Abstract
OBJECTIVE
Treatment of severe hypoglycemia outside of the hospital setting is limited to intramuscular glucagon requiring reconstitution prior to injection. The current study examined the safety and dose-response relationships of a needle-free intranasal glucagon preparation in youth aged 4 to <17 years.
RESEARCH DESIGN AND METHODS
A total of 48 youth with type 1 diabetes completed the study at seven clinical centers. Participants in the two youngest cohorts (4 to <8 and 8 to <12 years old) were randomly assigned to receive either 2 or 3 mg intranasal glucagon in two separate sessions or to receive a single, weight-based dose of intramuscular glucagon. Participants aged 12 to <17 years received 1 mg intramuscular glucagon in one session and 3 mg intranasal glucagon in the other session. Glucagon was given after glucose was lowered to <80 mg/dL (mean nadir ranged between 67 and 75 mg/dL).
RESULTS
All 24 intramuscular and 58 of the 59 intranasal doses produced a ≥25 mg/dL rise in glucose from nadir within 20 min of dosing. Times to peak plasma glucose and glucagon levels were similar under both intramuscular and intranasal conditions. Transient nausea occurred in 67% of intramuscular sessions versus 42% of intranasal sessions (P = 0.05); the efficacy and safety of the 2- and 3-mg intranasal doses were similar in the youngest cohorts.
CONCLUSIONS
Results of this phase 1, pharmacokinetic, and pharmacodynamic study support the potential efficacy of a needle-free glucagon nasal powder delivery system for treatment of hypoglycemia in youth with type 1 diabetes. Given the similar frequency and transient nature of adverse effects of the 2- and 3-mg intranasal doses in the two youngest cohorts, a single 3-mg intranasal dose appears to be appropriate for use across the entire 4- to <17-year age range.
Introduction
The Diabetes Control and Complications Trial conclusively demonstrated that lowering plasma glucose and HbA1c concentrations as close to normal as possible in adolescents and adults with type 1 diabetes (T1D) could prevent or delay the development of early microvascular complications but at the expense of a marked increase in the risk of severe hypoglycemic events causing seizure or loss of consciousness (1,2). Importantly, the T1D Exchange has demonstrated in our youth that although severe hypoglycemia is not greater with lower HbA1c levels (frequency of 4% and 7% in the past year for participants with HbA1c <7.0 and >9.0%, respectively), it nevertheless remains a serious threat irrespective of the level of glycemic control (3).
Fear of hypoglycemia can be a deterrent for patients and their families to strive for optimal metabolic control in youth with T1D (4,5), especially in parents of young children with T1D (6,7). While severe hypoglycemic events are alarming in and of themselves, fear of hypoglycemia may be increased by the daunting task of treating such events with currently available glucagon emergency kits. Owing to the propensity of glucagon to form fibrils once in aqueous solution (8), currently available glucagon emergency kits require reconstitution of lyophilized powder in a diluent immediately prior to intramuscular injection by family members or others who may not be well trained in or comfortable with giving injections.
Investigation of an intranasal route for administrating glucagon was first reported in 1983 (9). While a number of small studies yielded positive results in healthy volunteers and in adults and children with T1D, a product was never commercialized (9–15). We recently completed a trial of a novel dry powder glucagon formulation, packaged in an easy-to-use intranasal dispenser (Supplementary Fig. 1) in adults with T1D. That study showed that a 3-mg dose of intranasal glucagon was as effective as intramuscular glucagon in reversing insulin-induced hypoglycemia (16). Consequently, the current study was undertaken to assess the safety and pharmacokinetics and pharmacodynamics of intranasal glucagon compared with commercially available intramuscular glucagon in children and adolescents with T1D ranging in age from 4 to <17 years. We were particularly interested in whether adjustment of intranasal glucagon dosing would be needed based on age or weight for younger patients, as weight-based dosing is currently recommended for the intramuscular formulation; therefore, for children aged 4 to <12 years we assessed the exposure-response characteristics of both 2- and 3-mg intranasal doses.
