Larval sturgeon swimming capacity has never been assessed. We measured critical swimming velocity of larval green and white sturgeon, and summarized published juvenile critical swimming velocity data for all sturgeon species. Recommendations for anthropogenic water diversion facility flow management were developed from the data, emphasizing Californian green and white sturgeon conservation.
Keywords: Sturgeon, swimming, water diversion
Abstract
Little is known of the swimming capacities of larval sturgeons, despite global population declines in many species due in part to fragmentation of their spawning and rearing habitats by man-made water-diversion structures. Larval green (Acipenser medirostris) and white sturgeon (Acipenser transmontanus) inhabit the highly altered Sacramento–San Joaquin watershed, making them logical species to examine vulnerability to entrainment by altered water flows. The risk of larval sturgeon entrainment is influenced by the ontogeny of swimming capacity and dispersal timing and their interactions with water-diversion structure operations. Therefore, the aim of this study was to describe and compare the ontogeny and allometry of larval green and white sturgeon swimming capacities until completion of metamorphosis into juveniles. Despite the faster growth rates and eventual larger size of larval white sturgeon, green sturgeon critical swimming velocities remained consistently, though modestly, greater than those of white sturgeon throughout the larval life stage. Although behavioural interactions with water-diversion structures are also important considerations, regarding swimming capacity, Sacramento–San Joaquin sturgeons are most vulnerable to entrainment in February–May, when white sturgeon early larvae are in the middle Sacramento River, and April–May, when green sturgeon early larvae are in the upper river. Green sturgeon migrating downstream to the estuary and bays in October–November are also susceptible to entrainment due to their movements combined with seasonal declines in their swimming capacity. An additional inter-species comparison of the allometric relationship between critical swimming velocities and total length with several sturgeon species found throughout the world suggests a similar ontogeny of swimming capacity with growth. Therefore, although dispersal and behaviour differ among river systems and sturgeon species, similar recommendations are applicable for managers seeking to balance water demands with restoration and conservation of sturgeons worldwide.
Introduction
Several sturgeon life-history traits, such as longevity, late maturation, spawning migrations and long breeding intervals, make sturgeon species worldwide vulnerable to anthropogenic pressures (Rochard et al., 1990; Billard and Lecointre, 2001; Gessner et al., 2007; Mussen et al., 2014). Many sturgeon species spend all or part of their lives in coastal and inland systems, where habitat fragmentation by dams and other water-diversion structures is pervasive (Rochard et al., 1990; Billard and Lecointre, 2001; Williot et al., 2002). These structures can impede safe passage of migratory and resident fishes, interrupt watershed connectivity and adversely affect populations of many fish species (Xenopoulos et al., 2005; Arnekleiv et al., 2007; Caudill et al., 2007; Liermann et al., 2012), including sturgeons (Liermann et al., 2012). The ability of fish to navigate or avoid water-diversion structures is related to their behavioural responses to water flow (Hinch and Bratty, 2000) and swimming capacities (Hoover et al., 2005; Swanson et al., 2005; Adams et al., 2007; Boysen and Hoover, 2009). Sturgeons, in comparison to other anadromous fishes, may be particularly susceptible to altered flows around water-diversion structures due to their reduced swimming capacities (Peake et al., 1997; Deslauriers and Kieffer, 2012a) and unique behavioural responses to flow (Webb, 1986; Peake et al., 1997; Parsons et al., 2003; Hoover et al., 2005; Deslauriers and Kieffer, 2012a; Mussen et al., 2014). Due to the pervasiveness of water diversion from sturgeon rivers worldwide and the protected status of all 27 sturgeon species by at least one international or national government body (IUCN, 2014; SARA, 2014; USFWS, 2014), assessments of swimming abilities of sturgeons that encounter anthropogenic water-diversion structures are important to understanding potential impacts on sturgeon populations.
Green (Acipenser medirostris) and white sturgeon (Acipenser transmontanus) are anadromous and semi-anadromous fishes, respectively, that are protected in North America. Green sturgeon spawn in Oregon and California and are composed of at least two genetically distinct populations (Israel et al., 2009b). The Northern Distinct Population Segment (DPS), which is classified as a species of concern by the National Oceanic and Atmospheric Administration (NOAA) of the USA, includes all populations that spawn in rivers north of the Eel River of northwest California (Adams et al., 2007). Confirmed spawning locations for the Northern DPS are the Rogue River in Oregon and the Klamath River in northern California, though additional spawning locations are suspected (Adams et al., 2007). The Southern DPS is classified as threatened under the Endangered Species Act (ESA), and all suspected and confirmed spawning locations are within the Sacramento–San Joaquin (S-SJ) watershed in California (Adams et al., 2007). A review of the current distribution of green sturgeon and the spawning locations of both the Northern and Southern DPS has been provided by Beamesderfer et al. (2007).
White sturgeon are found in three major North American drainages, i.e. Fraser, Columbia and S-SJ. Populations in the Kootenay, Upper Fraser, Nechako and Columbia rivers are protected by the Canadian Species at Risk Act (SARA, 2014). The American ESA recognizes the Kootenay population as endangered, and though the S-SJ population is not classified, the American Fisheries Society identifies them as a conservation concern (Musick et al., 2000). White sturgeon are less marine oriented, with more life-history variation compared with green sturgeon. For example, the Kootenay population is landlocked and appears to disperse downstream more slowly than all other white sturgeon populations, which are semi-anadromous (McCabe and Tracy, 1993; Kynard and Parker, 2005; Kynard et al., 2010; McAdam, 2011). Details on the populations and distribution of white sturgeon throughout their range have been provided by Schreier et al. (2013).
Green and white sturgeon inhabiting the S-SJ watershed face a profoundly altered habitat. The hydrological regimen of the S-SJ watershed has been severely disrupted since the late 1800s, when hydraulic mining operations were pervasive throughout the central Sierra Nevada region (Cloern and Jassby, 2012). These changes have led to fish extinctions, extirpations (Moyle, 2002) and population declines (Stevens et al., 1985; Kimmerer et al., 2001; Moyle, 2002; Sommer et al., 2011). Despite the imminent threats to native species, conservation actions in the S-SJ watershed are challenging due to heavy societal water demands and use of the watershed as a resource. The S-SJ watershed supplies water to 25 million people and 1 million hectares of farmland (Service, 2007), facilitated by the construction of over 3300 water-diversion structures (Herren and Kawasaki, 2001), which divert more than 40% of the watershed drainage from the river system (Cloern and Jassby, 2012). Although many of these structures are not monitored for fish entrainment (i.e. being drawn in), anadromous fishes must pass by these diversion structures as they migrate, and the larval and juvenile fish of several species may be most susceptible to entrainment into diversions (Danley et al., 2002; Grimaldo et al., 2009). The spawning and rearing habitats of green and white sturgeon are located in the S-SJ watershed, and both species are susceptible to entrainment by the water diversion pumps operating in this watershed (Adams et al., 2007; Israel et al., 2009a; Mussen et al., 2014). Indeed, Californian water diversions have been implicated in the population declines of species such as Chinook salmon (Oncorhynchus tshawytscha; Moyle, 2002), delta smelt (Hypomesus transpacificus; Bennett, 2005), striped bass (Morone saxatilis; Stevens et al., 1985) and green sturgeon (Mussen et al., 2014). Additionally, entrainments of both green and white sturgeon are reported at state and federal pumping facilities (NOAA, 2005; Israel and Klimley, 2008; Israel et al., 2009a), with up to 10 000 white sturgeon reported in some years (Israel et al., 2009a).
For many fish species, relative year-class strength is most highly influenced by embryonic to larval stages (Bradford, 1992). Recruitment failure during these early life stages has been identified as a major bottleneck to other North American acipenserid species (Hardy and Litvak, 2004), and specifically to white sturgeon (Duke et al., 1999; Hildebrand et al., 1999). Larvae and juvenile green sturgeon appear to disperse downstream rapidly following emergence, after which they spend 0.5–4 years foraging throughout the watershed (Adams et al., 2007). Between ∼0.5 and 1.5 years of age, seawater tolerance (Allen and Cech, 2007, 2009, 2011) and a preference for high-salinity water (Poletto et al., 2013) develops, suggesting a predisposition to migrate to marine waters at this age. Laboratory studies of white sturgeon suggest that salinities of 20 ppt are stressful to juveniles (McEnroe and Cech, 1985; Tashjian et al., 2007). On the Columbia River, juvenile white sturgeon migrate seasonally up- and downstream, but have not been observed further downstream than the associated estuary (Parsley et al., 2008). Their intolerance of high salinities and migratory behaviour suggest that white sturgeon spend their entire juvenile life in their natal rivers. Therefore, anthropogenic alterations to their natal river systems, particularly for the dispersal and foraging stages of both larval and juvenile green and white sturgeon, are likely to have severe consequences at the population level. Little is known about the susceptibility of these two species to encounters with water-diversion structures during their larval life stage, and almost nothing is known of their ability to resist the altered water flows around diversions if they do encounter these facilities, though juvenile green sturgeon [30 cm and 150–200 days post-hatch (dph)] appear to be more vulnerable to impingement on diversion screens than white sturgeon of a similar size and age in sweeping flows of 20 and 37 cm s−1 (Poletto et al. 2014).
