We present a conceptual framework for conservation physiology intended to facilitate the application of physiological knowledge and concepts to conservation problems. The framework is focused on moving from knowledge to action, (e.g., policy, management interventions), which is essential if this mission-oriented discipline is to contribute meaningfully to evidence-based conservation and management.
Keywords: Ecology, global change, physiological tolerance, policy, resource management, restoration
Abstract
Current rates of biodiversity decline are unprecedented and largely attributed to anthropogenic influences. Given the scope and magnitude of conservation issues, policy and management interventions must maximize efficiency and efficacy. The relatively new field of conservation physiology reveals the physiological mechanisms associated with population declines, animal–environment relationships and population or species tolerance thresholds, particularly where these relate to anthropogenic factors that necessitate conservation action. We propose a framework that demonstrates an integrative approach between physiology, conservation and policy, where each can inform the design, conduct and implementation of the other. Each junction of the conservation physiology process has the capacity to foster dialogue that contributes to effective implementation, monitoring, assessment and evaluation. This approach enables effective evaluation and implementation of evidence-based conservation policy and management decisions through a process of ongoing refinement, but may require that scientists (from the disciplines of both physiology and conservation) and policy-makers bridge interdisciplinary knowledge gaps. Here, we outline a conceptual framework that can guide and lead developments in conservation physiology, as well as promote innovative research that fosters conservation-motivated policy.
Introduction
Global environmental change is leading to unprecedented levels of biodiversity loss (Rockstrom et al., 2009). Anthropogenic drivers of decline, including habitat alteration (Kerr and Deguise, 2004; Gallant et al., 2007), climate change (Pearson and Dawson, 2005; Monahan and Hijmans, 2008) and pollution (Menezes-Oliveira et al., 2013), perturb the physiological optimization of organisms (Carey, 2005; Pörtner and Farrell, 2008). When unchecked, effects of species decline can cascade through ecosystems and trophic communities (Duffy, 2003), leading to loss of specialist species (White and Kerr, 2007) or even changes in system state (Beisner et al., 2003). The complexity of threats and their concomitant interactions (Brook et al., 2008) require decisive and efficient conservation and management actions (Knight et al., 2006).
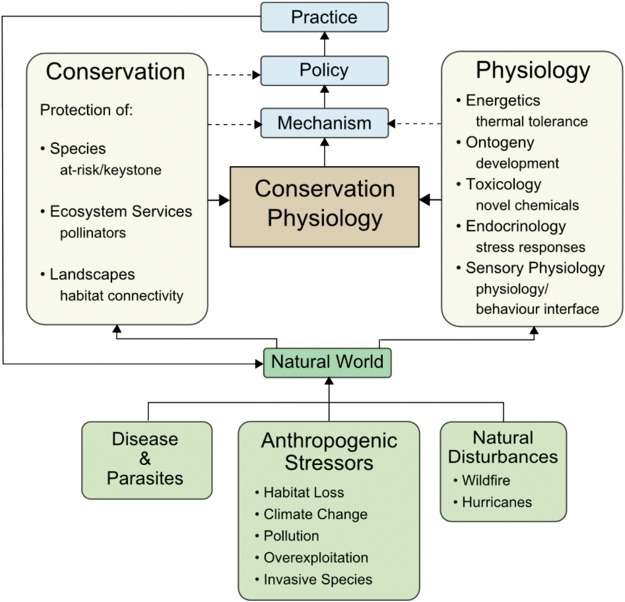
‘Conservation physiology’ integrates physiological perspectives into a broader conservation science (Fig. 1; Wikelski and Cooke, 2006). The merging of these two fields enables refinement of mechanistic knowledge that can be used to drive highly effective and specific policy recommendations. Conservation issues, broadly construed, include assessment of species and population viability, the anthropogenic threats that affect organisms, and intervention effectiveness. Prioritization of management interventions also falls under the umbrella of conservation. Physiology can be used to identify the sub-lethal and lethal effects that generate fitness decline. Thus, conservation physiology can be defined as a science that links global change effects on species abundance, dispersal and fitness to the physiological mechanisms that generate these declines and, in particular, the application of this knowledge to conservation efforts (Cooke et al., 2013b).
Figure 1:
The interaction between conservation and physiology, with notable sub-concepts and examples of applications for both fields.
Species are constrained in fitness and distribution by the range of environmental conditions they tolerate, which affects physiological performance and can alter population dynamics (Calow and Sibly, 1990; Spicer and Gaston, 1999; Ricklefs and Wikelski, 2002). Historically, human impacts on species and populations were most readily detected through assessment of declines, as with population censuses, which include measures of change in range size, distribution patterns, sex ratio and genetic diversity. These measures are time consuming and costly and, for some taxa, inaccurate, thereby detracting from conservation end-points (Hutchings and Baum, 2005). While the most immediately observed response to anthropogenic disturbance may be population declines, in most cases the response to any outside force starts at the level of a species' physiology. The extent to which monitoring-based approaches identify trends without clearly delineating their causes reduces the likelihood of achieving conservation management goals (Cooke and O'Connor, 2010). In many instances, conservation and management activities require frequent and rapid assessment of organismal response to interventions, yet decisions may be enacted in the absence of such scientific information (Salafsky et al., 2002; Sutherland et al., 2004; Dawson, 2011). Physiological measures can be incorporated into conservation as a means of overcoming these limitations (Wikelski and Cooke, 2006).
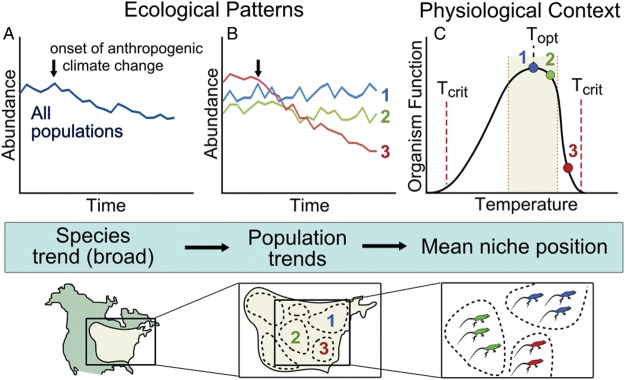
Physiological measurements provide additional mechanistic insights that may not be accessible from purely ecological studies (Fig. 2), enabling greater precision in detecting, attributing and predicting species' and individual responses to particular forms of environmental change (Wiens et al., 1993; Helmuth et al., 2005; Boyles et al., 2011; Seebacher and Franklin, 2012). Many ecological principles are based, at least in abstract terms, on physiological processes but, in general, focus on broad-scale patterns that are generalizable across a range of environments and ecological contexts (Levin, 1992). Traditional techniques detect responses at the population level, or at the individual level between generations (i.e. when measuring reproductive output). Physiological response to environmental conditions is inter- and intra-specific (Spicer and Gaston, 1999; Cooke et al., 2012), but may also be dependent on life stage (Pörtner and Farrell, 2008) or organismal responses that vary on diurnal or seasonal time scales (see Chown and Nicolson, 2004). Physiological techniques (i.e. monitoring stress hormones or whole-organism metrics of performance) can detect responses at the level of the individual at a very fine temporal resolution, as well as identify thresholds (Busch and Hayward, 2009) and vulnerability (Moritz and Agudo, 2013) to environmental stressors (and for the causal mechanism) that are relevant to the conservation issue. Given that optimization of physiological conditions relates to high fitness, while departures correspond to declines in organismal function and reproductive fitness (Arnold, 1983; Ricklefs and Wikelski, 2002; Pörtner and Farrell, 2008), physiology can be used to refine ecological mechanisms that have focused relevance to the conservation trajectories of species or populations.