Research Design and Methods
The study was conducted in the Clinical Research Center or outpatient infusion center affiliated with seven diabetes clinics within the T1D Exchange Clinic Network (17). The institutional review board of each participating institution approved the study protocol. Parents/guardians provided written informed consent, and assent from minors was obtained as required. Full protocol details are available on clinicaltrials.gov (clinical trial reg. no. NCT01997411).
Study Design
The study included three cohorts of children 4 to <8, 8 to <12, and 12 to <17 years of age. A sample size of 48 participants (12 in the older cohort and 18 in each of the two younger cohorts) was selected based on the U.S. Food and Drug Administration (FDA) guidance for pediatric pharmacokinetic studies, which indicates a standard approach is to administer either single or multiple doses of a drug to a relatively small (6–12) group of participants.
Participants 12 to <17 years of age were randomly assigned in a 1:1 ratio to receive either 3 mg intranasal glucagon or 1 mg intramuscular glucagon (GlucaGen HypoKit, Novo Nordisk) (18) for the first dosing visit, with the other glucagon preparation administered during the second dosing visit in a crossover fashion.
Studies in the 4- to <8- and 8- to <12-year age-groups were designed to compare the efficacy, safety, and pharmacokinetics of the 2-mg and 3-mg intranasal doses of glucagon with one another, as well as with weight-based dosing of intramuscular glucagon. For minimization of the number of dosing visits/procedures to a maximum of two, participants in the youngest cohort were randomly assigned to one of two groups in a 2:1 ratio. One group of 12 was studied twice, receiving 2 mg intranasal glucagon in one visit and 3 mg intranasal glucagon in the other visit in a double-blind, random order. The dosing visits were carried out within 7–28 days of each other, unless the second visit needed to occur later in order to accommodate the volume of blood required for some of the smaller participants. The other group (N = 6) was studied once, receiving a single weight-based dose of intramuscular glucagon. Intramuscular glucagon was administered per labeling guidelines with participants weighing <25 kg receiving a 0.5-mg dose and those ≥25 kg receiving a 1-mg dose (18).
During each visit, glucagon was given 5 min after the plasma blood glucose was <80 mg/dL, and successful treatment (the primary efficacy outcome) was defined as a ≥25 mg/dL rise in plasma glucose from nadir within 20 min of receiving glucagon without receipt of additional actions to increase the blood glucose level.
Eligibility Criteria
Participants included children meeting the following eligibility criteria: aged 4 to <17 years with T1D duration of at least 1 year and in good general health. Exclusion criteria included history of a severe hypoglycemic episode in the month prior to enrollment; pheochromocytoma or insulinoma; history of epilepsy or seizure disorder; cardiovascular, gastrointestinal, liver, or kidney disease; or use of medications such as β-blockers.
Study Medication
The intranasal AMG504-1 product (Locemia Solutions) contains 2 mg glucagon in 20 mg dry powder or 3 mg glucagon in 30 mg dry powder, depending on the dose. The nasal powder is administered with a single-use one-step dispensing device. The tip of the device was inserted in one nostril, and the dose was delivered by simply depressing a plunger connected to a piston that gently discharges the powder into the nostril (Supplementary Fig. 1). No inhalation or other cooperative measure is required from the patient, as absorption takes place through the nasal mucosa. An earlier phase 1 study showed that nasal congestion, with or without concomitant use of a decongestant, did not adversely affect glucagon pharmacokinetics or the glycemic response in otherwise healthy subjects given the 3-mg dose during and after recovery from a common cold (C.A. Piché, written communication). A phase 2 study established a dose response for intranasal glucagon that attained a maximal increase in blood glucose with the 3-mg dose, presumably due to saturable absorption across the nasal mucosa (19).
Intramuscular glucagon administered for study visits was the GlucaGen HypoKit (Novo Nordisk), which when reconstituted with the sterile water provided in the kit contains 1 mg/mL glucagon (18). Preparation and administration of the intramuscular glucagon was completed by a trained health care professional per the package guidelines, with weight-based dosing used when appropriate (18).
