Abstract
Tungiasis ensues from the penetration and burrowing of female sand fleas (Tunga spp.; Siphonaptera: Tungidae) in the skin of mammals. There are few case reports of severe tungiasis in goats and in these cases the Tunga species were not in most cases clearly identified. Two cases of severe tungiasis caused by Tunga penetrans in goat kids from tungiasis-endemic rural Uganda are reported. These are the first severe cases of tungiasis in goats reported from outside South America.
Keywords: clinical presentation, goat, Tunga penetrans, Uganda
Tungiasis is caused by female sand fleas (Tunga spp.) burrowing in the skin of mammals including humans. Although to date 14 species have been characterized in the genus Tunga, only three species, Tunga penetrans, Tunga trimamillata, and Tunga hexalobulata, are known to parasitize domestic animals (De Avelar et al. 2012, Linardi and Avelar 2014, Ezquiaga et al. 2015). Of the three species, only T. penetrans has been reported in Africa. In sub-Saharan Africa, the pathogen parasitizes humans and a wide range of animals (Ugbomoiko et al. 2008, Mutebi et al. 2015). T. trimamillata and T. hexalobulata, which have been reported only in South America, are the main sand flea species affecting domestic ruminants (Fioravanti et al. 2003, De Avelar et al. 2013, Linardi et al. 2013).
Principally, the life cycle of the T. penetrans consists of broadly two phases; off host development as well as the in situ development in the skin of homeothermic vertebrate hosts. In a suitable environment, off-host development progresses through eggs, larvae, pupae before adults emerge in about 17 d (Nagy et al. 2007, Pampiglione et al. 2009). A suitable environment has been characterized as one with dry and sandy soils containing some organic matter and away from direct sunlight with a temperature range of 20–31°C (Linardi et al. 2010). While both male and female adult fleas are hematophagous, only the females embed in the skin of a wide range of suitable homeothermic hosts, develop by neosomy, lay eggs, and eventually die in situ in about 30 d after penetration (Eisele et al. 2003, Feldmeier et al. 2007). Nevertheless, abnormal on-host metamorphosis has been reported (Thielecke and Feldmeier 2013). Normally, shortly after penetration, female sand fleas hypertrophy and increase in size by ∼2000 times within 2 wk (Eisele et al. 2003). During its development in host skin, T. penetrans evokes a severe inflammatory response which is often complicated by bacterial super infections which intensify the inflammation to cause severe morbidity.
There are few reported cases of goats infected by T. penetrans, of which, very few have presented with severe morbidity (Pampiglione et al. 2009) and all are exclusively from South America (Trentini et al. 2000, Gustinelli et al. 2006). This short communication presents two cases of severe manifestations of T. penetrans infection in goat kids detected during an epidemiological survey in rural impoverished communities in Busoga, Uganda (Mutebi et al. 2015).
Case Reports
Case 1
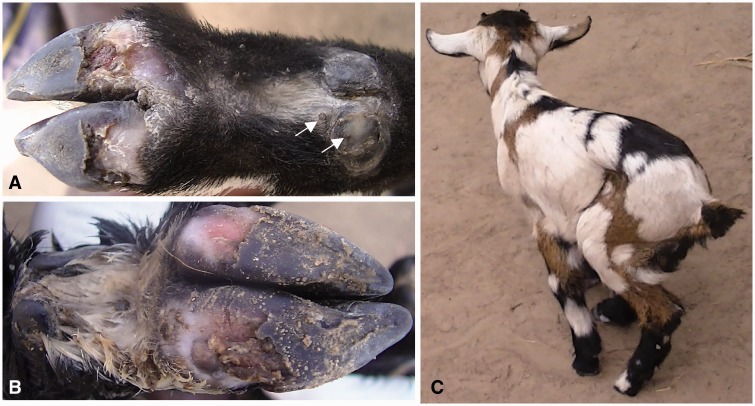
A 3-wk-old female goat kid with a history of weakness and inappetence was detected in Masolya Village, Bugiri District, Busoga, Uganda, where tungiasis is highly prevalent among humans. It was under free range management and neither anthelminthic nor ectoparasitic treatment had ever been administered. The kid was clinically anemic (pale mucous membranes), had a starry hair coat, and was also infested with Ctenocephalides canis. A total of 34 sand flea lesions were detected on the four limbs. According to the Fortaleza staging system (Eisele et al. 2003), 21 were viable (Stages IIa to IIIb) and 13 were dead (Stage IV). On the limbs, sand fleas were localized mainly on the coronary band of the claws but some were embedded on the soles of the hooves (Fig. 1A and B). The affected hoof wall was necrotic and had peeled off from the sole to expose the underlying soft tissue (Fig. 1B).