Figure 2:
Differences in attribution of causal relationships between conservation (A and B) and conservation physiology studies (B and C). Physiological knowledge of a species (or other system of interest) can increase the precision with which mechanisms for responses are identified. Here, climate warming is causing a species of conservation concern to experience gradual decline, when taken as an average across all populations (A). However, an examination of distinct populations for this species (B) shows that population 3 is declining rapidly, while populations 1 and 2 are not. Knowledge of the thermal tolerance of this species can help to explain this pattern (C); individuals from population 1 are at the optimal temperature for the species and, therefore, the population has not experienced temperature-related declines. Individuals of population 2 are experiencing temperatures that are not optimal, and their function is not maximized; however, they are within the tolerable range of temperature for the species and, therefore, are not experiencing significant population decline. Population 2 is at risk of accelerating decline due to climate change in the near future. Individuals of population 3 are experiencing temperatures warmer than the optimal tolerable range for the species (shaded in beige), leading to deterioration of function at the individual level, which extrapolates to population-level decline. These individuals are experiencing sub-lethal effects and are approaching the critical temperature at which mortality occurs. Population 3 is at risk of local extinction, which could increase endangerment risk for the species. Active management of the population is warranted, and could involve translocation, removal of dispersal barriers, etc.
The objective of this article is to outline a conceptual framework that details the role of conservation physiology in the larger conservation domain. This role includes discerning explicit physiological links between species fitness and environmental changes (Ricklefs and Wikelski, 2002; Tracy et al., 2006), particularly where these have practical benefits for species' conservation outcomes through policy relevance (Cooke and Suski, 2008; Cooke and O'Connor, 2010; Cooke et al., 2013b). Such contributions will then improve prospects for evidence-based decision-making (Sutherland et al., 2004). The framework we propose here is intended to guide, but not limit, developments in this emerging discipline.
Conservation physiology framework
Conservation physiology is an applied field that represents a solution-based approach to conservation and is a process of feedback between policy- and decision-makers and conservation physiologist practitioners. Physiology permits detection of incremental effects on species or population fitness, and this informs the decision-making process. This cycle of physiology informing conservation decision-making encourages an ongoing process of assessment, implementation, monitoring and evaluation. The integrated approach enables rapid modifications to conservation action based on changing conditions at any step in the process, because physiological knowledge identifies precise causal pathways for conservation issues and can detect sub-lethal effects (Fig. 3; adapted from Magnuszewski et al., 2010).
Figure 3:
Process of interaction between conservation, physiology and policy. (A) Ways in which physiological knowledge can contribute to the conservation policy development and implementation process (adapted from Magnuszewski et al., 2010). (B) Conservation, physiology and policy all provide feedback and input into each stage of the implementation and assessment process. Ongoing monitoring, assessment and evaluation increase the scientific weight of evidence and support decisive policy action.
Policy- and decision-makers should be considered the ultimate users of research findings in this context. As such, scientific findings need to translate into implementable solutions by being practical, repeatable and quantifiable. Cross-disciplinary collaboration and expertise improve integration and application of research findings within conservation management and policy (Meffe and Viederman, 1995). Policy-makers generate management decisions based on a combination of factors, including scientific research, societal views, normative values and socio-economic considerations (Gunningham et al., 1998). These factors contribute to the identification of conservation problems and the determination of how problems are addressed through policy. Linking stressors with their concomitant effects on biodiversity (or population) status is an integral part of this process and enables policy-makers and conservation managers to incorporate explicit predictions of species response into management decisions.
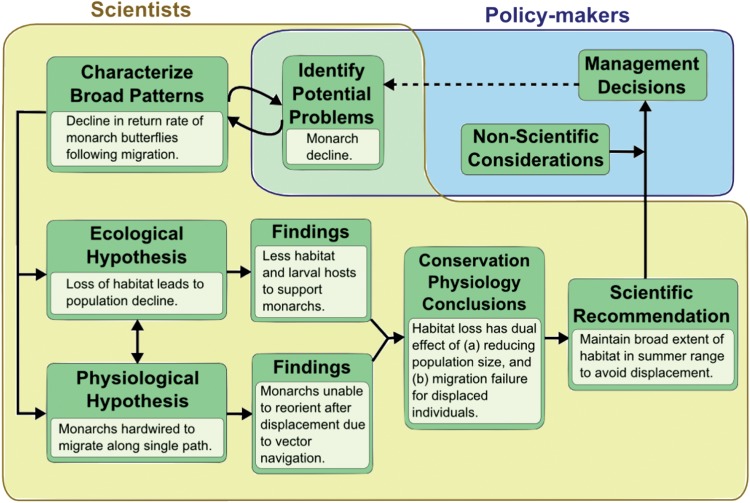
Policy-makers may give a lower priority to scientific information when there are competing jurisdictional and socio-economic concerns (Findlay et al., 2009). Improving the evidentiary weight of conservation research, as with the inclusion of physiology, can increase the likelihood that scientific information contributes to the policy process. When conservation issues have a clear physiological and mechanistic foundation, the scientific recommendations to policy-makers have higher levels of certainty, and non-evidence-based considerations that would lead to scientifically unsupported outcomes will less frequently play determining roles in policy development (Pullin et al., 2004; Sutherland et al., 2004). The conservation physiology framework is illustrated in Box A and Figure A. Our example of monarch butterfly (Danaus plexippus) decline across North America demonstrates the combined insights from ecological and physiological principles, which contribute to meaningful scientific recommendations that inform the conservation policy and decision-making process.
Box A: Conservation physiology conceptual framework: monarch butterfly case study.
