Restoration of degraded shrublands using native shrubs is a challenging task for conservation and management with continued climate change. Investigation of native shrub species from the Lower Rio Grande Valley, USA, showed physiological tolerance to potential future extreme soil water stress though with a gradient of water use strategies that are species specific.
Keywords: Gas exchange, soil salinity, soil water deficit, soil water potential, thorn shrubs
Abstract
The biogeographic distribution of plant species is inherently associated with the plasticity of physiological adaptations to environmental variation. For semi-arid shrublands with a legacy of saline soils, characterization of soil water-tolerant shrub species is necessary for habitat restoration given future projection of increased drought magnitude and persistence in these ecosystems. Five dominant native shrub species commonly found in the Lower Rio Grande Valley, TX, USA, were studied, namely Acacia farnesiana, Celtis ehrenbergiana, Forestiera angustifolia, Parkinsonia aculeata and Prosopis glandulosa. To simulate drought conditions, we suspended watering of healthy, greenhouse-grown plants for 4 weeks. Effects of soil salinity were also studied by dosing plants with 10% NaCl solution with suspended watering. For soil water deficit treatment, the soil water potential of P. glandulosa was the highest (−1.20 MPa), followed by A. farnesiana (−4.69 MPa), P. aculeata (−5.39 MPa), F. angustifolia (−6.20 MPa) and C. ehrenbergiana (−10.02 MPa). For the soil salinity treatment, P. glandulosa also had the highest soil water potential value (−1.60 MPa), followed by C. ehrenbergiana (−1.70 MPa), A. farnesiana (−1.84 MPa), P. aculeata (−2.04 MPa) and F. angustifolia (−6.99 MPa). Within the species, only C. ehrenbergiana and F. angustifolia for soil water deficit treatment and A. farnesiana for the salinity treatment had significantly lower soil water potential after 4 weeks of treatment (P < 0.05). We found that soil water potential, stomatal conductance and net photosynthesis of the species significantly reduced over time for both treatments (P < 0.05). We conclude that while all species exhibited capacities to withstand current water availability, some species demonstrated limited tolerance for extreme water stress that may be important for management of future shrub diversity in Lower Rio Grande Valley.
Introduction
Continued climate change, including increased temperature and decreased precipitation, may have rapid, widespread and long-lasting impacts on species composition and distribution in shrublands (Condit et al., 1995; McDowell et al., 2008; White et al., 2008). Further climate-imposed reduction in soil water availability is likely to intensify with changes in growth, survival and distribution of shrub species in these ecosystems (Grime, 1977; Archer, 1989; González-Rodríguez et al., 2004; Otieno et al., 2005; Gebrekirstos et al., 2006; Morgan et al., 2011). Water stress affects virtually all the physiological functions of plants related to photosynthesis and carbohydrate metabolism that affect their adaptation, growth and distribution (Grime 1977; Escos et al., 2000; Ditmarova et al., 2009). Co-existence of diverse shrub species in shrublands reflects adaptation plasticity with resource partitioning strategies among these species to cope with limited soil water availability (González-Rodríguez et al., 2004); therefore, shrub species in these ecosystems have evolved key morphological and physiological attributes required for adaptation to soil water deficit.
The suitability of shrub species for restoration and conservation of shrublands is related to water stress physiology of target plant species (Gebrekirstos et al., 2006). Plant water relation measures, such as water potential, gas exchange and intrinsic water use efficiency, are common physiological indices to determine the water stress and its effects on plants (Vertovec et al., 2001). Water constraints in shrublands are primarily affected by precipitation inputs and the evaporative environment. Excessive salt in soils also affects the water available to plants by decreasing the osmotic potential of the soil water. Irrigation from surface water increases the soil salt concentration because exclusion of salts by roots during transpiration coupled with low soil water recharge increases the salt concentration in the plant's root zone (McCoy, 1990; Archibald et al., 2006; Wiedenfeld, 2008). A high salt concentration in the soil negatively affects plant water uptake and maintenance of turgor (Maas and Hoffman, 1977).
In this study, we conducted a greenhouse study of five native, dominant shrub species from the Lower Rio Grande Valley (LRGV) of Texas to assess the effects of drought and soil salinity on soil water potential and gas-exchange properties. Within the LRGV, >95% of its original shrub cover was historically removed due to agricultural expansion, fuel wood harvest and other anthropogenic activities during the past century (Ewing and Best, 2004). Restoration at LRGV by land managers during the last two decades has resulted in limited replantation of native shrubs. The restoration programme is also aiming for the conservation of habitat of many endangered species, such as ocelots (Leopardus pardalis) and jaguarundis (Puma yagouaroundi), which use the dense shrubs as hiding cover. In addition to habitat, these shrublands are important carbon sinks (Navar-Chaidez, 2008). However, successful restoration of this shrubland requires an enhanced understanding of the limitations, tolerances and physiological responses to environmental factors of shrub species to avoid increased soil water deficit. The specific objectives of this study were as follows: (i) to compare the shrub species for their adaptation and tolerance to prolonged soil water stress imposed by soil water deficit and increased soil salinity; and (ii) to assess the conservation of water related to assimilation of carbon as a potential measure of water stress adaptation.
Materials and methods
Description of native shrub habitat
The LRGV is located in South Texas, USA, a part of the northern edge of the Tamaulipan Biotic province, which is an assemblage of unique shrubland ecosystems that extends from the Gulf of Mexico plains in south Texas to northern Mexico (Jahrsdoerfer and Leslie, 1988; Reid et al., 1990; González-Rodríguez et al., 2004). Soils of LRGV range from loamy fine sands to clays with deep, moderately fine textures formed in alluvial sediments (Jahrsdoerfer and Leslie, 1988; Wiedenfeld, 2008). Sea salt spray and irrigation water from the Rio Grande are the main sources of soil salinity in this site (Hendrickx et al., 1999; Foltescu et al., 2005; Wiedenfeld, 2008). The soil of LRGV contains between 800 and 900 mg l−1 total salt (Wiedenfeld, 2008). The climate is semi-arid and sub-tropical, with long, hot summers and short, mild winters (Eddy and Judd, 2003) with a mean of 330 frost-free days per year. The LRGV receives an annual average rainfall of 68.2 cm, with peak precipitation during September and October (Eddy and Judd, 2003). The yearly potential evapotranspiration is about 220 cm, with average mid-summer vapour pressure deficit values of 3.05 kPa (González-Rodríguez et al., 2004).
