Abstract
OBJECTIVE
Recent studies suggest that air pollution plays a role in type 2 diabetes (T2D) incidence and mortality. The underlying physiological mechanisms have yet to be established. We hypothesized that air pollution adversely affects insulin sensitivity and secretion and serum lipid levels.
RESEARCH DESIGN AND METHODS
Participants were selected from BetaGene (n = 1,023), a study of insulin resistance and pancreatic β-cell function in Mexican Americans. All participants underwent DXA and oral and intravenous glucose tolerance tests and completed dietary and physical activity questionnaires. Ambient air pollutant concentrations (NO2, O3, and PM2.5) for short- and long-term periods were assigned by spatial interpolation (maximum interpolation radius of 50 km) of data from air quality monitors. Traffic-related air pollution from freeways (TRAP) was estimated using the dispersion model as NOx. Variance component models were used to analyze individual and multiple air pollutant associations with metabolic traits.
RESULTS
Short-term (up to 58 days cumulative lagged averages) exposure to PM2.5 was associated with lower insulin sensitivity and HDL-to-LDL cholesterol ratio and higher fasting glucose and insulin, HOMA-IR, total cholesterol, and LDL cholesterol (LDL-C) (all P ≤ 0.036). Annual average PM2.5 was associated with higher fasting glucose, HOMA-IR, and LDL-C (P ≤ 0.043). The effects of short-term PM2.5 exposure on insulin sensitivity were largest among obese participants. No statistically significant associations were found between TRAP and metabolic outcomes.
CONCLUSIONS
Exposure to ambient air pollutants adversely affects glucose tolerance, insulin sensitivity, and blood lipid concentrations. Our findings suggest that ambient air pollutants may contribute to the pathophysiology in the development of T2D and related sequelae.
Introduction
Both ambient and traffic-related air pollutants have been associated with increased type 2 diabetes (T2D) incidence (1) and mortality (2). The underlying biological pathways for these effects have yet to be established. Only a few human studies have been performed and have suggested that short- and long-term exposure to air pollutants adversely affects key T2D-related pathways including glucose metabolism (3,4), insulin resistance (5–8), and dyslipidemia (4,9). While most previous studies have been conducted among Caucasians (3,7) and Asians (4,8), the effects in other populations with generally higher risk for T2D, such as Latinos (10) and African Americans (11), have not been adequately studied. Additionally, the informativeness of results from these studies is limited by the use of surrogate indices of insulin resistance, for example, HOMA of insulin resistance (HOMA-IR), which have well-documented limitations compared with detailed measures of insulin resistance such as intravenous glucose tolerance test and glucose clamps. A more direct assessment of these relationships requires approaches that use direct measures of insulin sensitivity and secretion.
In this study, we examined more detailed measurements of insulin sensitivity and secretion from a frequently sampled intravenous glucose tolerance test (FSIGT) and assessed their relationship with daily and monthly cumulative averaged exposures over various lagged periods up to 1 year for ambient (NO2, O3, and PM2.5) and traffic-related air pollution (TRAP) among 1,023 Mexican American women with a history of gestational diabetes mellitus (GDM) and their relatives. We hypothesized that short- and long-term air pollution exposures were associated with increased insulin resistance and lipid levels and decreased insulin secretion among Latino women with a history of GDM and their family members, who are at high risk of T2D.
Research Design and Methods
Study Participants
BetaGene participants were recruited from 2002 to 2008 and were Mexican American women with a confirmed diagnosis of GDM within the previous 5 years as well as their siblings or cousins (both sexes), all with fasting glucose levels <7 mmol/L. Women with previous GDM were identified from the Los Angeles County/University of Southern California Medical Center, Kaiser Permanente Southern California Medical Group, and obstetrical/gynecological clinics at local southern California hospitals. Children, parents, or husbands of women with a previous GDM diagnosis were not recruited in the study. Subjects who took medications for diabetes were excluded from the study. Details regarding recruitment have previously been described (12). All protocols for BetaGene were approved by the institutional review boards of participating institutions, and all participants provided written informed consent before participation.
Testing Procedures and Assays
Metabolic phenotypes were characterized in two separate visits to the Clinical Research Center at the University of Southern California. The first visit consisted of a physical examination, self-reported food frequency, and physical activity questionnaires (see Supplementary Data for details), a 2-h (75-g) oral glucose tolerance test (oGTT) (Supplementary Table 1), and fasting blood for lipid measurements (13). Participants with fasting glucose <7.0 mmol/L were invited for a second visit, which consisted of a DXA scan for direct measurement of percent body fat and an insulin-modified FSIGT for measurement of insulin sensitivity and β-cell function (Supplementary Table 1). DXA-measured percent body fat has been proven to be a more accurate measurement of adiposity than traditional BMI (14).
Ambient and TRAP Exposure Assessment
The air pollution and traffic exposures were assigned based on the participant’s residential address provided at the time of testing. Participant residence addresses were standardized and their locations were geocoded using Google Earth Pro (www.googleearth.com) and the TomTom’s EZ-Locate service (www.geocode.com). The addresses that were not recognized by Google Earth Pro were geocoded using the EZ-Locate service.