Study Procedures
Each glucagon-dosing visit was conducted after an overnight fast of at least 8 h. On arrival to the research center, an intravenous catheter was inserted in an arm vein for blood sampling. For participants using an insulin pump for diabetes management, the basal insulin infusion rate was increased by 25–50% to cause a gradual decline in plasma glucose in a procedure previously described by the Diabetes Research in Children Network (DirecNet) (20). Bolus doses of insulin equal to ∼1 h of the participant’s basal rate and further increases in basal insulin rate were administered, as needed, to achieve the target glucose of <80 mg/dL. Participants on injection therapy received their usual dose of long-acting insulin analog in the 24 h prior to the visit. Insulin was administered at a rate of 1 mU/kg/min i.v. to reach the target glucose of <80 mg/dL. A priming dose of 2–4 units i.v. insulin also was given if needed. For participants who arrived to the center with a plasma glucose <80 mg/dL, no additional insulin was administered and the randomized glucagon preparation was given immediately after intravenous access was obtained and baseline blood samples were collected.
Plasma glucose concentrations were measured every 5–10 min after the start of the study procedures using an FDA-approved glucose analyzer (YSI 2300, Yellow Springs Instruments, Yellow Springs, OH; Analox GM9, Analox Instruments, Lunenburg, MA; or HemoCue Glucose 201 Analyzer, Ängelholm, Sweden). Once the glucose concentration was <80 mg/dL, basal rates were returned to normal settings on the insulin pump or the intravenous insulin infusion was stopped. Five minutes later, blood samples for glucose and glucagon were collected and glucagon was administered (t = 0). Glucagon was delivered with the participant lying in a lateral recumbent position in the quadriceps muscle for the intramuscular administration or in a nare for the intranasal administration. Serial blood samples for glucose and glucagon were collected at t = 5, 10, 15, 20, 30, 40, 60, and 90 min. A reduced blood draw schedule was permitted for those participants of insufficient weight to accommodate the volume of blood required. Nasal and nonnasal symptom scores were ascertained at baseline and at t = 15, 30, 60, and 90 min after glucagon administration in all participants.
Biochemical Analysis
All samples for analysis of glucose and glucagon were collected on ice into tubes containing EDTA with the protease inhibitor aprotinin immediately added, centrifuged at 4°C, separated, and frozen at −80°C until subsequent analysis. Plasma glucose concentrations were measured by the glucose hexokinase method using an automated glucose analyzer (Roche Module P; Roche Diagnostics, Indianapolis, IN) and glucagon by a commercially available radioimmunoassay (Millipore, Billerica, MA) at the Northwest Lipid Research Laboratory (University of Washington, Seattle, WA).
Statistical Analysis
Separate analyses were conducted for each age cohort (4 to <8, 8 to <12, and 12 to <17 years old). All dosing visits in which glucagon was received were included in the analyses. One participant in the 12 to <17 year old cohort had the 3-mg intranasal dosing visit repeated owing to a device malfunction leading to insufficient administration of glucagon during the initial dosing visit (maximum glucagon concentration 327 pg/mL, which is 8% of the average glucagon concentration achieved in this dosing cohort). The results from this participant’s initial 3-mg intranasal dosing visit were not included in the efficacy analysis but were included in the safety analysis. The design defect that led to the device malfunction was corrected to prevent future malfunction.
All reported glucose and glucagon values are from the central laboratory (Northwest Lipid Research Laboratory). If the central laboratory glucose measurement was missing, the local plasma glucose measurement made using an FDA-approved glucose analyzer was used (this occurred for 11 of 738 [1.5%] glucose measurements). Nadir glucose concentration was defined as the minimum glucose measurement within 10 min after glucagon administration.