Justification for conservation physiology can be encapsulated elegantly by examining the plight of the monarch butterfly (Danaus plexippus; see Box Figure A). Ecological and conservation research demonstrates multiple aspects of life history as well as environmental requirements for the monarch butterfly. This species undergoes a multigenerational annual migration between southern Canada and Mexico. The monarch is designated as special concern in Canada (COSEWIC, 2013). Extensive loss of habitat in the overwintering and breeding grounds, climate change (Brower et al., 2012) and increases in genetically modified crops, along with concomitant increase of pesticide use to control milkweed, its larval food source (Zalucki and Lammers, 2010; Pleasants and Oberhauser, 2013), have led to rapid and drastic population declines. Anthropogenic threats are distinct to each life stage of the monarch butterfly. Implementing solutions at each life stage often requires ecological, behavioural and physiological observations.
Physiological research has yielded additional, compelling insights that improve the conservation prospects for this species. An experimental, 2500 km westward displacement of butterflies at the commencement of their autumn migration determined that individuals have a high sensitivity to displacement, such as occurs with habitat fragmentation, climate change and loss of larval food sources. Due to the vector navigational system used, monarchs that are displaced from summering locations are unable to reorient towards Mexico. Refinement of migration direction occurs only at the culmination of the autumn migration through exogenous factors (Mouritsen et al., 2013). Within the context of a conservation physiology framework, such information can be used to identify necessary conservation and policy action. Conservation research can identify whether populations are declining and can attribute these declines to specific anthropogenic threats. In this case, a purely conservation-based approach would generate a recommendation that habitat should be protected. Physiology identifies the precise physiological mechanism responsible for declines, leading to unambiguous solutions. In this specific instance, the vector navigation system used by monarch butterflies means that displacement from summering grounds translates into migration failure. Based on this research, conservation policies would focus on maintaining broad extents of habitat throughout the summering grounds. Given that monarchs undergo a multigenerational migration, there is a lag before effects of displacement (i.e. population loss) become apparent.
Conservation physiology builds on collaborative efforts from the fields of conservation, physiology and decision-makers using an iterative process of refinement that incorporates implementation, assessment and monitoring.
Box Figure A:
The general conservation physiology framework (dark green boxes) within the policy process (pale blue background), with supporting examples specific to monarch butterfly biology and management (light green boxes).
Applying the framework
Generally, conservation physiology can be applied in any case where knowledge of an organism's physiology improves the ability to predict or manipulate ecological patterns and their conservation outcomes. These organisms include bacteria, plants and animals in both aquatic and terrestrial environments. Relevant applications of conservation physiology include informing suitability of management interventions (i.e. ecosystem restoration or species translocation), viability assessments for endangered population and species recovery, and threat assessments that predict the effects of current and future anthropogenic drivers of biodiversity decline and relevant interventions on distribution and abundance (Wikelski and Cooke, 2006; Cooke and Suski, 2008). For instance, all of the International Union for the Conservation of Nature–Conservation Measures Partnership (IUCN-CMP) threat categories can be examined using conservation physiology (see Table 1). Conservation physiology has broad utility in research as well, such as evaluation of competing ecological hypotheses by differentiating between expected physiological mechanisms (Tracy et al., 2006). Additionally, physiological knowledge is now being used to assess the evolutionary basis for physiological adaptation in studies of phylogenetic niche conservatism and niche lability during climate change (Wiens and Graham, 2005). Among species with greater shared evolutionary history, trait-based responses to environmental changes are also likely to be shared, which may consequently lead to convergent responses to aspects of global change.
Table 1:
Potential application of conservation physiology research to IUCN-CMP threats
| IUCN-CMP threat category | Specific application | Physiological measures | Research |
|---|---|---|---|
| Residential and commercial development | Human land-use intensity effects on birds | Corticosterone and immunoglobulin | Chávez-Zichinelli et al. (2013) |
| Agriculture and aquaculture | Parasite incidence in aquaculture as an infective agent for wild salmon | Disturbance of ionoregulation | Brauner et al. (2012) |
| Energy production and mining | Aquatic pipeline crossing | Respiration, blood haematocrit and leucocrit, heart rate, etc. | Levesque and Dube (2007) |
| Transportation and service corridors | Effects of distance to road on bird species | Blood corticosterone levels | Dietz et al. (2013) |
| Biological resource use | Effects of logging and hunting on primates | Faecal glucorticoid metabolites | Rimbach et al. (2013) |
| Human intrusions and disturbance | Effects of tourism and food provisioning on endangered iguana | Dietary nutrition and endoparasitic infection | Knapp et al. (2013) |
| Natural system modifications | Mistiming of fire for red-backed fairy-wrens | Body mass and blood haemoglobin concentration | Murphy et al. (2010) |
| Invasive species, problematic species and diseases | Effects of season, humidity and sloughing on pathogens and infectious disease for frogs | Microbe abundance and recolonization rate | Cramp et al. (2014) |
| Pollution | Toxicity and mutagenicity post-oil spill | Photosynthetic activity of plankton, toxicity to microbes | Paul et al. (2013) |
| Geological events | Effects of volcanic mud exposure for fish | Phagocytic activity | Risjani et al. (2014) |
| Climate change and severe weather | Sensitivity to climate change across ontogenetic stages for endangered fish | Thermal and salinity limits, acclimatization states | Komoroske et al. (2014) |
Abbreviation: IUCN-CMP, International Union for the Conservation of Nature–Conservation Measures Partnership.
In applying a conservation physiology framework, there are four main considerations. First, changes in a species' environment can be linked to species decline (as measured through performance, fitness or stress response, among others) using physiological measures. The physiological measure provides an explicit link between rates of change for physiological function and species decline. Second, these physiological mechanisms may differ by species, even within the same taxonomic group, although the phenotypic response, in terms of fitness, may be similar. Third, conservation physiology is an applied research discipline that can be used to tailor policy and management to the specific physiological response pathway. This occurs within the broader context of policy development and implementation. Fourth, the field benefits the wider conservation decision-making context by increasing the weight of available scientific evidence.
(i) Physiological links between species fitness and environmental changes
Knowledge of species' physiological responses has the potential to aid in devising effective conservation solutions. Wildlife corridor use is an example that illustrates the unique physiological response that a species may exhibit in response to environmental stressors. Corridors have a long history in conservation, yet may be ineffective because of inadequate baseline data on their utility for their target species (Chetkiewicz et al., 2006). Research suggests that landscape use differs among individuals of a species based on physiological state. African elephants (Loxodonta africana) retreat to protected areas and corridors in response to human activities that cause physiological stress, as measured by faecal glucocorticoid metabolite hormones (Jachowski et al., 2013). Where humans and elephants co-occur and protected areas are not available, human–elephant conflict is common. In some situations, this has led to detusking of elephants, which impacts social hierarchy and nutrition for these animals (Mutinda et al., 2014). Strategic planning of corridors in regions with high human–elephant overlap is a more effective management tool and can provide elephants with a refuge that minimizes any potential conflict. Thus, physiological information can inform how and where protected areas are employed based on the level of anthropogenic pressure in the surrounding landscape.
