Ocean acidification is predicted to affect the performance of marine species, but little is known about the effects on sharks. We found that long-term exposure to elevated CO2 did not affect the epaulette shark, possibly because it experiences fluctuating environmental conditions in its shallow coral reef habitat.
Keywords: Climate change, ecophysiology, elasmobranch, hypoxia tolerance, ocean acidification
Abstract
Ocean acidification, resulting from increasing anthropogenic CO2 emissions, is predicted to affect the physiological performance of many marine species. Recent studies have shown substantial reductions in aerobic performance in some teleost fish species, but no change or even enhanced performance in others. Notably lacking, however, are studies on the effects of near-future CO2 conditions on larger meso and apex predators, such as elasmobranchs. The epaulette shark (Hemiscyllium ocellatum) lives on shallow coral reef flats and in lagoons, where it may frequently encounter short-term periods of environmental hypoxia and elevated CO2, especially during nocturnal low tides. Indeed, H. ocellatum is remarkably tolerant to short periods (hours) of hypoxia, and possibly hypercapnia, but nothing is known about its response to prolonged exposure. We exposed H. ocellatum individuals to control (390 µatm) or one of two near-future CO2 treatments (600 or 880 µatm) for a minimum of 60 days and then measured key aspects of their respiratory physiology, namely the resting oxygen consumption rate, which is used to estimate resting metabolic rate, and critical oxygen tension, a proxy for hypoxia sensitivity. Neither of these respiratory attributes was affected by the long-term exposure to elevated CO2. Furthermore, there was no change in citrate synthase activity, a cellular indicator of aerobic energy production. Plasma bicarbonate concentrations were significantly elevated in sharks exposed to 600 and 880 µatm CO2 treatments, indicating that acidosis was probably prevented by regulatory changes in acid–base relevant ions. Epaulette sharks may therefore possess adaptations that confer tolerance to CO2 levels projected to occur in the ocean by the end of this century. It remains uncertain whether other elasmobranchs, especially pelagic species that do not experience such diurnal fluctuations in their environment, will be equally tolerant.
Introduction
Anthropogenic CO2 emissions have caused an increase in atmospheric CO2 by almost 40% over the past 250 years (IPCC, 2013). The resulting rise from pre-industrialization levels (∼280 ppm) to 400 ppm in 2014 has occurred at a rate unprecedented for the past 800 000–1 000 000 years (Raven et al., 2005; Doney and Schimel, 2007; Lüthi et al., 2008). The oceans have absorbed more than 30% of the additional CO2 released by human activities, thus tempering the atmospheric rise in CO2 (Sabine et al., 2004; Sabine and Feely, 2007). However, the resulting rise in seawater CO2 partial pressure (PCO2) and the associated reduction in pH, called ocean acidification, is a significant threat to marine organisms and ecosystems (Hoegh-Guldberg et al., 2007; Fabry et al., 2008).
The reduced carbonate saturation state that accompanies lower seawater pH affects the ability of calcifying marine organisms to form carbonate shells and skeletons (Orr et al., 2005; Doney et al., 2009), but rising oceanic CO2 may also impact the respiratory physiology of many water-breathing organisms. Acid–base disturbances related to elevated environmental CO2 can reduce oxygen uptake and delivery, which could directly impact metabolic performance. Reductions in an organism's scope for aerobic metabolic performance can result in less energy being available for crucial life-history processes, such as growth and reproduction (Pörtner, 2008; Pörtner and Farrell, 2008). For instance, Humboldt squid (Dosidicus gigas) exhibit a 30% reduction in resting metabolic rate and a 45% decrease in activity upon exposure to projected near-future CO2 levels, owing to an impaired oxygen transport system, which would be predicted to reduce overall performance and compress their habitable depth range (Rosa and Seibel, 2008). In contrast, teleost fishes are expected to be physiologically well equipped to compensate pH and ion disturbances caused by high CO2 (Ishimatsu et al., 2008; Brauner and Baker, 2009). Nevertheless, interspecific variation is evident in the physiological responses of teleost fish to elevated CO2; for example, some fishes exhibit no change in aerobic scope in high-CO2 environments (Ishimatsu et al., 2008; Melzner et al., 2009a; Couturier et al., 2013), whereas others reduce aerobic scope (Munday et al., 2009) and some even increase aerobic scope (Couturier et al., 2013; Rummer et al., 2013a) when exposed to near-future CO2 levels. Consequently, the effects of ocean acidification on a broad range of species, including vulnerable and tolerant species, should be investigated in order to identify traits that will be important for individual performance and success in near-future oceans and predict changes in community structure (Melzner et al., 2009b).
In contrast to the growing body of knowledge about the effects of ocean acidification on teleost fishes, little is known about the impacts of rising levels of oceanic CO2 on elasmobranchs. Elasmobranchs buffer a pH disturbance, such as that associated with exposure to high CO2, in a manner similar to teleosts. Bicarbonate is accumulated in the blood, but in addition, elasmobranchs may also increase branchial ammonia excretion rates to ameliorate the acidosis further (Evans, 1982; King and Goldstein, 1983; Claiborne and Evans, 1992; Brauner and Baker, 2009; Tresguerres et al., 2010). The haemoglobin of elasmobranchs also has a much higher buffering capacity compared with that of most teleosts, and thus, O2 transport and aerobic performance may be less sensitive to pH disturbances (Berenbrink et al., 2005). Yet, it is thought that the resilience of elasmobranchs to acid–base disturbances is related largely to their sophisticated acid excretion processes at the gill (Wood et al., 1995). If elasmobranchs are notably tolerant to near-future CO2 conditions, this could potentially increase predation pressure and alter species compositions of marine environments.
The epaulette shark (Hemiscyllium ocellatum) exhibits exceptionally high tolerance to the severe hypoxia (low oxygen) that it routinely experiences while inhabiting shallow coral reef flats (Routley et al., 2002; Nilsson and Renshaw, 2004), and thus, it may not be surprising if this species is also tolerant to near-future CO2. However, acute responses may differ dramatically from the responses to long-term exposure; studies on H. ocellatum in response to anoxia or hypoxia have been following only minutes to hours of exposure (Wise et al., 1998; Renshaw et al., 2002; Routley et al., 2002; Chapman and Renshaw, 2009; Dowd et al., 2010; Speers-Roesch et al., 2012a). No study, to date, has examined how H. ocellatum responds to prolonged exposure to elevated CO2. Given that increased uptake of CO2 by the ocean will affect both the average CO2 levels and the magnitude of extreme CO2 levels (Shaw et al., 2013), it is important to consider longer-term responses to elevated CO2 beyond those that would be experienced on a diurnal basis (e.g. hours; Ohde and van Woesik, 1999; Compagno, 2002; Last and Stevens, 2009; Shaw et al., 2013). Thus, both the physiological sensitivity of the organism and the variations it may already be experiencing in its habitat are important when considering which species will exhibit positive or negative responses to rising ocean CO2 levels. However, it is also important to consider the relationship between environmental cues and other traits, such as behaviour—which is especially relevant to species like H. ocellatum—when considering the importance of phenotypic plasticity, because this could influence selection over the longer term (Marais and Chown, 2008).
We exposed H. ocellatum to near-future CO2 conditions for a minimum of 60 days and measured resting oxygen consumption rates and critical oxygen tensions as proxies for resting metabolic rate and sensitivity to hypoxia, respectively. In addition to whole-organism responses, we also measured or calculated haematological and tissue parameters, including plasma ionic (HCO3−, Cl−, Na+ and K+) and urea concentrations, haemoglobin (Hb), mean cell haemoglobin concentration (MCHC), haematocrit (Hct), spleen–somatic index (SSI) and citrate synthase activity in heart, brain and red muscle. The aim was to provide insight into the physiological parameters that may underpin changes in metabolic performance and sensitivity to hypoxia in this species. We hypothesized that H. ocellatum can physiologically tolerate elevated CO2 because it routinely experiences daily reductions in environmental O2 (Routley et al., 2002; Nilsson and Renshaw, 2004) and probably elevations in CO2. However, if CO2 tolerance is related to the diurnal patterns this species already experiences in their natural habitat, prolonged exposure (60 days) to elevated CO2 may negatively affect metabolic rate and hypoxia tolerance.