The proportion of participants in each treatment arm achieving at least a 25 mg/dL rise in central laboratory glucose above the glucose nadir within 20 min after receiving study glucagon, in the absence of additional actions to increase the blood glucose level, was computed. The maximum central lab glucose concentration after administration of study glucagon was calculated in each treatment arm. Summary statistics for blood glucose concentration at each time point across the dosing visit were computed within treatment arm. Missing glucose values and glucose values after receipt of intervention treatment were imputed using the Rubin method based on available glucose measurements, age cohort, and treatment arm.
Peak central laboratory glucagon concentration and time until administration of study glucagon until peak glucagon concentration (Tmax) were computed in each treatment arm. Summary statistics for blood glucagon concentration at each time point across the dosing visit were computed within treatment arm. Missing glucagon values were imputed using the Rubin method based on available glucagon measurements, age cohort, and treatment arm.
A treatment comparison of the occurrence of nausea with or without vomiting was completed, pooling the 2-mg intranasal and 3-mg intranasal study glucagon doses, using a generalized linear mixed model with random participant effect adjusting for age cohort. Similarly, a treatment comparison of the occurrence of head or facial pain also was completed using a generalized linear mixed model with random participant effect adjusting for age cohort.
Results are expressed as mean ± SD or median (interquartile range). Data analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
Participants
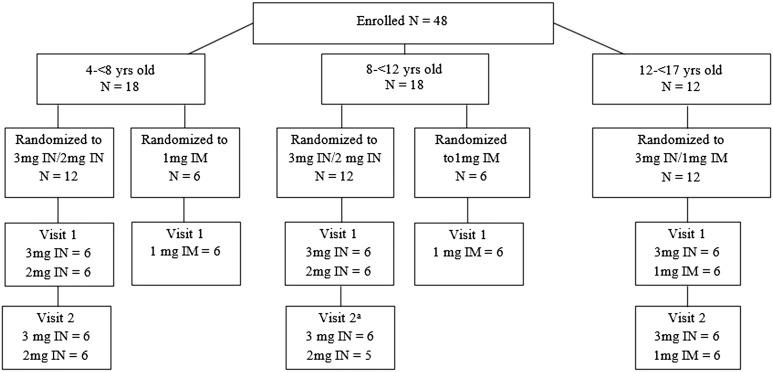
Forty-eight participants were enrolled in the study, and participant characteristics by age cohort are presented in Table 1. All but one participant completed each planned dosing visit; a 10-year-old withdrew from the study after the first dosing visit of 3 mg intranasal glucagon (Fig. 1).
Table 1.
Baseline demographic and clinical characteristics
| 4 to <8 years old (N = 18) | 8 to <12 years old (N = 18) | 12 to <17 years old (N = 12) | |
|---|---|---|---|
| Age (years) | 6.5 ± 1.2 | 11.1 ± 0.8 | 14.6 ± 1.6 |
| Female | 3 (17) | 8 (44) | 5 (42) |
| Non-Hispanic white | 18 (100) | 16 (89) | 10 (83) |
| Weight (kg) | 25.4 ± 5.2 | 43.2 ± 8.9 | 61.2 ± 13.8 |
| Duration of diabetes (years) | 2.8 (2.1, 3.8) | 4.6 (3.8, 6.7) | 5.9 (3.5, 8.0) |
| HbA1c (%)a | 8.1 ± 0.8 | 7.9 ± 0.9 | 8.2 ± 1.5 |
| HbA1c (mmol/mol)a | 65 ± 8.7 | 63 ± 9.8 | 66 ± 16.4 |
| Insulin pump use | 10 (56) | 16 (89) | 9 (75) |
| History of any prior severe hypoglycemic eventb | 6 (33) | 2 (11) | 5 (42) |
Data are mean ± SD, n (%), or median (25th, 75th percentile).
aHbA1c test performed locally;
bsevere hypoglycemic event defined as an episode that required third-party assistance for treatment.
Figure 1.
Study flowchart. aOne participant requested to withdraw prior to the second visit. IM, intramuscular; IN, intranasal; yrs, years.