Given that the ability of fishes to navigate or avoid water-diversion structures is related to their swimming capacity (Hoover et al., 2005; Swanson et al., 2005; Adams et al., 2007; Boysen and Hoover, 2009), one method to assess the susceptibility of fishes to altered water flows, such as those at or near water diversions, is to quantify critical swimming velocities (i.e. an index of prolonged swimming capacity, Ucrit; Brett, 1964; Beamish, 1978). To date, the prolonged swimming capacities of larval green and white sturgeon have never been assessed. However, studies suggest that both species forage most actively from ∼30 dph onwards and spend the majority of their time up to 30 dph inactively hiding within rocky substrate (Kynard and Parker, 2005; Kynard et al., 2005). The increased movement during this active foraging stage is most probably accompanied by increases in both swimming capacity and the potential to encounter water-diversion structures. Therefore, assessments of swimming abilities of these species during life stages that encounter water diversions are important to the management of these devices to minimize their impacts on sturgeon populations. In light of the paucity of information regarding swimming capacities of larval white and green sturgeon and their susceptibility to entrainment into water-diversion structures, the aim of the present study was to describe and compare the ontogeny of green and white sturgeon prolonged swimming capacities until completion of metamorphosis into juveniles, as well as to describe the allometry of swimming capacity throughout the juvenile life phase using swimming data in the published literature. We hypothesized that the similar lifestyles during the larval stage of these two species would be reflected in comparable swimming capacities throughout the larval life stage, but that the larger egg size of green sturgeon (Deng et al., 2002) would result in larger larvae and faster growth throughout the larval stage. Furthermore, the allometry of green and white sturgeon swimming performance was compared with those of other sturgeon species to investigate inter-species differences in the ontogeny of swimming capacity. Finally, understanding the ontogeny of swimming capacity in green and white sturgeon of early dispersal and migration age can be used to inform conservation managers of appropriate protective water-diversion flow limitations specific to the S-SJ watershed and according to season and location.
Materials and methods
Green and white sturgeon were reared in the laboratory at the Center for Aquatic Biology and Aquaculture, University of California, Davis (UC Davis). White sturgeon, obtained from Sterling Caviar and spawned in April 2012 and May 2013, were offspring of the F3 descendants of wild-caught Sacramento River sturgeon. Green sturgeon were tank spawned from first-generation domestic Klamath River broodstock in November 2011 and April 2013 following the methodology outlined by Van Eenennaam et al. (2001, 2012). For both species, the broodstock were spawned at 15°C, and eggs were reared at this temperature until 1 dph, when they were acclimated to 18 ± 0.5°C well water. At the onset of exogenous feeding (∼10–12 dph), green and white sturgeon larvae were fed ad libitum with Rangen (Buhl, ID, USA) salmonid starter moist feed. For each spawn, larvae were split into two 1-m-diameter flow-through tanks supplied with 18 ± 0.5°C, aerated well water (dissolved oxygen >8.2 mg l−1) creating flows of <5 cm s−1 and reared under a natural photoperiod (Van Eenennaam et al., 2001, 2012). Fish handling, rearing and swimming tests were performed in agreement with the UC Davis Institutional Animal Care and Use Committee protocol no. 17017.
Swimming performance
Protocols assessing Ucrit were carried out at 18.5 ± 0.5°C. Due to their small size and frequent feeding requirements (Mohseni et al., 2006), larval fish were fasted for 3–4 h before an individual fish was randomly chosen from alternating tanks and introduced into the swimming flume for a 30 min acclimation period. Swimming flumes were either a 1.5 l cylindrical flume or a 5 l rectangular, flat-bottomed flume (Loligo® Systems, Tjele, Denmark) equipped with a motor and a variable frequency driver. During the acclimation period, water velocity was 1 cm s−1. After 30 min, water velocity was increased to 10 cm s−1 and then increased in increments of 5 cm s−1 every 5 or 10 min, depending on the spawn year. During each increment, fish were monitored for swimming behaviour. If fish became impinged, which was defined as contact between the downstream screen of the chamber and a third of their body for any duration or less than a third of their body for 30 s, timers and flow were stopped for 2 min. After the 2 min rest, timers were restarted, and flow was resumed at the velocity at the time of impingement. On the third impingement at the same velocity increment, or failure to recommence swimming after the 2 min break, the fish was considered fatigued (Allen et al., 2006a). If a fish failed to successfully swim continuously through the first swimming velocity step by either holding station (avoiding swimming in the current) or impingement, that fish was considered to have low behavioural motivation to swim. These fish were not included in Ucrit calculations, but are reported in the results as non-participants.
Preliminary tests with sturgeon larvae of different sizes and ages dictated that the time interval and swimming flume size and design be modified to achieve successful swimming throughout larval development. The 2013 spawn (green and white sturgeon measured at ages 20–42 dph) were swum in the 1.5 l cylindrical swimming flume for 5 min intervals. The 2011 green sturgeon and 2012 white sturgeon spawns (measured at ages 34–60 dph) were swum in the 5 l rectangular, flat-bottomed flume (Hoover et al., 2011) for 10 min intervals. Comparisons of Ucrit for 30–40 dph 2013 spawns (swum in the 1.5 l tunnel at 5 min intervals) with the same-aged 2011 or 2012 spawns (swum in the 5 l tunnel at 10 min intervals) using a one-way ANOVA did not differ in either species (F1,22 = 1.053, P = 0.316 for green sturgeon; and F1,34 = 0.541, P = 0.467 for white sturgeon).
Manufacturer calibration of the 1.5 l swimming flume was verified using the dye technique recommended by Loligo®. For each dial setting, red food colouring was injected via the effluent flush line using a 60 ml syringe, and the time for the dye front to travel 20.5 cm to the downstream flow straightener was measured using a stopwatch accurate to the hundredth of a second. The 5 l swimming flume was calibrated using a portable Marsh-McBirney water flowmeter (model 201D; Hach, Loveland, CO, USA), set to a 2 s time constant. For each dial setting on the flume motor, three separate measurements of flow were recorded, and the mean for each dial setting was calculated. Critical swimming velocity was calculated according to the formula:
where Vf is the highest velocity at which the fish swam for the entire 10 or 5 min interval; Vi is the velocity increment (5 cm s−1); Tf is the duration of time the fish swam at the highest velocity attempted; and Ti is the time increment (10 or 5 min; Brett, 1964). Absolute Ucrit was expressed in centimetres per second, and relative Ucrit was calculated by dividing absolute Ucrit of individual fish by total length (TL; in centimetres) of that fish, and expressed as body lengths per second (BL s−1). The small fish size relative to tunnel cross-sectional area (≤5%) precluded the requirement for solid blocking effect adjustments (Bell and Terhune, 1970). Values of Ucrit for the 72 green and 87 white sturgeon reported here were used both in the regression of larval Ucrit values (in centimetres per second) with days post-hatch and in determination of the allometric exponents described in the ‘Data analysis and statistics’ section below.
Growth
Following completion of swimming tests, all fish were euthanized by buffered anaesthetic overdose (MS-222, 1 g l−1) and the wet mass (in grams), girth (circumference at the opercula, in centimetres) and TL measured.
Data analyses and statistics
Wet mass, TL and Ucrit of larval green and white sturgeon, 20–60 dph, were evaluated for species differences by comparing the linear regression of TL, mass and absolute Ucrit (in centimetres per second) with days post-hatch and Ucrit (in centimetres per second) with TL.
In order to explore the allometric relationship between TL and relative Ucrit (in body lengths per second) and absolute Ucrit (in centimetres per second) in green and white sturgeon, we independently combined the larval green and white sturgeon Ucrit values measured in this study with other Ucrit data (N. A. Fangue, unpublished data) and published data mined from the literature for white and green sturgeon of larger sizes (Table 1). Important Ucrit study parameters, such as temperature, velocity and time increments and fish age, are also summarized. The relationship between relative Ucrit (in body lengths per second) and TL was fitted to a power function, and the allometric exponents for green and white sturgeon were independently determined as the slope of the linear regression of the log of TL vs. the log of Ucrit (in body lengths per second). Although not included in calculations of allometric exponents, published Ucrit data for seven other sturgeon species from nine studies were plotted for comparison with green and white sturgeon data (Table 1).