(ii) Specificity of species–environmental links
Conservation efforts must often be tailored not only to the specific anthropogenic pressures but also to the species of concern and the physiological response mechanism. Thermal tolerance is a key determinant of species' fitness (Terblanche et al., 2011) and distribution (Root, 1988; Sinclair et al., 2003a), yet is governed by highly specific physiological mechanisms that vary by species. Climate change impacts are a rapidly developing area for conservation physiology studies (Monahan and Hijmans, 2008; Chown et al., 2010), with the expectation that ranges for many species will expand poleward as temperatures warm at their cool thermal limits. For instance, freeze tolerance is a key strategy for ectotherms to survive sub-zero temperatures (Sinclair et al., 2003a), such as for Isabella tiger moth (Pyrrharctia isabella) pupae. These organisms control the freezing process via a combination of ice-nucleating proteins and intra-cellular antifreeze (Marshall and Sinclair, 2012). For the Isabella tiger moth caterpillar, diminished snow cover due to climate warming increases exposure to prolonged sub-zero temperatures, yet because cold exposure induces freezing, metabolic expenditure is suppressed for a longer period, and emerging pupae have greater mass and higher fitness (Marshall and Sinclair, 2012). For freeze-tolerant ectotherms, there are two main causes of cold-induced mortality. Temperatures that drop below critical thresholds will cause severe tissue damage that translates into temperature-based northern range limits (Bale, 2002), but repeated cycles of freezing and thawing, as would be expected with increased weather fluctuations due to climate change, also cause tissue damage and lower survival (Marshall and Sinclair, 2011).
Other ectotherms, such as the invasive emerald ash borer (Agrilus planipennis fairmaire), are freeze avoidant (Crosthwaite et al., 2011). These organisms use a combination of strategies, such as removal of ice-nucleating agents from cells and tissues, as well as increasing their supercooling capacity and using intra-cellular antifreeze to prevent ice crystal formation (Bale, 2002). Rapid lowering of temperature renders freeze-avoidant strategies ineffective (Sinclair et al., 2003b). The emerald ash borer has undergone significant poleward range expansion since it was first observed in North America in 2002 (Venette and Abrahamson, 2010). The invasion front is limited by cold temperatures (<− 30°C), which reduce the intensity of ash infestation by decreasing emerald ash borer densities (DeSantis et al., 2013).
In general and in the short-term, climate warming is likely to reduce barriers to poleward range expansion for both the Isabella tiger moth and the emerald ash borer by increasing overwintering survival and fitness (Crosthwaite et al., 2011; Williams et al., 2012). Long-term trends in warming will promote continued range expansion for the emerald ash borer, yet for the Isabella tiger moth this will eventually lead to reduced fitness if freezing cannot be maintained through the overwintering period. The physiological mechanisms that govern ecological responses for the Isabella tiger moth and emerald ash borer are markedly different. In practice, climatic extremes and rates of warming exert species-specific effects through distinctive physiological mechanisms (Bale and Hayward, 2010). Given that conservation focuses on altering outcomes for target organisms through either ameliorating conditions (and/or reducing barriers to fitness) for beneficial species or increasing barriers to fitness for invasive and pest species, knowledge of species-specific physiological mechanisms (and the manipulation thereof) has high applicability in policy.
(iii) Physiology as a method of promoting effective application of conservation
As an example of the utility of conservation physiology to policy, upstream relocation of Chinook salmon was once considered an effective method to enable fish bypass of water-diversion dams, and was incorporated into fish rescue strategies (see Mosser et al., 2013). Lack of hydrological connectivity as well as increased water temperatures due to dam structures and climate warming contribute to high mortality for economically significant species. Conservation management decisions were previously based on the assumption that any intervention that improved connectivity would have a net benefit for the species (Hilborn, 2006). Fish relocation has low efficacy, but the reason was not determined until physiological impacts were examined. For salmon, cessation of migration occurs when upper thermal limits are exceeded, which may precipitate management interventions, such as upstream relocation. However, once upper thermal limits are exceeded, upstream relocation will have no impact, because the fish do not survive to reproduce (Mosser et al., 2013). Among juvenile Chinook salmon, relocation hinders the physiological mechanisms responsible for homing and orientation during adult migration (Keefer et al., 2008; Keefer and Caudill, 2012). Prior to these studies, capture and relocation was considered a viable conservation strategy for Chinook salmon (Mullen, 1987). In this case study, expensive management policies were implemented prior to the elaboration of mechanisms affecting relocation success rates. Conservation physiology research elucidated effects of relocation strategies, which led to entirely different management strategies, such as timing relocation efforts prior to temperatures exceeding critical limits, as well as decommissioning diversion dams and installing fish screens. The end result is a scientific recommendation that is far more likely to influence decision-makers, even though the costs of implementation are sometimes very high.
(iv) Informing decision-making through conservation physiology
Conservation physiology has the capacity to improve decision-making within the process of conservation policy development, implementation and assessment. Generally, conservation policies mandate particular management goals. The likelihood of conservation action (or inaction) reflects urgency, funding, jurisdiction and the potential impact of decisions or policies on stakeholders (Salafsky and Redford, 2013). Nevertheless, management interventions are unlikely to succeed if the causes of declines cannot be identified clearly. Conservation physiology contributes to potential management success by improving understanding of how stressors diminish the likelihood of species and individual survival (thereby identifying the proximate causes of population decline), predicting response to conservation actions and providing tools for evaluating and monitoring the effectiveness of a given action or regulation through time. Constraints to policy implementation (i.e. public views, economic considerations or competing interests, etc.) characterize the types of conservation physiology research that are considered feasible; however, researchers in the field should also strive to investigate what would be considered appropriate in the absence of constraints.
Physiological knowledge reduces uncertainty, which improves policy implementation. A minimal standard of evidence is required in any decision-making process where there is an assessment of risk. The acceptable standard of evidence changes based on perceived risk. Insufficient evidentiary strength and consistency is a common problem in conservation research and, inevitably, means that research fails to inform policy and management recommendations (Busch and Hayward, 2009). In cases where conservation strategies have had few marginal benefits (Ferraro and Pattanayak, 2006), this may be partly due to the lack of information about specific understanding of how and why species respond to human activities (Stewart et al., 2005). Limited funding resources for conservation projects, when coupled with a low return on investment in terms of effectiveness (Sutherland et al., 2004), leave room for the decision process to be driven by values and economic considerations that argue against action (Findlay et al., 2009; Mooers et al., 2010).