Plasma Glucose and Glucagon Responses
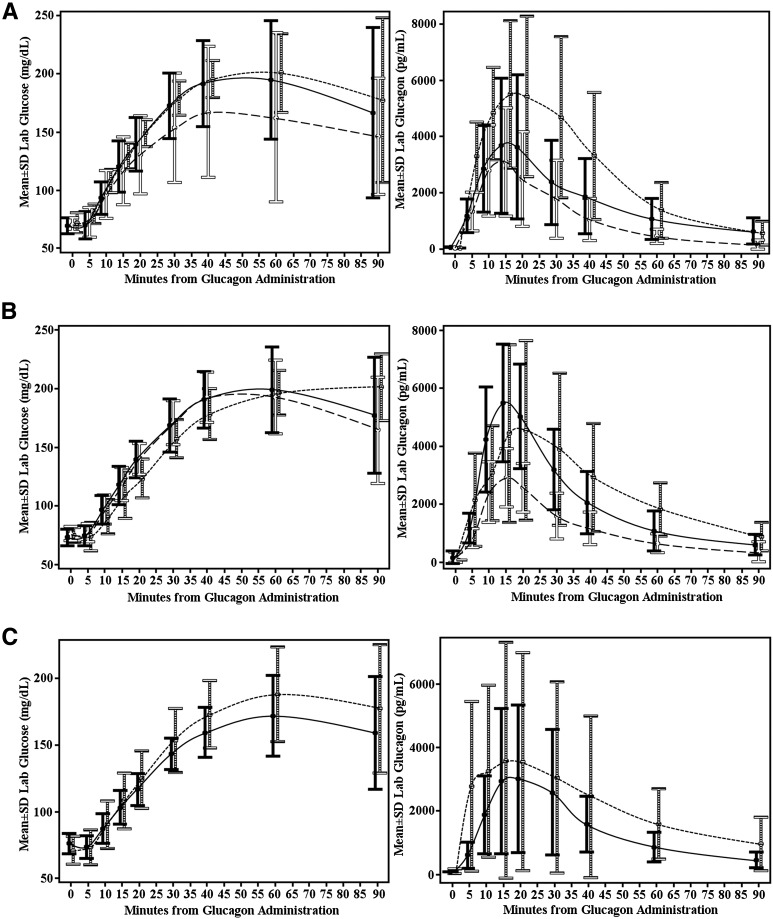
Results showed that at all dosing visits the insulin infusion successfully lowered mean nadir plasma glucose levels when stratified by age and formulation used, as shown in Table 2, to a range of 67–75 mg/dL across age cohorts for the intranasal glucagon visits and 69–72 mg/dL across age cohorts for the intramuscular glucagon visits. The primary outcome of a ≥25 mg/dL rise in plasma glucose within 20 min after glucagon administration was achieved in all 24 intramuscular dosing visits and in 58 of the 59 intranasal dosing visits (Table 2). The one exception was a 6-year-old boy who blew his nose immediately after administration of the 2-mg intranasal dose. This resulted in a peak glucagon level of 324 pg/mL, which was 10-fold less than the mean level detected with the 2-mg intranasal dose administered to other participants in this age-group (Table 2). A 72 mg/dL increase in plasma glucose after 20 min was achieved in the same patient after the 3-mg intranasal dose. The mean maximal glucose concentrations achieved in all successful intranasal dosing visits ranged between 178 and 208 mg/dL across age cohorts, with mean maximal glucose in the intramuscular dosing visits ranging between 194 and 211 mg/dL across the age cohorts (Table 2).
Table 2.
