Abstract
Seasonal timing is assumed to involve the circadian clock, an endogenous mechanism to track time and measure day length. Some debate persists, however, and aphids were among the first organisms for which circadian clock involvement was questioned. Inferences about links to phenology are problematic, as the clock itself is little investigated in aphids. For instance, it is unknown whether aphids possess diurnal rhythms at all. Possibly, the close interaction with host plants prevents independent measurements of rhythmicity. We reared the pea aphid Acyrthosiphon pisum (Harris) on an artificial diet, and recorded survival, moulting, and honeydew excretion. Despite their plant-dependent life style, aphids were independently rhythmic under light–dark conditions. This first demonstration of diurnal aphid rhythms shows that aphids do not simply track the host plant’s rhythmicity.
Keywords: circadian clock, hourglass clock, Acyrthosiphon pisum, artificial diet, photoperiodism
Throughout latitudes and altitudes, day- and night-time temperatures and their respective durations vary, and species are adapted to make best use of the temporal niches via diurnal or nocturnal activity (Bennie et al. 2014). Diurnal rhythms can dictate whether species meet, and can contribute to presence or absence of biotic interactions (Stich and Lampert 1981, Fleury et al. 2000). Due to the relevance of a correct timing, nearly all organisms examined so far possess an endogenous mechanism called circadian clock (Moore-Ede et al. 1982, but see Lu et al. 2010), and the circadian clock affects all major physiological processes (Moore-Ede et al. 1982).
One remaining question is whether the clock is involved in seasonal timing (phenology) via day length (photoperiod) measurements (Bunning 1936). Supporters of clock involvement have proposed two models, which describe how phase relations of clock and environment (external coincidence, Pittendrigh and Minis 1964), or of multiple clocks (internal coincidence, Pittendrigh and Minis 1972) could govern photoperiodism. Numerous studies indeed correlated clock gene expression with photoperiodism (Schultz and Kay 2003), including in hemipterans (Ikeno et al. 2010), but this correlation can be at least partially attributed to research bias in favor of clock genes (Bradshaw and Holzapfel 2010). Hence, despite accumulating correlative evidence, the debate is still not fully settled (Danks 2005).
An alternative to clock involvement in photoperiodism is the hour glass model (Garner and Allard 1920). In this model, steady, clock-independent accumulation of a molecule triggers a response upon reaching a threshold. Aphids played a prominent role in the discussion of clock involvement in photoperiodism, as they were seen as first evidence for such an hour glass model (Lees 1973). Careful re-evaluation contradicted this view, and suggested that aphid photoperiodism depends on the circadian clock (Hardie and Vaz Nunes 2001). The clock was proposed to damp quickly, i.e., disappear within few cycles. However, very little empirical data are available about damping or other properties of the aphid clock itself, let alone studies on how the clock affects aphid behavior. Before settling the argument on circadian clock involvement in photoperiodism, the first logical question is whether aphids have a diurnal rhythm driven by an endogenous clock.
The lack of research on diurnal rhythms of aphids may in part be explained by the high degree of food specialization, which complicates studies of an independent rhythm. Aphids are known for their remarkable phenotypic plasticity, and asexual forms (morphs) can bear sexual offspring if induced by a short photoperiod (Lees 1973). Because the sexual offspring is less dependent on host plants, experiments with various aphid species have been conducted on such sexual morphs (Eisenbach and Mittler 1980, Thieme and Dixon 1996). However, if the ultimate aim is the link of circadian clock and photoperiodism, tests on long-day (asexual) aphids are needed. While some experiments have been also conducted on asexual morphs, the aphids were always held on living plants (Gomez et al. 2006, Cortes et al. 2010, Taylor et al. 2012), so the changing C:N ratio of the plant might have entrained the aphid, i.e., food has reset the circadian clock. Only one study has elegantly disentangled plant and aphid clocks by subjecting both to different light–dark rhythms (Hodgson and Lane 1981), but such a protocol cannot be extended to constant darkness. Instead of relying on different morphs or on plants, we chose to raise aphids independent of their host plants. Even though artificial diets have been developed for the pea aphid (Febvay et al. 1988), they have never been used to resolve this problem. We thus asked whether aphids have a diurnal rhythm by measuring honeydew excretion and moult on an artificial diet, as these phenomena likely represent clock output.