Greenhouse experiment
In order to assess species responses to soil water deficit and soil salinity, we chose five dominant native shrub species from the LRGV for greenhouse study. We cultivated plants of Acacia farnesiana (L.) Willd. [Fabaceae], Celtis ehrenbergiana (Klotzsch) Liebm. [Ulmaceae], Forestiera angustifolia Torr. [Oleaceae], Parkinsonia aculeata L. [Fabaceaa] and Prosopis glandulosa Torr. [Fabaceae] grown from seeds acquired from LRGV in a greenhouse.
Twelve plants (0.5–1.2 m in height) of each species were grown in 5 l pots filled with commercial potting soil and watered by drip-irrigation every day. Each plant was fertilized with commercial fertilizer (∼3.5 g, Osmocote NPK – 18:6:10; Scotts-Siera Horticultural Product Company, Marysville, OH, USA) added to each pot every 3 months. Out of the 12 plants per species, four plants of each species were randomly selected to be used as controls, with continued daily irrigation. Four of the remaining plants were exposed to soil water deficit treatment by suspension of watering for 4 weeks, referred to as the soil water deficit (SWD) treatment. The remaining four plants of each species were subjected to suspended water for 4 weeks coupled with soil salt treatments, referred to as the salinity treatment. The salt treatment consisted of initial dosing of the potting soil with 680 ml of 0.86 m NaCl to acclimate the plant to the added salt. Next, plants were then irrigated with the addition of 680 ml of 1.72 m NaCl at the beginning of the second week of the experiment. The higher concentration of NaCl solution was equivalent to twice the amount of salt currently present in LRGV soil. In order to avoid biases associated with potential irradiance levels in the greenhouse, the plants were repositioned within the greenhouse each week. The temperature of the greenhouse was maintained at ∼22°C, with relative humidity of 70–90%.
Soil water potential measurements
The soil water potential (SWP) of each plant pot exposed to the SWD and salinity treatments was measured in continuous mode using a WP4-T Dew Point Potentiameter (Decagon Devices Inc. 2007, Pullman, WA, USA) every week for 4 weeks from the beginning of treatments (from 28 December 2011 to 25 January 2012). Each sample was prepared with ∼8.00 g soil taken at predawn, 8–10 cm below the top of each individual plant pot.
Gas-exchange measurements
Measurements of midday net photosynthetic rate (Pn) and stomatal conductance (gs) of water vapour were taken for each plant using a CI-340 Hand-Held Photosynthetic System (CID Inc., 2008, Camas, WA, USA) at midday after SWP measurement. The intensity of photosynthetic active radiation was maintained above 800 μmol m−2 s−1 during the gas-exchange measurements, because plants grown in open environments require this amount of photosynthetic active radiation for active photosynthesis. For each leaf sample measured, leaf area was measured using Adobe Acrobat Professional 8 (Adobe Systems Inc., 2011) after digitally scanning each leaf (Visoneer OneTouch 9420 Scanner). The leaf area was used to calculate gs and Pn per unit area.
Statistical analysis
We used repeated-measures ANOVA to determine the effects of species, time, treatments and their interactions on SWP, gs and Pn, followed by Tukey's post hoc multiple comparisons to analyse the differences between the species and between the time points within the treatments. Pair-wise comparison was used to analyse the differences within the species and between the treatments (control vs. SWD or salinity) in terms of SWP, gs and Pn. The significance level was set at P = 0.05.
Results
Soil water potential
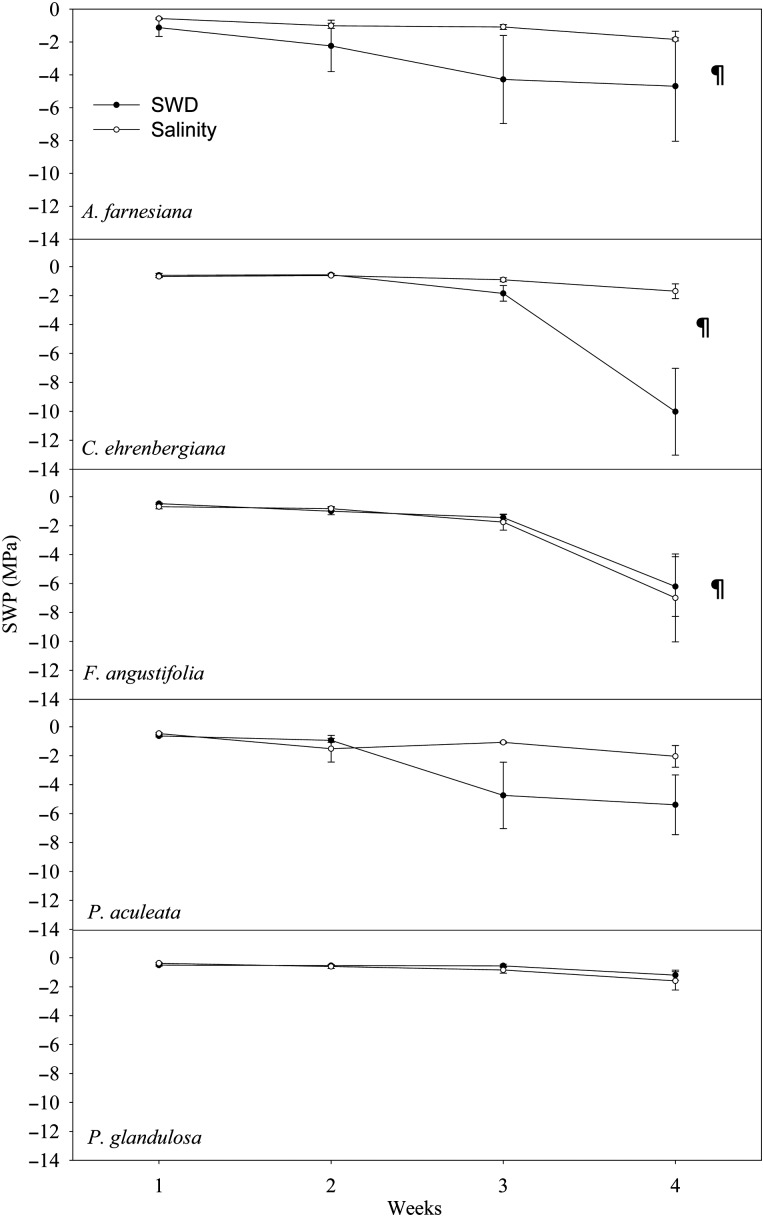
For the SWD treatment, the water potential of C. ehrenbergiana soil in weeks 1 (P = 0.004), 2 (P = 0.004) and 3 (P = 0.012) was significantly higher compared with that in week 4 (Fig. 1). The water potential of F. angustifolia soil in weeks 1 (P = 0.010), 2 (P = 0.019) and 3 (P = 0.032) was significantly higher compared with that in week 4 (Fig. 1). For the salinity treatment, the water potential of A. farnesiana soil in weeks 1 (P = 0.001), 2 (P = 0.002) and 3 (P = 0.001) was significantly higher compared with that in week 4 (Fig. 1).