Table 1:
Summary of values used for green and white sturgeon allometric exponent determinantions and literature values of Ucrit for other sturgeon species
| Species | TL [cm (mean ± SEM)] |
Ucrit |
n | Temp. (°C) | Speed increment (cm s−1) | Time interval (min) | Age (dph) | |
|---|---|---|---|---|---|---|---|---|
| BL s−1 | cm s −1 | |||||||
| Siberian sturgeon | 58.4 (0.6)1 | 1.8 (<0.1) | 105.5 (0.0) | 4 | 24 | 10 | 10 | 600 |
| 64.3 (0.9)1 | 1.7 (0.1) | 106.3 (0.1) | 7 | 24 | 10 | 10 | 600 | |
| Shortnose sturgeon | 7.1 (<0.1)2 | 3.2 (0.2) | 22.3 (0.6) | 71 | 15 | 3 | 20 | y-o-y |
| 19.4 (0.1)2,3 | 1.5 (0.1) | 29.5 (1.3) | 6 | 10–25 | 5 | 30 | 255 | |
| Lake sturgeon | 554 | 1.2 | 65 | 14 | 10 | |||
| Amur sturgeon | 18.8 (0.3)5 | 2.0 (0.1) | 36.8 (1.9) | 18 | 20 | 0.25 BL s−1 | 30 | |
| Chinese sturgeon | 13.7 (2.0)6 | 2.6 (<0.1) | 36.0 (5.0) | 2 | 16–25 | 10 | 20 | 75–195 |
| 24.5 (2.4)6 | 2.3 (0.1) | 55.5 (2.5) | 2 | 10–25 | 10 | 20 | 75–360 | |
| 35.36 | 2.0 | 70.0 | 1 | 10–16 | 10 | 20 | 255–360 | |
| 40.56 | 2.1 | 85.0 | 1 | 10–16 | 10 | 20 | 255–360 | |
| 54.8 (1.3)1 | 1.4 (<0.1) | 77.8 (1.5) | 7 | 24 | 10 | 10 | 600 | |
| 62.2 (0.7)1 | 1.3 (<0.1) | 81.8 (3.8) | 5 | 24 | 10 | 10 | 600 | |
| Pallid sturgeon | 21.4 (0.3)7 | 1.7 (0.1) | 35.9 (1.2) | 8 | 20 | 5 | 30 | 180 |
| Shovelnose sturgeon | 23.1 (0.3)7 | 1.6 (1.2) | 37.0 (1.4) | 2 | 20 | 5 | 30 | 180 |
| 57.0 (0)8 | 1.79 (0.2) | 102.0 (14.0) | 2 | 16 | 10 | 15 | ||
| 67.2 (1.4)8 | 1.4 (0.2) | 90.9 (14.8) | 3 | 16 | 10 | 15 | ||
| Green sturgeon | 4.3 (0.2)9a | 8.5 (0.4) | 35.7 (1.7) | 32 | 18–19 | 5 | 5 | 20–42 |
| 6.5 (0.2)9b | 7.1 (0.2) | 45.3 (1.5) | 40 | 18–19 | 5 | 10 | 34–60 | |
| 15.4 (0.6)10a | 2.9 (0.1) | 43.2 (1.3) | 25 | 18–19 | 10 | 20 | 73–177 | |
| 22.1 (0.4)10a | 2.2 (0.1) | 48.1 (1.3) | 27 | 18–19 | 10 | 20 | 73–177 | |
| 22.2 (0.6)11 | 2.4 (0.1) | 52.9 (1.2) | 20 | 18–19 | 5 | 5 | ||
| 34.7 (0.6)10b | 1.4 (0.1) | 4.8 (1.5) | 22 | 18–19 | 10 | 20 | 73–177 | |
| 44.1 (0.7)10b | 1.0 (0.1) | 44.9 (4.0) | 9 | 18–19 | 10 | 20 | 73–177 | |
| 49.4 (0.6)12 | 1.2 (0.5) | 57.5 (2.5) | 53 | 18–19 | 10 | 30 | 320–360 | |
| 68.3 (2.7)13 | 1.2 (0.1) | 79.2 (4.9) | 11 | 19 | 10 | 20 | 340–360 | |
| White sturgeon | 4.7 (0.2)9a | 5.5 (0.2) | 25.2 (1.1) | 43 | 18–19 | 5 | 5 | 20–42 |
| 8.0 (0.4)9c | 4.6 (0.2) | 35.3 (1.4) | 44 | 18–19 | 5 | 10 | 34–60 | |
| 24.8 (0.8)11 | 2.6 (0.1) | 64.2 (1.6) | 23 | 18–19 | 5 | 5 | ||
| 34.214 | 1.6 | 56.4 | 1 | 11–12.5 | 5 | 15 | ||
| 38.3 (0.3)11 | 1.8 (0.1) | 69.2 (2.2) | 3 | 18–19 | 5 | 5 | ||
Abbreviation: BL, body length; dph, days post-hatch; TL, total length; Ucrit, critical swimming velocity; y-o-y, young of the year.
4Peake et al. (1995), cited by Adams et al. (1997). 5Cai et al. (2013). 6He et al. (2013).
7Adams et al. (2003). 8Adams et al. (1997). 9aPresent study, 2013 spawned.
9bPresent study, 2011 spawned. 9cPresent study, 2012 spawned.
10aAllen et al. (2006a) saltwater-intolerant size range.
10bAllen et al. (2006a) saltwater-tolerant range.
11N. A. Fangue, unpublished data. 12Miller et al. (2014).
13Mayfield and Cech Jr (2004). 14Counihan and Frost (2011).
Linear regressions were performed using lm in R (http://www.r-project.org; see Table 2 for regression table), and statistical significance was considered at α = 0.05 for all analyses.
Table 2:
Regression table for relationships between total length (TL; in centimetres), mass (in grams) and critical swimming velocity [Ucrit (in body lengths per second)] and days post-hatch (dph) of green and white sturgeon
| Variable | β (±SEM) | t | P value | |
|---|---|---|---|---|
| TL | dph | 0.189 (0.010) | 12.99 | <0.001 |
| species | −2.503 (0.530) | −4.72 | <0.001 | |
| species × dph | 0.091 (0.013) | 6.85 | <0.001 | |
| F3,159 = 311; adjusted r2 = 0.852; P < 0.001 | ||||
| Mass | dph | 0.130 (0.009) | 5.62 | <0.001 |
| species | −3.576 (0.565) | −6.33 | <0.001 | |
| species × dph | 0.113 (0.0142) | 8.00 | <0.001 | |
| F3,159 = 146.2; adjusted r2 = 0.729; P < 0.001 | ||||
| Ucrit | dph | 0.673 (0.068) | 8.40 | <0.001 |
| species | −7.977 (4.426) | −1.80 | 0.073 | |
| species × dph | −0.086 (0.111) | −0.77 | 0.441 | |
| F3,159 = 82; adjusted r2 = 0.598; P < 0.001 | ||||
| Ucrit | TL | 2.686 (0.339) | 9.45 | <0.001 |
| species | −4.125 (3.456) | −1.19 | 0.234 | |
| species × dph | −1.836 (0.573) | −3.20 | 0.002 | |
| F3,159 = 103.9; adjusted r2 = 0.598; P < 0.001 | ||||
A multiplication sign signifies an interaction.
Results
Growth
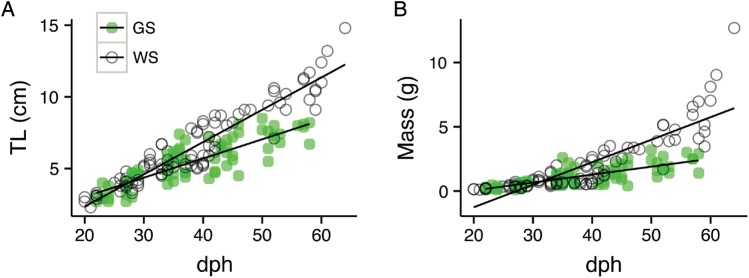
Although the green and white sturgeon were a similar size early in the larval stage, the growth rate was greater for larval white than green sturgeon, resulting in larger white sturgeon at 25 and 32 dph for TL and mass comparisons, respectively (Fig. 1A and B). The equations for the total length vs. dph regressions were y = 0.35 + 0.134x and y = −2.16 + 0.225x for larval green and white sturgeon, respectively. The equations for mass vs. days post-hatch regressions were y = −1.19 + 0.062x and y = −4.77 + 0.175x for larval green and white sturgeon, respectively (Fig. 1A and B). Overall, white sturgeon rates of increase in mass and TL with age were similar, whereas for green sturgeon the TL increased at double the rate of mass (Fig. 1A and B).
Figure 1.
Ontogeny of length (TL, in centimetres; A) and mass (in grams; B) in larval green (GS, green circles, n = 72) and white sturgeon (WS, open circles, n = 87) from 20 to 60 days post-hatch (dph). The equations for the total length vs. days post-hatch regressions were y = 0.35 + 0.134x with an r2 of 0.715 and y = −2.16 + 0.225x with an r2 of 0.884 for larval green and white sturgeon, respectively. The equations for mass vs. days post-hatch regressions were y = −1.19 + 0.062x with an r2 of 0.586 and y = −4.77 + 0.175x with an r2 of 0.732 for larval green and white sturgeon, respectively.
Swimming performance
Larval green and white sturgeon appeared to possess similar motivation to swim in the swim tunnel. Success rates for achieving continuous swimming in the swim tunnel for at least one interval were 87 and 86%, respectively, for green sturgeon (2011 spawned) and white sturgeon (2012 spawned), and 79 and 78%, respectively, for green and white sturgeon spawned in 2013.
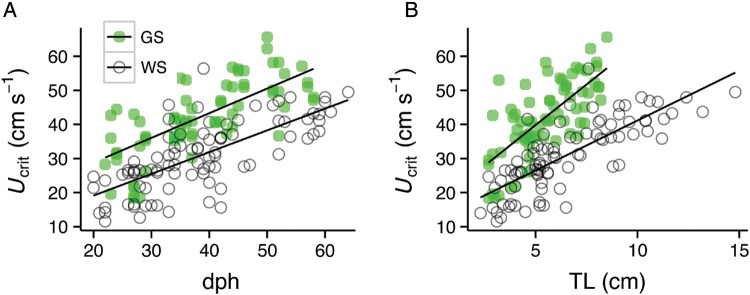
Despite the slower growth of larval green sturgeon, ontogeny of absolute Ucrit (in centimetres per second) did not differ between larval green and white sturgeon (Fig. 2A). The slope of increase in Ucrit (in centimetres per second) with days post-hatch did not differ significantly between green and white sturgeon (P = 0.073; Table 2), but, due to a significantly larger intercept (P < 0.001), green sturgeon Ucrit (in centimetres per second) was consistently greater than that of white sturgeon at the same age (Fig. 2A). The slope of increase in Ucrit (in centimetres per second) with TL also did not differ significantly between green and white sturgeon (P = 0.234; Table 2), but there was a significant interaction between species and TL (P = 0.002; Table 2). Similar to the relationship between Ucrit (in centimetres per second) and days post-hatch, the intercept of the relationship between Ucrit (in centimetres per second) and TL for green sturgeon was also significantly greater than that for white sturgeon (P < 0.001; Table 2), resulting in green sturgeon Ucrit (in centimetres per second) being consistently greater than that of white sturgeon at the same TL (Fig. 2B).