Materials and methods
Experimental animals
Epaulette sharks (Hemiscyllium ocelatum) were collected from the northern Great Barrier Reef by Northern Barrier and Cairns Marine (Cairns, Queensland, Australia) and transported to James Cook University (JCU). Five to six individuals were placed in each of six 700 l tanks in a recirculating seawater system. Individuals were measured [standard length, 33.38 ± 7.29 cm (mean ± SD)] to ensure an equal distribution of sizes among tanks. Unique fin clips along the margins of pectoral, pelvic and dorsal fins were used for individual identification. Shelter was provided in the form of PVC pipe sections placed within each tank. Food was provided once every 24 h and consisted of 4% of shark biomass per tank in raw prawn meat. There was no indication that any individuals or treatment groups were eating less than this amount throughout the duration of the study. Sharks were acclimated to laboratory conditions for at least 4 weeks prior to commencing CO2 treatments.
Experimental CO2 conditions
The experimental system comprised three 8000 l recirculating seawater systems, each set to simulate one of the following three CO2 treatments: control (∼390 µatm); medium (∼600 µatm); and high (∼880 µatm). Carbon dioxide levels were achieved and maintained by CO2 infusion of seawater in 3000 l sumps attached to each recirculating seawater system. The pHNBS (National Bureau of Standards scale) levels were set to match target CO2 concentrations and maintained using a CO2-infusing system (Aqua Medic GmbH, Bissendorf, Germany). If the pH rose above the set point, an electronic solenoid initiated the system to deliver a steady stream of CO2 into a diffuser within the corresponding sump. Carbon dioxide-equilibrated seawater from each system was delivered to two replicate 700 l tanks (∼25 l min−1) per treatment. Each tank contained five or six sharks, as described above. This central approach of pH manipulation allowed for stability in seawater pH and PCO2 within the holding tanks. Tanks were covered with transparent plastic sheeting to reduce CO2 loss to the atmosphere.
The pHNBS was measured daily (Hach, model #HQ40d) in each tank to ensure that it remained within ±0.05 of desired levels. Temperatures were also measured daily and maintained at 28.5°C by automated heater/chillers attached to each seawater system. Salinity and alkalinity were measured on a weekly basis. Total alkalinity (TA) was estimated using Gran titrations and certified reference materials (Dr A. G. Dickson, Scripps Institution of Oceanography). Average seawater PCO2 was calculated using these parameters in CO2SYS (Pierrot et al., 2006) and using constants from Dickson and Millero (1987) (Table 1).
Table 1:
Mean values for PCO2, pH, total alkalinity, salinity and temperature over the course of the CO2 exposure period
| Treatment | Tank number | PCO2 (μatm) | pH | Total alkalinity (μmol kg−1) | Salinity (ppt) | Temperature (°C) |
|---|---|---|---|---|---|---|
| Control | 1 | 397 ± 6.5 | 8.16 ± 0.006 | 2145 ± 4.7 | 35.6 ± 0.07 | 28.6 ± 0.05 |
| Control | 2 | 384 ± 6.8 | 8.18 ± 0.006 | 2145 ± 4.7 | 35.6 ± 0.07 | 28.4 ± 0.04 |
| Medium | 1 | 614 ± 16.6 | 8.00 ± 0.009 | 2095 ± 5.1 | 35.9 ± 0.07 | 28.7 ± 0.05 |
| Medium | 2 | 608 ± 16.5 | 8.00 ± 0.009 | 2095 ± 5.1 | 35.9 ± 0.07 | 28.6 ± 0.05 |
| High | 1 | 876 ± 14.6 | 7.86 ± 0.006 | 2079 ± 5.3 | 36.0 ± 0.03 | 28.7 ± 0.03 |
| High | 2 | 861 ± 14.4 | 7.87 ± 0.006 | 2079 ± 5.3 | 36.0 ± 0.03 | 28.7 ± 0.04 |
Total alkalinity was measured weekly for each treatment condition, and temperature was measured daily for each tank within each treatment. Means were calculated for each treatment over the entire experimental period and are given for each holding tank, ±SEM. Abbreviation: PCO2, seawater carbon dioxide partial pressure.
Sharks were introduced to the CO2 treatments following 30 days acclimation to laboratory holding conditions and were then maintained in their respective CO2 treatment conditions for a minimum of 60 days prior to physiological experimentation.
Experimental protocol
Resting oxygen consumption rates
Resting O2 consumption rates (ṀO2Rest) were determined for sharks following 60–68 days of exposure to control (n = 10), medium (n = 12) and high (n = 11) CO2 conditions and a 48 h fasting period using an intermittent-flow respirometry system with purpose-built respirometry chambers. Animals were transferred individually into the cylindrical 11 or 15 l respirometry chambers (depending on animal body size) submerged in a temperature-controlled aquarium (28.5°C) within each animal's respective experimental CO2 conditions and habituated to the chamber for 12 h before oxygen consumption measurements commenced. Submersible pumps were fitted to each chamber to supply a continuous water flow (1300 l h−1; WEIPRO WH-2000; Yongcheng Aquarium Co., Ltd, Guangdong, China) from the surrounding water bath through the chambers. During respirometry trials, a digital relay timer (MFRT-1 Multi Function Recycling Timer; Xiamen SUPERPRO Technology Co., Ltd, Xiamen, Fujian, China) was used to stop water flow for 15 or 20 min and then resume flushing for 15 min over a total period of 12 h. The intervals of interrupted water flow were short enough to ensure that oxygen within the chambers did not fall below 80% saturation at any time, while flush periods were long enough to eliminate accumulation of metabolic CO2 and allow water oxygen levels to return to 100% saturation (Steffensen et al., 1984; Steffensen, 1989). A second pump (1300 l h−1; WEIPRO WH-2000) was connected to each respirometry chamber to recirculate water continuously within the chamber, thus ensuring complete mixing and homogeneous water PO2 (PWO2). Contactless spots (2 mm) with oxygen-sensitive REDFLASH dye were adhered to the inside of glass tubes connected to the recirculating pumps on each respirometer. These spots were then linked to a Firesting Optical Oxygen Meter (Pyro Science e. K., Aachen, Germany) via 5 m fibre-optic cables to record continuously (0.5 Hz) the temperature-compensated O2 concentration (in milligrams per litre) of the water within each chamber over the 12 h period of time. The 0 and 100% oxygen levels of the Firesting oxygen meter were calibrated using 0 and 100% air-saturated seawater. At the end of each trial, the wet mass was taken for each shark [232.47 ± 117.98 g (mean ± SD)] prior to release back to experimental holding conditions.