Figure 1.
Average soil water potential (SWP) of five native shrub species over 4 weeks for soil water deficit (SWD) and salinity treatments. ¶ Soil of C. ehrenbergiana and F. angustifolia had a significantly lower water potential value at week 4 for both treatments and A. farnesiana had a significantly lower water potential at week 4 of salinity treatment.
The SWP was significantly different among the species for both treatments (Table 1). The SWP of species was significantly reduced by treatment and time for both experiments (Table 1). The interaction between species and treatment, species and time, and time and treatment significantly reduced the SWP of species in salinity treatment (Table 1). The post hoc analysis showed that SWP was significantly higher in weeks 1–3 compared with that in week 4 of SWD treatment (P = 0.001). During this period, there was a higher SWP in week 1 than in weeks 2 (P = 0.008) and 3 (P = 0.012). During the 4 weeks of SWD treatment, SWP was significantly higher in week 2 compared with week 3 (P = 0.023). For the salinity treatment, weeks 1 (P = 0.001), 2 (P = 0.001) and 3 (P = 0.001) had significantly higher SWP values compared with week 4. During this period, week 1 had significantly higher SWP values compared with week 3 (P = 0.001).
Table 1.
Effects of species, treatments, time and their interactions on soil water potential, photosynthetic rate and stomatal conductance of five native species for control, soil water deficit and salinity experiments
| Source | Significance level |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| SWP |
gs |
Pn |
|||||||
| Control | SWD | Salinity | Control | SWD | Salinity | Control | SWD | Salinity | |
| Species | – | 0.001 | 0.001 | n.s. | n.s. | n.s. | n.s. | 0.024 | n.s. |
| Treatment | – | 0.001 | 0.002 | n.s. | 0.001 | 0.001 | n.s. | 0.001 | 0.001 |
| Time | – | 0.003 | 0.001 | n.s. | 0.008 | 0.004 | n.s. | 0.001 | 0.002 |
| Species × treatment | – | n.s. | 0.001 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Species × time | – | n.s. | 0.001 | n.s. | n.s. | 0.002 | n.s. | n.s. | n.s. |
| Treatment × time | – | n.s. | 0.001 | n.s. | 0.001 | n.s. | n.s. | n.s. | n.s. |
P values were determined by repeated-measures ANOVA at 95% confidence intervals; n.s. represents non-significant results at P = 0.05 significance level.
Abbreviations: gs, stomatal conductance; Pn, photosynthetic rate; SWD, soil water deficit; and SWP, soil water potential.
Gas exchange
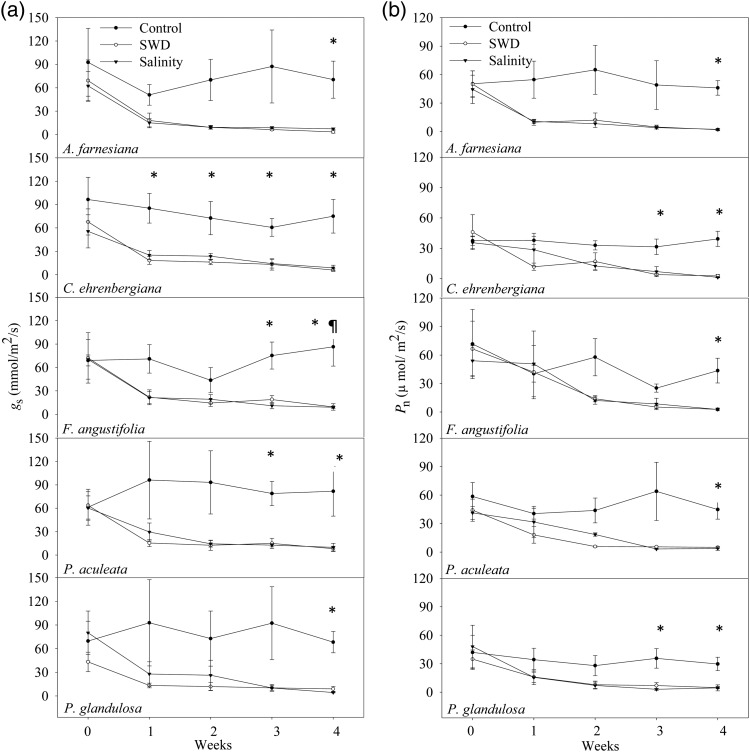
The average gs value of control plants of all species remained constant throughout the experiment, with no significant difference between species or over time. For the SWD treatment, A. farnesiana had significantly lower gs than control plants at week 4 (P = 0.023; Fig. 2a). Celtis ehrenbergiana had significantly lower gs values compared with control plants at weeks 1 (P = 0.008), 2 (P = 0.028), 3 (P = 0.001) and 4 (P = 0.01; Fig. 2a). Compared with control plants, F. angustifolia had significantly lower gs at weeks 3 (P = 0.012) and 4 (P = 0.027; Fig. 2a). At weeks 3 (P = 0.003) and 4 (P = 0.011), P. aculeata had significantly lower gs values than that of control plants (Fig. 2a). When compared with control plants, P. glandulosa had significantly lower gs at week 4 (P = 0.025; Fig. 2a).
