same reasons as in the case of Diplonema LSU, namely the extreme divergence of the corresponding mito-rRNAs 36, 38.
Mechanisms of mitochondrial gene expression
In all euglenozoans, mtDNA is transcribed into polycistrons, which undergo endonucleolytic cleavage and further editing or processing (or both) into translatable mRNAs ( Figure 4). In kinetoplastids, the majority of mt-encoded genes exist in a cryptic form, as their corresponding transcripts have to undergo extensive post-transcriptional RNA editing of the uridine insertion/deletion type (for recent reviews, see 19, 27, 28). In Diplonema, only a few insertions of blocks of uridines have been documented initially, but the recent comprehensive count amounts to ~200 31, 32, 35. RNA editing in Trypanosoma and related flagellates is extremely complex, as the insertions and deletions of hundreds and dozens of uridines, respectively, are performed by a multitude of gRNAs and several protein complexes that interact in a highly dynamic manner 28, 39. Upon the addition of complex poly-U/A tails 27, 28 ( Figure 4), the fully edited transcripts are translated on protein-rich and rRNA-poor ribosomes 40, but the role of the additional 45S ribosomal subunit of unique protein composition is still unclear 41. Likely owing to their extreme hydrophobicity, only a few of the de novo synthesized mt proteins have been observed 42, 43.
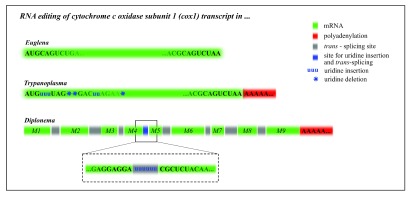
Figure 4. The comparison of RNA processing of the cox1 transcript in representative euglenozoans.
In contrast to Trypanoplasma borreli and Diplonema papillatum, the cytochrome c oxidase subunit 1 (cox1) transcript of Euglena gracilis (in green) does not undergo RNA editing, splicing, or polyadenylation prior to its translation. In Trypanoplasma, cox1 undergoes RNA editing in the form of numerous uridine insertions (small blue “u”) and deletions (blue star), followed by polyadenylation (in red). The Diplonema cox1 transcript is formed by trans-splicing of nine small fragments called modules M1 thru M9 (in green), which is accompanied by the insertion of six uridines between the modules M4 and M5. Finally, the transcript is polyadenylated and translated on mitochondrial ribosomes.
The post-transcriptional processing is very different in Diplonema, where fragments of genes transcribed from individual DNA circles are trans-spliced 31, 33. The genome and mitoproteome of Diplonema may eventually shed light on this unique processing, but so far the proteins (and potentially also small RNAs) involved in it remain completely unknown. In any case, it is highly likely that the fully trans-spliced mRNAs are in organello translated (our unpublished data). Indeed, the recently described simplicity of mt mRNA processing in Euglena 38 and likely other euglenids is in stunning contrast with the baroque complexity of RNA editing or trans-splicing (or both) in kinetoplastids and diplonemids ( Figure 4 and Table 1).
Table 1. Architecture and gene content of mitochondrial genomes of representative euglenozoans and humans.
| Trypanosoma brucei |
Diplonema
papillatum |
Euglena gracilis | Human | |
|---|---|---|---|---|
| Type of mitochondrial cristae | Discoidal | Discoidal | Discoidal | Tubular |
| Mode of life | Parasitic | Phagotrophic
osmotrophic |
Mixotrophic
heterotrophic autotrophic |
Heterotrophic |
| Habitat | Insect gut
mammalian bloodstream |
Marine | Freshwater | Predominantly
terrestral |
| Genome structure | Circular | Circular | Linear | Circular |
| protein-coding genes | 18 | 10 | 7* | 13 |
| rRNA (SSU/LSU) | +/+ | +/+ | +/? | +/+ |
| tRNA | - | - | - | 22 |
| Chromosome size | Maxicircles: ~20.0 kb
Minicircles: ~1.0 kb |
Class A: 6.0 kb
Class B: 7.0 kb |
~1.0 to ~9.0 kb | 16.6 kb |
| Genome copy number | Maxicircles: ~dozens
Minicircles: ~5,000 |
~100 | ? | ~50 to 1×10 5 |
| mRNA polyadenylation | Yes | Yes | No | Yes |
| Trans-splicing | No | Yes | No | No |
| Uridine insertions | Yes | Yes | No | No |
| Uridine deletions | Yes | No | No | No |
| Introns | No | No | No | No |
*Seven complete genes, together with multiple gene fragments.