Weight of evidence represents a systematic approach to quantifying uncertainty (Sutherland et al., 2004). To generate recommendations that advance conservation objectives, research findings must first contribute to a minimal weight of evidence (Thompson et al., 2005) and, second, contribute to transparent evaluation of implemented recommendations (Ferraro and Pattanayak, 2006; Guyatt et al., 2008). Effective study design is one of the most critical factors used to generate the high-impact evidentiary standards and evaluation of outcomes (Sutherland et al., 2004; Carey, 2005). Enhanced evidentiary quality occurs with consideration of effect size, consistency of results across multiple studies, precision and publication bias (for a more detailed discussion of these and other considerations, see Guyatt et al., 2008). Conservation physiology has the potential to increase the scientific contribution to policy development by providing an experimental or pseudo-experimental design that identifies not only the mechanism for effects but also the precise relationship between the rate of environmental change and species fitness (Carey, 2005; Cooke et al., 2013a). In doing so, conservation physiology promotes research application in a management and policy context.
Challenges
Conservation physiology, as a new field, faces a number of hurdles; among the most consequential is the need to improve the applicability of physiological data and measurements to conservation. An additional challenge, where theoretical insights may be particularly critical, is the need to discover ways to ‘scale up’ from physiological observations to ecological pattern (Levin, 1992; Cooke and O'Connor, 2010; Cooke et al., 2014). Differences in the scale of investigation between the two fields can lead to difficulties of extrapolation, particularly if the examined end-point varies substantially within and between populations and species. Finally, translating discovery at the conservation and physiological interface into management application is the final and, arguably, best test of success for this field. Like conservation biology itself, conservation physiology is a mission-oriented discipline (e.g. Soulé, 1985).
Physiological measurements and tools necessary to overcome such hurdles should be non-invasive, non-lethal and, ideally, involve rapid assessment (Cooke and O'Connor, 2010). Obtaining reliable baseline data is problematic in many fields; however, two options are to improve data accessibility through data sharing (Wolkovich et al., 2012) and to employ time series and rate-of-change study designs (Cooke and O'Connor, 2010). Furthermore, the scope of effectiveness for conservation physiology improves if individual- and population-level effects (as measured through physiological investigations) are linked to the species and communities of conservation concern.
Improved education for physiologists, conservationists and policy-makers on the policy process and conservation needs is essential and will foster higher impact collaboration (Cooke and O'Connor, 2010). The inclusion of managers and policy-makers in conservation physiology research will improve stakeholder and individual participation and the likelihood that research results will be applied. While there need not be an expectation that every conservation physiological research outcome will find direct policy application, policy relevance and impact should, nevertheless, remain a key consideration in conservation physiology research. To facilitate this, research should be accessible to conservation practitioners (Pullin et al., 2004; Stewart et al., 2005) and a greater emphasis placed on interpretive scientific skills.
Collaboration is an integral component of conservation physiology but is not without attendant challenges. Overlap in terminology changes for physiology and conservation did not generally increase following the initial coining of the term ‘conservation physiology’ (Lennox and Cooke, 2014). When both sides of the conservation physiology discipline can view the findings of the other, it leads to a mutual awareness of contributions (Sutherland et al., 2013), a need highlighted and partly addressed by the newly created journal, Conservation Physiology. The highly specialized knowledge base required for physiology, conservation, and policy and management decision-making demands collaborative efforts. This, in turn, generates strong and relevant scientific knowledge that can inform conservation decision-making.
Conclusions
Here, we have outlined a conceptual framework for merging conservation and physiology that we argue will yield improved conservation decision-making. Within the broader suite of processes that make up conservation policy development and implementation, this application of physiological knowledge is most useful to informing development of the following aspects: (i) overall policies that respond to a conservation problem; (ii) on-the-ground adaptive management actions that effectively accomplish the conservation objectives mandated by those policies; and (iii) evaluation tools and techniques that characterize the effectiveness of both of these at mitigating conservation problems. The strength of the conservation physiology framework arises from an integrative approach with an applied focus. This translates into improved dialogue and input between practitioners of conservation, physiology and policy, where each informs the design, conduct and implementation of conservation physiology research.
Given that physiological research investigates causal response mechanisms to changes in optimal environmental conditions, where shifts in organismal condition relative to physiological requirements affect overall functioning and fitness (Tracy et al., 2006), conservation physiology can rigorously inform the decision process for policy and management (Carey, 2005). Conservation and policy needs identify critical research questions for conservation physiologists; physiology reveals the mechanistic underpinnings of behaviour and performance (Cooke et al., 2014), thereby identifying new policy needs to promote conservation. In a world of pervasive human influence on the natural world, there is a growing need for conservation research to produce strong and decisive evidence for the consequences to natural systems (Rudd et al., 2011). Conservation physiology is uniquely poised to meet this challenge.
Funding
This work was supported by the Natural Sciences and Engineering Research Council (Discovery Grant Program, S.J.C. and J.T.K.; Post Doctoral Fellows Program, C.M.O.; Canada Graduate Scholarship, C.M.R.) and by the Ontario Graduate Scholarship Program (L.E.C.). This work was further supported by the Canada Research Chair Program (S.J.C.), the University Research Chair Program at the University of Ottawa (J.T.K.) and the University of Ottawa Excellence Scholarship Program (L.E.C. and C.M.R.). Additional funding sources include the E.B. Eastburn program (C.M.O.) and Conservation International (D.L.).
Acknowledgements
L.E.C. would like to dedicate her portion of this manuscript to her children, Robin and Julian. Comments from anonymous reviewers have greatly improved this paper. This paper is a product of a graduate course on Advances in Conservation, Physiology & Behaviour offered through the Ottawa-Carleton Institute of Biology.