Figure 2.
Ontogeny of larval green and white sturgeon critical swimming velocity (Ucrit, in centimetres per second) vs. days post-hatch (A) and total length (B) through the larval life stage. The equations of the regressions for Ucrit (in centimetres per second) vs. days post-hatch were y = 14.34 + 0.724x with an r2 of 0.469 and y = 6.36 + 0.638x with an r2 of 0.497 for larval green (n = 72) and white sturgeon (n = 87), respectively. The equations of the regressions for Ucrit (in centimetres per second) vs. days post-hatch were y = 15.97 + 4.766x with an r2 of 0.508 and y = 11.84 + 2.930x with an r2 of 0.603 for larval green (n = 72) and white sturgeon (n = 87), respectively.
Green and white sturgeon Ucrit allometry
As predicted, relative Ucrit (in body lengths per second) vs. TL in juvenile green and white sturgeon decreased with increasing TL, and the relationship took the form of a power function (Fig. 3A). Also as expected, absolute Ucrit (in centimetres per second) increased with TL for both species (Fig. 3B). The allometric exponent, which is the slope of decrease in relative Ucrit (in body lengths per second) with growth, was greater for green (−0.83) than white sturgeon (−0.42). In sturgeons <5 cm long, green sturgeon relative Ucrit (in body lengths per second) was twice that of white sturgeon. However, due to the more rapid decrease in green sturgeon relative Ucrit (in body lengths per second) with increasing length, white sturgeon relative Ucrit (in body lengths per second) began to exceed that of green sturgeon in fish that were between 12 and 20 cm TL. When relative Ucrit values (in body lengths per second) of other sturgeon species were plotted with the green and white sturgeon allometric curves, both larval green and larval white sturgeon <20 cm in length appeared to have greater Ucrit (in body lengths per second) than those of other sturgeon species of the same size (Fig. 3A). This difference disappeared in sturgeons >20 cm in length.
Figure 3.
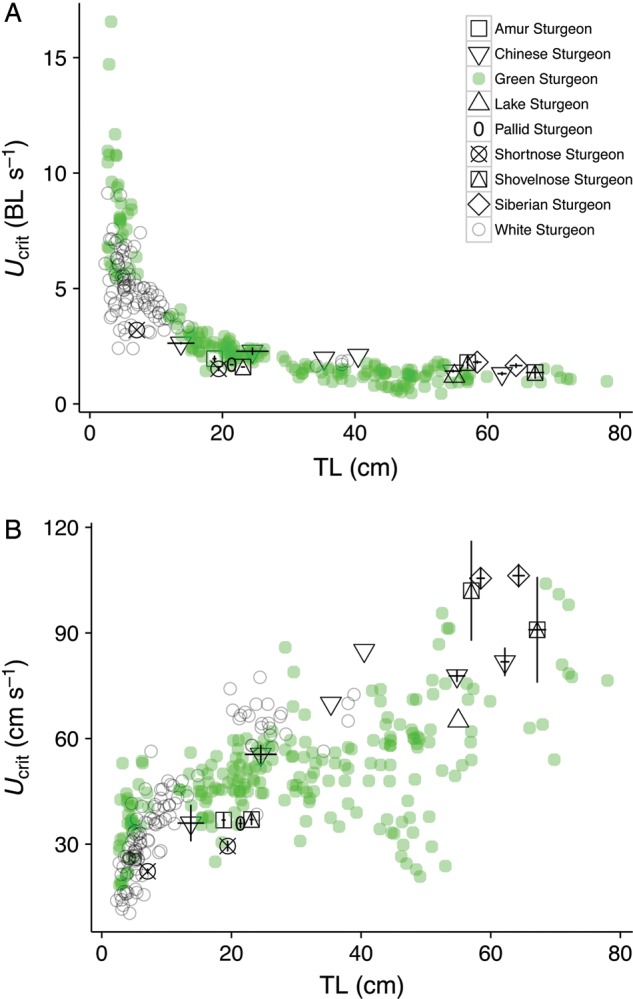
Allometry of green and white sturgeon and other Acipenser and Scaphirhynchus species' (see Table 1 for citations) relative critical swimming velocity [Ucrit, in body lengths (BL) per second; A] and absolute Ucrit (in centimetres per second; B) vs. total length using data from this experiment and published and unpublished sturgeon Ucrit data. The Ucrit points for other sturgeon species represent means (±SEM), and citations and values are listed in Table 1. The temperature range across all studies was 10–25°C. Green and white sturgeon relative Ucrit (in body lengths per second) changed with TL according to the function y = 3.34x−0.82 (r2 = 0.89; F1,234 = 1997; P < 0.001) and y = 2.31x −0.42 (r2 = 0.47; F1,93 = 84.5; P < 0.001), respectively.
Discussion
This first assessement of the swimming capacities of larval green and white sturgeon revealed biological differences between the two species, which alone are not expected to dictate remarkably different conservation strategies around water-diversion structures. Furthermore, we report the first attempt at determining the allometric relationship between swimming capacity and size in sturgeons, in which we found potential inter-species differences.
Although there are no published swimming capacity values for larval green and white sturgeon with which to compare Ucrit results, the sizes of green sturgeon reported here generally fell within the mass and length ranges reported in previous studies performed at similar temperatures. Green sturgeon at the end of the larval stage (60 dph) were of similar masses (range, 1.5–2.7 g) and lengths (range, 6–7.5 cm) to previously reported late-larval green sturgeon masses (range, 1.5–3 g) and lengths (range, 6.2–9.4 cm; Deng et al., 2002; Allen et al., 2006b). White sturgeon, in contrast, tended to be slightly larger than previously reported. Although the early larval (20 dph) white sturgeon weight range of 0.2–0.4 g overlapped with white sturgeon of a similar age (20–30 dph) of 0.09–0.23 g (Deng et al., 2003, 2009), late larval white sturgeon were slightly larger than previously reported. White sturgeon metamorphosing into juveniles (45 dph) in a previous study averaged 4.5 cm in length and had a mean weight of 1 g (Deng et al., 2002), compared with the length and weight ranges of 7–9 cm and 2.5–3.2 g, respectively, for white sturgeon of a similar age in the present study. The larger size of late larval stage white sturgeon in this study is consistent with previously reported inter-family and inter-individual variability for green and white sturgeon (Van Eenennaam et al., 2005; Linares-Casenave et al., 2013) which may explain the ontological differences between the two species observed here. Replication of larval ontogeny studies is needed to strengthen support for these differences.
All larval growth studies discussed here, including this one, were performed at water temperatures ranging from 18 to 19°C. The effects of temperature on the growth of larval green and white sturgeon between 20 and 60 dph have never been assessed, but the growth of older, larger green sturgeon (>144 dph) increases with increasing temperature up to 15°C (Mayfield and Cech, 2004). We expect that similar effects of temperature on growth would occur in larval sturgeons. Therefore, given that size influences Ucrit (Figs 2 and 3), temperature would most probably affect Ucrit of larval sturgeons. This effect would probably also be influenced by the direct effects of temperature on swimming performance, which result in increased swimming speeds at higher temperatures, up to a high-temperature limit (Mayfield and Cech, 2004; Allen et al., 2006a; Deslauriers and Kieffer, 2012b).
Absolute swimming capacity (i.e. expressed in centimetres per second) generally increases with increasing body size in fishes (Bainbridge, 1960; Brett, 1965; He, 1986), and absolute Ucrit measured in both larval green and larval white sturgeon increased with size, as expected (Figs 2B and 3B). Also as expected, absolute Ucrit values of larval green and larval white sturgeon increased at similar rates with growth through the larval life stage. However, Ucrit values of larval green sturgeon aged 20–60 dph were consistently ∼10 cm s−1 greater than those for white sturgeon of the same age (Fig. 2A).
Unlike absolute swimming capacity, relative swimming capacity (i.e. expressed as body lengths per second) tends to decrease with size according to a power relationship in fishes (Bainbridge, 1960; Brett, 1965; He, 1986). The exponent of the power function relating maximal sprint swimming speed with body length has been determined as −1.09, −0.58 and −0.71 for sprinting common dace (Leuciscus leuciscus), rainbow trout (Oncorynchus mykiss) and goldfish (Carassius auratus), respectively (Bainbridge, 1960). For prolonged swimming capacity, the allometric exponent appears to range from −0.43 to −0.53, based on measurements on sockeye salmon (Oncorynchus nerka; calculated from Brett, 1965) and saithe (Pollachius virens; He, 1986). To our knowledge, the relationship between swimming capacity and body size has not been examined for any sturgeon species. Considering the unique body and tail morphology of these basal actinopterygians, which theoretically results in inefficient swimming kinetics (Webb, 1986; Qu et al., 2013), we hypothesized that sturgeon species would possess a unique allometric exponent.