Fig. 1.
Tungiasis in Case 1. Stage III lesions on coronary band of the dew claw (arrows) and bilateral hoof wall loss of the front limb (A). Dead sand fleas, necrosis, and loss of hoof wall tissues of the hind leg (B). Infected goat kid with lameness (C).
Sites with viable lesions were painful on palpation and the kid exhibited shifting limb lameness while moving (Fig. 1C). It spent most of the time lying down but its body temperature was within the normal range (38.9°C). The household also had a mature goat (dam to the affected kid), a sow, a dog, and a cat. The sow, the dog, and all four household members were infected by T. penetrans but the mature goat and the cat did not have any sand flea lesions. Goats were housed in a kitchen with earthen floor and the homestead was generally of poor sanitation with household waste being disposed off in the garden close to the compound.
Case 2
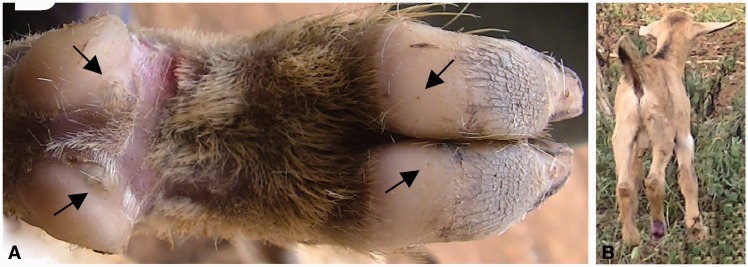
A 3-wk-old male goat kid was detected from Busindha Village, Bugiri District in which human tungiasis is endemic. It was kept under free range management and had no history of ectoparasite or helminth control measures. It had six viable (Stages IIa to IIIb) embedded sand fleas which were exclusively on the right hind leg at the coronary band of both hooves and the accessory digits. The kid was neither anemic (as per the physical examination) nor was it pyrexic (body temperature 38.4°C). The area around the attachment sites of Stage III lesions on the coronary band of the dew claws was edematous (Fig. 2A, arrows) and very painful on touch as evidenced by limb retraction and bleating on touch.
Fig. 2.
Tungiasis in Case 2. Right hind leg of a goat kid with viable sand fleas (A) surrounded by edematous zones at the coronary band of claws (arrows). Goat kid with swinging lameness (B).
The affected limb was raised off the ground when the kid was moving (swinging lameness; Fig. 2B). On the same compound, there were three adult goats (≥1 yr), one sow, one cat, and eight chickens. The other goats, the four examined chickens, and a cat were not affected but 12 out of 13 household members had tungiasis. The affected goat kid was roaming on the compound during day time whereas other goats were tethered in bushes. However, it was housed during the night with other goats in a goat shade on the compound which had a dusty floor.
Intervention
Affected kids were dewormed with Wormicid 150 (levamisole 150 mg; Cosmos Limited, Nairobi City, Kenya) and Supona aerosol (chlorfenvinphos 4.8%, dichlorphos 0.75%, and gentian violet 0.145%; Pfizer Laboratories (Pty) Limited, Sandton, 2196, South Africa) was applied on the affected sites to kill the embedded sand fleas and control secondary bacterial infections. One viable sand flea (Stage III) was extracted from each of the goats, fixed in 70% ethanol, and transported to the laboratory at the College of Veterinary Medicine, Animal Resources and Bio-security, Makerere University and the Freie Universität, Berlin, Germany, for morphological identification based on features described previously (De Avelar et al. 2012, Ezquiaga et al. 2015). The chitin of the anterior hypertrophic abdominal segment of the gravid fleas had an appearance reminiscent of a clover leaf on light and electron microscopy hence identified as T. penetrans (Mutebi et al. 2015).