Critical oxygen tension
Upon completion of ṀO2Rest measurements, sharks were permitted to recover in their respective CO2 treatment conditions for ∼3 weeks. Then, the same respirometers used to determine ṀO2Rest were used to determine the critical oxygen tension (Pcrit) for the same sharks exposed to control (n = 9), medium (n = 12) and high CO2 (n = 10). By this point, sharks would have been exposed to their respective experimental conditions for 85–92 days. Prior to measurements, sharks were fasted for 48 h before being introduced to the cylindrical 11–15 l respirometry chambers. Then, the ṀO2 of each animal was monitored for a minimum of 4 h using an intermittent flush cycle (15 min flush–15 min closed) so that stable ṀO2Rest was achieved prior to commencing the hypoxia experiment. The respirometers were then sealed by turning off flush pumps and closing previously installed ball-valves downstream of the flush pumps. Oxygen levels in the chamber were monitored continuously (0.5 Hz) and allowed to decrease to at least 0.8 mg l−1 to ensure that the critical oxygen tension for each individual was recorded (based on estimates from Routley et al., 2002). The changes in water pH and PCO2 that occur when using closed respirometry for a short period of time have been shown previously to have no effect on Pcrit in fish (Henriksson et al., 2008). After this oxygen concentration was achieved, the aforementioned flush cycle was reinstated such that O2 levels within each respirometer could quickly return to normoxic conditions (100% air-saturated seawater).
Haematological and tissue analyses
Following Pcrit measurements, animals were returned to their treatment tanks to recover for ∼1 week. After this time, blood was sampled from sharks exposed to control (n = 8), medium (n = 10) and high (n = 8) CO2 conditions by inserting a 23 gauge needle posterior to the cloaca into the caudal vein and collecting the blood (<1% of body volume) into heparinized syringes. Animals were then euthanized by severing the spinal cord using the method described by Speers-Roesch et al. (2012b) so that tissues could be sampled. Whole blood [Hb] was determined using the HemoCue® (Hb 201 System, Australia Pty Ltd) with 10 µl of whole blood and was reported as grams per 100 ml using a calibration curve according to Clark et al. (2008) corrected for tropical reef species by Rummer et al. (2013b). The Hct was determined by centrifuging 60 µl of whole blood in heparinized microcapillary tubes for 3 min at 17 000g and calculated as the ratio of packed red blood cells to total blood volume (as a percentage). Both [Hb] and Hct were used to calculate the MCHC. The spleen was dissected from each shark and weighed to the nearest 0.001 g. The SSI was calculated as the ratio of the spleen to body mass (as a percentage). Plasma was flash frozen immediately in liquid nitrogen and then stored at −80°C until analysis for [HCO3−] via colorometrically linked enzyme assay and for [Na+], [K+], [Cl−] (1:1 dilution with deionized water) and [urea] (1:19 dilution with deionized water) via ion-specific electrodes (ISE; Beckman Coulter System AU480). Heart, brain and red muscle samples were also collected and frozen in liquid N2 for citrate synthase enzyme analysis according to McClelland et al. (2005). Briefly, frozen tissues were homogenized in a standard buffer solution containing 5 mm EDTA, 0.1% Triton X-100, 0.2 mm dithiothreitol and 50 mm Hepes (adjusted to pH 7.4) and stored at −80°C. The citrate synthase assay buffer contained (mm): 20 Tris (pH 8.0), 0.1 5,5-dithiobis (2-nitrobenzoic acid) and 0.3 acetyl-CoA. The reaction was initiated by the addition of 0.5 mm oxaloacetate, and absorbance was measured for 5 min at 412 nm. Control samples were assayed without oxaloacetate to control for background hydrolase activity.
Calculations and statistical analyses
Raw text files created for the Firesting recordings were imported offline into LabChart version 6.1.3 (ADInstruments, Colorado Springs, CO, USA), which was used to analyse data. A modified version of equations from Bushnell et al. (1994) and Schurmann and Steffensen (1997) was used to calculate ṀO2Rest (in milligams per kilogram per hour). To do this, the average of the shallowest 10% of slopes [change in O2 concentration over a period of 15–20 min (in milligams of O2 per litre per second) in between flushing cycles] was determined for each individual shark. From this, the appropriate proportion of background O2 consumption, which was measured 2–3 h before and after each trial for each respirometer and assumed linear, was subtracted. This value was then multiplied by the volume of the respirometer (in litres; minus the volume of the fish), all of which was divided by the mass of the fish (in kilograms). Respirometers were cleaned daily to ensure that background (microbial) respiration did not exceed 10% of the ṀO2Rest of the sharks. Means and SEM for ṀO2Rest were calculated for each of the three CO2 treatments.
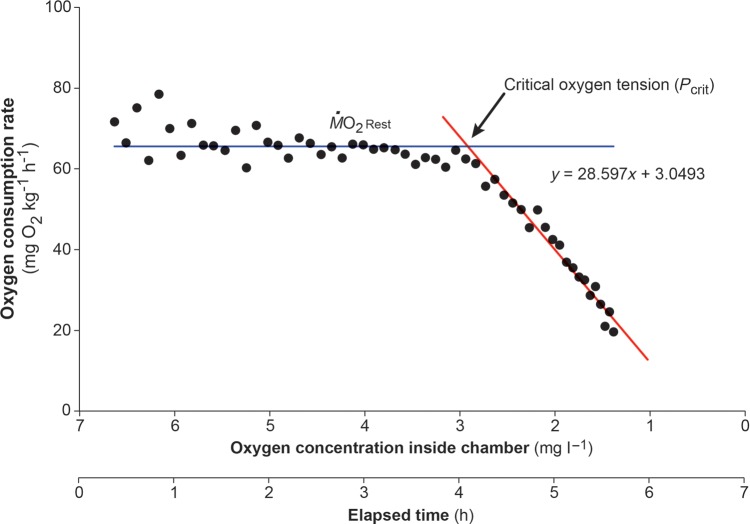
A similar data extraction and calculation protocol was followed for determining Pcrit. Again, ṀO2Rest was calculated for each shark from the shallowest 10% of slopes that were recorded prior to sealing the respirometer. Then, the mean slope for every 5 min period of time while the respirometer was sealed was extracted (usually 20–30 slopes), and ṀO2 values were calculated from those slopes. To determine Pcrit, all ṀO2 values were plotted against the oxygen concentration within the chamber for each shark. A horizontal (regression) line was fitted to the mean ṀO2Rest prior to sealing the respirometer. Then, a linear regression was applied to all of the points that consecutively fell below ṀO2Rest once the respirometer had been sealed. The point at which both regression lines intersected was reported as the critical oxygen tension or Pcrit (in milligams of O2 per litre) for that individual (Fig. 1; Ott et al., 1980; Nilsson et al., 2004; Collins et al., 2013). Means and SEM for Pcrit were calculated for each CO2 treatment.
Figure 1:
Representative trace illustrating the changes in oxygen consumption rate of an individual epaulette shark (Hemiscyllium ocellatum) as the oxygen concentration of the water decreased and the time over which this occurred. The parallel line represents the resting oxygen consumption rate. After the respirometry chamber was sealed, the oxygen consumption rate began to decrease below resting levels. The diagonal line is a trend line, with the intersection of both lines demarcating the critical oxygen tension (Pcrit).
Nested ANOVA, with holding tanks nested within CO2 treatments, was first used to test whether there was a significant effect of holding tank on mean ṀO2Rest (in milligams per kilogram per hour) or mean Pcrit (in milligams of O2 per litre). As there was no significant effect of tanks on either parameter, data from the two tanks within treatments were pooled for further analyses. ANCOVA was used to compare ṀO2Rest among the three CO2 treatments, with standard length as a covariate. To compare Pcrit among treatment groups, a robust regression analysis was performed with standard length as a covariate. Robust regression analysis was chosen over ANCOVA for Pcrit analysis due to potential outliers that could otherwise be solely responsible for significant outcomes. The removal of such outliers was rejected owing to the relatively small sample size. Instead, robust regression weighs values differently based on their chance of being an outlier. Hence, the further away a single data point was from the mean, the less influential it became for the statistical outcome of the analysis. There was no interaction between the main effect (CO2) and the covariate in either analysis; therefore, to increase statistical power, the analyses were run again without this term included. Standard length was not included in haematological and tissue data analyses because it had no significant effect on the outcomes. ANOVAs were then used together with Holm–Sidak post hoc tests to compare haematological and tissue parameters between animals acclimated to control, medium and high CO2 conditions. Statistical significance was accepted when P < 0.05. All analyses were carried out using S-Plus (TIBCO Software Inc., Palo Alto, CA, USA).