Materials and Methods
Aphids are relatively immobile, so monitoring locomotor activity is currently not possible. We therefore decided to quantify feeding and moulting. To do so, we counted exuviae and honeydew drops, which are the excess sugar excreted after feeding from the plant sap. Although artificial diets are well-suited to monitor activity uncoupled of the host, there is also a considerable limitation: Artificial diets deteriorate within 2–3 d (van Emden and Harrington 2007), so the diet has to be renewed within <2 d. Furthermore, even with regularly renewed diet, food intake is reduced on artificial diets (van Emden and Harrington 2007), and we noticed a relatively low amount of honeydew production of about 1 drop per aphid and day, requiring large sampling intervals.
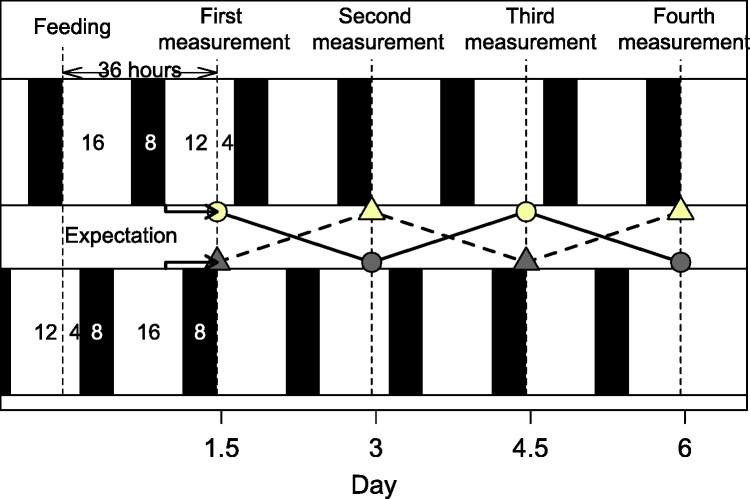
These problems prompted us to design an admittedly complex experiment (see also Fig. 1). Aphids were held continuously under a photoperiod of 16:8 (L:D) h to avoid induction of sexual morphs. We renewed the diet every 1.5 d, either shortly after lights-on, or shortly before lights-off, and counted accumulated honeydew and exuviae. Thus, the aphids experienced either one night (16 h light + 8 h darkness + 12 h light = 28:8 L:D, treatment “L”) or two nights (4 L + 8D + 16 L + 8D = 20:16 L:D, treatment “D”) between measurements. This feeding protocol also avoided entrainment (synchronization of the clock) with the feeding rhythm, as food was provided in the morning or evening in an alternating manner. To reduce the influence of any diurnal disturbance, we replicated the experiment in another chamber, which was phase-shifted by 12 h. We hence expected subsequent increases and decreases in activity with opposing patterns in the two chambers (see also Fig. 1).
Fig.1.
Experimental setup. The experiments were conducted in parallel in two climate chambers. Both chambers were set to a continuous photoperiod of 16:8 (L:D) h but differ in phase. Over the first 36 h after start of the experiment (first dashed line, “feeding”) one chamber received 28 h of light (16L + 8D + 12 L, treatment “L”), whereas the other received 20 h of light (4L + 8D + 16L + 8D, treatment “D”). Accumulated honeydew and exuviae were counted at the end of the 36-h period (dashed line). Measurements were repeated four times, so that the aphids in each chamber received alternating amounts of night time (8–16–8–16 in the upper chamber). Accumulated honeydew and exuviae (activity) of the two chambers (circles and triangles) were expected to depend on treatment (bright vs. dark), and hence to be in opposing directions in the two chambers.