In a follow-up 1 wk after the treatment, the kid in Case 1 was reported to have died 4 d after treatment following a short-clinical course of illness characterized by anorexia and a mucoid nasal discharge. No viable sand fleas were detected and healing by re-epithelialization had taken place when the kid in Case 2 was re-examined 1 wk post-treatment. The kid had also regained a normal gait.
Discussion
Data on tungiasis-associated morbidity in animals is scarce. Regarding goats, only a few cases have been described in South America (Trentini et al. 2000, Gustinelli et al. 2006). Most of these were attributed to T. trimamillata, which was initially misidentified as T. penetrans (Linardi et al. 2013). An epidemiological study in Uganda during which these two cases were identified, detected tungiasis in only two goat kids from two villages out of 848 goats examined from 10 villages (Mutebi et al. 2015). In general, it appears that goats represent rather uncommon hosts of T. penetrans in sub-Saharan Africa. This may be attributed to the tough horny hoof wall and thick skin which increase as the goats mature and move on rough surfaces resulting into a considerable barrier for penetrating sand fleas. This may explain why mature goats on the same compound were not infected. However, goat kids still have soft hoof walls and thinner stratum corneum. In this case, the affected kids were the only young goats (≤5 mo) on the compounds on which they were detected.
Necrosis of the hoof wall in one kid could be attributed to a dense cluster of sand fleas exerting pressure on tissues at the site of penetration. In addition, the goat kid may have rubbed the itchy lesions against hard surfaces further aggravating the necrosis. The site of extensive necrosis (soles of the hooves, Fig. 1A and B) further supports this assertion since the sole of the hoof is the area that goats can easily rub against hard surfaces.
In humans and pigs, the degree of morbidity parallels the intensity of infection (Feldmeier et al. 2004). From the cases presented it appears that in young goats, even a few lesions can induce severe morbidity as indicated by severe inflammation, tissue necrosis, and lameness. Therefore, tungiasis should be included among the differential diagnoses whenever hoof necrosis and lameness are detected in goats in endemic areas. The cause of death of one of the kids was not ascertained. However, from the clinical history obtained, respiratory complications such as pneumonia are implicated.
As demonstrated in this article, animal tungiasis can cause severe morbidity. It is also an important epidemiological factor in human tungiasis in sub-Saharan Africa (Mutebi et al. 2015). Therefore, ectoparasite control should be encouraged among animal owners. However, an effective compound is yet to be identified. The chlorfenvinphos and dichlorphos in the Supona wound spray appeared to be tungicidal at least in one kid and the antibacterial effects of gentian violet presumably helped to curb super infections thus facilitating healing. However, these effects need to be validated through controlled studies.
Conclusion
Tungiasis can cause severe morbidity in goats especially kids manifesting with variable degrees of lameness. Therefore, tungiasis should be considered a differential diagnosis when lameness and hoof lesions are detected in goats in endemic areas.
Acknowledgments
The Management of High Heights Initiatives and the goat owners are appreciated for their support. No specific funding was received for this study. The cases described were detected during an epidemiological survey of animal tungiasis which was supported by Bayer Animal Health, Leverkusen, German and the German Academic Exchange Service (DAAD). Data are part of Francis Mutebi s thesis.