References
- 1.Arnold SJ. (1983) Morphology, performance and fitness. Amer Zool 23: 347–361. [Google Scholar]
- 2.Bale JS. (2002) Insects and low temperatures: from molecular biology to distributions and abundance. Philos Trans R Soc Lond B Biol Sci 357: 849–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bale JS, Hayward SL. (2010) Insect overwintering in a changing climate. J Exp Biol 213: 980–994. [DOI] [PubMed] [Google Scholar]
- 4.Beisner BE, Haydon DT, Cuddington K. (2003) Alternative stable states in ecology. Front Ecol Environ 1: 376–382. [Google Scholar]
- 5.Boyles JG, Seebacher F, Smit B, McKechnie AE. (2011) Adaptive thermoregulation in endotherms may alter responses to climate change. Integr Comp Biol 51: 676–690. [DOI] [PubMed] [Google Scholar]
- 6.Brauner CJ, Sackville M, Gallagher Z, Tang S, Nendick L, Farrell AP. (2012) Physiological consequences of the salmon louse (Lepeophtheirus salmonis) on juvenile pink salmon (Oncorhynchus gorbuscha): implications for wild salmon ecology and management, and for salmon aquaculture. Philos Trans R Soc Lond B Biol Sci 367: 1770–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brook BW, Sodhi NS, Bradshaw CJA. (2008) Synergies among extinction drivers under global change. Trends Ecol Evol 23: 453–460. [DOI] [PubMed] [Google Scholar]
- 8.Brower LP, Taylor OR, Williams EH, Slayback DA, Zubieta RR, Ramírez MI. (2012) Decline of monarch butterflies overwintering in Mexico: is the migratory phenomenon at risk? Insect Conserv Diver 5: 95–100. [Google Scholar]
- 9.Busch DS, Hayward LS. (2009) Stress in a conservation context: a discussion of glucocorticoid actions and how levels change with conservation-relevant variables. Biol Conserv 142: 2844–2853. [Google Scholar]
- 10.Calow P, Sibly RM. (1990) A physiological basis of population processes: ecotoxicological implications. Funct Ecol 4: 283–288. [Google Scholar]
- 11.Carey C. (2005) How physiological methods and concepts can be useful in conservation biology. Integr Comp Biol 45: 4–11. [DOI] [PubMed] [Google Scholar]
- 12.Chávez-Zichinelli CA, MacGregor-Fors I, Quesada J, Rohana PT, Romano MC, Valdéz R, Schondube JE. (2013) How stressed are birds in an urbanizing landscape? Relationships between the physiology of birds and three levels of habitat alteration. Condor 115: 84–92. [Google Scholar]
- 13.Chetkiewicz C-LB, Clair CCS, Boyce MS. (2006) Corridors for conservation: integrating pattern and process. Annu Rev Ecol Evol Syst 37: 317–342. [Google Scholar]
- 14.Chown SL, Nicolson S. (2004) Insect Physiological Ecology: Mechanisms and Patterns. Oxford University Press, New York. [Google Scholar]
- 15.Chown SL, Hoffmann AA, Kristensen TN, Angilletta MJ, Jr, Stenseth NC, Pertoldi C. (2010) Adapting to climate change: a perspective from evolutionary physiology. Climate Res 43: 3–15. [Google Scholar]
- 16.Cooke SJ, O'Connor CM. (2010) Making conservation physiology relevant to policy makers and conservation practitioners. Conserv Lett 3: 159–166. [Google Scholar]
- 17.Cooke SJ, Suski CD. (2008) Ecological restoration and physiology: an overdue integration. Bioscience 58: 957–968. [Google Scholar]
- 18.Cooke SJ, Hinch SG, Donaldson MR, Clark TD, Eliason EJ, Crossin GT, Raby GD, Jeffries KM, Lapointe M, Miller K, et al. (2012) Conservation physiology in practice: how physiological knowledge has improved our ability to sustainably manage Pacific salmon during up-river migration. Philos Trans R Soc Lond B Biol Sci 367: 1757–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooke SJ, Blumstein DT, Buchholtz R, Caro T, Fernández-Juricic E, Franklin CE, Metcalfe J, O'Connor CM, St Clair CCC, Sutherland WJ. et al. (2013a) Physiology, behavior and conservation. Physiol Biochem Zool 87: 1–14. [DOI] [PubMed] [Google Scholar]
- 20.Cooke SJ, Sack L, Franklin CE, Beardall J, Wikelski M, Chown SL. (2013b) What is conservation physiology? Perspectives on an increasingly integrated and essential science. Conserv Physiol 1: doi:10.1093/conphys/cot001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooke SJ, Killen SS, Metcalfe JD, McKenzie DJ, Mouillot D, Jørgensen C, Peck MA. (2014) Conservation physiology across scales: insights from the marine realm. Conserv Physiol 2: doi:10.1093/conphys/cou024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.COSEWIC (2013) Canadian Wildlife Species at Risk. Committee on the Status of Endangered Wildlife in Canada. http://www.Cosewic.Gc.Ca/eng/sct0/rpt/rpt_csar_e.cfm [Google Scholar]
- 23.Cramp RL, McPhee RK, Meyer EA, Ohmer ME, Franklin CE. (2014) First line of defence: the role of sloughing in the regulation of cutaneous microbes in frogs. Conserv Physiol 2: doi:10.1093/conphys/cou012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crosthwaite JC, Sobek S, Lyons DB, Bernards MA, Sinclair BJ. (2011) The overwintering physiology of the emerald ash borer, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae). J Insect Physiol 57: 166–173. [DOI] [PubMed] [Google Scholar]
- 25.Dawson TP. (2011) Beyond predictions: biodiversity conservation in a changing climate. Science 332: 53–58. [DOI] [PubMed] [Google Scholar]
- 26.DeSantis RD, Moser WK, Gormanson DD, Bartlett MG, Vermunt B. (2013) Effects of climate on emerald ash borer mortality and the potential for ash survival in North America. Agr Forest Meteorol 178: 120–128. [Google Scholar]
- 27.Dietz MS, Murdock CC, Romero LM, Ozgul A, Foufopoulos J. (2013) Distance to a road is associated with reproductive success and physiological stress response in a migratory landbird. Wilson J Ornithol 125: 50–61. [Google Scholar]
- 28.Duffy JE. (2003) Biodiversity loss, trophic skew and ecosystem functioning. Ecol Lett 6: 680–687. [Google Scholar]
- 29.Ferraro PJ, Pattanayak SK. (2006) Money for nothing? A call for empirical evaluation of biodiversity conservation investments. PLoS Biol 4: e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Findlay CS, Elgie S, Giles B, Burr L. (2009) Species listing under Canada's Species at Risk Act. Conserv Biol 23: 1609–1617. [DOI] [PubMed] [Google Scholar]