The ratio of the treatments depends on the amount of honeydew produced during lights-on (x) and during lights-off (y):
| (1) |
Therefore, we expected the ratio of the two treatments to range between 0.5 (x = 0, night active) and 1.4 (y = 0, day active), and to be 1 if aphids prefer neither day nor night (x = y). We repeated the measurements four times. We provided a holidic diet with 20% w/v sucrose and defined amounts of amino acids, vitamins, and trace metals. The diet is based on diet A0 by Febvay et al. (1988), but uses 10 mM nicotinic acid instead of nicotine amide; a full recipe can be found in Supp. Table 1 (online only). We fed it to clone LL01, an asexual green alfalfa biotype originally from the Lusignan area, kindly provided by G. Febvay (INRA Lyon, France). Following a technique by Mittler and Dadd (1963), aphids were held in 35- by 10-mm Petri dishes, which were covered with two stretched parafilm M membranes with 250 µl diet in between. Per chamber we placed 14 replicates of 30 newly born nymphs in Petri dishes. The parents of the nymphs were light-entrained under a photoperiod of 16:8 (L:D) h (i.e., the circadian clock was synchronized with the light–dark cycle), but reared on plants. We started the first measurement period at 4 d age. Every 1.5 d (during lights-on in both chambers), we placed surviving nymphs into new Petri dishes with new food and counted the accumulated honeydew and exuviae. We counted the number of honeydew drops but did not estimate the volumes due to the low visibility and the small sizes; we noticed, however, no trend in drop diameters, and variability in drop volumes can be considered low (Auclair 1958). The two climate chambers (Sanyo MLR-H series) provided 18.1 ± 0.9°C and 81.3 ± 2.8 humidity and a photoperiod of 16:8 (L:D) h at 19.7 ± 0.7 klux.
Statistics were performed with R 3.1.1 (R Core Team 2014). We applied a mixed-effects model including chamber, time, and their interaction as factors, and Petri dish as random term. As we expected alternating slopes (Fig. 1), a significant interaction term with reversing slopes would evidence rhythmicity. We corrected honeydew excretion for the number of surviving aphids. We additionally corrected honeydew excretion for moulting aphids, which are not expected to produce honeydew. This yielded the combined activity estimate .
Results
Honeydew excretion was overall low, with one to three drops per aphid per 1.5 d, but the nutrient uptake was sufficient for experimental animals to develop into adults and to survive for 2 wk. Survival declined over time (65–89% survival rates between measurements), leaving on average 38% (11.4 ± 0.4 aphids) at the fourth measurement (see Supp. Fig. 1 [online only]). Under a photoperiod of 16:8 (L:D) h, survival, moulting, and honeydew excretion individually did not significantly alternate with changing treatments, but the slopes of the combined activity estimate crossed significantly (i.e., significant statistical interaction, Tables 1 and 2; Fig. 2). This interaction conforms to our prediction of diurnal rhythmicity. In the L treatments (with 28 h light in 36 h), the median of observed activity (drops per nonmoulting survivor) was 1.80 drops, 31% higher than in the D treatments (with 20 h light in 36 h, 1.37 drops). The ratio of L/D = 1.31 allows estimates on how active aphids were during lights-on and during lights-off (x and y in eq. 1). For instance, if aphids were purely day-active (x = 1, y = 0), one would expect activity for 28 h in the L treatment and for 20 h in the D treatment, so that the ratio L/D between the treatments would be 28/20 = 1.4, or 40% higher in the L treatment. Solving eq. (1) with L/D = 1.31 yields 1 y = 7.2 x. We conclude that aphids are seven times more active during day than during night.
Table 1.
ANOVA results
| Response | Factor | df | F | P | Significance levela |
|---|---|---|---|---|---|
| Survival | Chamber | 1,26 | 1.40 | 0.25 | |
| Time | 3,77 | 85.70 | <0.0001 | *** | |
| Chamber:time | 3,77 | 1.59 | 0.20 | ||
| Moulting | Chamber | 1,26 | 1.19 | 0.29 | |
| Time | 3,77 | 3.24 | 0.03 | * | |
| Chamber:time | 3,77 | 1.77 | 0.16 | ||
| Honeydew | Chamber | 1,26 | 0.08 | 0.78 | |
| Time | 3,69 | 5.37 | <0.01 | ** | |
| Chamber:time | 3,69 | 2.43 | 0.07 | ‘ | |
| Activity | Chamber | 1,26 | 0.99 | 0.33 | |
| Time | 1,69 | 1.27 | 0.29 | ||
| Chamber:time | 1,69 | 3.02 | 0.04 | * |
The three responses aphid survival, moulting, and honeydew excretion were combined to one estimate that expresses activity as honeydew excretion per nonmoulting survivor. We expected a significant interaction of chamber and time (see main text).
a Significance levels: ***p < 0.001; **p < 0.01; *p < 0.05; ‘p < 0.1.