The body and tail shape of sturgeons differs from that of the typical, faster swimming actinopterygians and more closely resembles that of chondrichthyans. Differing from the more derived actinopterygian homocercal caudal fin, the sturgeon caudal fin is heterocercal, which, for lake sturgeon, has been shown to generate 66% less thrust than for the homocercal caudal fin of trout (Webb, 1986). Further reductions in acipenserid swimming efficiency arise from increased drag due to the sturgeon spindle-shaped body form and rough body surface (Webb, 1986), in comparison to the smooth surfaces and streamlined fusiform body shape of more derived fishes. This inefficiency in body design results in reduced burst, prolonged and sustained swimming capacities compared with those of salmonids (Peake et al., 1997; Deslauliers and Kieffer, 2012a). In combination with the behavioural tendency of sturgeons to station hold at high water velocities (Webb, 1986; Parsons et al., 2003; Hoover et al., 2005; Deslauliers and Kieffer, 2012a), these aspects of sturgeon morphology have the potential to alter the allometric exponent for the length–swimming capacity relationship for sturgeons compared with other actinopterygians.
Indeed, the allometric exponent for green, but not white sturgeon, as determined by relating Ucrit and length of larval sturgeons from this study and juvenile sturgeons from previous studies, differed from that of other actinopterygians. The allometric exponent for all known Ucrit (in body lengths per second) data for larval to 80-cm-long green and white sturgeon shows the allometric relationship between Ucrit (in body lengths per second) and length of white sturgeon to have an exponent of −0.42, which is more like that of sockeye salmon (calculated from Brett, 1965) and saithe (He, 1986), compared with the exponent of −0.83 for green sturgeon, which more closely resembles the exponent for sprint swimming in common dace and goldfish (Bainbridge, 1960). Although these dissimilarities could be an artifact of the lack of Ucrit values for 40- to 80-cm-long white sturgeon, they may also reflect differences in the ontogeny of green and white sturgeon prolonged swimming capacities.
Sometime between the completion of metamorphosis into juveniles (∼60 dph and at a TL of 7–8 and 9–11 cm for green and white sturgeon, respectively) and the onset of green sturgeon tolerance of full-strength sea water (∼130 dph and at a TL of ∼25 cm; Allen and Cech, 2007; Allen et al., 2009, 2011), white sturgeon Ucrit (in centimetres per second) began to exceed that of green sturgeon. This transition appears to be due to a shallower slope of increase in Ucrit with length of green compared with white sturgeon during the early juvenile stage. The timing of this transition is consistent with previously reported evidence of a seasonal reduction in swimming capacity of green sturgeon undergoing physiological changes associated with preparation for downstream migration to estuarine or marine waters (Allen et al., 2006a). More swimming data on white sturgeon of the 40–80 cm size range are required to determine whether this difference between green and white sturgeon Ucrit is maintained throughout the life of these two species.
Although the paucity of published Ucrit values for other sturgeon species across a significant size range prevents comparison of green and white sturgeon allometric exponents with those of other Acipenser species, early juvenile green and white sturgeon appear to have superior swimming capacities compared with other Acipenser species at this life stage. The Ucrit (in centimetres per second) of Chinese sturgeon (Acipenser sinensis), pallid sturgeon (Scaphirhynchus albus), shovelnose sturgeon (Scaphirhynchus platorynchus), Amur sturgeon (Acipenser schrenckii) and shortnose sturgeon (Acipenser brevirostrum) with lengths of ∼20 cm or less lie below the green and white sturgeon allometric curves (Table 1 and Fig. 3A and B). Sturgeons >30 cm long appear to lie on the green sturgeon allometric curve. Thus, despite their potentially higher prolonged swimming capacity during the larval and early juvenile stage, green and white sturgeon have similar swimming capacities to other Acipenser species during later juvenile and adult life stages. It should be noted, however, that comparisons of Ucrit values among studies have inherent challenges, because test temperatures (Mayfield and Cech, 2004; Allen et al., 2006a) and end-point criteria for exhaustion, as well as the chosen time intervals and speed increments of the tests (Beamish, 1978; Hammer, 1995), are not standardized and can have substantial effects on Ucrit measurements. It is possible that methodological differences may affect exhaustion end-points, thereby influencing Ucrit values across Acipenser studies.
The stronger swimming capacities of larval and early juvenile green and white sturgeon may reflect demands for relatively vigorous swimming activity during early life stages of these species. Laboratory-based studies suggest that early juvenile white sturgeon from the Sacramento River undergo an active dispersal, with strong swimming behaviour (Kynard and Parker, 2005). Late larval green sturgeon have also been shown to swim actively up- and downstream while foraging in laboratory-based studies, and early juveniles actively migrate downstream to over-wintering grounds (Kynard et al., 2005). Chinese sturgeon, in contrast, discontinue laboratory-observed migratory behaviour early in their life history, before the larval life stage begins (Zhuang et al., 2002), and pallid and shovelnose sturgeon both exhibit passive drifting migratory behaviour in the laboratory (Kynard et al., 2002), which suggests that their foraging phase may not require a high swimming capacity. Shortnose sturgeon are amphidromous (Bemis and Kynard, 1997); this primarily freshwater lifestyle may not require the swimming capacity of the anadromous green and semi-anadromous white sturgeon. Contrary to this interpretation of anadromous sturgeons having greater swimming capacity than non-anadromous sturgeons during the early juvenile phase, Amur sturgeon, which are anadromous (Bemis and Kynard, 1997), possess a lower swimming capacity than green sturgeon at the early juvenile life stage. In the laboratory, this species exhibits migratory behaviour lasting much of their larval stage (Zhuang et al., 2003). Perhaps Amur sturgeon also experience a drop in swimming ability related to physiological preparation for entry into saline waters that is seen in green sturgeon with the onset of migration to brackish and salt water (Allen et al., 2006a). Thus, it seems that the strong swimming ability of larval and early juvenile green and white sturgeon could be reflective of their life-history strategies.
Based on the results of this study and previously published laboratory behavioural and field observational studies, we have developed seasonal recommendations of water flow velocities likely to overwhelm larval green and white sturgeon at water-diversion facilities. We caution that these recommendations are based solely on capacity to maintain position in water flows, and encourage them to be considered in conjunction with species- and life-stage-specific behavioural responses to water flows and water-diversion structures. Unfortunately, sturgeon water-diversion-structure behavioural interaction studies are limited; however, juvenile green sturgeon appear to lack avoidance behaviour when encountering water-diversion structures (Mussen et al., 2014).
Green sturgeon larval and juvenile migration and behaviour
The threatened Southern Distinct Population Segment of green sturgeon spawn primarily in the upper reaches of the Sacramento River from April to May (Adams et al., 2007; Heublein et al., 2008). Based on laboratory studies, it appears that hatched green sturgeon, unlike other acipenserids, which are typically transiently pelagic immediately after hatch, exclusively hide in rocks at the river bottom until initiation of exogenous feeding at 10 dph. At this stage, they begin a 10 day nocturnally active dispersal downstream to foraging sites in the middle reaches of the river (Kynard et al., 2005). Once dispersed to foraging sites, larval green sturgeon appear to forage nocturnally on the river bottom and hide in the rocks during the day until ∼100 dph or the autumn, when they begin another active downstream migration (Kynard et al., 2005). At this age, green sturgeon have demonstrated tolerance of and preference for near full-strength seawater (Allen and Cech, 2007; Allen et al., 2009, 2011; Poletto et al., 2013) and are therefore likely to migrate to the S-SJ Delta or San Pablo and San Francisco bays. The physiological preparation process for this migration from fresh to saltwater has been associated with reductions in Ucrit of juvenile (0.5- to 1.5-year-old) green sturgeon (Allen et al., 2006a).
Green sturgeon conservation recommendations
During their initial dispersal migration in spring, the tiny 10 dph larval green sturgeon are probably vulnerable to being overwhelmed by water-diversion intake flows. At 20–25 dph (∼2 cm TL), green sturgeon Ucrit ranged from 20 to 53 cm s−1, and the linear regression predicted a mean Ucrit of 29 cm s−1. Swimming capacity consistently increased with days post-hatch (Fig. 2), which suggests that younger, migrating green sturgeon are unlikely to have swimming capacities this high. Therefore, nighttime flows at water-diversion structures likely to be encountered by green sturgeon in the upper and middle reaches of the Sacramento River from May through the summer should be limited to 29 cm s−1, assuming that they detect the diversion flows and can avoid them behaviourally. Mussen et al. (2014) showed that larger juvenile green sturgeon are easily entrained in a simulated water-diversion intake structure.
By 55 dph, when green sturgeon were ∼7 cm long and expected to remain nocturnally foraging in the middle reaches of the river, mean Ucrit predicted from the linear regression increased 1.9-fold to 54 cm s−1 (Fig. 2) and ranged from 46 to 58 cm s−1. This suggests that diversion structures in the middle reaches of the Sacramento River should be limited to maximal velocities of 54 cm s−1 during the night from July until the following May spawn, assuming that they detect the diversion flows and can avoid them behaviourally.
The Ucrit of juvenile green sturgeon changes slowly with growth, such that with a 6-fold increase in total length from 8 to 50 cm, mean Ucrit increased only 1.2-fold, from 50 to 60 cm s−1 (Fig. 3B). In addition to this modest increase, Ucrit values of 50-cm-long sturgeon are highly variable, ranging from 20 to 75 cm s−1, with nearly a quarter of the sturgeon at this size swimming < 40 cm s−1. As this size corresponds to the range exhibiting reductions in swimming capacity thought to be related to downstream migration to saltwater (Allen et al., 2006a), a corresponding reduction in maximal diversion velocities to 40 cm s−1 may be important to protect migrating juvenile green sturgeon through the middle and lower reaches of the Sacramento River and the Delta and Bays in October and November (Mussen et al., 2014).