- 31.Gallant AL, Klaver RW, Casper GS, Lannoo MJ. (2007) Global rates of habitat loss and implications for amphibian conservation. Copeia 2007: 967–979. [Google Scholar]
- 32.Gunningham N, Grabosky PN, Sinclair D. (1998) Smart Regulation: Designing Environmental Policy, Vol 514 Clarendon Press, Oxford. [Google Scholar]
- 33.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Schünemann H, GRADE Working Group. (2008) What is “quality of evidence” and why is it important to clinicians? BMJ 336: 995–999B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helmuth B, Kingsolver JG, Carrington E. (2005) Biophysics, physiological ecology, and climate change: does mechanism matter? Annu Rev Physiol 67: 177–201. [DOI] [PubMed] [Google Scholar]
- 35.Hilborn R. (2006) Faith-based fisheries. Fisheries 31: 554–555. [Google Scholar]
- 36.Hutchings JA, Baum JK. (2005) Measuring marine fish biodiversity: temporal changes in abundance, life history and demography. Philos Trans R Soc Lond B Biol Sci 360: 315–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jachowski DS, Montgomery RA, Slotow R, Millspaugh JJ. (2013) Unravelling complex associations between physiological state and movement of African elephants. Funct Ecol 27: 1166–1175. [Google Scholar]
- 38.Keefer ML, Caudill CC. (2012) A review of adult salmon and steelhead straying with an emphasis on Columbia River populations. In D.O.F.a.W. Resources, ed., College of Natural Resources, University of Idaho. [Google Scholar]
- 39.Keefer ML, Caudill CC, Peery CA, Lee SR. (2008) Transporting juvenile salmonids around dams impairs adult migration. Ecol Appl 18: 1888–1900. [DOI] [PubMed] [Google Scholar]
- 40.Kerr JT, Deguise I. (2004) Habitat loss and the limits to endangered species recovery. Ecol Lett 7: 1163–1169. [Google Scholar]
- 41.Knapp CR, Hines KN, Zachariah TT, Perez-Heydrich C, Iverson JB, Buckner SD, Halach SC, Lattin CR, Romero LM. (2013) Physiological effects of tourism and associated food provisioning in an endangered iguana. Conserv Physiol 1: doi:10.1093/conphys/cot032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knight AT, Cowling RM, Campbell BM. (2006) An operational model for implementing conservation action. Conserv Biol 20: 408–419. [DOI] [PubMed] [Google Scholar]
- 43.Komoroske LM, Connon RE, Lindberg J, Cheng BS, Castillo G, Hasenbein M, Fangue A. (2014) Ontogeny influences sensitivity to climate change stressors in an endangered fish. Conserv Physiol 2: doi:10.1093/conphys/cou008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lennox R, Cooke SJ. (2014) State of the interface between conservation and physiology: a bibliometric analysis. Conserv Physiol 2: doi:10/1093/conphys/cou003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levesque LM, Dube MG. (2007) Review of the effects of in-stream pipeline crossing construction on aquatic ecosystems and examination of Canadian methodologies for impact assessment. Environ Monit Assess 132: 395–409. [DOI] [PubMed] [Google Scholar]
- 46.Levin SA. (1992) The problem of pattern and scale in ecology. Ecology 73: 1943–1967. [Google Scholar]
- 47.Magnuszewski P, Sodomkova K, Slob A, Muro M, Sendzimir J, Pahl-Wostl C. (2010) Report on conceptual framework for science-policy barriers and bridges. Final version 22.12.2010 of deliverable No. 1.1 of the EC FP7 project PSI-connect. EC contract No. 226915. July 2010, Delft, The Netherlands. [Google Scholar]
- 48.Marshall KE, Sinclair BJ. (2011) The sub-lethal effects of repeated freezing in the woolly bear caterpillar Pyrrharctia isabella. J Exp Biol 214: 1205–1212. [DOI] [PubMed] [Google Scholar]
- 49.Marshall KE, Sinclair BJ. (2012) Threshold temperatures mediate the impact of reduced snow cover on overwintering freeze-tolerant caterpillars. Naturwissenschaften 99: 165–166. [DOI] [PubMed] [Google Scholar]
- 50.Meffe GK, Viederman S. (1995) Combining science and policy in conservation biology. Wildl Soc Bull 23: 327–332. [Google Scholar]
- 51.Menezes-Oliveira VB, Scott-Fordsmand JJ, Soares A, Amorim MJB. (2013) Effects of temperature and copper pollution on soil community – extreme temperature events can lead to community extinction. Environ Toxicol Chem 32: 2678–2685. [DOI] [PubMed] [Google Scholar]
- 52.Monahan WB, Hijmans RJ. (2008) Ecophysiological constraints shape autumn migratory response to climate change in the North American field sparrow. Biol Lett 4: 595–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mooers AO, Doak DF, Findlay CS, Green DM, Grouios C, Manne LL, Rashvand A, Rudd MA, Whitton J. (2010) Science, policy, and species at risk in Canada. Bioscience 60: 843–849. [Google Scholar]
- 54.Moritz C, Agudo R. (2013) The future of species under climate change: resilience or decline? Science 341: 504–508. [DOI] [PubMed] [Google Scholar]
- 55.Mosser C, Thompson L, Strange J. (2013) Survival of captured and relocated adult spring-run Chinook salmon (Oncorhynchus tshawytscha) in a Sacramento River tributary after cessation of migration. Environ Biol Fish 96: 405–417. [Google Scholar]
- 56.Mouritsen H, Derbyshire R, Stalleicken J, Mouritsen OO, Frost BJ, Norris DR. (2013) An experimental displacement and over 50 years of tag-recoveries show that monarch butterflies are not true navigators. Proc Natl Acad Sci USA 110: 7348–7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mullen JW. (1987) Status and propagation of Chinook salmon in the mid-Columbia river through 1985. In U.S.F.a.W. Service, ed., Fisheries Assistance Office, Washington, DC, USA. [Google Scholar]
- 58.Murphy SA, Legge SM, Heathcote J, Mulder E. (2010) The effects of early and late-season fires on mortality, dispersal, physiology and breeding of red-backed fairy-wrens (Malurus melanocephalus). Wildl Res 37: 145–155. [Google Scholar]
- 59.Mutinda M, Chenge G, Gakuya F, Otiende M, Omondi P, Kasiki S, Soriguer RC, Alasaad S. (2014) Detusking fence-breaker elephants as an approach in human-elephant conflict mitigation. PLoS ONE 9: e91749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paul JH, Hollander D, Coble P, Daly KL, Murasko S, English D, Basso J, Delaney J, McDaniel L, Kovach CW. (2013) Toxicity and mutagenicity of Gulf of Mexico waters during and after the Deepwater Horizon oil spill. Environ Sci Technol 47: 9651–9659. [DOI] [PubMed] [Google Scholar]