Ambient air quality information for 2002–2008 in California was obtained from the U.S. Environmental Protection Agency’s Air Quality System (http://www.epa.gov/ttn/airs/airsaqs) data and additionally from data collected for the southern California Children’s Health Study (CHS) (15). The O3 and NO2 data were collected using Federal Reference Method (FRM) monitors. The data on PM2.5 were primarily collected using FRM or Federal Equivalent Method monitors; however, CHS continuous PM data were used when no FRM or Federal Equivalent Method monitors were available from the Air Quality System. The 20–30 km spacing of the air monitoring network in southern California provides good characterization of the pollution gradients across the populated areas. The daily average air quality data were spatially mapped to the residence locations using inverse distance-squared interpolation. The data from up to four air quality measurement stations were included in each interpolation. Because of the regional nature of O3, NO2, and PM2.5 concentrations, a maximum interpolation radius of 50 km was used for all pollutants. However, when a residence (n = 352) was located within 5 km of one or more stations with valid observations, the interpolation was based solely on the values from the nearest monitor. The average distance from residences to the nearest monitor was 6.6, 6.9, and 10.7 km for O3, NO2, and PM2.5, respectively. Average air pollution concentrations were assigned for the 90 days and 12 months prior to each subject’s FSIGT test date. Sufficient data were available to make assignments for >99% of the subjects.
Dispersion modeled estimates of ambient concentrations from local on-road vehicle emissions were used to characterize the annual average TRAP exposure (16). The CALINE4 line source dispersion model (16) was applied using Tele Atlas/GDT traffic volumes for 2009, the EMFAC2007 vehicle emission factors for 2009, and measured hourly wind speed and direction data for 11 geographic subregions in southern California. The model was used to estimate NOx concentrations contributed by traffic on freeways within 5-km radius buffers for 874 residences with accurate geocodes. We have found that this metric is a strong predictor of local-scale variation in observed NOx concentrations in southern California (17).
Contextual Variables
Contextual variables were collected to characterize the socioeconomic status (SES) of participants (Supplementary Data). Demographic data including median household income, poverty rate, unemployment rate, and proportion of respondents over age 25 years with highest attained education were obtained from the U.S. Census Bureau website (http://www.census.gov/). Fast foods, grocery stores, and parks and recreation areas were extracted from Esri’s Business Analyst database, and a crime index was calculated based on crime data extracted from Esri’s Community Analyst database (version 2013) at the zip-code level.
Data Analysis
Insulin sensitivity (SI), the acute insulin response to glucose (AIRg) during the first 10 min of the FSIGT, insulin metabolic clearance rate (MCR), and insulin fractional disappearance rate (FDR) were determined using the Millennium version of the Bergman minimal model (18). Disposition index (DI), a measure of pancreatic β-cell compensation for insulin resistance, was computed as the product of SI and AIRg. For comparison with other studies, the approximation formulae of HOMA models (19) were also used to estimate insulin resistance from plasma fasting glucose and insulin concentrations at the time of the FSIGT.
Fasting and postchallenge insulin from oGTTs; SI, AIRg, DI, MCR, and FDR from FSIGTs; and HDL cholesterol–to–LDL cholesterol (HDL-C–to–LDL-C) ratio and triglycerides were log transformed to approximate normal distributions prior to analysis. Geometric means were presented for these variables. Daily (0–90 days) and monthly (1–12 months) cumulative averages of air pollution concentrations over increasing lagged time periods before FSIGT test date were calculated by averaging the summation of air pollution concentrations during the lagged time period over the number of days or months during that period. Associations of daily and monthly cumulative average ambient air pollutants and annual average TRAP NOx prior to FSIGT date with diabetes-related traits were assessed under a variance components framework using SOLAR (version 4.3.1) to account for correlations among related individuals within families. Ascertainment correction was used in SOLAR to adjust for potential overestimation of association estimates from women with previous GDM in our family-based study design. Because no prior knowledge exists for critical exposure lagged periods for various metabolic outcomes and because models for cumulative averaged exposures over consecutive lag periods were not considered as nested (20), the Akaike information criterion (AIC) was used as a systematic tool to select the short-term ambient air pollutant exposure periods (0–60 days cumulative lagged average) with the best model fit for the different outcomes. Association results for these selected short-term exposure periods, and 12-month average exposures as representatives of long-term exposures, are presented in the tables. Estimates for continuous exposures were scaled to 1-SD unit for each air pollutant. Age, sex, percent body fat, contextual variables, total minutes of physical activity per week, total daily caloric intake, dietary antioxidants intakes including vitamin C and β-carotene intakes, an indicator variable for the year of FSIGT testing date, and the season of FSIGT testing date were included as covariates to adjust for potential confounding effect. The four seasons were defined as winter (December to February), spring (March to May), summer (June to August), and fall (September to November). Multipollutant models were used to test the joint impact of air pollutant combinations (21). The lagged periods of short-term cumulative averages of air pollutants in these multipollutant models were selected by AIC from previous single pollutant models.
Results
A total of 1,023 participants with complete oGTTs and FSIGTs and at least one of the air pollution measurements were included in this study. The mean age was 34.5 years (range 17.9–65.6), mean BMI was 29.7 kg/m2, and mean percent body fat was 33.9% (Table 1). A total of 694 (67.8%) participants were female, 211 (20.6%) women had a diagnosis of GDM during a prior pregnancy, and 804 (78.5%) subjects were overweight or obese with BMI ≥25 kg/m2. The geographic areas where our participants lived had 22% households below the federally designated poverty rate, and 20% of people aged >25 years had highest attained education at or below high school (Table 1). The distributions of concentrations of 30-day lagged cumulative average and 12-month average ambient pollutants are presented in Table 2. These concentrations are consistent with reports from other studies in southern California (22). Correlations among ambient air pollutants are presented in Table 2. Ambient NO2 was negatively correlated with O3 and positively correlated with PM2.5 for both short- and long-term measurements.