Why are mitochondrial genomes in Euglenozoa so diverse?
Soon after its discovery, RNA editing in kinetoplastids was explained as a remnant of the RNA world 44. A more plausible explanation postulates that gene fragmentation in the euglenozoan last common ancestor was “the seed of future chaos”, leading to the emergence of extremely complex post-transcriptional mechanisms, differing in each sister lineage, yet eventually correcting the scrambling on the post-transcriptional level 45. In fact, it was argued that the kinetoplastid RNA editing is a prime example of “irremediable complexity”, a rampant mechanism that does not provide any selective advantage yet fixes the problem 39, 46. The recent finding of a mt genome in Euglena 38 implies that the irreversible scrambling originally implied for the mtDNA of the euglenozoan last common ancestor 45 did happen at a later stage in evolution, probably in the predecessor of diplonemids and kinetoplastids. Although despite the available sequence data the mutual relationships among the three euglenozoan lineages remain unresolved, we can predict, on the basis of their mt genomes and transcriptomes, that the mostly free-living photosynthetic euglenids constitute the earliest offshoot of the long euglenozoan branch.
Acknowledgments
The scanning electron micrographs of Diplonema and Trypanosoma were kindly provided by Binnypreet Kaur and Eva Horáková (Biology Centre, České Budějovice), respectively.
Funding Statement
This work was funded by Gordon and Betty Moore Foundation grant GBMF4983 and Czech Grant Agency 15-21974S to JL.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; referees: 2 approved]
References
- 1. Adl SM, Simpson AG, Lane CE, et al. : The revised classification of eukaryotes. J Eukaryot Microbiol. 2012;59(5):429–93. 10.1111/j.1550-7408.2012.00644.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lukeš J, Skalický T, Týč J, et al. : Evolution of parasitism in kinetoplastid flagellates. Mol Biochem Parasitol. 2014;195(2):115–22. 10.1016/j.molbiopara.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 3. Hampl V, Hug L, Leigh JW, et al. : Phylogenomic analyses support the monophyly of Excavata and resolve relationships among eukaryotic "supergroups". Proc Natl Acad Sci U S A. 2009;106(10):3859–64. 10.1073/pnas.0807880106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Vargas C, Audic S, Henry N, et al. : Ocean plankton. Eukaryotic plankton diversity in the sunlit ocean. Science. 2015;348(6237):1261605. 10.1126/science.1261605 [DOI] [PubMed] [Google Scholar]
- 5. Lukeš J, Flegontova O, Horák A: Diplonemids. Curr Biol. 2015;25(16):R702–4. 10.1016/j.cub.2015.04.052 [DOI] [PubMed] [Google Scholar]
- 6. Tielens AG, van Hellemond JJ: Surprising variety in energy metabolism within Trypanosomatidae. Trends Parasitol. 2009;25(10):482–90. 10.1016/j.pt.2009.07.007 [DOI] [PubMed] [Google Scholar]
- 7. Zíková A, Hampl V, Paris Z, et al. : Aerobic mitochondria of parasitic protists: Diverse genomes and complex functions. Mol Biochem Parasitol. 2016; pii: S0166-6851(16)30015-9. 10.1016/j.molbiopara.2016.02.007 [DOI] [PubMed] [Google Scholar]
- 8. Marande W, Lukes J, Burger G: Unique mitochondrial genome structure in diplonemids, the sister group of kinetoplastids. Eukaryot Cell. 2005;4(6):1137–46. 10.1128/EC.4.6.1137-1146.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith DR, Keeling PJ: Mitochondrial and plastid genome architecture: Reoccurring themes, but significant differences at the extremes. Proc Natl Acad Sci U S A. 2015;112(33):10177–84. 10.1073/pnas.1422049112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pawlowski J, Audic S, Adl S, et al. : CBOL protist working group: barcoding eukaryotic richness beyond the animal, plant, and fungal kingdoms. PLoS Biol. 2012;10(11):e1001419. 10.1371/journal.pbio.1001419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gray MW: Diversity and evolution of mitochondrial RNA editing systems. IUBMB Life. 2003;55(4–5):227–33. 10.1080/1521654031000119425 [DOI] [PubMed] [Google Scholar]