Results
Resting oxygen consumption rates
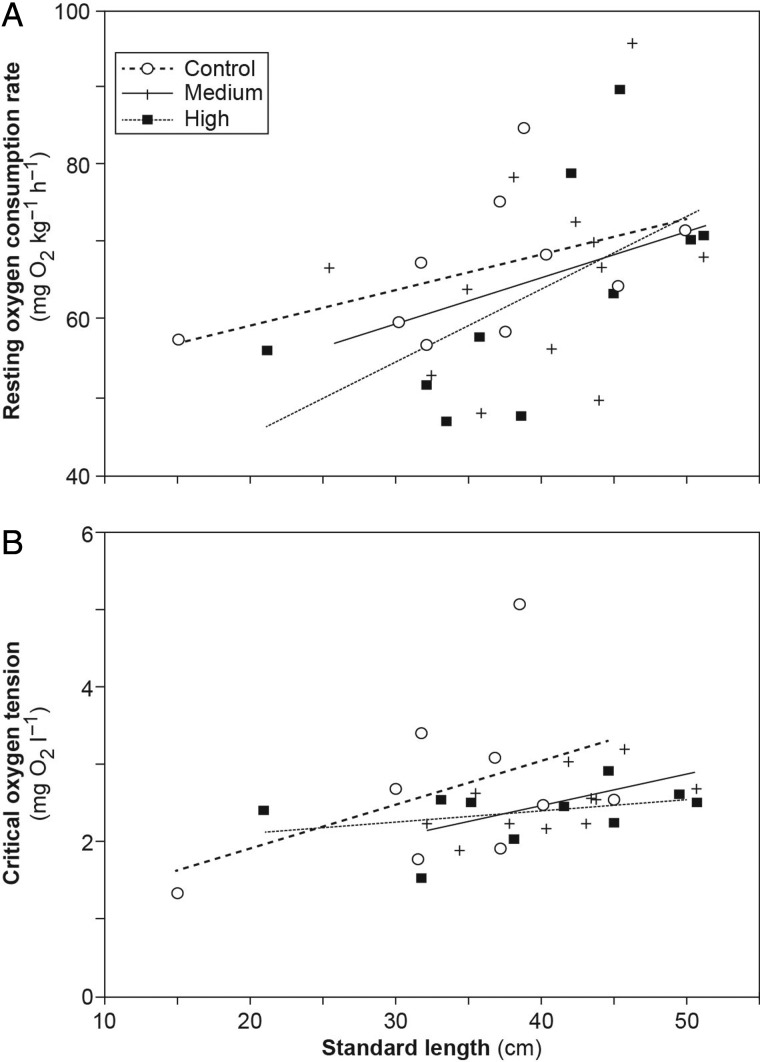
There were no significant differences in ṀO2Rest values between CO2 treatment groups (F2,28 = 0.578; P = 0.568). However, ṀO2Rest depended on the standard length of the individuals, with larger animals having a higher ṀO2Rest than smaller animals (F2,28 = 6.70; P = 0.0151; Fig. 2A). Values for ṀO2Rest ranged from 46.8 to 95.4 mg O2 kg−1 h−1 with a mean of 65.2 ± 2.13 mg O2 kg−1 h −1 across all treatments.
Figure 2:
The relationship between resting oxygen consumption rate (A) and critical oxygen tension (Pcrit; B) with the standard length of individual sharks from control, medium and high CO2 treatment groups. Please refer to Materials and methods for further details regarding calculations.
Critical oxygen tension
The Pcrit values did not differ significantly between CO2 treatment groups (t4,26 = − 0.170; P = 0.866). However, standard length had a significant effect on the Pcrit of individuals (t4,26 = 2.26; P = 0.0323; Fig. 2B), with larger animals reaching Pcrit at a higher seawater O2 concentration than smaller animals. The Pcrit values ranged from 1.32 to 5.07 mg O2 l−1 with a mean of 2.51 ± 0.122 mg O2 l−1 across all treatment groups.
Haematology and tissue samples
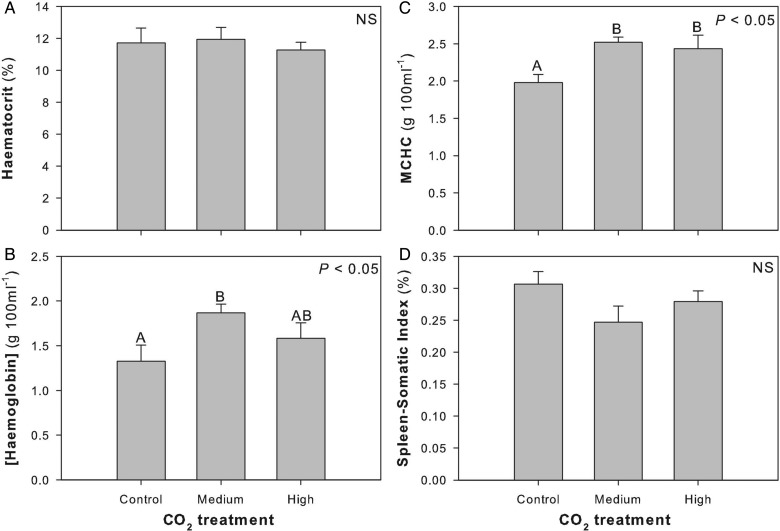
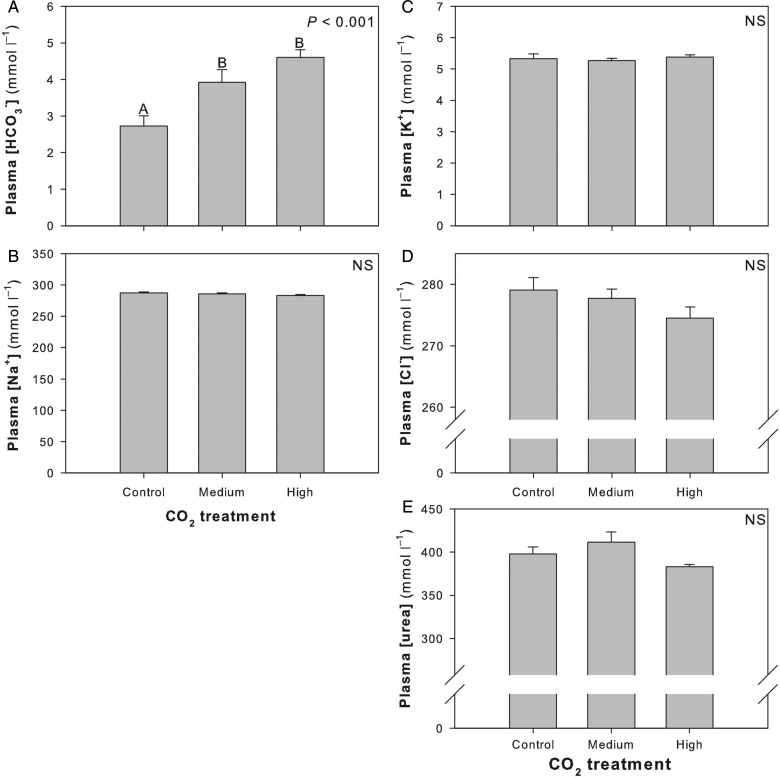
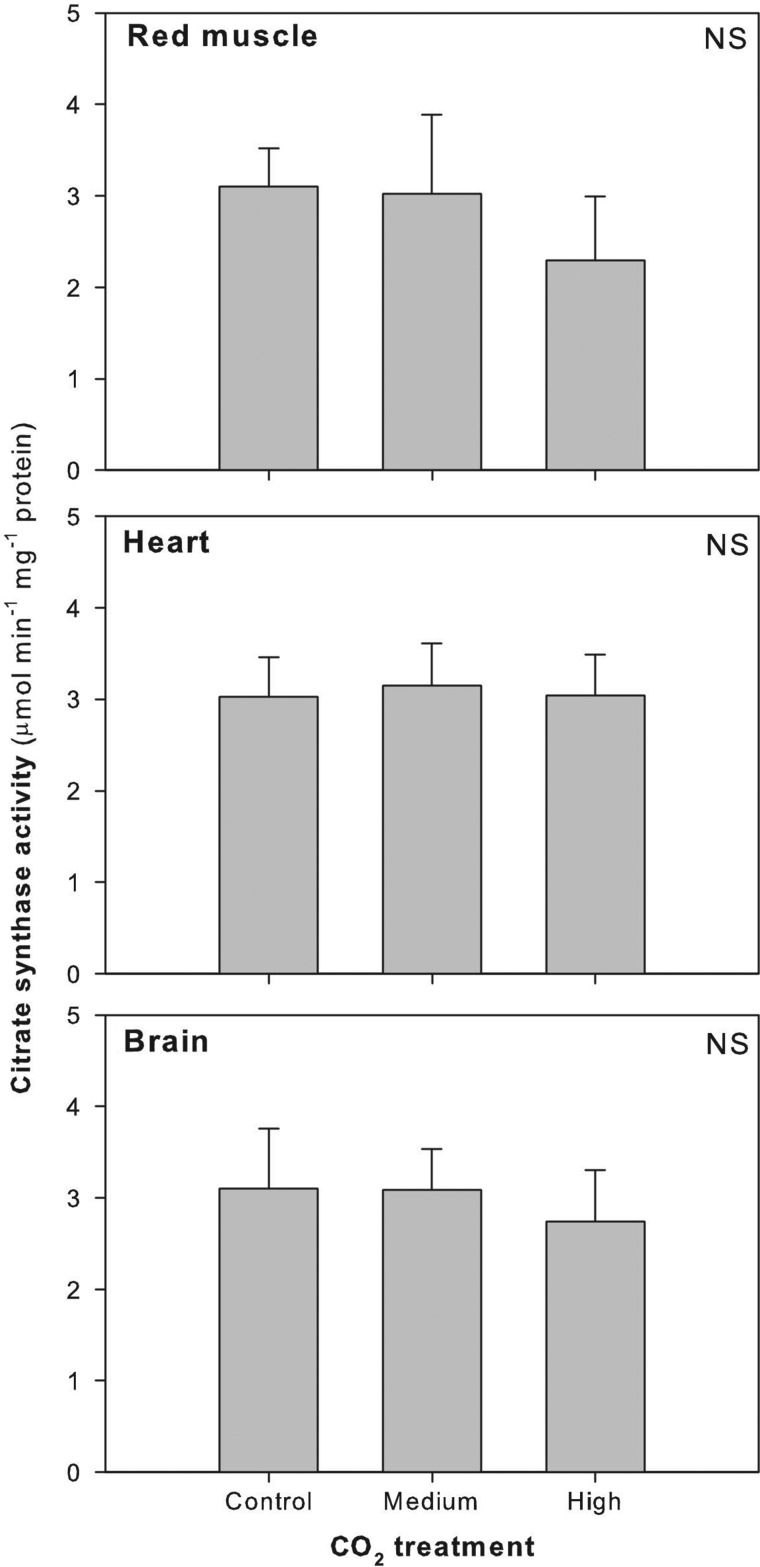
No significant differences were detected in Hct between CO2 treatment groups (F2,22 = 0.214; P = 0.809; Fig. 3A). There was a significant increase in [Hb] between the control and the medium CO2 treatment groups (F2,23 = 3.447; P = 0.048; Fig. 3B), an elevation that was maintained with the high CO2 treatment group for MCHC values (F2,21 = 5.067; P = 0.0160; Fig. 3C). Although not significant, there was a trend toward decreased SSI with high CO2 exposure (F2,22 = 2.050; P = 0.153; Fig. 3D). There was a significant increase in plasma [HCO3−] in both the medium and high CO2 treatment groups (F2,21 = 10.893; P < 0.001; Fig. 4A). However, there was no difference in plasma [Na+], [K+], [Cl−] or [urea] between control and CO2 treatment groups ([Na+], F2,21 = 1.543, P = 0.237, Fig. 4B; [K+], F2,21 = 0.247, P = 0.783, Fig. 4C; [Cl−], F2,21 = 1.697, P = 0.207, Fig. 4D; and [urea], F2,21 = 2.907, P = 0.077, Fig. 4E). Citrate synthase activity did not change significantly between control and CO2 treatment groups in red muscle (F2,16 = 0.371; P = 0.696), heart (F2,18 = 0.0238; P = 0.976) or brain (F2,19 = 0.131; P = 0.878; Fig. 5).
Figure 3:
Changes in haematocrit (A), haemoglobin concentration (B), mean cell haemoglobin concentration (MCHC; C), and spleen–somatic index (D) after sharks were exposed to control, medium or high CO2 for ∼90 days. Different letters within a panel demarcate significant differences between treatment groups, and statistical significance is noted in the top right corner of each panel. Abbreviation: NS, not significant.
Figure 4:
Changes in plasma parameters after sharks were exposed to control, medium or high CO2 for ∼90 days. Different letters within a panel demarcate significant differences between treatment groups, and statistical significance is noted in the top right corner of each panel. Abbreviation: NS, not significant.