Figure 2.
Average values of stomatal conductance (gs; a) and photosynthetic rate (Pn; b) for five native shrub species with 95% confidence intervals showing the result of control, SWD and salinity treatments, respectively. * Significantly lower gs and Pn of each species compared with its control plants for SWD and salinity treatments. ¶ Significantly lower gs value of F. angustifolia for salinity treatment only at week 4.
For the salinity treatment, A. farnesiana had significantly lower gs values compared with that of control plants at week 4 (P = 0.012; Fig. 2a). When compared with control plants, C. ehrenbergiana had significantly lower gs values at weeks 1 (P = 0.015), 2 (P = 0.049), 3 (P = 0.001) and 4 (P = 0.012; Fig. 2a). At week 4, F. angustifolia had significantly lower gs values than that of control plants (P = 0.022; Fig. 2a). At weeks 3 (P = 0.012) and 4 (P = 0.003), P. aculeata had significantly lower gs values compared with control plants (Fig. 2a). Compared with control plants, P. glandulosa had significantly lower gs at week 4 (P = 0.005, Fig. 2a).
The gs values of the species were significantly reduced when analysed by treatment and time for SWD and salinity treatments (Table 1). The interaction of time and treatment significantly reduced the gs of the species for SWD treatment (Table 1). The interaction of species and time had significant effects on gs of the species for salinity treatment (Table 1). The post hoc analysis showed that gs of the species was significantly reduced over time; week 0 had significantly higher values compared with those of weeks 1 (P = 0.001), 2 (P = 0.001), 3 (P = 0.001) and 4 of SWD (P = 0.001). There were significantly higher gs values in week 1 (P = 0.007) than in week 4 (P = 0.024) during this period of SWD treatment. For the salinity treatment, there was a significantly higher gs value in week 0 (P = 0.001) than in weeks 1 (P = 0.001), 2 (P = 0.002), 3 (P = 0.001) and 4 (P = 0.001). During this period, there was a significantly higher gs value in week 1 than in weeks 3 (P = 0.023) and 4 (P = 0.005) of the salinity treatment.
The average Pn value of all control plants remained unchanged throughout the experiment, with no significant difference between species or over time. For the SWD treatment, A. farnesiana (P = 0.001), F. angustifolia (P = 0.029) and P. aculeata (P = 0.003) had significantly lower Pn values at week 4 compared with that of control plants (Fig. 2b). Celtis ehrenbergiana had significantly lower Pn values at weeks 3 (P = 0.013) and 4 (P = 0.001) compared with control plants (Fig. 2b). At week 3 (P = 0.024) and 4 (P = 0.009), P. glandulosa had significantly lower Pn values compared with that of the control plants (Fig. 2b).
For the salinity treatment, we found that A. farnesiana (P = 0.001), F. angustifolia (P = 0.018) and P. aculeata (P = 0.003) had significantly lower Pn values at week 4 compared with the control plants (Fig. 2b). At weeks 3 and 4, C. ehrenbergiana had significantly lower Pn values (P = 0.03 and P = 0.013, respectively) compared with the control plants (Fig. 2b). At weeks 3 (P = 0.012) and 4 (P = 0.008), P. glandulosa had significantly lower Pn values compared with the control plants (Fig. 2b).
The Pn was significantly different only among the plant species in the SWD treatment (Table 1). The Pn of species was significantly reduced by treatment and time for SWD and salinity treatments (Table 1). The post hoc analysis showed that there was a significantly higher Pn of the species in week 0 than in weeks 1 (P = 0.003), 2 (P = 0.002), 3 (P = 0.001) and 4 (P = 0.001) for the SWD treatment. For the salinity treatment, there was a significantly higher Pn in week 0 compared with weeks 2 (P = 0.001), 3 (P = 0.001) and 4 (P = 0.001). During this period, there was a significantly higher Pn value in week 1 than in weeks 3 (P = 0.046) and 4 (P = 0.009). During the 4 weeks of salinity treatment, there was a significantly higher Pn value in week 2 than in week 4 (P = 0.001).
Discussion
The significant reduction of SWP values during the experiment due to the effects of drought and soil salinity indicates that soil water availability to the plants was reduced over time. The experiment showed that shrub species can survive with a soil water potential as low as −10.02 MPa (e.g. C. ehrenbergiana). Plant species that continue to function with a higher SWP may have specific adaptations to rehydrate plant cells more efficiently from available soil water or minimize the transpiration rate to conserve available soil moisture (Ritchie and Hinckley, 1975; Wan and Sosebee, 1991). Several studies have shown that SWP equilibrates with leaf water potential due to low atmospheric demand for water during the pre-dawn period (Morgan, 1984; Saliendra et al., 1995; Sellin, 1996; González-Rodríguez et al., 2000; Gebrekirstos et al., 2006). However, areas with high minimal temperatures may have transpiration at night where pre-dawn leaf water potential does not equilibrate with SWP (Donovan et al., 1999, 2001, 2003; Bucci et al., 2004; Howard et al., 2009). Other factors responsible for this disequilibrium may include low osmotic potential of plant species (Berger et al., 1996), hydraulic resistance to the soil–plant pathway (Myers and Neales, 1984), smaller plant size (Brown and Archer, 1990), night-time growth-induced reduction in cell water potential (Boyer, 1995) and soil moisture heterogeneity (Ourcival et al., 1994).