Table 2.
Means (±SEM) of estimates for diurnal rhythms
| Response | Treatment | First measurement | Second measurement | Third measurement | Fourth measurement |
|---|---|---|---|---|---|
| Survival | L | 25.79 (±1.45) | 21.57 (±1.56) | 12.93 (±0.90) | 12.64 (±0.95) |
| D | 26.85 (±1.51) | 19.86 (±1.06) | 18.07 (±1.26) | 10.14 (±0.93) | |
| Moulting | L | 11.93 (±1.08) | 6.57 (±0.79) | 6.00 (±0.59) | 5.79 (±0.68) |
| D | 10.31 (±1.21) | 5.79 (±0.52) | 6.57 (±0.64) | 3.64 (±0.37) | |
| Honeydew | L | 28.46 (±3.91) | 37.86 (±2.95) | 16.07 (±2.95) | 12.00 (±1.57) |
| D | 24.46 (±3.02) | 23.09 (±1.73) | 14.46 (±1.51) | 8.00 (±1.23) | |
| Activity | L | 2.44 (±0.50) | 2.69 (±0.17) | 2.77 (±0.52) | 1.68 (±0.20) |
| D | 1.50 (±0.14) | 1.84 (±0.15) | 1.39 (±0.15) | 1.39 (±0.22) |
Activity is the combined estimate of honeydew excretion per nonmoulting survivor. The four measurements were taken after 1.5, 3, 4.5, and 6 d, and correspond to the dashed lines in Fig. 1. Treatment L corresponds to 28 h light and 8 h darkness, Treatment D corresponds to 20 h light and 16 h darkness as described in Fig. 1 and in the main text.
Fig. 2.
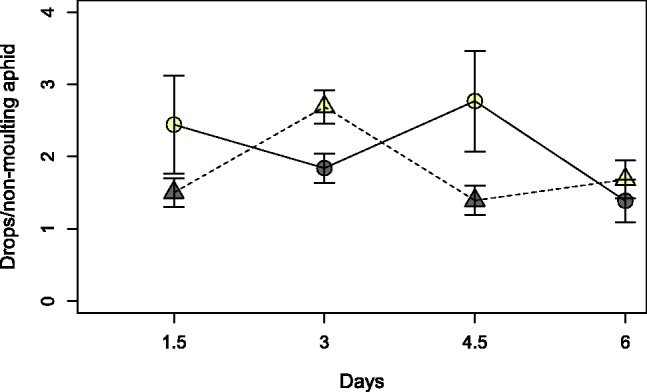
Honeydew excretion per nonmoulting aphid in changing day:night ratios (see also Fig. 1). Colour coding is the same as in Fig. 1, i.e., aphids from the two chambers (circles and triangles) that received the L treatment (28 h light, 8 h darkness) are presented in yellow, whereas aphids in the D treatment (20 h light, 16 h darkness) are presented in grey. Error bars indicate SEM.
Discussion
The experiment is to our knowledge the first to measure aphid diurnal rhythms independent of host plants by feeding artificial diets. Although independent diurnal rhythms have been observed in sexual morphs, after photoperiodic induction (Eisenbach and Mittler 1980, Thieme and Dixon 1996), in all studies on asexual morphs aphids were reared on living plants (Gomez et al. 2006, Cortes et al. 2010, Taylor et al. 2012). Aphids can be described as plant parasites (The International Aphid Genomics Consortium 2010), so aphids might well hitch-hike the plant rhythm instead of using the light–dark cycle (LD). The present study indicates that this is not the case (evidenced by the statistical significant interaction term), and that aphids have diurnal rhythms even on constant food sources.
Common to all studies in various species is an activity peak during day time, and our results show that this is generally also true for the pea aphid. However, further experiments are needed to determine how the activity distributes over the course of the day. Knowledge of the activity pattern of aphids has implications for pest control, because it assists more specific treatment with insecticide in circadian manner (Hooven et al. 2009). Furthermore, it is interesting to know whether the plant modulates the aphid rhythmicity, because exploitation of diurnal changes in host receptivity and quality can lead to coevolution among the circadian clocks (Goodspeed et al. 2012, Martinez-Bakker and Helm 2015). Changes in diurnal timing are also accompanied by changes in the abiotic conditions, which aphid experience. For example, day-activity might be an explanation of fitness constraints under short days (Joschinski et al. 2015). Overall, our study lays the foundation for future studies on aphid diurnal rhythms and the interaction with their host plants.