Summary of glucose and glucagon concentrations
| 4 to <8 years old | 8 to <12 years old | 12 to <17 years old | ||||||
|---|---|---|---|---|---|---|---|---|
| IM |
2 mg IN |
3 mg IN |
IM |
2 mg IN |
3 mg IN |
IM |
3 mg IN |
|
| N | 6 | 12 | 12 | 6 | 11 | 12 | 12 | 12 |
| Plasma glucose at nadir (mg/dL)a | 71 ± 8 | 68 ± 9 | 67 ± 10 | 72 ± 12 | 75 ± 7 | 71 ± 6 | 69 ± 11 | 73 ± 9 |
| Plasma glucose immediately prior to glucagon administration ≤70 mg/dL | 3 (50) | 6 (50) | 7 (58) | 1 (17) | 3 (27) | 6 (50) | 7 (58) | 1 (8) |
| ≥25 mg/dL rise in glucose by 20 minc | 6 (100) | 11 (92) | 12 (100) | 6 (100) | 11 (100) | 12 (100) | 12 (100) | 12 (100) |
| Time until all participants experienced rise in glucose ≥25 mg/dL (min)d | 10 | 20 | 15 | 20 | 20 | 15 | 20 | 20 |
| Mean rise in plasma glucose at 20 min (mg/dL) | 77 ± 10 | 60 ± 31 | 70 ± 20 | 49 ± 13 | 64 ± 11 | 67 ± 15 | 54 ± 14 | 41 ± 10 |
| Maximum plasma glucose (mg/dL)b | 211 ± 27 | 189 ± 54 | 208 ± 44 | 205 ± 24 | 201 ± 28 | 206 ± 32 | 194 ± 33 | 178 ± 27 |
| Cmax glucagon (pg/mL) | 6,343 ± 2,029 | 3,531 ± 1,762 | 4,033 ± 2,435 | 4,817 ± 3,086 | 2,952 ± 1,024 | 5,832 ± 2,106 | 4,382 ± 3,771 | 3,186 ± 2,294 |
| Tmax glucagon (min) | 17 (5, 30) | 15 (10, 20) | 17 (10, 60) | 17 (5, 30) | 15 (10, 20) | 15 (10, 30) | 17 (5, 30) | 20 (15, 30) |
Data are mean ± SD, n (%), or median (25th, 75th percentile). One participant completed only one of two scheduled dosing visits: a 10-year-old who withdrew from the study after the first dosing visit of 3 mg intranasal glucagon. Cmax, peak central laboratory glucagon concentration; IM, intramuscular; IN, intranasal.
aMinimum central laboratory glucose value measured within 10 min after glucagon administration;
bmaximum glucose after administration of glucagon;
cthe one participant who failed to achieve a ≥25 mg/dL rise in glucose blew his nose immediately after 2 mg intranasal dose administration and had a peak glucagon of only 324 pg/mL;
dexcludes the one participant who blew his nose and did not experience a ≥25 mg/dL rise in glucose.
Plasma glucagon levels increased rapidly within 5 min of intranasal and intramuscular glucagon administration (Fig. 2), and peak mean glucagon concentrations across age cohorts ranged between 2,952 and 5,832 pg/mL for the intranasal cohort and 4,382 and 6,343 pg/mL for the intramuscular cohort. Further details regarding peak mean glucagon concentrations based on age and formulation of glucagon used are presented in Table 2. Moreover, the median time to the Tmax was ≤20 min for both intramuscular and intranasal glucagon formulation and in all three age-groups.
Figure 2.
A: Glucose and glucagon concentrations over time according to treatment arm: 4 to <8 years old. Solid black bars and line, 3 mg intranasal; solid white bars and long dashed line, 2 mg intranasal; horizontal black-and-white striped bars and short dashed line, intramuscular. B: Glucose and glucagon concentrations over time according to treatment arm: 8 to <12 years old. Solid black bars and line, 3 mg intranasal; solid white bars and long dashed line, 2 mg intranasal; horizontal black-and-white striped bars and short dashed line, intramuscular. C: Glucose and glucagon concentrations over time according to treatment arm: 12 to <17 years old. Solid black bars and line, 3 mg intranasal; horizontal black-and-white striped bars and short dashed line, intramuscular. Lab, laboratory.