References Cited
- De Avelar D. M., Linhares A. X., Linardi P. M. 2012. A new species of Tunga (Siphonaptera: Tungidae) from Brazil with a key to the adult species and neosomes. J. Med. Entomol. 49: 23–28. [DOI] [PubMed] [Google Scholar]
- De Avelar D. M., Filho E. J. F., Linardi P. M. 2013. A new species of tunga (siphonaptera: Tungidae) parasitizing cattle from Brazil. J. Med. Entomol. 50: 679–684. [DOI] [PubMed] [Google Scholar]
- Eisele M., Heukelbach J., Marck E. V., Mehlhorn H., Meckes O., Franck S., Feldmeier H. 2003. Investigations on the biology, epidemiology, pathology and control of Tunga penetrans in Brazil: I. Natural history of tungiasis in man. Parasitol. Res. 90: 87–99. [DOI] [PubMed] [Google Scholar]
- Ezquiaga M. C., Linardi M. P., De Avelar D. M., Lareschi M. 2015. A new species of Tunga perforating the osteoderms of its armadillo host in Argentina and redescription of the male of Tunga penetrans. Med. Vet. Entomol. 29: 196–204. [DOI] [PubMed] [Google Scholar]
- Feldmeier H., Eisele M., Marck E. V., Mehlhorn H., Ribeiro R., Heukelbach J. 2004. Investigations on the biology, epidemiology, pathology and control of Tunga penetrans in Brazil: IV. Clinical and histopathology. Parasitol. Res. 94: 275–282. [DOI] [PubMed] [Google Scholar]
- Feldmeier H., Witt L., Schwalfenberg S., Linardi P. M., Ribeiro R. A., Capaz R. A. C., Marck E. V., Meckes O., Mehlhorn H., Mencke N., Heukelbach J. 2007. Investigations on the biology, epidemiology, pathology and control of Tunga penetrans in Brazil. VI. Natural history of the infestation in laboratory-raised Wistar rats. Parasitol. Res. 102: 1–13. [DOI] [PubMed] [Google Scholar]
- Fioravanti M. L., Pampiglione S., Trentini M. 2003. A second species of Tunga (Insecta, Siphonaptera) infecting man; Tunga trimamillata. Parasite 10: 282–283. [PubMed] [Google Scholar]
- Gustinelli A., Fioravanti M. L., Fabbri V., Trentini M., Onore G., Caffara M., Pampiglione S. 2006. Pathology of Tunga trimamillata (Siphonaptera, Tungidae) in animals, pp. 6–11. In, ICOPA XI, 2006, Glagow. [Google Scholar]
- Linardi P. M., Avelar D. M. D. 2014. Neosomes of tungid fleas on wild and domestic animals. Parasitol. Res. 113: 3517–3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linardi P. M., De Avelar D. M., Facury Filho E. J. 2013. Establishment of Tunga trimamillata (Siphonaptera: Tungidae) in Brazil. Parasitol. Res. 112: 3239–3242. [DOI] [PubMed] [Google Scholar]
- Linardi P. M., Calheiros C. M. L., Campelo-Junior E. B., Duarte E. M., Heukelbach J., Feldmeier H. 2010. Occurence of off-host stages of Tunga penetrans (Siphonaptera) in various environments in Brazil. Ann. Trop. Med. Parasit. 104: 337–345. [DOI] [PubMed] [Google Scholar]
- Mutebi F., Krücken J., Feldmeier H., Waiswa C., Mencke N., Sentongo E., von-Samson-Himmelstjerna G. 2015. Animal reservoirs of zoonotic tungiasis in endemic rural villages of Uganda. PLoS Negl. Trop. Dis. 9: e0004126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy N., Abari E., D’Haese J., Calheiros C., Heukelbach J., Mencke N., Feldmeier H. 2007. Investigations on the life cycle and morphology of Tunga penetrans in Brazil. Parasitol. Res. 101: S233–S242. [DOI] [PubMed] [Google Scholar]
- Pampiglione S., Fioravanti M. L., Gustinelli A., Onore G., Montovani B., Luchetti A., Trentini M. 2009 Sand flea (Tunga spp.) infections in man and domestic animals: state of the art. Med. Vet. Entomol. 23: 172–186. [DOI] [PubMed] [Google Scholar]
- Thielecke M., Feldmeier H. 2013. The fate of the embedded virgin sand flea Tunga penetrans: hypothesis, self-experimentation and photographic sequence. Travel. Med. Infect. Dis. 11: 440–443. [DOI] [PubMed] [Google Scholar]
- Trentini M., Pampiglione S., Gianneto S., Finocchiaro B. 2000. Observations about specimens of Tunga spp. (Siphonaptera: Tungidae) extracted from goats in Ecuador. Parassitologia 45: 65. [Google Scholar]
- Ugbomoiko U. S., Ariza L., Heukelbach J. 2008. Pigs are the most important animal reservoirs for Tunga penetrans (jigger flea) in rural Nigeria. Trop. Doct. 38: 226–227. [DOI] [PubMed] [Google Scholar]