Swimming capacity of green sturgeon larger than 70 cm has not been assessed. Therefore, lower Sacramento River and Delta diversion structures from which juvenile green sturgeon are expected to encounter flows should be limited to maximal flows of 54 cm s−1, assuming that they detect the diversion flows and can avoid them behaviourally.
White sturgeon larval and juvenile migration and behaviour
Most Sacramento River white sturgeon spawning appears to be limited to between Colusa (river km 231) and Verona, California (river km 160; Kolhorst, 1976). Laboratory studies suggest that immediately upon hatching, white sturgeon hatchlings disperse passively downstream for 10 days (Kynard and Parker, 2005). From 10 to 28 dph, white sturgeon forage with gradually increasing activity and progress from hiding within rocky substrate to complete use of open bottom, additionally spending some time at the surface or within the water column (Kynard and Parker, 2005). Active foraging appears to continue until 50 dph, when white sturgeon behaviour in the laboratory suggests a second downstream migration (Kynard and Parker, 2005). Larvae have been found as far downstream as Suisun Bay, but such far-downstream larval sightings occur in high-flow years when larvae have probably been overwhelmed by flows and flushed downstream (Stevens and Miller, 1970).
White sturgeon conservation recommendations
Hatchling white sturgeon are likely to be highly susceptible to entrainment into water-diversion structures during the passive dispersal phase, because these fish have virtually no ability to resist diversion flows during this life stage. Middle to lower Sacramento River diversion structures in the vicinity of white sturgeon spawning sites should be highly regulated when adults are spawning (February–May).
Due to their high foraging activity, 30 dph larval white sturgeon, which are located throughout the lower reaches of the Sacramento River from March to June, are likely to be vulnerable to entrainment into water-diversion structures. At 35 dph (∼5.5 cm TL), white sturgeon Ucrit values ranged from 22 to 45 cm s−1, and the linear regression equation predicted a Ucrit of 29 cm s−1 (Fig. 2), suggesting that the majority of the population, if able to detect and avoid diversion flows, would not be vulnerable to water-diversion flow rates lower than 29 cm s−1. Later in the summer, as larval white sturgeon grow and metamorphose into juveniles (60 dph, ∼10 cm TL), their swimming capacity, and therefore their ability to escape diversion structure flows, increased slightly to 41–48 cm s−1, with the linear regression predicting a Ucrit of 45 cm s−1 (Fig. 2). At this developmental stage, some sturgeon have also been found in the Bay (Stevens and Miller, 1970) and, therefore, potentially the Delta as well. Therefore, lower Sacramento River and Delta diversion structures potentially encountered by foraging larval white sturgeon from March to June should be limited to maximal flows of 29 cm s−1. These limits can probably be increased further in the autumn to a maximum of 50 cm s−1 as fish grow and increase swimming capacity (Figs 2 and 3) until the next spawn in the following February. Although Ucrit values of 115 cm s−1 were achieved by 95-cm-long (7 kg) white sturgeon (personal communication from Nguyen, Jackson, and Peterson), fish of this size are likely to be >1 year old. Through the winter months, neither size nor swimming capacity is expected to increase, so it is unlikely that white sturgeon spending their first winter in the Sacramento River are able to hold position in water flows >50 cm s−1.
We developed season-specific recommendations for flow limitations around water-diversion structures of the Sacramento River and its Delta by integrating laboratory-based findings on the ontogeny of green and white sturgeon with swimming capacity and behavioural information (Table 3).
Table 3:
Overview of flow-tolerance limitations of green and white sturgeon throughout the Sacramento–San Joaquin watershed according to location and time of year, based on critical swimming velocity data
| Upper river | Middle river | Lower river/delta/bays | |
|---|---|---|---|
| January | <50 cm s−1 | ||
| February | WS early larvae | ||
| March | |||
| April | GS early larvae | ||
| May | |||
| June | GS and WS 29 cm s−1 | ||
| July | WS 45 cm s−1 | ||
| August | GS 50 cm s−1 | ||
| September | |||
| October | <50 cm s−1 | GS 40 cm s−1 | |
| November | |||
| December | |||
Abbreviations: GS, green sturgeon; WS, white sturgeon. Green sections demarcate tolerable water velocities of ≥50 cm s−1; red sections demarcate presence of life stages which are predicted to be intolerant of even very low water velocities; and yellow sections signify recommended water flow velocity limitations to protect present life stages. Behavioural (e.g. avoidance) considerations are not part of this analysis, and they remain an important topic for future research.
Throughout the entire Sacramento River system from December to February, sturgeons present are unlikely to be overwhelmed by flows below 50 cm s−1. This period can be extended to April for the upper reaches, where green sturgeon tend to spawn. From February to June, populations of highly susceptible larval sturgeons with limited to no swimming ability are present within the river system. This is of particular concern in the middle to lower reaches from February until April, when young white sturgeon are expected to be drifting passively downstream from spawning sites. In the upper reaches, susceptible larval green sturgeon with limited swimming capabilities are expected to be present in April and May. By the end of May, green sturgeon larvae in the upper reaches are expected to have developed sufficient swimming ability to resist flows up to 29 cm s−1, but white sturgeon in the middle to lower reaches are not expected to develop similar swimming capacities until June. By July, although green sturgeon present throughout the upper to lower reaches are likely to be able to resist water flows >50 cm s−1, white sturgeon in the lower reaches remain limited to flows of 45 cm s−1 until September. In October, as juvenile green sturgeon develop saltwater tolerance and begin their downstream migration to the estuaries and bays, their swimming capacity drops, requiring a 2 month (October and November) 40 cm s−1 limit of flows around water-diversion structures in the middle and lower Sacramento River and the Delta and bays.
Similar conservation recommendations can be applied to other acipenserid species, with adjustments for dispersal behaviour, river site usage and behavioural responses to flows and diversion structures. The slightly greater absolute Ucrit (in centimetres per second) of early juvenile green and white sturgeon compared with other sturgeon species suggests that acipenserids in general require lower velocities than green and white sturgeon around diversion structures, if they are to resist entrainment volitionally. However, it is also important to consider the behavioural responses of the different sturgeons and their life stages to water-diversion structures. The complete lack of published larval Ucrit data for any acipenserid species before this study underscores the urgency for studies on this vulnerable life stage of this globally imperiled family.
Acknowledgements
Thank you to B. Williamson, F. La Luz, C. Turcotte, K. Ho, T. Agosta, E. Zarri and I. Chau for assistance in fish swimming measurements and daily animal care. J. Van Eenennaam provided technical assistance with fish spawning, and E. Hallen and P. Lutes from the Center for Aquatic Biology and Aquaculture assisted with fish rearing. Wild green sturgeon broodstock were provided by the Yurok tribe, and white sturgeon were donated by Sterling Caviar. S. Lee and L. Haller generously contributed their time to help rear these fish. J.B.P. was supported through a National Science Foundation Graduate Student Fellowship. The research was funded by the California Department of Fish and Wildlife's Ecosystem Restoration Program [E0783004, E1183017 to N.A.F.], the United States Bureau of Reclamation [R10AC20012 to N.A.F. and J.J.C.Jr] and the UC Davis Agricultural Experiment Station [2098-H to N.A.F.].