- 12. Smith DR: The past, present and future of mitochondrial genomics: have we sequenced enough mtDNAs? Brief Funct Genomics. 2016;15(1):47–54. 10.1093/bfgp/elv027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flegontov P, Michálek J, Janouškovec J, et al. : Divergent mitochondrial respiratory chains in phototrophic relatives of apicomplexan parasites. Mol Biol Evol. 2015;32(5):1115–31. 10.1093/molbev/msv021 [DOI] [PubMed] [Google Scholar]
- 14. Burger G, Gray MW, Forget L, et al. : Strikingly bacteria-like and gene-rich mitochondrial genomes throughout jakobid protists. Genome Biol Evol. 2013;5(2):418–38. 10.1093/gbe/evt008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roy J, Faktorová D, Lukes J, et al. : Unusual mitochondrial genome structures throughout the Euglenozoa. Protist. 2007;158(3):385–96. 10.1016/j.protis.2007.03.002 [DOI] [PubMed] [Google Scholar]
- 16. Lukes J, Guilbride DL, Votýpka J, et al. : Kinetoplast DNA network: evolution of an improbable structure. Eukaryot Cell. 2002;1(4):495–502. 10.1128/EC.1.4.495-502.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jensen RE, Englund PT: Network news: the replication of kinetoplast DNA. Annu Rev Microbiol. 2012;66:473–91. 10.1146/annurev-micro-092611-150057 [DOI] [PubMed] [Google Scholar]
- 18. Povelones ML: Beyond replication: division and segregation of mitochondrial DNA in kinetoplastids. Mol Biochem Parasitol. 2014;196(1):53–60. 10.1016/j.molbiopara.2014.03.008 [DOI] [PubMed] [Google Scholar]
- 19. Verner Z, Basu S, Benz C, et al. : Malleable mitochondrion of Trypanosoma brucei. Int Rev Cell Mol Biol. 2015;315:73–151. 10.1016/bs.ircmb.2014.11.001 [DOI] [PubMed] [Google Scholar]
- 20. Liu B, Liu Y, Motyka SA, et al. : Fellowship of the rings: the replication of kinetoplast DNA. Trends Parasitol. 2005;21(8):363–9. 10.1016/j.pt.2005.06.008 [DOI] [PubMed] [Google Scholar]
- 21. Borst P, Fase-Fowler F, Weijers PJ, et al. : Kinetoplast DNA from Trypanosoma vivax and T. congolense. Mol Biochem Parasitol. 1985;15(2):129–42. 10.1016/0166-6851(85)90114-8 [DOI] [PubMed] [Google Scholar]
- 22. Sloof P, de Haan A, Eier W, et al. : The nucleotide sequence of the variable region in Trypanosoma brucei completes the sequence analysis of the maxicircle component of mitochondrial kinetoplast DNA. Mol Biochem Parasitol. 1992;56(2):289–99. 10.1016/0166-6851(92)90178-M [DOI] [PubMed] [Google Scholar]
- 23. Shapiro TA, Englund PT: The structure and replication of kinetoplast DNA. Annu Rev Microbiol. 1995;49:117–43. 10.1146/annurev.mi.49.100195.001001 [DOI] [PubMed] [Google Scholar]
- 24. Benne R, De Vries BF, Van den Burg J, et al. : The nucleotide sequence of a segment of Trypanosoma brucei mitochondrial maxi-circle DNA that contains the gene for apocytochrome b and some unusual unassigned reading frames. Nucleic Acids Res. 1983;11(20):6925–41. 10.1093/nar/11.20.6925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Simpson L, Neckelmann N, de la Cruz VF, et al. : Comparison of the maxicircle (mitochondrial) genomes of Leishmania tarentolae and Trypanosoma brucei at the level of nucleotide sequence. J Biol Chem. 1987;262(13):6182–96. [PubMed] [Google Scholar]
- 26. Koslowsky D, Sun Y, Hindenach J, et al. : The insect-phase gRNA transcriptome in Trypanosoma brucei. Nucleic Acids Res. 2014;42(3):1873–86. 10.1093/nar/gkt973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aphasizhev R, Aphasizheva I: Mitochondrial RNA editing in trypanosomes: small RNAs in control. Biochimie. 2014;100:125–31. 10.1016/j.biochi.2014.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Read LK, Lukeš J, Hashimi H: Trypanosome RNA editing: the complexity of getting U in and taking U out. Wiley Interdiscip Rev RNA. 2016;7(1):33–51. 10.1002/wrna.1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alfonzo JD, Blanc V, Estévez AM, et al. : C to U editing of the anticodon of imported mitochondrial tRNA Trp allows decoding of the UGA stop codon in Leishmania tarentolae. EMBO J. 1999;18(24):7056–62. 