Figure 5:
Changes in red muscle, heart and brain citrate synthase enzyme activity after sharks were exposed to control, medium or high CO2 for ∼90 days. Statistical significance is noted in the top right corner of each panel. Abbreviation: NS, not significant.
Discussion
Long-term exposure to near-future CO2 conditions did not significantly affect metabolic performance or hypoxia sensitivity of epaulette sharks. In contrast, changes in [Hb] and MCHC were evident after ∼90 days of exposure to 600 µatm CO2 levels, and plasma [HCO3−] was elevated in both the moderate and high CO2 treatment groups, suggesting that physiological adjustments were being made to cope with elevated CO2 at the level of oxygen transport and ion regulation. However, there was no increase in metabolic capacity at the level of the mitochondria, as indicated by the lack of change in citrate synthase activity. Our findings suggest that, for this reef-inhabiting benthic elasmobranch, neither the energetic costs of basic maintenance nor sensitivity to hypoxia may be compromised in the elevated CO2 conditions projected for the end of this century.
The compensatory mechanisms used by H. ocellatum to maintain resting metabolic rates in normoxic and hypoxic conditions after prolonged exposure to elevated CO2 may be linked to maintaining oxygen uptake and delivery and ion regulation. Following ∼90 days of CO2 exposure, epaulette sharks exhibited a significant increase in [Hb] and MCHC. Short-term changes in haematological parameters have been documented in teleosts and elasmobranchs following capture, cannulation and exercise (Soivio et al., 1973; Wood et al., 1977; Turner et al., 1983; Wells et al., 1986), upon acclimation to elevated temperature (adult horn sharks, Heterodontus francisci; Neale et al., 1977) and in response to anoxia (grey carpet shark, Chiloscyllium punctatum, and epaulette shark; Chapman and Renshaw, 2009). In teleosts, acute changes can be associated with adrenergic red blood cell (RBC) swelling (Caldwell et al., 2006), a mechanism in place to protect RBC pH and oxygen transport during stress, but not known to occur in elasmobranchs (Berenbrink et al., 2005). Both teleosts and elasmobranchs do, however, use their spleen to produce and store RBCs (Turner et al., 1983; Fänge and Nilsson, 1985; Lai et al., 2006) and can contract it to increase the proportion of RBCs in the circulation (Ken-Ichi, 1988; Lai et al., 2006), presumably to aid in oxygen transport (Jensen et al., 1992). We observed a decrease, although non-significant, in the SSI in sharks exposed to both medium and high CO2, suggesting that splenic contraction(s) may have occurred at some point during the CO2 exposure period. Periodic splenic contractions could also increase the proportion of immature RBCs in circulation, which could explain the slight increase in MCHC without significant changes in Hct. The temporal scale of splenic RBC release and subsequent increases in erythropoietin, the glycoprotein responsible for regulating RBC numbers, is well understood for teleosts (Lai et al., 2006) and could be similar in elasmobranchs exposed to elevated CO2 over extended periods of time, which is worth further investigation.