Table 1.
Selected characteristics of BetaGene study participants with complete FSIGT, oGTT, and air pollution measurements
| N | Mean | SD | Median | 25th, 75th quantiles | |
|---|---|---|---|---|---|
| Age (years) | 1,023 | 34.5 | 8.1 | 34.3 | 28.8, 39.5 |
| BMI (kg/m2) | 1,023 | 29.7 | 6.2 | 28.7 | 25.5, 32.7 |
| Percent body fat | 1,013 | 33.9 | 8.6 | 35.3 | 27.5, 40.4 |
| Measurements obtained from FSIGT | |||||
| SI (×10−5 min−1 per pmol/L)‡ | 1,023 | 4.27 | 2.33 | 4.42 | 3.07, 6.21 |
| AIRg (pmol/L × 10 min)‡ | 1,023 | 2,840 | 2,171 | 2,490 | 1,436, 4,258 |
| DI (SI × AIRg)‡ | 1,023 | 11,565 | 8,021 | 11,232 | 6,647, 17,782 |
| Insulin MCR (mL/min/m2)‡ | 1,022 | 8.2 | 5.5 | 9.1 | 5.8, 12.9 |
| Insulin FDR (min−1 × 100)‡ | 1,022 | 18.3 | 11.8 | 20.0 | 13.2, 27.6 |
| Measurements obtained from oGTT | |||||
| Fasting glucose (mmol/L) | 1,023 | 5.1 | 0.7 | 5.1 | 4.8, 5.4 |
| 2-h glucose (mmol/L) | 1,023 | 7.6 | 2.2 | 7.4 | 6.1, 8.8 |
| Fasting insulin (pmol/L)‡ | 1,022 | 43.1 | 29.7 | 42.0 | 24.0, 72.0 |
| 2-h insulin (pmol/L)‡ | 1,022 | 347.1 | 274.5 | 360.0 | 222.0, 606.0 |
| HOMA-IR (mmol/L × mU/L)‡ | 1,022 | 1.61 | 1.2 | 1.61 | 0.95, 2.70 |
| Lipids§ | |||||
| Cholesterol (mg/dL)‖ | 1,008 | 174.6 | 34.0 | 171.0 | 152.0, 196.0 |
| HDL-C (mg/dL)‖ | 1,008 | 46.4 | 11.1 | 45.0 | 39.0, 52.5 |
| LDL-C (mg/dL)‖ | 989 | 105.3 | 28.6 | 103.0 | 85.6, 123.2 |
| HDL-C–to–LDL-C ratio × 100‡ | 989 | 44.8 | 16.6 | 43.9 | 34.2, 56.2 |
| Triglycerides (mg/dL)ঠ| 1,008 | 96.7 | 59.1 | 98.0 | 64.0, 141.0 |
| Physical activity and dietary intakes | |||||
| Total weekly physical activity (min/week) | 984 | 501 | 690 | 217 | 45, 600 |
| Total daily caloric intake (kcal/day)‡ | 987 | 2,323 | 1,024 | 2,291 | 1,749, 2,922 |
| Daily vitamin C intake (mg/day)‡ | 987 | 153.9 | 102.9 | 157.1 | 102.5, 232.0 |
| Daily β-carotene intake (μg/day)‡ | 987 | 5,167 | 3,940 | 5,719 | 3,219, 8,529 |
| Contextual variables | |||||
| Proportions of households below federally designated poverty rate# | 1,023 | 0.22 | 0.12 | 0.21 | 0.12, 0.29 |
| Proportion of respondents aged >25 years with highest attained education# | |||||
| No education | 1,023 | 0.07 | 0.05 | 0.07 | 0.03, 0.10 |
| ≤High school | 1,017 | 19.88 | 92.07 | 5.79 | 2.34, 12.65 |
| Some college or technical school | 1,023 | 0.23 | 0.10 | 0.22 | 0.14, 0.31 |
| >4 years of college | 1,023 | 0.11 | 0.10 | 0.08 | 0.04, 0.15 |
‡Log transformation was applied and geometric means (SDs) are presented.
§Lipid concentrations were measured using fasting blood.
‖For conversion of measurements from conventional units to Système International (SI) units, multiply by a conversion factor of 0.02586.
¶For conversion of measurements from conventional units to Système International units, multiply by a conversion factor of 0.01129.
#Different geographic information system tools (intersect, dissolve and calculate field) were applied to calculate the proportion of the census block groups that fell within each of the 300 m buffers for each of the geocoded addresses.
Table 2.
Air pollution concentrations prior to the FSIGT tests and pairwise correlations among air pollutants of the BetaGene cohort
| Air pollutant exposures | N | Mean (SD)§ | Pairwise correlations† |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 30-day cumulative average |
Annual average |
TRAP | |||||||
| PM2.5 | NO2 | O3 | PM2.5 | NO2 | O3 | ||||
| 30-day cumulative average | |||||||||
| PM2.5 (µg/m3) | 992 | 16.8 (5.5) | 1 | 0.44 (<0.001) | −0.02 (0.64) | 0.48 (<0.001) | 0.28 (<0.001) | 0.04 (0.25) | 0.01 (0.83) |
| NO2 (ppb) | 994 | 24.1 (6.8) | 1 | −0.37 (<0.001) | 0.56 (<0.001) | 0.71 (<0.001) | −0.31 (<0.001) | 0.14 (<0.001) | |
| O3 (ppb) | 996 | 43.4 (13.9) | 1 | −0.07 (0.019) | −0.16 (<0.001) | 0.38 (<0.001) | −0.09 (0.010) | ||
| Annual average | |||||||||
| PM2.5 (µg/m3) | 1,016 | 17.4 (3.6) | 1 | 0.78 (<0.001) | −0.30 (<0.001) | 0.14 (<0.001) | |||
| NO2 (ppb) | 1,018 | 25.6 (6.4) | 1 | −0.51 (<0.001) | 0.22 (<0.001) | ||||
| O3 (ppb) | 1,023 | 40.8 (6.8) | 1 | −0.18 (<0.001) | |||||
| TRAP modeled as freeway NOx (ppb) | 874 | 17.0 (13.8) | 1 | ||||||
ppb, parts per billion.