- 61.Pearson RG, Dawson TP. (2005) Long-distance plant dispersal and habitat fragmentation: identifying conservation targets for spatial landscape planning under climate change. Biol Conserv 123: 389–401. [Google Scholar]
- 62.Pleasants JM, Oberhauser KS. (2013) Milkweed loss in agricultural fields because of herbicide use: effect on the monarch butterfly population. Insect Conserv Divers 6: 135–144. [Google Scholar]
- 63.Pörtner HO, Farrell AP. (2008) Ecology, physiology and climate change. Science 322: 690–692. [DOI] [PubMed] [Google Scholar]
- 64.Pullin AS, Knight TM, Stone DA, Charman K. (2004) Do conservation managers use scientific evidence to support their decision-making? Biol Conserv 119: 245–252. [Google Scholar]
- 65.Ricklefs RE, Wikelski M. (2002) The physiology/life-history nexus. Trends Ecol Evol 17: 462–468. [Google Scholar]
- 66.Rimbach R, Link A, Heistermann M, Gómez-Posada C, Galvis N, Heymann EW. (2013) Effects of logging, hunting, and forest fragment size on physiological stress levels of two sympatric ateline primates in Colombia. Conserv Physiol 1: doi:10/1093/conphys/cot031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Risjani Y, Yunianta, Couteau J, Minier C. (2014) Cellular immune responses and phagocytic activity of fishes exposed to pollution of volcano mud. Mar Environ Res 96: 73–80. [DOI] [PubMed] [Google Scholar]
- 68.Rockstrom J, Steffen W, Noone K, Persson A, Chapin FS, Lambin EF, Lenton TM, Scheffer M, Folke C, Schellnhuber HJ. et al. (2009) A safe operating space for humanity. Nature 461: 472–475. [DOI] [PubMed] [Google Scholar]
- 69.Root T. (1988) Energy constraints on avian distributions and abundances. Ecology 69: 330–339. [Google Scholar]
- 70.Rudd MA, Beazley KF, Cooke SJ, Fleishman E, Lane DE, Mascia MB, Roth R, Tabor G, Bakker JA, Bellefontaine T. et al. (2011) Generation of priority research questions to inform conservation policy and management at a national level. Conserv Biol 25: 476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salafsky N, Redford KH. (2013) Defining the burden of proof in conservation. Biol Conserv 166: 247–253. [Google Scholar]
- 72.Salafsky N, Margoluis R, Redford KH, Robinson JG. (2002) Improving the practice of conservation: a conceptual framework and research agenda for conservation science. Conserv Biol 16: 1469–1479. [Google Scholar]
- 73.Seebacher F, Franklin CE. (2012) Determining environmental causes of biological effects: the need for a mechanistic physiological dimension in conservation biology. Philos Trans R Soc Lond B Biol Sci 367: 1607–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sinclair BJ, Addo-Bediako A, Chown SL. (2003a) Climatic variability and the evolution of insect freeze tolerance. Biol Rev 78: 181–195. [DOI] [PubMed] [Google Scholar]
- 75.Sinclair BJ, Vernon P, Klok CJ, Chown SL. (2003b) Insects at low temperatures: an ecological perspective. Trends Ecol Evol 18: 257–262. [Google Scholar]
- 76.Soulé ME. (1985) What is conservation biology? Bioscience 35: 727–734. [Google Scholar]
- 77.Spicer J, Gaston K. (1999) Physiological Diversity: Ecological Implications. Wiley-Blackwell, MA, USA. [Google Scholar]
- 78.Stewart GB, Coles CF, Pullin AS. (2005) Applying evidence-based practice in conservation management: lessons from the first systematic review and dissemination projects. Biol Conserv 126: 270–278. [Google Scholar]
- 79.Sutherland WJ, Pullin AS, Dolman PM, Knight TM. (2004) The need for evidence-based conservation. Trends Ecol Evol 19: 305–308. [DOI] [PubMed] [Google Scholar]
- 80.Sutherland WJ, Spiegelhalter D, Burgman MA. (2013) Twenty tips for interpreting scientific claims. Nature 503: 335–337. [DOI] [PubMed] [Google Scholar]
- 81.Terblanche JS, Hoffmann AA, Mitchell KA, Rako L, le Roux PC, Chown SL. (2011) Ecologically relevant measures of tolerance to potentially lethal temperatures. J Exp Biol 214: 3713–3725. [DOI] [PubMed] [Google Scholar]
- 82.Thompson B, Diamond KE, McWilliam R, Snyder P, Snyder SW. (2005) Evaluating the quality of evidence from correlational research for evidence-based practice. Except Children 71: 181–194. [Google Scholar]
- 83.Tracy CR, Nussear KE, Esque TC, Dean-Bradley K, Tracy CR, DeFalco LA, Castle KT, Zimmerman LC, Espinoza RE, Barber AM. (2006) The importance of physiological ecology in conservation biology. Integr Comp Biol 46: 1191–1205. [DOI] [PubMed] [Google Scholar]
- 84.Venette RC, Abrahamson M. (2010) Cold hardiness of emerald ash borer, Agrilus planipennis: a new perspective. In black ash symposium: proceedings of the meeting, 25–27 May 2010. Bemidji, MN, USA. United States Department of Agriculture, Forest Service, Chippewa National Forest, 5 pp. http://www.fs.usda.gov/Internet/FSEDOCUMENTS/stelprdb5191794.pdf [Google Scholar]
- 85.White PJT, Kerr JT. (2007) Human impacts on environment–diversity relationships: evidence for biotic homogenization from butterfly species richness patterns. Glob Ecol Biogeogr 16: 290–299. [Google Scholar]
- 86.Wiens JJ, Graham CH. (2005) Niche conservatism: integrating evolution, ecology, and conservation biology. Ann Rev Ecol Evol Syst 36: 519–539. [Google Scholar]
- 87.Wiens JA, Stenseth NC, Vanhorne B, Ims RA. (1993) Ecological mechanisms and landscape ecology. Oikos 66: 369–380. [Google Scholar]
- 88.Wikelski M, Cooke SJ. (2006) Conservation physiology. Trends Ecol Evol 21: 38–46. [DOI] [PubMed] [Google Scholar]
- 89.Williams CM, Marshall KE, MacMillan HA, Dzurisin JDK, Hellmann JJ, Sinclair BJ. (2012) Thermal variability increases the impact of autumnal warming and drives metabolic depression in an overwintering butterfly. PLoS ONE 7: e34470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wolkovich EM, Regetz J, O'Connor MI. (2012) Advances in global change research require open science by individual researchers. Glob Change Biol 18: 2102–2110. [Google Scholar]
- 91.Zalucki MP, Lammers JH. (2010) Dispersal and egg shortfall in Monarch butterflies: what happens when the matrix is cleaned up? Ecol Entomol 35: 84–91. [Google Scholar]