Plasma [HCO3−] was elevated in sharks upon 90 days of exposure to elevated CO2, which indicates some level of long-term acid–base compensation. This finding is supported by studies by Deigweiher et al. (2008), in which acclimation to elevated CO2 over 6 weeks in a marine teleost resulted in upregulation of Na+/HCO3− cotransporters (NBC1) and Na+–K+-ATPase at higher densities. Given the relationship between bicarbonate availability and synthesis of urea (the predominant osmolyte used by most elasmobranchs), acid–base compensatory mechanisms could have affected [urea] and therefore the efficiency of osmoregulatory pathways (Wood et al., 1995). As [urea] did not change with CO2 exposure, this may not be problematic at the CO2 levels used here and/or over the 90 day duration. The activity of citrate synthase, the first enzyme of the Krebs cycle located within the mitochondria, can be a good indicator of aerobic capacity. Unchanged citrate synthase activity after prolonged CO2 exposure further suggests that there is no limitation at the level of aerobic energy production in any of the tested tissues (McClelland et al., 2005). Although there may have been no changes to aerobic capacity, changes may have been occurring in anaerobic pathways (e.g. activity of lactate dehydrogenase, the last enzyme of anaerobic glycolysis) to maintain energy production. This would be worthy of further investigation. As Esbaugh et al. (2012) suggest, species that are already adapted to low levels of CO2 may no longer rely on traditional short-term acid–base compensation strategies but instead use morphological changes (e.g. gill permeability, diffusion distances) or alter chemical equilibrium constraints in the blood over longer periods to maintain oxygen transport.
While there were no changes in metabolic performance in the sharks upon long-term CO2 exposure, there was an unexpected pattern of mass-specific metabolic rates, with larger sharks exhibiting higher mass-specific metabolic rates than smaller sharks. This contradicts the usual pattern exhibited by ectotherms, but may be related to their feeding patterns. For example, we examined sharks ranging in size from ∼20 to 50 cm. However, we used a set 48 h fasting period prior to determining oxygen consumption rates and prior to blood and tissue sampling because of their small size and benthic lifestyle and previous feeding patterns while in captivity. It could have been that 48 h was sufficient fasting time for the smaller animals but not for the larger animals of that size range (Wood et al., 2007). Therefore, the larger animals could have been exhibiting slightly increased metabolic rates due to specific dynamic action, which could also mask any acid–base processes occurring due to CO2 exposure. The relationship between acid–base disturbances originating from feeding and those due to elevated water CO2 has ecological relevance and should be investigated in future studies.
Environment and lifestyle play an important role in physiological tolerance to changing environmental conditions (Pörtner and Farrell, 2008), and this study confirms that H. ocellatum is no exception. It is already known that H. ocellatum exhibits the lowest value of Pcrit shown for any elasmobranch tested to date, suggesting an exceptional tolerance to short-term hypoxia, which is unique among chondrichthyans (Wise et al., 1998; Routley et al., 2002). Hemiscyllium ocellatum occupies shallow reef platforms that are subject to dramatic diurnal fluctuations in environmental O2 and CO2 conditions (Routley et al., 2002; Diaz and Breitburg, 2009; Last and Stevens, 2009). During calm nights, the low O2 tension encountered on coral reefs can drop below 10% air saturation (Routley et al., 2002), usually as a result of respiration by reef organisms and especially during nocturnal low tides. This can also result in elevations in PCO2, which have been reported to exceed 1000 µatm on shallow reef flats at night (Ohde and van Woesik, 1999; Shaw et al., 2013). The CO2 levels may even be higher in caves, reef crevices and restricted-flow habitats, which are used by H. ocellatum for shelter (Compagno, 2002; Last and Stevens, 2009). Indeed, diurnal or acute fluctuations in O2 and CO2 may play a role in signalling metabolism in species using such habitats. However, acute responses often differ dramatically from responses to prolonged exposure, and it is important to make this distinction. The increased uptake of CO2 by the ocean will affect both the average CO2 level and the magnitude of extreme CO2 fluctuations (Ohde and van Woesik, 1999; Shaw et al., 2013). This makes our finding that H. ocellatum exhibited no change in metabolic performance, including sensitivity to hypoxia, after prolonged exposure to projected future CO2 levels even more important.
Adaptation to life on shallow reef platforms and lagoons may be the key to species like H. ocellatum for maintaining performance in projected future CO2 concentrations (Melzner et al., 2009b). While noteworthy, what was previously known about the physiological tolerance of the epaulette shark to challenging environmental conditions was related to acute exposure of minutes to hours. This is extremely relevant to a shelter-seeking, benthic, reef-dwelling species like the epaulette shark that would experience such conditions burrowing into coral caves to avoid predation or to exploit food sources, activities vital to biological fitness. Pelagic shark species, many of which function as apex predators in their respective environments (Last and Stevens, 2009), however, do not typically exhibit shelter-seeking behaviours in areas that would experience the routine fluctuations in water chemistry experienced by H. ocellatum and therefore may not tolerate prolonged periods of elevated CO2. Given that increased uptake of CO2 by the ocean may mean that the high CO2 levels that the epaulette shark may already routinely experience could be the new average ocean CO2 levels, some species may be able to tolerate future conditions better. Future studies should investigate the importance of fluctuating environmental conditions in shaping an organism's tolerance. Differential effects on functional groups could impact predator–prey dynamics, affect the population structure of elasmobranchs and other aquatic organisms inhabiting coral reefs and, ultimately, impact ecosystem health. Investigating both sensitive and tolerant species from an array of habitat types would help to tease apart the role of the environment from other factors, including evolutionary history and behaviour, all of which is important when considering conservation measures under future climate change scenarios.
Funding
This work was supported by funding from the School of Marine and Tropical Biology (D.D.U.H., P.L.M.); the School of Earth and Environmental Science (C.A.S., M.R.H.); AIMS@JCU (D.D.U.H., M.R.H.); and the Australian Research Council Centre of Excellence for Coral Reef Studies (J.L.R., P.L.M.).
Acknowledgements
We thank the Marine and Aquaculture Research Facility Units (MARFU) at James Cook University for the technical support and excellent research facilities, Professor Rhondda Jones and Geoffrey Collins for advice and helpful discussions, as well as K. Corkill and L. Davies for technical support. We also greatly appreciate the efforts of three anonymous reviewers whose thorough and insightful edits, comments and suggestions have helped to make this a much stronger story.