Based on the results of observed SWP, we categorized A. farnesiana, C. ehrenbergiana and F. angustifolia as water stress-sensitive species, because the soil of these species significantly reduced the water potential during the experiments. On the contrary, P. aculeata and P. glandulosa were categorized as water stress-tolerant species, because we did not observe significant loss of soil water potential for these species. For C. ehrenbergiana, the large decrease in SWP may be associated with high transpiration of this species, which also had higher initial stomatal conductance. Hence, plants that can reduce transpiration during drought may have higher survivorship in comparison to the species with higher transpiration rates. Soil water stress-tolerant species can withstand extreme dehydration of the protoplasm (González-Rodríguez et al., 2011). These species continue to maintain gas exchange under strongly negative plant water potential by maintaining osmotic potentials or by accumulating solutes or by both mechanisms (Morgan, 1984; Gebrehiwot et al., 2005).
Higher SWP with saline soils suggests that salinity may reduce water loss from species during a prolong water stress period. Higher SWP during soil water stress suggests that solute concentration in the root zone may reduce water loss from plants during relatively longer periods of water stress (Kozlowski, 1997). Salt accumulation in the root zone of these species may result in an increased solute concentration in the xylem content due to mobilization of cell sap from cells en route to reduce water loss, resulting in inefficient water extraction from the soil (Gollan et al., 1985; González-Rodríguez et al., 2011). The species grown in soil with higher water potential can maintain higher plant water potential compared with other coexisting species in an identical environment that might involve osmotic adjustments during the soil water stress (Morgan, 1984; Tezara et al., 1999; Chaves et al., 2003). Such species tolerate soil salinity by absorbing ions from the soil that are sequestered in cell vacuoles or synthesized into compatible solute in the cytoplasm (Kozlowski, 1997). Significantly lower SWP in F. angustifolia and higher SWP in P. glandulosa compared with the other species after 4 weeks of the salinity treatments may be connected to higher and lower transpiration rates, respectively, for these species.
In the field, water stress-tolerant species may be phreatophytic by necessity. For example, P. glandulosa may extract water from soil through a deep rooting system that differentiates it from species with a shallow rooting system (e.g. A. farnesiana, C. ehrenbergiana and F. angustifolia). Plant strategies to cope with water stress may be related to a deep vs. shallow rooting system in competitive field environments (González-Rodríguez et al., 2011). The advantage of allocating biomass in deep root systems by the phreatophytic species may be to avoid competition for available water in the upper soil surface with shallow-rooted species. However, the greenhouse study showed that water stress-tolerant responses are related to the water conservation strategy rather than the phreatophytic characteristics. Such species have the capacity to reduce the transpiration rate with decreasing soil water availability (Wan and Sosebee, 1991). These characteristics of P. glandulosa partly explain its increased dominance in drought-prone shrublands of Texas over the past century (Brown and Archer, 1999).
The findings of this study showed that a significant reduction in gs occurred earlier than in SWP of some species. This suggests that a small reduction in the available soil water affects gas exchange of these plants. Mechanisms for this may be excessive root production of abscisic acid during drought, which is delivered to leaves by transpirative flow and triggers stomatal closure to avoid tissue dehydration (Gowing et al., 1993; Trejo et al., 1993; Montagu and Woo, 1999). The complex interactions of other environmental factors, such as leaf water potential, xylem hydraulic conductivity, plant nutritional status, xylem sap pH and leaf-to-air vapour pressure deficit, were also reported to influence stomatal control during soil water stress (Dai et al., 1992; Tardieu and Davies, 1992; Salleo et al., 2000; Medrano et al., 2002). The salt concentration in the rooting zone reduces root hydraulic conductance; hence, it reduces the amount of water flow from the roots to the upper portion of the canopy, causing a reduction in gs (González-Rodríguez et al., 2004). The SWP can be even lower in the field compared with a controlled environment that may increase variation in the stomatal response to imposed drought effects (Davies, 1977; Dawyer and Stewart, 1985).
We also found that gs was affected by each of the treatments ∼1 week earlier than that of net photosynthesis in three of the species, suggesting that soil water stress affects water vapour conductance independent of photosynthesis. Plants may show both stomatal and non-stomatal limitation of photosynthesis during water stress (Noormets et al., 2001). Acacia farnesiana demonstrated stomatal control of photosynthesis, where lower gs and photosynthesis occurred in the same week for this species. For the remaining species, our data suggest non-stomatal inhibition of photosynthesis due to increased CO2 reduction in the chloroplast, as well as reduced efficiency of ribulose biphosphate regeneration due to inactivated electron transport via shrinkage of intercellular spaces (Iyengar and Reddy, 1996). The gs of most species decreases with high salinity, as we observed (Parida et al., 2004), which is likely to induce closure of stomata with restricted availability of internal CO2 for carboxylation (Brugnoli and Björkman, 1992). Also, the leaf area changes associated with loss of turgor may affect cell wall properties, with a decrease in net photosynthesis being associated with deformation of the cellular structure (Franco et al., 1997; González-Rodríguez et al., 2004). However, more investigation is required to determine how stomatal or non-stomatal reductions in photosynthesis occur for the shrub species tested in this study.
We found significantly reduced values of Pn of all species that occurred before decreases in SWP values in both treatments. In a resource-limited ecosystem, low accumulation of water and minerals available for investment in the photosynthetic apparatus may significantly reduce the photosynthesis of species that maintain a higher plant water potential (Soyza et al., 2004). Water stress imposed due to prolonged soil water deficit and soil salinity may affect the growth and survival of plants due to a reduction in gas exchange, although this may vary greatly among species (Bolarín et al., 1991). A potential reason for the lower photosynthesis found due to the treatments might be reduced ATP synthesis, with overall lower rates of cellular metabolism (Tezara et al., 1999). Reduction in photosynthesis due to soil salinity is accompanied by dysfunction in protein and nucleic acid metabolism and enzymatic activities (Kozlowski and Pallardy, 2002). However, we found no difference in net photosynthesis among the species for both treatments, indicating that the gas-exchange capacity of these species is broadly affected by soil water deficit.