†Pearson correlation r (P values) are presented.
§Means and SDs of air pollutant concentrations are presented.
Associations for Short-term (Daily Cumulative Average) Ambient Air Pollutant Exposures
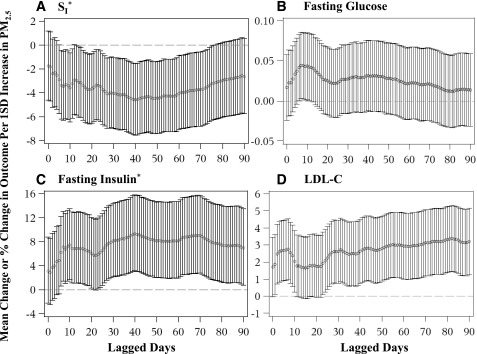
We found robust associations of individual ambient air pollutants with diabetes-related outcomes (Fig. 1 and Supplementary Figs. 1–5). The associations of lagged daily cumulative averages of PM2.5 with SI were significantly negative from 6 to 76 days before FSIGT date. Although mostly not statistically significant, the positive association between PM2.5 and fasting glucose was elevated at lagged day 7 and then declined but remained positive from day 15 until the end of the 90-day period. After adjustment for age, sex, percent body fat, seasonality, and contextual variables, higher daily cumulative averages of PM2.5 over the lagged periods selected by AIC (up to 58 days prior) were associated with lower SI, MCR, FDR, and HDL-C–to–LDL-C ratio and higher fasting glucose and insulin, HOMA-IR, total cholesterol, and LDL-C (all P ≤ 0.036) (Table 3). As an example, a 1-SD (5.1 μg/m3) increase in 0–40 days’ PM2.5 was significantly associated with a 4.9% decrease in SI. This association effect size was similar to the impact of a one-unit increase in percent body fat or BMI on decreasing SI (Supplementary Table 3). Higher NO2 over various lagged periods (up to 37 days prior) was associated with lower SI, MCR, and FDR and higher fasting glucose and insulin and HOMA-IR (all P ≤ 0.023) (Table 3). No significant associations were found between O3 and diabetes-related outcomes (data not shown). Additionally adjustment for physical activity, total daily caloric intake, daily vitamin C and β-carotene intakes, and the year indicator of FSIGT testing date did not significantly influence the results (data not shown).
Figure 1.
Regression associations between cumulative averages of daily PM2.5 and selected metabolic outcomes over 0–90 days prior to FSIGT date. Associations were adjusted for age, sex, percent body fat, contextual variables, and seasons of FSIGT measurements. Mean changes in fasting glucose and LDL-C and mean percent changes in SI and fasting insulin per 1-SD change in PM2.5 and 95% CIs are presented in four outcome panels: SI (×10−5 min−1 per pmol/L) (A), fasting glucose (mmol/L) (B), fasting insulin (pmol/L) (C), and LDL-C (mg/dL) (D). *Outcomes were log transformed in the association analysis.
Table 3.
Regression associations between PM2.5 and NO2 with diabetes-related metabolic traits among 1,023 BetaGene participants
| Exposure outcomes | PM2.5 |
NO2 |
||||
|---|---|---|---|---|---|---|
| Short-term* |
Annual average† |
Short-term* |
Annual average† |
|||
| Lagged period* | β (P)‡ | β (P)§ | Lagged period* | β (P)‡ | β (P)§ | |
| Measurements obtained from FSIGT | ||||||
| SI (×10−5 min−1 per pmol/L)‖ | 40 | −4.60 (0.003) | −1.63 (0.32) | 7 | −3.81 (0.023) | −1.84 (0.29) |
| AIRg (pmol/L × 10 min)‖ | 34 | 1.23 (0.30) | −0.05 (0.97) | 37 | 1.08 (0.42) | −0.54 (0.68) |
| DI (SI × AIRg)‖ | 8 | −1.25 (0.32) | −0.40 (0.76) | 3 | −1.13 (0.40) | −1.37 (0.33) |
| Insulin MCR (mL/min/m2)‖ | 36 | −5.65 (0.004) | −3.86 (0.06) | 37 | −6.55 (0.003) | −5.06 (0.017) |
| Insulin FDR (min−1 × 100)‖ | 58 | −5.77 (0.003) | −5.06 (0.012) | 37 | −7.93 (<0.001) | −6.87 (0.001) |
| Measurements obtained from oGTT | ||||||
| Fasting glucose (mmol/L) | 7 | 0.04 (0.036) | 0.08 (<0.001) | 12 | 0.06 (0.012) | 0.07 (0.005) |
| 2-h glucose (mmol/L) | 3 | 0.06 (0.36) | −0.05 (0.51) | 56 | −0.11 (0.18) | −0.07 (0.37) |
| Fasting insulin (pmol/L)‖ | 40 | 9.31 (0.003) | 5.84 (0.07) | 32 | 8.41 (0.013) | 4.48(0.18) |
| 2-h insulin (pmol/L)‖ | 57 | 2.92 (0.24) | 0.78 (0.76) | 4 | 2.90 (0.26) | 2.48 (0.35) |
| HOMA-IR (mmol/L × mU/L)‖ | 40 | 6.99 (0.002) | 5.81 (0.016) | 32 | 6.63 (0.009) | 4.58 (0.07) |
| Lipids** | ||||||
| Cholesterol (mg/dL)†† | 3 | 2.25 (0.034) | 1.98 (0.10) | 4 | 1.09 (0.35) | 0.45 (0.72) |
| HDL-C (mg/dL)†† | 4 | −0.35 (0.32) | −0.15 (0.70) | 45 | −0.80 (0.058) | −0.72 (0.08) |
| LDL-C (mg/dL)†† | 4 | 2.66 (0.003) | 2.07 (0.043) | 5 | 1.58 (0.12) | 1.04 (0.33) |
| HDL-C–to–LDL-C ratio × 100‖ | 7 | −3.17 (0.005) | −2.38 (0.06) | 30 | −2.10 (0.12) | −2.56 (0.05) |
| Triglycerides (mg/dL)‖‡‡ | 14 | −1.59 (0.40) | −2.35 (0.26) | 17 | −2.12 (0.31) | −1.56 (0.47) |
Boldface P values indicate regression estimates were statistically significant (P < 0.05).