References
- Berenbrink M, Koldkjær P, Kepp O, Cossins AR. (2005) Evolution of oxygen secretion in fishes and the emergence of a complex physiological system. Science 307: 1752–1757. [DOI] [PubMed] [Google Scholar]
- Brauner CJ, Baker DW. (2009) Patterns of acid-base regulation during exposure to hypercarbia in fishes. In Glass ML, Wood SC, eds, Cardio-Respiratory Control in Vertebrates. Springer, Heidelberg, pp 43–63. [Google Scholar]
- Bushnell P, Steffensen J, Schurmann H, Jones D. (1994) Exercise metabolism in two species of cod in arctic waters. Polar Biol 14: 43–48. [Google Scholar]
- Caldwell S, Rummer JL, Brauner CJ. (2006) Blood sampling techniques and storage duration: effects on the presence and magnitude of the red blood cell β-adrenergic response in rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol A Mol Integr Physiol 144: 188–195. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Renshaw G. (2009) Hematological responses of the grey carpet shark (Chiloscyllium punctatum) and the epaulette shark (Hemiscyllium ocellatum) to anoxia and re–oxygenation. J Exp Zool A Ecol Genet Physiol 311: 422–438. [DOI] [PubMed] [Google Scholar]
- Claiborne JB, Evans DH. (1992) Acid-base balance and ion transfers in the spiny dogfish (Squalus acanthias) during hypercapnia: a role for ammonia excretion. J Exp Zool 261: 9–17. [Google Scholar]
- Clark TD, Eliason EJ, Sandblom E, Hinch SG, Farrell AP. (2008) Calibration of a hand-held haemoglobin analyser for use on fish blood. J Fish Biol 73: 2587–2595. [Google Scholar]
- Collins GM, Clark TD, Rummer JL, Carton AG. (2013) Hypoxia tolerance is conserved across genetically distinct sub-populations of an iconic, tropical Australian teleost (Lates calcarifer). Conserv Physiol 1: doi:10.1093/conphys/cot029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagno LJ. (2002) Sharks of the World: An Annotated and Illustrated Catalogue of Shark Species Known to Date. Vol. 2, Bullhead, Mackeral and Carpet Sharks (Heterodontiformes, Lamniformes and Orecto-lobiformes) Rome, Food and Agriculture Organization of the United Nations [Google Scholar]
- Couturier CS, Stecyk JAW, Rummer JL, Munday PL, Nilsson GE. (2013) Species-specific effects of near-future CO2 on the respiratory performance of two tropical prey fish and their predator. Comp Biochem Physiol A Mol Integr Physiol 166: 482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deigweiher K, Koschnick N, Pörtner H-O, Lucassen M. (2008) Acclimation of ion regulatory capacities in gills of marine fish under environmental hypercapnia. Am J Physiol Regul Integr Comp Physiol 295: R1660–R1670. [DOI] [PubMed] [Google Scholar]
- Diaz RJ, Breitburg DL. (2009) The hypoxic environment. Fish Physiol 27: 1–23. [Google Scholar]
- Dickson A, Millero F. (1987) A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res 34: 1733–1743. [Google Scholar]
- Doney SC, Schimel DS. (2007) Carbon and climate system coupling on timescales from the Precambrian to the Anthropocene. Ann Rev Environ Resour 32: 31–66. [Google Scholar]
- Doney SC, Fabry VJ, Feely RA, Kleypas JA. (2009) Ocean acidification: the other CO2 problem. Annu Rev Mar Sci 1: 169–192. [DOI] [PubMed] [Google Scholar]
- Dowd WW, Renshaw GM, Cech JJ, Jr, Kultz D. (2010) Compensatory proteome adjustments imply tissue-specific structural and metabolic reorganization following episodic hypoxia or anoxia in the epaulette shark (Hemiscyllium ocellatum). Physiol Genomics 42: 93–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esbaugh AJ, Heuer R, Grosell M. (2012) Impacts of ocean acidification on respiratory gas exchange and acid–base balance in a marine teleost, Opsanus beta. J Comp Physiol B 182: 921–934. [DOI] [PubMed] [Google Scholar]
- Evans DH. (1982) Mechanisms of acid extrusion by two marine fishes: the teleost, Opsanus beta, and the elasmobranch, Squalus acanthias. J Exp Biol 97: 289–299. [Google Scholar]
- Fabry VJ, Seibel BA, Feely RA, Orr JC. (2008) Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J Mar Sci 65: 414–432. [Google Scholar]
- Fänge R, Nilsson S. (1985) The fish spleen: structure and function. Experientia 41: 152–158. [DOI] [PubMed] [Google Scholar]
- Henriksson P, Mandic M, Richards JG. (2008) The osmorespiratory compromise in sculpins: impaired gas exchange is associated with freshwater tolerance. Physiol Biochem Zool 81: 310–319. [DOI] [PubMed] [Google Scholar]
- Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD, Sale PF, Edwards AJ, Caldeira K, et al. (2007) Coral reefs under rapid climate change and ocean acidification. Science 318: 1737–1742. [DOI] [PubMed] [Google Scholar]
- IPCC (2013) Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM, eds. Cambridge, UK and New York, NY, USA, Cambridge University Press. [Google Scholar]
- Ishimatsu A, Hayashi M, Kikkawa T. (2008) Fishes in high-CO2, acidified oceans. Mar Ecol Prog Ser 373: 295–302. [Google Scholar]
- Jensen FB, Nikinmaa M, Weber RE. (1993) Environmental perturbations of oxygen transport in teleost fishes: causes, consequences and compensations. In Rankin JC, Jensen F, eds, Fish Ecophysiology. Springer, The Netherlands, pp 161–179. [Google Scholar]
- Ken-Ichi Y. (1988) Contraction of spleen in exercised freshwater teleost. Comp Biochem Physiol A Physiol 89: 65–66. [Google Scholar]
- King PA, Goldstein L. (1983) Renal ammoniagenesis and acid excretion in the dogfish, Squalus acanthias. Am J Physiol Regul Integr Comp Physiol 245: R581–R589. [DOI] [PubMed] [Google Scholar]
- Lai JC, Kakuta I, Mok HO, Rummer JL, Randall DJ. (2006) Effects of moderate and substantial hypoxia on erythropoietin levels in rainbow trout kidney and spleen. J Exp Biol 209: 2734–2738. [DOI] [PubMed] [Google Scholar]
- Last PR, Stevens JD. (2009) Sharks and Rays of Australia. Cambridge, MA, USA, Harvard University Press. [Google Scholar]
- Lüthi D, Le Floch M, Bereiter B, Blunier T, Barnola J-M, Siegenthaler U, Raynaud D, Jouzel J, Fischer H, Kawamura K. (2008) High-resolution carbon dioxide concentration record 650,000–800,000 years before present. Nature 453: 379–382. [DOI] [PubMed] [Google Scholar]
- Marais E, Chown SL. (2008) Beneficial acclimation and the Bogert effect. Ecol Lett 11: 1027–1036. [DOI] [PubMed] [Google Scholar]
- McClelland GB, Dalziel AC, Fragoso NM, Moyes CD. (2005) Muscle remodeling in relation to blood supply: implications for seasonal changes in mitochondrial enzymes. J Exp Biol 208: 515–522. [DOI] [PubMed] [Google Scholar]
- Melzner F, Göbel S, Langenbuch M, Gutowska MA, Pörtner H-O, Lucassen M. (2009a) Swimming performance in Atlantic cod (Gadus morhua) following long-term (4–12 months) acclimation to elevated seawater PCO2. Aquat Toxicol 92: 30–37. [DOI] [PubMed] [Google Scholar]
- Melzner F, Gutowska M, Langenbuch M, Dupont S, Lucassen M, Thorndyke MC, Bleich M, Pörtner HO. (2009b) Physiological basis for high CO2 tolerance in marine ectothermic animals: pre-adaptation through lifestyle and ontogeny? Biogeosciences 6: 2313–2331. [Google Scholar]