Only seedlings of the plant species were tested in this experiment. Some research indicates that growth form and plant size may influence the physiological responses of species to environmental factors. For example, Pn and gs of seedlings showed lower values compared with larger plants of the same species at more arid locations (Brown and Archer, 1990; Knapp and Fahnestock, 1990; Donavan and Ehleringer, 1991, 1992). However, seedlings of plant species grown in wet soil may have higher gs when compared with larger plants of same species (Donavan and Ehleringer, 1991). The variation may be attributed to the shallow rooting system of younger plants in the field and the smaller taproot for water storage (Brown and Archer, 1990; Knapp and Fahnestock, 1990). Hence, larger plants may show potential to avoid environmental stress that may have stronger and more negative effects on smaller plants of the same species.
In semi-arid shrublands, such as the LRGV, precipitation has been gradually declining over the past century, with increased magnitude and duration of drought periods (Porporato et al., 2001). Coupled with inherently saline soils from both the legacy of agricultural irrigation and airborne inputs from the ocean, the inter-specific variations in soil water potential and gas-exchange values recorded during the experiment suggest that the species showed differences in their capacity to withstand a wider range of soil water status. All species studied are potential candidates for continued restoration and conservation of these degraded shrubland ecosystems. However, maintaining future diversity for Tamaulipan shrublands may be difficult given the range of water stress responses shown for this common group of shrubs species.
Conclusion
We found physiological evidence that the soil water potential of the five co-occurring shrub species decreased in response to progressive water stress, affecting their gas-exchange capacity. Water stress due to drought and salinity affects inter-specific differences in water relation and gas-exchange characteristics of these species. Our findings suggest that the coexisting plants have developed tolerance strategies to soil water stress, but the extent can be species specific. Our results also indicated that soil water potential drives gas-exchange limitation in some species and that photosynthesis in some species may be controlled by non-stomatal mechanisms of tissue water and solute concentration. From our experiments, while all five species exhibited capacities to withstand current water availability, some species have limited tolerances for extreme water stress.
Acknowledgements
This research was made possible through the US Fish and Wildlife Services (FWS Agreement Number: 201819J608) with special support by Kelly McDowell and Mitch Stenberg. Additional funding was provided by Folmar Research Fund of Baylor University. We thank the three anonymous reviewers for their helpful comments and suggestions on the manuscript.
References
- 1.Archer S. (1989) Have southern Texas Savannas been converted to woodlands in recent history? Am Nat 134: 545–561. [Google Scholar]
- 2.Archibald RD, Harper RJ, Fox JED, Silberstein RP. (2006) Tree performance and root-zone salt accumulation in three dryland Australian plantations. Agrofores Syst 66: 191–204. [Google Scholar]
- 3.Berger A, Grouzis M, Fournier C. (1996) The water status of six woody species coexisting in the Sahel (Ferlo, Senegal). J Trop Ecol 12: 607–627. [Google Scholar]
- 4.Bolarín MC, Fernández FG, Cruz V, Cuartero J. (1991) Salinity tolerance in four wild tomato species using vegetative yield-salinity response curves. J Am Soc Hort Sci 116: 286–290. [Google Scholar]
- 5.Boyer JS. (1995) Measuring the Water Status of Plants and Soils. Academic Press, San Diego. [Google Scholar]
- 6.Brown JR, Archer S. (1990) Water relation of perennial grass and seedling vs adult woody plants in a subtropical savanna, Texas, USA. Oikos 57: 366–374. [Google Scholar]
- 7.Brown JR, Archer S. (1999) Shrub invasion of grassland: recruitment is continuous and not regulated by herbaceous biomass and density. Ecology 80: 2385–2396. [Google Scholar]
- 8.Brugnoli E, Björkman O. (1992) Growth of cotton under continuous salinity stress: influence on allocation pattern, stomatal and non-stomatal components of photosynthesis and dissipation of excess light energy. Planta 187: 335–347. [DOI] [PubMed] [Google Scholar]
- 9.Bucci SJ, Scholz FB, Godstein G, Meinzer FC, Hinojosa JA, Hoffmann WA, Franco AC. (2004) Processes preventing nocturnal equilibration between leaf and soil water potential in tropical savanna woody species. Tree Physiol 24: 1119–1127. [DOI] [PubMed] [Google Scholar]
- 10.Chaves MM, Maroco JP, Pereira JS. (2003) Understanding plant responses to drought — from genes to the whole plant. Funct Plant Biol 30: 239–264. [DOI] [PubMed] [Google Scholar]
- 11.Condit R, Hubbell SP, Foster RB. (1995) Mortality rates of 205 neotropical tree and shrub species and the impact of a severe drought. Ecol Monogr 65: 419–439. [Google Scholar]
- 12.Dai Z, Edwards GE, Ku MSB. (1992) Control of photosynthesis and stomatal conductance in Ricinus communis L. (castor bean) by leaf to air vapor pressure deficit. Plant Physiol 99: 1426–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies WJ. (1977) Stomatal responses to water stress and light in plants grown in controlled environments and in the field. Crop Sci 5: 735–740. [Google Scholar]