*Various cumulative average daily lagged periods were selected for different outcomes as short-term exposures using AIC to achieve best model fitting.
†12-month average ambient air pollutant exposures were selected as representative of long-term exposures.
‡Associations of short-term exposures to air pollutants with metabolic traits were adjusted for age, sex, percent body fat, seasonality, and contextual variables. For outcomes including fasting and 2-h glucose, total cholesterol, HDL-C, and LDL-C, β represents the absolute changes in the outcome associated with 1-SD change of the exposure variables. For other log-transformed outcomes, β represents the percent change in the outcome associated with 1-SD change of the exposure variables. P values were derived from likelihood ratio tests.
§Associations between 12-month average pollutants levels and metabolic traits were adjusted for age, sex, percent body fat, and contextual variables.
‖Variables were log transformed in the association analysis.
**Lipid concentrations were measured using fasting blood.
††For conversion of measurements from conventional units to Système International (SI) units, multiply by a conversion factor of 0.02586.
‡‡For conversion of measurements from conventional units to Système International units, multiply by a conversion factor of 0.01129.
The effects of short-term PM2.5 exposure with SI varied by percent body fat (interaction P = 0.024). A 1% increase in percent body fat increased the negative association between PM2.5 and SI by 8%. As an example, after categorization of the cohort into normal body fat percentage (<25% for males and <32% for females) and obese (≥25% for males and ≥32% for females) based on the criteria of the American Council on Exercise (23), higher daily cumulative lag PM2.5 exposure (over 0–40 days) was significantly associated with lower SI within the obese group (mean change in SI per 1-SD increase of PM2.5 = −6.6%, P = 0.003). However, the effect size of the association was much smaller and not statistically significant in the normal body fat percentage group (mean percent change in SI per 1-SD increase of PM2.5 = −1.8%, P = 0.45). No other significant interactions were found between short-term periods of cumulative daily average air pollutants and age, sex, and percent body fat for the associations between air pollutants and metabolic outcomes.
Associations for Long-term (12-Month Average) Ambient Air Pollutant Exposures
Longer-term association patterns were assessed using monthly cumulative averages of air pollutant exposures. Associations between PM2.5 and SI were significantly negative in the 2-month period prior to the FSIGT and then nonsignificantly negative for the remainder of the 12-month period. The association between PM2.5 and fasting glucose was significantly positive at month 5–6 and remained significantly positive over 12 months (Supplementary Figs. 6–11). Individual 12-month average concentrations were used to estimate the effects of long term chronic exposure levels. Higher 12-month average PM2.5 was associated with higher fasting glucose, HOMA-IR, and LDL-C and lower FDR (P ≤ 0.043) after adjustment for age, sex, percent body fat, and contextual variables (Table 3 and Supplementary Figs. 6 and 7). Higher annual average NO2 was associated with higher fasting glucose and lower MCR and FDR (P ≤ 0.017) (Supplementary Figs. 8 and 9). The association between 12-month average PM2.5 with LDL-C and total cholesterol was reduced by 13% with each 1% increase in percent body fat (interaction P = 0.009 and 0.034, respectively). No other significant interactions were found between 12-month average ambient air pollutants and age, sex, percent body fat, or history of GDM.
Associations for TRAP and Multiple Pollutants
No significant associations were found between TRAP modeled as freeway NOx and metabolic outcomes after adjustment for age, sex, percent body fat, and contextual variables (Supplementary Table 3). Associations of short- and long-term PM2.5 with metabolic outcomes did not change substantially after adjustment for TRAP (Supplementary Table 3).