References
- Adams PB, Grimes C, Hightower JE, Lindley ST, Moser ML, Parsley MJ. (2007) Population status of North American green sturgeon, Acipenser medirostris. Environ Biol Fish 79: 339–356. [Google Scholar]
- Adams SR, Parsons GR, Hoover JJ, Killgore KJ. (1997) Observations of swimming ability in shovelnose sturgeon (Scaphirhynchus platorynchus). J Freshw Ecol 12: 631–633. [Google Scholar]
- Adams SR, Adams GL, Parsons GR. (2003) Critical swimming speed and behavior of juvenile shovelnose sturgeon and pallid sturgeon. Trans Am Fish Soc 132: 37–41. [Google Scholar]
- Allen PJ, Cech J., Jr (2007) Age/size effects on juvenile green sturgeon, Acipenser medirostris, oxygen consumption, growth, and osmoregulation in saline environments. Environ Biol Fish 79: 211–229. [Google Scholar]
- Allen PJ, Hodge B, Werner I, Cech J., Jr (2006a) Effects of ontogeny, season, and temperature on the swimming performance of juvenile green sturgeon (Acipenser medirostris). Can J Fish Aquat Sci 63: 1360–1369. [Google Scholar]
- Allen PJ, Nicholl M, Cole S, Vlazny A, Cech J., Jr (2006b) Growth of larval to juvenile green sturgeon in elevated temperature regimes. Trans Am Fish Soc 135: 89–96. [Google Scholar]
- Allen PJ, Cech J, Jr, Kültz D. (2009) Mechanisms of seawater acclimation in a primitive, anadromous fish, the green sturgeon. J Comp Physiol B 179: 903–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen PJ, McEnroe M, Forostyan T, Cole S, Nicholl MM, Hodge B, Cech J., Jr (2011) Ontogeny of salinity tolerance and evidence for seawater-entry preparation in juvenile green sturgeon, Acipenser medirostris. J Comp Physiol B 181: 1045–1062. [DOI] [PubMed] [Google Scholar]
- Arnekleiv JV, Kraabøl M, Museth J. (2007) Efforts to aid downstream migrating brown trout (Salmo trutta L.) kelts and smolts passing a hydroelectric dam and a spillway. Hydrobiologia 582: 5–15. [Google Scholar]
- Bainbridge R. (1960) Speed and stamina in three fish. Exp Biol 37: 129–153. [Google Scholar]
- Beamesderfer RC, Simpson ML, Kopp GJ. (2007) Use of life history information in a population model for Sacramento green sturgeon. Environ Biol Fish 79: 315–337. [Google Scholar]
- Beamish FWH. (1978) Swimming capacity. In WW Hoar, DJ Randall, eds, Fish Physiology, Vol. VII, Locomotion, Academic Press Inc, New York, pp 101–172. [Google Scholar]
- Bell WH, Terhune LBD. (1970) Water tunnel design for fisheries research. Fish Res Board Canada Tech Rep 195: 1–69. [Google Scholar]
- Bemis WE, Kynard B. (1997) Sturgeon rivers: an introduction to acipenseriform biogeography and life history. Environ Biol Fish 48: 167–183. [Google Scholar]
- Bennett WA. (2005) Critical assessment of the delta smelt population in the San Francisco estuary, California. San Francisco Estuary and Watershed Science 3(2): Article 1. http://escholarship.org/uc/search?entity=jmie_sfews;volume=3;issue=2. [Google Scholar]
- Billard R, Lecointre G. (2001) Biology and conservation of sturgeon and paddlefish. Rev Fish Biol Fish 10: 355–392. [Google Scholar]
- Boysen KA, Hoover JJ. (2009) Swimming performance of juvenile white sturgeon (Acipenser transmontanus): training and the probability of entrainment due to dredging. J Appl Ichthyol 25: 54–59. [Google Scholar]
- Bradford MJ. (1992) Precision of recruitment predictions from early life stages of marine fishes. Fish Bull 90: 439–453. [Google Scholar]
- Brett JR. (1964) The respiratory metabolism and swimming performance of young sockeye salmon. J Fish Res Board Can 21: 1183–1964. [Google Scholar]
- Brett JR. (1965) The relation of size to rate of oxygen consumption and sustained swimming speed of sockeye salmon (Oncorhynchus nerka). J Fish Res Board Can 22: 1491–1501. [Google Scholar]
- Cai L, Taupier R, Johnson D, Tu Z, Liu G, Huang Y. (2013) Swimming capability and swimming behavior of juvenile Acipenser schrenckii. J Exp Zool A Ecol Genet Physiol 319: 149–155. [DOI] [PubMed] [Google Scholar]
- Caudill CC, Daigle WR, Keefer ML, Boggs CT, Jepson MA, Burke BJ, Zabel RW, Bjornn TC, Peery CA. (2007) Slow dam passage in adult Columbia River salmonids associated with unsuccessful migration: delayed negative effects of passage obstacles or condition-dependent mortality? Can J Fish Aquat Sci 64: 979–995. [Google Scholar]
- Cloern JE, Jassby AD. (2012) Drivers of change in estuarine-coastal ecosystems: discoveries from four decades of study in San Francisco Bay. Rev Geophys 50: 1–33. [Google Scholar]
- Counihan TD, Frost CN. (2011) Influence of externally attached transmitters on the swimming performance of juvenile white sturgeon. Trans Am Fish Soc 128: 965–970. [Google Scholar]
- Danley ML, Mayr SD, Young PS, Cech JJ. (2002) Swimming performance and physiological stress responses of splittail exposed to a fish screen. North Am J Fish Manag 22: 1241–1249. [Google Scholar]
- Deng DF, Koshio S, Yokoyama S, Bai SC, Shao Q, Cui Y, Hung SSO. (2003) Effects of feeding rate on growth performance of white sturgeon (Acipenser transmontanus) larvae. Aquaculture 217: 589–598. [Google Scholar]
- Deng DF, Wang C, Lee S, Bai S, Hung SSO. (2009) Feeding rates affect heat shock protein levels in liver of larval white sturgeon (Acipenser transmontanus). Aquaculture 287: 223–226. [Google Scholar]
- Deng X, Van Eenennaam JP, Doroshov SI. (2002) Comparison of early life stages and growth of green and white sturgeon. Am Fish Soc Symp 28: 237–248. [Google Scholar]
- Deslauriers D, Kieffer JD. (2011) The influence of flume length and group size on swimming performance in shortnose sturgeon Acipenser brevirostrum. J Fish Biol 79: 1146–1155. [DOI] [PubMed] [Google Scholar]
- Deslauriers D, Kieffer JD. (2012a) Swimming performance and behaviour of young-of-the-year shortnose sturgeon (Acipenser brevirostrum) under fixed and increased velocity swimming tests. Can J Zool 351: 345–351. [Google Scholar]
- Deslauriers D, Kieffer JD. (2012b) The effects of temperature on swimming performance of juvenile shortnose sturgeon (Acipenser brevirostrum). J Appl Ichthyol 28: 176–181. [Google Scholar]
- Duke S, Anders P, Ennis G, Hallock R, Hammond J, Ireland S, Laufle J, Lauzier R, Lockhard L, Matotz B, et al. (1999) Recovery plan for Kootenai River white sturgeon (Acipenser transmontanus). J Appl Ichthyol 15: 157–163. [Google Scholar]
- Gessner J, Van Eenennaam JP, Doroshov SI. (2007) North American green and European Atlantic sturgeon: comparisons of life histories and human impacts. Environ Biol Fish 79: 397–411. [Google Scholar]
- Grimaldo LF, Sommer T, Van Ark N, Jones G, Holland E, Moyle PB, Herbold B, Smith P. (2009) Factors affecting fish entrainment into massive water diversions in a tidal freshwater estuary: can fish losses be managed? North Am J Fish Manag 29: 1253–1270. [Google Scholar]
- Hammer C. (1995) Fatigue and exercise tests with fish. Comp Biochem Physiol A Physiol 112: 1–20. [Google Scholar]
- Hardy RS, Litvak MK. (2004) Effects of temperature on the early development, growth, and survival of shortnose sturgeon, Acipenser brevirostrum, and Atlantic sturgeon, Acipenser oxyrhynchus, yolk-sac larvae. Environ Biol Fish 70: 145–154. [Google Scholar]
- He P. (1986) Swimming performance in three species of marine fish and some aspects of swimming in fish gears. PhD thesis. Aberdeen University. [Google Scholar]
- He X, Lu S, Liao M, Zhu X, Zhang M, Li S, You X, Chen J. (2013) Effects of age and size on critical swimming speed of juvenile Chinese sturgeon Acipenser sinensis at seasonal temperatures. J Fish Biol 82: 1047–1056. [DOI] [PubMed] [Google Scholar]
- Herren JR, Kawasaki SS. (2001) Inventory of water diversions in four geographic areas in California's Central Valley. Fish Bulletin 179. Contributions to the Biology of Central Valley Salmonids, Vol. 2 http://www.dfg.ca.gov/fish/Resources/Reports/Bulletin179_V2.asp. [Google Scholar]
- Heublein JC, Kelly JT, Crocker CE, Klimley AP, Lindley ST. (2008) Migration of green sturgeon, Acipenser medirostris, in the Sacramento River. Environ Biol Fish 84: 245–258. [Google Scholar]
- Hildebrand L, McLeod C, McKenzie S. (1999) Status and management of white sturgeon in the Columbia River in British Columbia, Canada: an overview. J Appl Ichthyol 15: 164–172. [Google Scholar]
- Hinch SG, Bratty J. (2000) Effects of swim speed and activity pattern on success of adult sockeye salmon migration through an area of difficult passage. Trans Am Fish Soc 129: 598–606. [Google Scholar]
- Hoover JJ, Killgore KJ, Clarke DG, Smith H, Turnage A, Beard J. (2005) Paddlefish and sturgeon entrainment by dredges: swimming performance as an indicator of risk. DOER Technical Notes Collection (ERDC TN-DOER-E22), US Army Engineer Research and Development Center, Vicksburg, MS, USA. http://el.erdc.usace.army.mil/dots/doer/pubs.cfm?Topic=TechNote&Code=doer. [Google Scholar]
- Hoover JJ, Collins J, Boysen KA, Katzenmeyer AW, Killgore KJ. (2011) Critical swimming speeds of adult shovelnose sturgeon in rectilinear and boundary-layer flow. J Appl Ichthyol 27: 226–230. [Google Scholar]