- 14.Dawyer LM, Stewart DW. (1985) Water stress conditioning of corn (Zea mays) in the field and the greenhouse. Can J Bot 63: 704–710. [Google Scholar]
- 15.de Soyza AG, Killingbeck KT, Whitford WG. (2004) Plant water relations and photosynthesis during and after drought in a Chihuahuan desert arroyo. J Arid Environ 59: 27–39. [Google Scholar]
- 16.Ditmarova L, Kurjak D, Palmroth S, Kmet J, Strelcova K. (2009) Physiological responses of Norway spruce (Picea abies) seedlings to drought stress. Tree Physiol 30: 205–213. [DOI] [PubMed] [Google Scholar]
- 17.Donavan LA, Ehleringer JR. (1991) Ecophysiological differences among juvenile and reproductive plants of several woody species. Oecologia 86: 594–597. [DOI] [PubMed] [Google Scholar]
- 18.Donavan LA, Ehleringer JR. (1992) Contrasting water use patterns among size and life-history classes of a semi-arid shrub. Funct Ecol 6: 482–488. [Google Scholar]
- 19.Donovan LA, Grise DJ, West JB, Pappert RA, Alder NN, Richards JH. (1999) Predawn disequilibrium between plant and soil water potentials in two cold-desert shrubs. Oecologia 120: 209–217. [DOI] [PubMed] [Google Scholar]
- 20.Donovan LA, Linton MJ, Richards JH. (2001) Predawn plant water potential does not necessarily equilibrate with soil water potential under well-watered conditions. Oecologia 129: 328–335. [DOI] [PubMed] [Google Scholar]
- 21.Donovan LA, Linton MJ, Richards JH. (2003) Magnitude and mechanisms of disequilibrium between predawn plant and soil water potentials in desert shrubs. Ecology 84: 463–470. [Google Scholar]
- 22.Eddy MR, Judd FW. (2003) Phenology of Acacia berlandieri, A. minuata, A. rigidula, A. schaffneri, and Chloroleucon ebano in the Lower Rio Grande Valley of Texas during a drought. Southwest Nat 48: 321–332. [Google Scholar]
- 23.Escos J, Alados CL, Pugnaire FI, Puigdefabregas J, Elmen J. (2000) Stress resistance strategy in an arid land shrub: interaction between developmental instability and fractal dimension. J Arid Environ 45: 325–336. [Google Scholar]
- 24.Ewing K, Best C. (2004) South Texas Tamaulipan thornscrub restoration experiment measures growth of planted woody vegetation. Ecol Res 22: 11–17. [Google Scholar]
- 25.Foltescu VL, Pryor SC, Bennet C. (2005) Sea salt generation, dispersion and removal on the regional scale. Atmos Environ 39: 2123–2133. [Google Scholar]
- 26.Franco JA, Fernández JA, Bañón S, González A. (1997) Relationship between the effects of salinity on seedling leaf area and fruit yield of six muskmelons cultivars. J Hort Sci 32: 642–647. [Google Scholar]
- 27.Gebrehiwot K, Muys B, Haile M, Mitloehner R. (2005) The use of plant water relations to characterize tree species and sites in the drylands of northern Ethopia. J Arid Environ 60: 581–592. [Google Scholar]
- 28.Gebrekirstos A, Teketay D, Fetene M, Mitlöhner R. (2006) Adaptation of five co-occurring tree and shrub species to water stress and its implication in restoration of degraded lands. For Ecol Manage 229: 259–267. [Google Scholar]
- 29.Gollan T, Turner NC, Schulze E-D. (1985) The response of stomata and leaf gas exchange to vapor pressure deficits and soil water content. III. In the sclerophyllous woody species Nerium olaender. Oecologia 65: 356–362. [DOI] [PubMed] [Google Scholar]
- 30.González-Rodríguez H, Cantú-Silva I, Gómez-Meza MV, Jordan WR. (2000) Seasonal plant water relationships in Acacia berlandieri. Arid Soil Res Rehab 14: 343–357. [Google Scholar]
- 31.González-Rodríguez H, Cantú-Silva IC, Gómez-Meza MV, Lozano RGR. (2004) Plant water relations of thornscrub shrub species, north-eastern Mexico. J Arid Environ 58: 483–503. [Google Scholar]
- 32.González-Rodríguez H, Cantú-Silva I, Ramírez-Lozano RG, Gómez-Meza MV, Sarquis-Ramírez J, Coria-Gil N, Cervantes-Montoya JR, Maiti RK. (2011) Xylem water potentials of native shrubs from northeastern Mexico. Acta Agr Scand, Section B - Soil Plant Sci 61: 214–219. [Google Scholar]
- 33.Gowing DJG, Jones HG, Davies WJ. (1993) Xylem-transported abscisic acid: the relative importance of its mass and its concentration in the control of stomatal aperture. Plant Cell Environ 16: 453–459. [Google Scholar]
- 34.Grime JP. (1977) Evidence for existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am Nat 111: 1169–1194. [Google Scholar]
- 35.Hendrickx JMH, Harrison JB, Beekma J, Rodribuez-Marin G. (1999) Salinity management in the Rio Grande Bosque. USDA Forest Service Proceeding RMRS-P-7, pp 68–71. [Google Scholar]
- 36.Howard AR, Van Iersel MW, Richards JH, Donovan LA. (2009) Night-time transpiration can decrease hydraulic redistribution. Plant Cell Environ 32: 1060–1070. [DOI] [PubMed] [Google Scholar]
- 37.Iyengar ERR, Reddy MP. (1996) Photosynthesis in high salt-tolerant plants. In Pessarakli M, ed, Handbook of Photosynthesis. Marcel Decker, Inc, Baton Rose, USA, pp 56–65. [Google Scholar]
- 38.Jahrsdoerfer SE, Leslie DM. (1988) Tamaulipan brushland of the Lower Rio Grande Valley of Texas: Description, human impacts, and management options. Biological Report 88 US Fish and Wildlife Service, US Department of Interior. [Google Scholar]
- 39.Knapp AK, Fahnestock JT. (1990) Influence of plant size on the carbon and water relations of Cucurbita foetidissima HBK. Funct Ecol 4: 789–797. [Google Scholar]