10.1093/emboj/18.24.7056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alfonzo JD, Söll D: Mitochondrial tRNA import--the challenge to understand has just begun. Biol Chem. 2009;390(8):717–22. 10.1515/BC.2009.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vlcek C, Marande W, Teijeiro S, et al. : Systematically fragmented genes in a multipartite mitochondrial genome. Nucleic Acids Res. 2011;39(3):979–88. 10.1093/nar/gkq883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kiethega GN, Yan Y, Turcotte M, et al. : RNA-level unscrambling of fragmented genes in Diplonema mitochondria. RNA Biol. 2013;10(2):301–13. 10.4161/rna.23340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marande W, Burger G: Mitochondrial DNA as a genomic jigsaw puzzle. Science. 2007;318(5849):415. 10.1126/science.1148033 [DOI] [PubMed] [Google Scholar]
- 34. Valach M, Burger G, Gray MW, et al. : Widespread occurrence of organelle genome-encoded 5S rRNAs including permuted molecules. Nucleic Acids Res. 2014;42(22):13764–77. 10.1093/nar/gku1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moreira S, Valach M, Aoulad-Aissa M, et al. : Novel modes of RNA editing in mitochondria. Nucleic Acids Res. 2016;44(10):4907–19. 10.1093/nar/gkw188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Spencer DF, Gray MW: Ribosomal RNA genes in Euglena gracilis mitochondrial DNA: fragmented genes in a seemingly fragmented genome. Mol Genet Genomics. 2011;285(1):19–31. 10.1007/s00438-010-0585-9 [DOI] [PubMed] [Google Scholar]
- 37. Tessier LH, van der Speck H, Gualberto JM, et al. : The cox1 gene from Euglena gracilis: a protist mitochondrial gene without introns and genetic code modifications. Curr Genet. 1997;31(3):208–13. 10.1007/s002940050197 [DOI] [PubMed] [Google Scholar]
- 38. Dobáková E, Flegontov P, Skalický T, et al. : Unexpectedly Streamlined Mitochondrial Genome of the Euglenozoan Euglena gracilis. Genome Biol Evol. 2015;7(12):3358–67. 10.1093/gbe/evv229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lukeš J, Archibald JM, Keeling PJ, et al. : How a neutral evolutionary ratchet can build cellular complexity. IUBMB Life. 2011;63(7):528–37. 10.1002/iub.489 [DOI] [PubMed] [Google Scholar]
- 40. Zíková A, Panigrahi AK, Dalley RA, et al. : Trypanosoma brucei mitochondrial ribosomes: affinity purification and component identification by mass spectrometry. Mol Cell Proteomics. 2008;7(7):1286–96. 10.1074/mcp.M700490-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ridlon L, Škodová I, Pan S, et al. : The importance of the 45 S ribosomal small subunit-related complex for mitochondrial translation in Trypanosoma brucei. J Biol Chem. 2013;288(46):32963–78. 10.1074/jbc.M113.501874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Horvath A, Kingan TG, Maslov DA: Detection of the mitochondrially encoded cytochrome c oxidase subunit I in the trypanosomatid protozoan Leishmania tarentolae. Evidence for translation of unedited mRNA in the kinetoplast. J Biol Chem. 2000;275(22):17160–5. 10.1074/jbc.M907246199 [DOI] [PubMed] [Google Scholar]
- 43. Škodová-Sveráková I, Horváth A, Maslov DA: Identification of the mitochondrially encoded subunit 6 of F 1F O ATPase in Trypanosoma brucei . Mol Biochem Parasitol. 2015;201(2):135–8. 10.1016/j.molbiopara.2015.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cech TR: RNA editing: world's smallest introns? Cell. 1991;64(4):667–9. 10.1016/0092-8674(91)90494-J [DOI] [PubMed] [Google Scholar]
- 45. Flegontov P, Gray MW, Burger G, et al. : Gene fragmentation: a key to mitochondrial genome evolution in Euglenozoa? Curr Genet. 2011;57(4):225–32. 10.1007/s00294-011-0340-8 [DOI] [PubMed] [Google Scholar]
- 46. Gray MW, Lukes J, Archibald JM, et al. : Cell biology. Irremediable complexity? Science. 2010;330(6006):920–1. 10.1126/science.1198594 [DOI] [PubMed] [Google Scholar]