Although ambient air pollutants were correlated (Table 2), we assessed the joint impact of two pollutants with low or moderate pairwise correlations in a multipollutant model for their relationship with metabolic outcomes. When short-term NO2 and PM2.5 were included in a multipollutant model, the associations of short-term PM2.5 with SI, fasting insulin, and LDL-C–to–HDL-C ratio were robust and remained statistically significant; however, the short-term NO2 associations with SI, fasting glucose and insulin, and HOMA-IR were all attenuated by >25% and no longer statistically significant (all P ≥ 0.15). Adjustment for short-term O3 or 12-month average NO2 and O3 had little effect on short-term PM2.5 associations with SI, fasting insulin, LDL-C, or LDL-C–to–HDL-C ratio (all regression coefficients changed ≤21%, all P < 0.047).
The median residential distance from the nearest air quality monitor for PM2.5 was 6.6 km (range 0.1–49.9). Because data quality of ambient air pollutant concentrations may vary by the distance between residential address and the nearest air quality monitor, sensitivity analyses among 931 subjects within 10 km away from the nearest monitor were conducted. Our main observations about PM2.5 associations with insulin sensitivity and serum lipids were robust (Supplementary Table 4).
Conclusions
In this study of Mexican Americans, many of whom were young obese adults genetically related to women with a history of GDM, exposure to ambient air pollutants was associated with a spectrum of adverse metabolic outcomes related to T2D pathophysiology. We observed that participants exposed to higher short-term average PM2.5 concentrations had lower insulin sensitivity, insulin clearance, and HDL-C–to–LDL-C ratios and higher fasting glucose and insulin, HOMA-IR, total cholesterol, and LDL-C. Higher annual average PM2.5 exposure was significantly associated with higher fasting glucose, HOMA-IR, and LDL-C and lower insulin clearance. The magnitudes of effect from a 1-SD difference of PM2.5 on metabolic outcomes were similar compared with the impact of a one-unit change in percent body fat or BMI on the same metabolic outcomes. Percent body fat significantly modified associations of short-term PM2.5 exposure with SI. No significant associations were observed between TRAP and metabolic outcomes.
One hallmark of T2D is decreased insulin sensitivity. Several clinical studies in children (5,7) and adults (6,8) have found positive associations between air pollutants and insulin resistance. While these studies used a surrogate measure of insulin sensitivity, such as HOMA-IR, which is calculated from a single fasting measure and confounded by changes in insulin secretion, our study used a detailed measure of insulin sensitivity and provides robust evidence that short-term exposure to PM2.5 is associated with lower insulin sensitivity. Our results showing that obesity and short-term exposure to PM2.5 have a synergistic impact on insulin sensitivity suggest that PM2.5 may have a larger effect on a system already stressed by increased levels of body fat.
Recent studies suggest possible mechanisms that may explain the association between air pollutants, particularly PM2.5, and diabetes and insulin resistance. Studies of obese mice (24) and healthy humans (25) have found air pollutant exposures including PM2.5 and diesel exhaust alter endothelial function, which has been implicated in reduced insulin sensitivity and peripheral glucose uptake. The link between air pollutants and insulin resistance may also involve low-grade systemic inflammation and inflammation in visceral adipose tissue (26) and the hypothalamus (27) as indicated by increased tumor necrosis factor-α (28), interleukin-6 (28,29), fibrinogen (29), white blood cell counts (30), and microglial/astrocyte reactivity (27). The exposure of PM2.5 has also been shown to increase endoplasmic reticulum stress pathways and hepatic inflammation including upregulation of c-Jun N-terminal kinase 1/2 pathways (31,32) and abnormal Insulin receptor substrate phosphorylation (32), which can further lead to peripheral and hepatic insulin resistance (33). Additionally, increased sympathetic nervous system (34) and hypothalamic-pituitary-adrenal (HPA) axis activity (35) mediated by acute exposures to PM may also play a role in observed associations between PM2.5 and insulin resistance. Our findings support the hypothesis that short-term exposure to PM2.5 contributes to increased insulin resistance.
Along with insulin sensitivity, β-cell compensation for insulin resistance characterizes the predisposition of T2D. Increased insulin resistance also increases β-cell stress, which gradually causes β-cell dysfunction as T2D progresses over time (36). The observed association between PM2.5 and serum lipid levels in this cohort also suggests that PM2.5 may contribute to the lipotoxicity of β-cells (37). Therefore, we hypothesized that PM2.5 is inversely related to β-cell function. However, no significant associations were found between exposures to air pollutants and β-cell function, which could be due to increased insulin response to compensate for increased insulin resistance associated with PM2.5 exposure. Studies in older individuals who have experienced longer period of environmental stress may be needed to fully assess the role of PM on β-cell function.
Recent studies also found positive associations between ambient and traffic-related air pollutants and impaired glucose metabolism (3,4), with most studies assessing the impact of acute exposures. A study in Taiwan documented that elevated 5-day average O3 was associated with increased fasting glucose (4). Additionally, higher 3-day average PM10 and O3 were associated with increased HbA1c (4). In the current study, we found that higher short- and long-term exposure to PM2.5 was associated with higher fasting glucose, additionally suggesting a chronic impact of PM2.5 on glucose metabolism. However, we did not find significant associations of O3 or TRAP with metabolic outcomes. The lack of significant findings could be because we excluded patients with diabetes from our cohort, which limited our power to detect significant associations. However, our results shed light on the impact of air pollution on T2D-related metabolic traits among people with and people without prediabetes, which will be important for early prevention of T2D in the high-risk population. Also, the geographic differences and the different methods used for TRAP estimations among different studies could complicate comparisons of results from our study to other studies.