- 40.Kozlowski TT. (1997) Response of woody plants to flooding and salinity. Tree Physiol Monogr 1: 1–29. [Google Scholar]
- 41.Kozlowski TT, Pallardy SG. (2002) Acclimation and adaptive responses of woody plants to environmental stresses. Bot Rev 68: 270–334. [Google Scholar]
- 42.Maas EV, Hoffman GJ. (1977) Crop salt tolerance–current assessment. J Irrig Drain Eng 103: 115–134. [Google Scholar]
- 43.McCoy TW. (1990) Evaluation of ground-water resources in the Lower Rio Grande Valley, Texas. Texas Water Development Board, Report 316, 48 pp. [Google Scholar]
- 44.McDowell NG, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, et al. (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178: 719–739. [DOI] [PubMed] [Google Scholar]
- 45.Medrano H, Escalona JM, Bota J, Gulías J, Flexas J. (2002) Regulation of photosynthesis of C3 plants to progressive drought: stomatal conductance as a reference parameter. Ann Bot 89: 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montagu KD, Woo KC. (1999) Recovery of tree photosynthetic capacity from seasonal drought in the wet–dry tropics: the role of phyllode and canopy processes in Acacia auriculiformis. Aust J Plant Physiol 26: 135–145. [Google Scholar]
- 47.Morgan JA, LeCain DR, Pendall E, Blumenthal D, Kimball BA, Carrillo Y, Williams DG, Heisler-White J, Dijkstra FA, West M. (2011) C4 grasses prosper as carbon dioxide eliminates desiccation in warmed semi-arid grassland. Nature 476: 202–205. [DOI] [PubMed] [Google Scholar]
- 48.Morgan JM. (1984) Osmoregulation and drought stress in higher plants. Annu Rev Plant Physiol 35: 299–319. [Google Scholar]
- 49.Myers BA, Neales TF. (1984) Seasonal changes in the water relations of Eucalyptus behriana F. Muell, and E. microcarpa (Maiden) in the field. Aust J Plant Bot 32: 495–510. [Google Scholar]
- 50.Navar-Chaidez JDJ. (2008) Carbon fluxes resulting from land-use change in the Tamaulipan thornscrub of northern Mexico. Carbon Balance Manag 3: 6 doi:10.1186/1750-0680-3-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noormets A, Sober A, Pell EJ, Dickson RE, Podila GK, Sober J, Isebrands JG, Karnosky DF. (2001) Stomatal and non-stomatal limitation to photosynthesis in two trembling aspen (Populus tremuloides Michx.) clones exposed to elevated CO2 and/or O3. Plant Cell Environ 24: 327–336. [Google Scholar]
- 52.Otieno DO, Schmidt MWT, Adiku S, Tenhaunen J. (2005) Physiological and morphological responses to water stress in two Acacia species from contrasting habitats. Tree Physiol 25: 361–371. [DOI] [PubMed] [Google Scholar]
- 53.Ourcival JM, Floret C, Le Floch E, Pontanier R. (1994) Water relations between two perennial species in the steppes of southern Tunisia. J Arid Environ 28: 333–350. [Google Scholar]
- 54.Parida AK, Das AB, Mohanty P. (2004) Defense potentials to NaCl in a mangrove, Bruguiera parviflora: differential changes of isofoms of some antioxidative enzymes. J Plant Physiol 161: 531–542. [DOI] [PubMed] [Google Scholar]
- 55.Porporato A, Laio F, Ridolfi L, Rodriguez-Iturbe I. (2001) Plants in water-control ecosystems: active role in hydrologic processes and response to water stress III. Vegetation water stress. Adv Water Resour 24: 725–744. [Google Scholar]
- 56.Reid N, Marroquin J, Beyer-Munzel P. (1990) Utilization of shrubs and trees for browse, fuelwood and timber in the Tamaulipan thornscrub, northeastern Mexico. For Ecol Manage 36: 61–79. [Google Scholar]
- 57.Ritchie GA, Hinckley TM. (1975) The pressure chamber as an instrument for ecological research. Adv Ecol Res 9: 165–254. [Google Scholar]
- 58.Saliendra NZ, Sperry JS, Comstock JP. (1995) Influence of leaf water status on stomatal response to humidity, hydraulic conductance, and soil drought in Betula occidentalis. Planta 196: 357–366. [Google Scholar]
- 59.Salleo S, Nardini A, Pitt F, Lo Gullo MA. (2000) Xylem cavitation and hydraulic control of stomatal conductance in Laurel (Laurus nobilis L.). Plant Cell Environ 23: 71–79. [Google Scholar]
- 60.Sellin A. (1996) Base water potential of Picea abies as a characteristic of the soil water status. Plant Soil 184: 273–280. [Google Scholar]
- 61.Tardieu F, Davies WJ. (1992) Stomatal response to ABA is a function of current plant water status. Plant Physiol 98: 540–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tezara W, Mitchell VJ, Driscoll SD, Lawlor DW. (1999) Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 401: 914–917. [Google Scholar]
- 63.Trejo CL, Davies WJ, Ruiz L del MP. (1993) Sensitivity of stomatal to abscisic acid: an effect of the mesophyll. Plant Physiol 102: 497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vertovec M, Sakçali S, Ozturk M, Salleo S, Giacomich P, Feoli E, Nardini A. (2001) Diagnosing plant water status as a tool for quantifying water stress on a regional basis in Mediterranean drylands. Ann For Sci 58: 113–125. [Google Scholar]
- 65.Wan C, Sosebee RE. (1991) Water relations and transpiration of honey mesquite on 2 sites in west Texas. J Range Manage 44: 156–160. [Google Scholar]
- 66.White JD, Gutzwiller KJ, Barrow CW, Randall LJ, Swint P. (2008) Modeling mechanisms of vegetation change due to fire in a semi-arid ecosystem. Ecol Modell 214: 181–200. [Google Scholar]
- 67.Wiedenfeld B. (2008) Effects of irrigation water salinity and electrostatic water treatment for sugarcane production. Agri Water Manage 95: 85–88. [Google Scholar]