- Israel JA, Klimley AP. (2008) Life history conceptual model for North American green sturgeon (Acipenser medirostris). https://nrm.dfg.ca.gov/documents/ContextDocs.aspx?sub=DRERIP_Documents_Models. [Google Scholar]
- Israel J, Drauch A, Gingras M. (2009a) Life history conceptual model for white sturgeon. https://nrm.dfg.ca.gov/documents/ContextDocs.aspx?sub=DRERIP_Documents_Models. [Google Scholar]
- Israel JA, Bando KJ, Anderson EC, May B. (2009b) Polyploid microsatellite data reveal stock complexity among estuarine North American green sturgeon (Acipenser medirostris). Can J Fish Aquat Sci 66: 1491–1504. [Google Scholar]
- IUCN (2014) IUCN Red List of Threatened Species. Version 2013.2. www.iucnredlist.org. [Google Scholar]
- Kimmerer WJ, Cowan JH, Miller LW, Rose KA. (2001) Analysis of an estuarine striped bass population: effects of environmental conditions during early life. Estuaries 24: 557–575. [Google Scholar]
- Kolhorst DW. (1976) Sturgeon spawning in the Sacramento River in 1973, as determined by distribution of larvae. Calif Fish Game 62: 32–40. [Google Scholar]
- Kynard B, Parker E. (2005) Ontogenetic behavior and dispersal of Sacramento River white sturgeon, Acipenser transmontanus, with a note on body color. Environ Biol Fish 74: 19–30. [Google Scholar]
- Kynard B, Zhuang P, Zhang L, Zhang T, Zhang Z. (2002) Ontogenetic behavior and migration of Volga River Russian sturgeon, Acipenser gueldenstaedtii, with a note on adaptive significance of body color. Environ Biol Fish 65: 411–421. [Google Scholar]
- Kynard B, Parker E, Parker T. (2005) Behavior of early life intervals of Klamath River green sturgeon, Acipenser medirostris, with a note on body color. Environ Biol Fish 72: 85–97. [Google Scholar]
- Kynard B, Parker E, Kynard B. (2010) Ontogenetic behavior of Kootenai River white sturgeon, Acipenser transmontanus, with a note on body color: a laboratory study. Environ Biol Fish 88: 65–77. [Google Scholar]
- Liermann CR, Nilsson C, Robertson J, Ng RY. (2012) Implications of dam obstruction for global freshwater fish diversity. Bioscience 62: 539–548. [Google Scholar]
- Linares-Casenave J, Werner I, Van Eenennaam JP, Doroshov SI. (2013) Temperature stress induces notochord abnormalities and heat shock proteins expression in larval green sturgeon (Acipenser medirostris Ayres 1854). J Appl Ichthyol 29: 958–967. [Google Scholar]
- McAdam SO. (2011) Effects of substrate condition on habitat use and survival by white sturgeon (Acipenser transmontanus) larvae and potential implications for recruitment. Can J Fish Aquat Sci 68: 812–822. [Google Scholar]
- McCabe GTJ, Jr, Tracy CA. (1993) Spawning and early life history Acipenser transmontanus, in the lower Columbia River. Fish Bull 92: 760–772. [Google Scholar]
- McEnroe M, Cech J., Jr (1985) Osmoregulation in juvenile and adult white sturgeon, Acipenser transmontanus. Environ Biol Fish 14: 23–30. [Google Scholar]
- Mayfield RB, Cech J., Jr (2004) Temperature effects on green sturgeon bioenergetics. Trans Am Fish Soc 133: 961–970. [Google Scholar]
- Miller EA, Froehlich HE, Cocherell DE, Thomas MJ, Cech J, Jr, Klimley AP, Fangue NA. (2014) Effects of acoustic tagging on juvenile green sturgeon incision healing, swimming performance, and growth. Environ Biol Fish 97: 647–658. [Google Scholar]
- Mohseni M, Pourkazemi M, Bahmani M, Falahatkar B, Pourali HR, Salehpour M. (2006) Effects of feeding rate and frequency on growth performance of yearling great sturgeon, Huso huso. J Appl Ichthyol 22Suppl s1: 278–283. [Google Scholar]
- Moyle PB. (2002) Inland Fishes of California. University of California Press, Berkeley, CA, USA, 253 pp. [Google Scholar]
- Musick JA, Harbin MM, Berkeley SA, Burgess GH, Eklund AM, Findley L, Gilmore RG, Golden JT, Ha DS, Huntsman GR, et al. (2000) Marine, estuarine, and diadromous fish stocks at risk of extinction in North America (exclusive of Pacific salmonids). Fisheries 25: 6–30. [Google Scholar]
- Mussen TD, Cocherell D, Poletto JB, Reardon JS, Hockett Z, Ercan A, Bandeh H, Kavvas ML, Cech J, Jr, Fangue NA. (2014) Unscreened water-diversion pipes pose an entrainment risk to the threatened green sturgeon, Acipenser medirostris. PLoS ONE 9: e86321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOAA (2005) Green sturgeon (Acipenser medirostris) status review update. Biological Review team, Santa Cruz Laboratory. Southwest Fisheries Science Center, NOAA Fisheries. http://www.nmfs.noaa.gov/pr/species/fish/greensturgeon.htm. [Google Scholar]
- Parsley MJ, Popoff ND, Wright CD, van der Leeuw BK. (2008) Seasonal and diel movements of white sturgeon in the Lower Columbia River. Trans Am Fish Soc 137: 1007–1017. [Google Scholar]
- Parsons GR, Hoover JJ, Killgore JK. (2003) Effect of pectoral fin ray removal on station-holding ability of shovelnose sturgeon. North Am J Fish Manag 23: 742–747. [Google Scholar]
- Peake S, Beamish FWH, McKinley RS, Scruton DA, Katopodis C. (1997) Relating swimming performance of lake sturgeon, Acipenser fulvescens, to fishway design. Can J Fish Aquat Sci 1366: 1361–1366. [Google Scholar]
- Poletto JB, Cocherell DE, Klimley AP, Cech J, Jr, Fangue NA. (2013) Behavioural salinity preferences of juvenile green sturgeon Acipenser medirostris acclimated to fresh water and full-strength salt water. J Fish Biol 82: 671–685. [DOI] [PubMed] [Google Scholar]
- Poletto JB, Cocherell DE, Ho N, Cech J, Jr, Klimley AP, Fangue NA. (2014) Juvenile green sturgeon (Acipenser medirostris) and white sturgeon (Acipenser transmontanus) behavior near water-diversion fish screens: experiments in a laboratory swimming flume. Can J Fish Aquat Sci 71: 1030–1038. [Google Scholar]
- Qu Y, Duan M, Yan J, Feng G, Liu J, Zhang L, Zhuang P. (2013) Effects of lateral morphology on swimming performance in two sturgeon species. J Appl Ichthyol 29: 310–315. [Google Scholar]
- Rochard E, Castelnaud G, Lepage M. (1990) Sturgeons (Pisces: Acipenseridae); threats and prospects. J Fish Biol 37: 123–132. [Google Scholar]
- SARA (2014) Species at Risk Act Public Registry. www.registrelep-sararegistry.gc.ca. [Google Scholar]
- Schreier AD, Mahardja B, May B. (2013) Patterns of population structure vary across the range of the white sturgeon. Trans Am Fish Soc 142: 1273–1286. [Google Scholar]
- Service RF. (2007) Delta blues, California style. Science 317: 442–445. [DOI] [PubMed] [Google Scholar]
- Sommer T, Armor C, Baxter R, Breuer R, Brown L, Culberson S, Feyrer F, Gingras M, Herbold B, Mueller-Solger A, et al. (2011) The collapse of pelagic fishes in the Upper San Francisco estuary. Fisheries 32: 270–277. [Google Scholar]
- Stevens DE, Miller LW. (1970) Distribution of sturgeon larvae in the Sacramento-San Joaquin River system. Calif Fish Game 56: 80–86. [Google Scholar]
- Stevens DE, Kohlhorst DW, Miller LW, Kelley DW. (1985) The decline of striped bass in the Sacramento-San Joaquin estuary, California. Trans Am Fish Soc 114: 12–30. [Google Scholar]
- Swanson C, Young PS, Cech J., Jr (2005) Close encounters with a fish screen: integrating physiological and behavioral results to protect endangered species in exploited ecosystems. Trans Am Fish Soc 134: 1111–1123. [Google Scholar]
- Tashjian D, Cech J, Jr, Hung SSO. (2007) Influence of dietary l-selenomethionine exposure on the survival and osmoregulatory capacity of white sturgeon in fresh and brackish water. Fish Physiol Biochem 33: 109–119. [Google Scholar]
- USFWS (2014) US Fish and Wildlife Services. Endangered Species. http://www.fws.gov/endangered/. [Google Scholar]
- Van Eenennaam JP, Webb MAH, Deng X, Doroshov SI, Mayfield RB, Cech J, Jr, Hillemeier DC, Willson TE. (2001) Artificial spawning and larval rearing of Klamath River green sturgeon. Trans Am Fish Soc 130: 159–165. [Google Scholar]
- Van Eenennaam JP, Linares-Casenave J, Deng X, Doroshov SI. (2005) Effect of incubation temperature on green sturgeon embryos, Acipenser medirostris. Environ Biol Fish 68: 145–154. [Google Scholar]
- Van Eenennaam JP, Linares-Casenave J, Doroshov SI. (2012) Tank spawning of first generation domestic green sturgeon. J Appl Ichthyol 28: 505–511. [Google Scholar]
- Webb W. (1986) Kinematics of lake sturgeon, Acipenser fulvescens, at cruising speeds. Can J Zool 64: 2137–2141. [Google Scholar]
- Williot P, Arlati G, Chebanov M, Gulyas T, Kasimov R, Kirschbaum F, Patriche N, Pavlovskaya LP, Poliakova L, Pourkazemi M, et al. (2002) Status and management of Eurasian sturgeon: an overview. Int Rev Hydrobiol 87: 483–506. [Google Scholar]
- Xenopoulos MA, Lodeg DM, Alcamo J, Marker M, Schulze K, Van Vuurens DP. (2005) Scenarios of freshwater fish extinctions from climate change and water withdrawal. Glob Chang Biol 11: 1557–1564. [Google Scholar]
- Zhuang P, Kynard B, Zhang L, Zhang T, Cao W. (2002) Ontogenetic behavior and migration of Chinese sturgeon, Acipenser sinensis. Environ Biol Fish 65: 83–97. [Google Scholar]
- Zhuang P, Kynard B, Zhang L, Zhang T, Cao W. (2003) Comparative ontogenetic behavior and migration of kaluga, Huso dauricus, and Amur sturgeon, Acipenser schrenckii, from the Amur River. Environ Biol Fish 66: 37–48. [Google Scholar]