Dyslipidemias, characterized by decreased HDL-C, increased LDL-C and triglycerides, have been associated with elevated exposure to air pollutants (4,9). A study in Taiwanese adults showed increased PM10 was associated with elevated triglycerides, and reduced HDL (4). A study among asthmatic patients found that a 1 μg/m3 increase of coarse PM resulted in a 4.8% increase in triglycerides and a 1.2% increase in VLDL (9). Consistent with these studies, our results indicate that both short- and long-term exposure to PM2.5 are positively associated with total cholesterol and LDL-C and negatively associated with HDL-C–to–LDL-C ratio.
The current study has several strengths. First, our sample consists of a large cohort of Mexican Americans, a high-risk group for metabolic disease, with an oGTT to assess glucose tolerance and FSIGT-based detailed measures of insulin sensitivity and secretion. Using the latter, we were able to apply physiological models to assess pancreatic β-cell function, which was first tested for its association with air pollution in our study. Second, both short- and long-term ambient air pollution exposures were measured, which allowed us to investigate the different impact of short- and long-term exposures on metabolic outcomes. Third, both ambient and TRAP exposures were estimated, allowing an assessment of the independent effects of ambient air pollutants as well as traffic-related pollution on metabolic outcomes.
Our findings need to be interpreted in light of our study design and analytic approach. First, our study only recruited Mexican American adults coming from families with a history of GDM, and a large proportion of our participants were overweight or obese. This may limit the generalizability of our results to other racial/ethnic populations and people with genetically lower risk of T2D. Second, lifetime residential histories were not available for longer-term exposure assignments, and personal air pollution exposure levels were not monitored in this study, which is likely to result in nondifferential misclassification, leading to attenuations of association estimates. Additionally, the cross-sectional and observational design of our study precludes us from examining the dynamic impact of air pollution on the change in metabolic traits and drawing any causal relationship from our results. Third, although census-tract level contextual variables were used to adjust for socioeconomic factors in the analysis, individual-level information on SES was not available. However, in other studies in Los Angeles (38), regional pollutants such as PM2.5 were not strongly correlated with SES, so residual confounding by SES is not likely to be a major threat to the validity in this study. Lastly, data were not available on some covariates of interest, such as ambient temperatures, sleep, glycemic index, noise, smoking history, and indoor and outdoor temperatures; time-activity patterns; and other indicators of indoor sources of air pollution and air exchange rates, such as heaters, gas stoves, and ventilation.
In conclusion, our study indicates that exposure to ambient air pollutants may adversely affect diabetes-related metabolism including glucose intolerance, insulin resistance, and dyslipidemia in Mexican American adults at high risk of T2D. Remarkably, the impact of PM2.5 on T2D-related traits was comparable to the influence of obesity on these traits. Our findings indicate that ambient air pollution may play as important a role as obesity in the development of T2D and related sequelae in this metabolically high-risk population.
Supplementary Material
Article Information
Funding. This work was supported by National Institutes of Health grants DK-061628, M01-RR-0043, and UL1-TR000130, American Diabetes Association Research Award Clinical Research grant 7-09-CT-09, the Southern California Environmental Health Sciences Center funded by the National Institute of Environmental Health Sciences (grant 5P30ES007048), National Institute of Environmental Health Sciences grant 5P01ES011627, and the Hastings Foundation.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. Z.C. and F.D.G. conducted the analyses and wrote the manuscript. M.T.S., R.M.W., A.H.X., T.A.B., F.L., J.P.W., E.T., and F.D.G. contributed to study design and data collection. M.T.S., C.T.-C., R.M.W., A.H.X., T.A.B., R.H., T.M.B., F.L., and J.P.W. edited the manuscript and contributed to discussion. All authors reviewed the manuscript. Z.C. and F.D.G. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 13–17 June 2014.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc15-1795/-/DC1.
References
- 1.Chen H, Burnett RT, Kwong JC, et al. Risk of incident diabetes in relation to long-term exposure to fine particulate matter in Ontario, Canada. Environ Health Perspect 2013;121:804–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brook RD, Cakmak S, Turner MC, et al. Long-term fine particulate matter exposure and mortality from diabetes in Canada. Diabetes Care 2013;36:3313–3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teichert T, Vossoughi M, Vierkötter A, et al. Association between traffic-related air pollution, subclinical inflammation and impaired glucose metabolism: results from the SALIA study. PLoS One 2013;8:e83042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chuang KJ, Yan YH, Cheng TJ. Effect of air pollution on blood pressure, blood lipids, and blood sugar: a population-based approach. J Occup Environ Med 2010;52:258–262 [DOI] [PubMed]
- 5.Thiering E, Cyrys J, Kratzsch J, et al. Long-term exposure to traffic-related air pollution and insulin resistance in children: results from the GINIplus and LISAplus birth cohorts. Diabetologia 2013;56:1696–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brook RD, Xu X, Bard RL, et al. Reduced metabolic insulin sensitivity following sub-acute exposures to low levels of ambient fine particulate matter air pollution. Sci Total Environ 2013;448:66–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelishadi R, Mirghaffari N, Poursafa P, Gidding SS. Lifestyle and environmental factors associated with inflammation, oxidative stress and insulin resistance in children. Atherosclerosis 2009;203:311–319 [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Hong YC. GSTM1, GSTT1, and GSTP1 polymorphisms and associations between air pollutants and markers of insulin resistance in elderly Koreans. Environ Health Perspect 2012;120:1378–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeatts K, Svendsen E, Creason J, et al. Coarse particulate matter (PM2.5-10) affects heart rate variability, blood lipids, and circulating eosinophils in adults with asthma. Environ Health Perspect 2007;115:709–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kjos SL, Peters RK, Xiang A, Henry OA, Montoro M, Buchanan TA. Predicting future diabetes in Latino women with gestational diabetes. Utility of early postpartum glucose tolerance testing. Diabetes 1995;44:586–591 [DOI] [PubMed] [Google Scholar]