We are well aware that the rhythm in diurnal behavior is no evidence for circadian clock involvement yet, as it needs also continuation (“free-runs”) under constant conditions (Moore-Ede et al. 1982). Future experiments need to test aphid rhythmicity under constant darkness, and in particular need to quantify how the oscillation of the clock damps out. On the one hand, studies suggest an hour glass mechanism, i.e., no clock involvement, in aphids (Lees 1973); on the other hand, correlative evidence from other species suggests that this clock mechanism is not the norm (e.g., Ikeno et al. 2010). These apparent differences could be united by a quickly damping clock (Hardie and Vaz Nunes 2001), and a damped clock might be exemplified by damping activity under constant darkness. The molecular mechanism of the aphid clock has been investigated, and some parts of the core clockwork (CRY and the PER/TIM feedback loop) are indeed undergoing accelerated changes (Cortes et al. 2010). Our current protocol, which requires feeding every 36 h to maintain high honeydew excretion rates, does not yet allow working under constant darkness, because feeding in darkness proved impossible. Hopefully further advances in rearing methods will allow studying aphid clock properties in depth. Yet, the demonstration of independent diurnal behavior is a crucial first step in understanding aphid clocks.
In conclusion, we showed that pea aphids produce honeydew and moult during daytime, and maintain the rhythm independently of host plants. We think that pea aphids are worth investigating for the involvement of clock and photoperiodism in aphid physiology, and our study with artificial diets are a first step in understanding its mechanisms.
Supplementary Data
Supplementary data are available at Journal of Insect Science online.
Acknowledgments
We thank Gerard Febvay for information on diet preparation and providing their aphid clone, and Barbara Helm for advice on the manuscript. Funding was provided by the German Research Foundation (DFG), collaborative research center SFB 1047 “Insect timing,” Projects A1 and C3. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References Cited
- Auclair J. L. 1958. Honeydew excretion in the pea aphid, Acyrthosiphon pisum (Harr.) (Homoptera: Aphididae). J. Insect Physiol. 2: 330–337. [Google Scholar]
- Bennie J. J., Duffy J. P., Inger R., Gaston K. J. 2014. Biogeography of time partitioning in mammals. Proc. Natl. Acad. Sci. USA 111: 13727–13732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw W. E., Holzapfel C. M. 2010. What season is it anyway? Circadian tracking vs. photoperiodic anticipation in insects. J. Biol. Rhythms 25: 155–165. [DOI] [PubMed] [Google Scholar]
- Bunning E. 1936. Die endogene Tagesrhythmik als Grundlage der photoperiodischen Reaktion. Berichte der Deutschen Botanischen Gesellschaft 54: 590–607. [Google Scholar]
- Cortes T., Ortiz-Rivas B., Martinez-Torres D. 2010. Identification and characterization of circadian clock genes in the pea aphid Acyrthosiphon pisum. Insect Mol. Biol. 19: 123–139. [DOI] [PubMed] [Google Scholar]
- Danks H. V. 2005. How similar are daily and seasonal biological clocks? J. Insect Physiol. 51: 609–619. [DOI] [PubMed] [Google Scholar]
- Eisenbach J., Mittler T. E. 1980. An aphid circadian rhythm: Factors affecting the release of sex pheromone by oviparae of the greenbug, Schizaphis graminum. J. Insect Physiol. 26: 511–515. [Google Scholar]
- Febvay G., Delobel B., Rahbé Y. 1988. Influence of the amino acid balance on the improvement of an artificial diet for a biotype of Acyrthosiphon pisum (Homoptera: Aphididae). Can. J. Zool. 66: 2449–2453. [Google Scholar]