- Munday PL, Crawley NE, Nilsson GE. (2009) Interacting effects of elevated temperature and ocean acidification on the aerobic performance of coral reef fishes. Mar Ecol Prog Ser 388: 235–242. [Google Scholar]
- Neale NL, Honn KV, Chavin W. (1977) Hematological responses to thermal acclimation in a cold water squali-form (Heterodontus francisci Girard 1984). J Comp Physiol 115: 215–222. [Google Scholar]
- Nilsson GE, Renshaw GM. (2004) Hypoxic survival strategies in two fishes: extreme anoxia tolerance in the North European crucian carp and natural hypoxic preconditioning in a coral-reef shark. J Exp Biol 207: 3131–3139. [DOI] [PubMed] [Google Scholar]
- Nilsson GE, Hobbs J-P, Munday PL, Östlund-Nilsson S. (2004) Coward or braveheart: extreme habitat fidelity through hypoxia tolerance in a coral-dwelling goby. J Exp Biol 207: 33–39. [DOI] [PubMed] [Google Scholar]
- Ohde S, van Woesik R. (1999) Carbon dioxide flux and metabolic processes of a coral reef, Okinawa. Bull Mar Sci 65: 559–576. [Google Scholar]
- Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, Feely RA, Gnanadesikan A, Gruber N, Ishida A, Joos F, et al. (2005) Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437: 681–686. [DOI] [PubMed] [Google Scholar]
- Ott ME, Heisler N, Ultsch GR. (1980) A re-evaluation of the relationship between temperature and the critical oxygen tension in freshwater fishes. Comp Biochem Physiol A Physiol 67: 337–340. [Google Scholar]
- Pierrot D, Lewis E, Wallace DWR. (2006) MS Excel Program Developed for CO2 System Calculations. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, TN, USA. http://cdiac.ornl.gov/ftp/co2sys/CO2SYS_calc_XLS_v2.1/: [Google Scholar]
- Pörtner HO. (2008) Ecosystem effects of ocean acidification in times of ocean warming: a physiologist's view. Mar Ecol Prog Ser 373: 203–217. [Google Scholar]
- Pörtner HO, Farrell AP. (2008) Physiology and climate change. Science 322: 690–692. [DOI] [PubMed] [Google Scholar]
- Raven J, Caldeira K, Elderfield H, Hoegh-Guldberg O, Liss P, Riebesell U, Shepherd J, Turley C, Watson A. (2005) Ocean Acidification Due to Increasing Atmospheric Carbon Dioxide. The Royal Society, London, UK. [Google Scholar]
- Renshaw GMC, Kerrisk CB, Nilsson GE. (2002) The role of adenosine in the anoxic survival of the epaulette shark, Hemiscyllium ocellatum. Comp Biochem Physiol B Biochem Mol Biol 131: 133–141. [DOI] [PubMed] [Google Scholar]
- Rosa R, Seibel BA. (2008) Synergistic effects of climate-related variables suggest future physiological impairment in a top oceanic predator. Proc Natl Acad Sci USA 105: 20776–20780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routley MH, Nilsson GE, Renshaw GMC. (2002) Exposure to hypoxia primes the respiratory and metabolic responses of the epaulette shark to progressive hypoxia. Comp Biochem Physiol A Mol Integr Physiol 131: 313–321. [DOI] [PubMed] [Google Scholar]
- Rummer JL, McKenzie DJ, Innocenti A, Supuran CT, Brauner CJ. (2013a) Enhanced muscle oxygen delivery may represent the incipient function of the Root effect in ray-finned fishes. Science 340: 1327–1329. [DOI] [PubMed] [Google Scholar]
- Rummer JL, Stecyk JAW, Couturier CS, Watson S-A, Nilsson GE, Munday PL. (2013b) Elevated CO2 enhances aerobic scope of a coral reef fish. Conserv Physiol 1: doi:10.1093/conphys/cot023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabine CL, Feely RA. (2007) The oceanic sink for carbon dioxide. Chapter 3. In Reay D, Hewitt N, Grace J, Smith K, eds, Greenhouse Gas Sinks, CABI Publishing, Oxfordshire, UK, pp 31–49. [Google Scholar]
- Sabine CL, Feely RA, Gruber N, Key RM, Lee K, Bullister JL, Wanninkhof R, Wong CS, Wallace DWR, Til-brook B. (2004) The oceanic sink for anthropogenic CO2. Science 305: 367–371. [DOI] [PubMed] [Google Scholar]
- Schurmann H, Steffensen JF. (1997) Effects of temperature, hypoxia and activity on the metabolism of juvenile Atlantic cod. J Fish Biol 50: 1166–1180. [Google Scholar]
- Shaw EC, McNeil BI, Tilbrook B, Matear R, Bates ML. (2013) Anthropogenic changes to seawater buffer capacity combined with natural reef metabolism induce extreme future coral reef CO2 conditions. Glob Change Biol 19: 1632–1641. [DOI] [PubMed] [Google Scholar]
- Soivio A, Nyholm K, Westman K. (1973) Notes on haematocrit determinations on rainbow trout, Salmo gairdneri. Aquaculture 2: 31–35. [Google Scholar]
- Speers-Roesch B, Richards JG, Brauner CJ, Farrell AP, Hickey AJR, Wang YS, Renshaw GMC. (2012a) Hypoxia tolerance in elasmobranchs. I. Critical oxygen tension as a measure of blood oxygen transport during hypoxia exposure. J Exp Biol 215: 93–102. [DOI] [PubMed] [Google Scholar]
- Speers-Roesch B, Brauner CJ, Farrell AP, Hickey AJR, Renshaw GMC, Wang YS, Richards JG. (2012b) Hypoxia tolerance in elasmobranchs. II. Cardiovascular function and tissue metabolic responses during progressive and relative hypoxia exposures. J Exp Biol 215: 103–114. [DOI] [PubMed] [Google Scholar]
- Steffensen JF. (1989) Some errors in respirometry of aquatic breathers: how to avoid and correct them. Fish Physiol Biochem 6: 49–59. [DOI] [PubMed] [Google Scholar]
- Steffensen JF, Johansen K, Bushnell PG. (1984) An automated swimming respirometer. Comp Biochem Physiol A Physiol 79: 437–440. [Google Scholar]
- Tresguerres M, Parks SK, Salazar E, Levin LR, Goss GG, Buck J. (2010) Bicarbonate-sensing soluble adenylyl cyclase is an essential sensor for acid/base homeostasis. Proc Natl Acad Sci USA 107: 442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JD, Wood CM, Höbe H. (1983) Physiological consequences of severe exercise in the inactive benthic flathead sole (Hippoglossoides elassodon): a comparison with the active pelagic rainbow trout (Salmo gairdneri). J Exp Biol 104: 269–288. [Google Scholar]
- Wells R, McIntyre R, Morgan A, Davie P. (1986) Physiological stress responses in big gamefish after capture: observations on plasma chemistry and blood factors. Comp Biochem Physiol A Physiol 84: 565–571. [DOI] [PubMed] [Google Scholar]
- Wise G, Mulvey JM, Renshaw GM. (1998) Hypoxia tolerance in the epaulette shark (Hemiscyllium ocellatum). J Exp Zool 281: 1–5. [Google Scholar]
- Wood CM, McMahon B, McDonald D. (1977) An analysis of changes in blood pH following exhausting activity in the starry flounder, Platichthys stellatus. J Exp Biol 69: 173–185. [DOI] [PubMed] [Google Scholar]
- Wood CM, Pärt P, Wright PA. (1995) Ammonia and urea metabolism in relation to gill function and acid–base balance in a marine elasmobranch, the spiny dogfish (Squalus acanthias). J Exp Biol 198: 1545–1558. [DOI] [PubMed] [Google Scholar]
- Wood CM, Kajimura M, Bucking C, Walsh PJ. (2007) Osmoregulation, ionoregulation and acid–base regulation by the gastrointestinal tract after feeding in the elasmobranch (Squalus acanthias). J Exp Biol 210: 1335–1349. [DOI] [PubMed] [Google Scholar]