- 11.Marshall MC., Jr Diabetes in African Americans. Postgrad Med J 2005;81:734–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe RM, Allayee H, Xiang AH, et al. Transcription factor 7-like 2 (TCF7L2) is associated with gestational diabetes mellitus and interacts with adiposity to alter insulin secretion in Mexican Americans. Diabetes 2007;56:1481–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Black MH, Fingerlin TE, Allayee H, et al. Evidence of interaction between PPARG2 and HNF4A contributing to variation in insulin sensitivity in Mexican Americans. Diabetes 2008;57:1048–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah NR, Braverman ER. Measuring adiposity in patients: the utility of body mass index (BMI), percent body fat, and leptin. PLoS One 2012;7:e33308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gauderman WJ, Urman R, Avol E, et al. Association of improved air quality with lung development in children. N Eng J Med 2015;372:905–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benson PE. A review of the development and application of the CALINE3 and 4 models. Atmos Environ B Urban Atmos 1992;26:379–390 [Google Scholar]
- 17.Franklin M, Vora H, Avol E, et al. Predictors of intra-community variation in air quality. J Expo Sci Environ Epidemiol 2012;22:135–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther 2003;5:1003–1015 [DOI] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 20.Akaike H. Information theory and an extension of the maximum likelihood principle. In Second International Symposium on Information Theory. Budapest, Hungary, Akadémiai Kiado, p. 267–281 [Google Scholar]
- 21.Urman R, McConnell R, Islam T, et al. Associations of children’s lung function with ambient air pollution: joint effects of regional and near-roadway pollutants. Thorax 2014;69:540–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berhane K, Zhang Y, Linn WS, et al. The effect of ambient air pollution on exhaled nitric oxide in the Children’s Health Study. Eur Respir J 2011;37:1029–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Council on Exercise. What are the guidelines for percentage of body fat loss? Available from http://www.acefitness.org/acefit/healthy-living-article/60/112/what-are-the-guidelines-for-percentage-of/ [article online], 2009. Accessed 26 June 2015
- 24.Sun Q, Wang A, Jin X, et al. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA 2005;294:3003–3010 [DOI] [PubMed] [Google Scholar]
- 25.Mills NL, Törnqvist H, Robinson SD, et al. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation 2005;112:3930–3936 [DOI] [PubMed] [Google Scholar]
- 26.Rajagopalan S, Brook RD. Air pollution and type 2 diabetes: mechanistic insights. Diabetes 2012;61:3037–3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu C, Fonken LK, Wang A, et al. Central IKKβ inhibition prevents air pollution mediated peripheral inflammation and exaggeration of type II diabetes. Part Fibre Toxicol 2014;11:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Q, Yue P, Deiuliis JA, et al. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 2009;119:538–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rückerl R, Greven S, Ljungman P, et al.; AIRGENE Study Group . Air pollution and inflammation (interleukin-6, C-reactive protein, fibrinogen) in myocardial infarction survivors. Environ Health Perspect 2007;115:1072–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen JC, Schwartz J. Metabolic syndrome and inflammatory responses to long-term particulate air pollutants. Environ Health Perspect 2008;116:612–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rui W, Guan L, Zhang F, Zhang W, Ding W. PM2.5 -induced oxidative stress increases adhesion molecules expression in human endothelial cells through the ERK/AKT/NF-κB-dependent pathway. J Appl Toxicol 2016;36:48–59 [DOI] [PubMed]
- 32.Zheng Z, Xu X, Zhang X, et al. Exposure to ambient particulate matter induces a NASH-like phenotype and impairs hepatic glucose metabolism in an animal model. J Hepatol 2013;58:148–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boura-Halfon S, Zick Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am J Physiol Endocrinol Metab 2009;296:E581–E591 [DOI] [PubMed] [Google Scholar]
- 34.Balasubramanian P, Sirivelu MP, Weiss KA, et al. Differential effects of inhalation exposure to PM2.5 on hypothalamic monoamines and corticotrophin releasing hormone in lean and obese rats. Neurotoxicology 2013;36:106–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomson EM, Vladisavljevic D, Mohottalage S, Kumarathasan P, Vincent R. Mapping acute systemic effects of inhaled particulate matter and ozone: multiorgan gene expression and glucocorticoid activity. Toxicol Sci 2013;135:169–181 [DOI] [PMC free article] [PubMed]
- 36.Xiang AH, Wang C, Peters RK, Trigo E, Kjos SL, Buchanan TA. Coordinate changes in plasma glucose and pancreatic β-cell function in Latino women at high risk for type 2 diabetes. Diabetes 2006;55:1074–1079 [DOI] [PubMed] [Google Scholar]
- 37.Unger RH, Zhou YT. Lipotoxicity of beta-cells in obesity and in other causes of fatty acid spillover. Diabetes 2001;50(Suppl. 1):S118–S121 [DOI] [PubMed] [Google Scholar]
- 38.Millstein J, Gilliland F, Berhane K, et al. Effects of ambient air pollutants on asthma medication use and wheezing among fourth-grade school children from 12 Southern California communities enrolled in The Children’s Health Study. Arch Environ Health 2004;59:505–514 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.