- Fleury F., Allemand R., Vavre F., Fouillet P., Boulétreau M. 2000. Adaptive significance of a circadian clock: Temporal segregation of activities reduces intrinsic competitive inferiority in Drosophila parasitoids. Proc. R. Soc. Lond., Ser. B: Biol. Sci. 267: 1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner W. W., Allard H. A. 1920. Effect of the relative length of day and night and other factors of the environment on growth and reproduction in plants. J. Agric. Res. 18: 553–606. [Google Scholar]
- Gomez S. K., Oosterhuis D. M., Hendrix D. L., Johnson D. R., Steinkraus D. C. 2006. Diurnal pattern of aphid feeding and its effect on cotton leaf physiology. Environ. Exp. Bot. 55: 77–86. [Google Scholar]
- Goodspeed D., Chehab E. W., Min-Venditti A., Braam J., Covington M. F. 2012. Arabidopsis synchronizes jasmonate-mediated defense with insect circadian behavior. Proc. Natl. Acad. Sci. 109: 4674–4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie J., Vaz Nunes M. 2001. Aphid photoperiodic clocks. J. Insect Physiol. 47: 821–832. [DOI] [PubMed] [Google Scholar]
- Hodgson C. J., Lane I. R. 1981. Some effects of photoperiod on larviposition and fresh weight-gain in Myzus persicae. Physiol. Entomol. 6: 21–25. [Google Scholar]
- Hooven L. A., Sherman K. A., Butcher S., Giebultowicz J. M. 2009. Does the clock make the poison? Circadian variation in response to pesticides. PLoS ONE 4: e6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeno T., Tanaka S., Numata H., Goto S. 2010. Photoperiodic diapause under the control of circadian clock genes in an insect. BMC Biol. 8: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joschinski J., Hovestadt T., Krauss J. 2015. Coping with shorter days: Do phenology shifts constrain aphid fitness? PeerJ 3: e1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees A. D. 1973. Photoperiodic time measurement in the aphid Megoura viciae. J. Insect Physiol. 19: 2279–2316. [Google Scholar]
- Lu W. Q., Meng Q. J., Tyler N. J. C., Stokkan K. A., Loudon A. S. I. 2010. A circadian clock is not required in an arctic mammal. Curr. Biol. 20: 533–537. [DOI] [PubMed] [Google Scholar]
- Martinez-Bakker M., Helm B. 2015. The influence of biological rhythms on host–parasite interactions. Trends Ecol. Evol. 30: 314–326. [DOI] [PubMed] [Google Scholar]
- Mittler T. E., Dadd R. H. 1963. Studies on the artificial feeding of the aphid Myzus persicae (Sulzer)—I. Relative uptake of water and sucrose solutions. J. Insect Physiol. 9: 623–645. [DOI] [PubMed] [Google Scholar]
- Moore-Ede M. C., Sulzman F. M., Fuller C. A. 1982. The clocks that time us. Harvard University Press, Cambridge, MA. [Google Scholar]
- Pittendrigh C. S., Minis D. H. 1964. The entrainment of circadian oscillations by light and their role as photoperiodic clocks. Am. Nat. 98: 261–294. [Google Scholar]
- Pittendrigh C. S., Minis D. H. 1972. Circadian systems: longevity as a function of circadian resonance in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 69: 1537–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. 2014. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Schultz T. F., Kay S. A. 2003. Circadian clocks in daily and seasonal control of development. Science 301: 326–328. [DOI] [PubMed] [Google Scholar]
- Stich H.-B., Lampert W. 1981. Predator evasion as an explanation of diurnal vertical migration by zooplankton. Nature 293: 396–398. [Google Scholar]
- Taylor S. H., Parker W. E., Douglas A. E. 2012. Patterns in aphid honeydew production parallel diurnal shifts in phloem sap composition. Entomol. Exp. Appl. 142: 121–129. [Google Scholar]
- The International Aphid Genomics Consortium. 2010. Genome sequence of the pea aphid Acyrthosiphon pisum. PLoS Biol. 8: e1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieme T., Dixon A. F. G. 1996. Mate recognition in the Aphis fabae complex: Daily rhythm of release and specificity of sex pheromones. Entomol. Exp. Appl. 79: 85–89. [Google Scholar]
- Van Emden H. F., Harrington R. 2007. Aphids as Crop Pests, Cabi, Wallingford, United Kingdom. [Google Scholar]