Adverse Events
Adverse events stratified by age cohort and glucagon formulation and dosage are provided in Table 3, with full details available in Supplementary Table 1. Nausea, with or without vomiting, occurred in 67% of participants who received intramuscular glucagon compared with 43% for the 3-mg intranasal dose and 39% for the 2-mg intranasal dose (P = 0.05 intramuscular vs. intranasal). Head/facial discomfort was reported by 24% of participants receiving the 3-mg intranasal dose, 17% receiving the 2-mg intranasal dose, and 13% receiving intramuscular (P = 0.30 intramuscular vs. intranasal).
Table 3.
Adverse events by treatment arm within age cohort
| 4 to <8 years old | 8 to <12 years old | 12 to <17 years old | ||||||
|---|---|---|---|---|---|---|---|---|
| Adverse events |
IM |
2 mg IN |
3 mg IN |
IM |
2 mg IN |
3 mg IN |
IM |
IN |
| N | 6 | 12 | 12 | 6 | 11 | 12 | 12 | 13 |
| One or more events | 5 (83) | 6 (50) | 5 (42) | 6 (100) | 5 (46) | 6 (50) | 7 (58) | 9 (69) |
| Gastrointestinala,b | 5 (83) | 5 (42) | 5 (42) | 5 (83) | 4 (36) | 6 (50) | 6 (50) | 6 (46) |
| Headachea | 0 | 2 (17) | 1 (8) | 2 (33) | 2 (18) | 4 (33) | 1 (8) | 4 (31) |
| Nasala,c | 0 | 1(8) | 2 (17) | 0 | 0 | 1 (8) | 0 | 3 (23) |
| Oculara,d | 0 | 0 | 0 | 0 | 1(9) | 0 | 0 | 2 (15) |
| Sensory/paina,e | 2 (33) | 1 (8) | 0 | 3 (50) | 0 | 0 | 0 | 0 |
| Othera,f | 1 (17) | 1 (8) | 0 | 1 (17) | 0 | 0 | 0 | 0 |
One serious adverse event was reported in which a 7-year-old participant (intramuscular treatment) experienced a hypoglycemic event after receiving a bolus of insulin with lunch. The participant received 90 g oral carbohydrates and made a full recovery. One participant in the 12- to <17-year-old group had a repeat 3-mg intranasal glucagon dosing visit owing to a device malfunction leading to insufficient receipt of glucagon during the initial visit; both dosing visits were included in the safety analysis. IM, intramuscular; IN, intranasal.
aN (%) of participants with at least 1 occurrence of the adverse event group;
bincludes abdominal pain (upper), diarrhea, nausea, and vomiting;
cincludes nasal congestion, nasal discomfort, sneezing, and rhinalgia;
dincludes eye irritation, lacrimation increase, and ocular discomfort;
eincludes catheter site pain and injection site discomfort;
fincludes hypoglycemia, tachycardia, and dizziness.
Conclusions
The current study was designed as a phase 1, pharmacokinetic, and pharmacodynamic dose-finding study of a new, needle-free, dry powder intranasal glucagon preparation for treatment of hypoglycemia in youth with T1D. Plasma glucagon concentrations rapidly increased to supraphysiologic levels in a similar fashion after both intranasal and intramuscular glucagon dosing, accompanied by a rise in glucose of ≥25 mg/dL within 20 min of administration in all of the intramuscular dosing visits and 58 of 59 intranasal doses. The single failed 2-mg intranasal response observed is thought to have resulted from not receiving the intended dose of glucagon, as the participant blew his nose immediately after the product was administered. In most situations, it is highly unlikely that patients requiring emergency glucagon therapy for treatment of severe hypoglycemia will have such a problem. Importantly, as intranasal glucagon is absorbed passively through the nasal mucosa, no cooperation from an individual requiring rescue glucagon therapy is required.
Another aim of the study was to evaluate whether the intranasal dose of glucagon should be adjusted based on age or body weight, as is recommended for intramuscular glucagon (18,21). In participants who were <12 years old, the plasma glucose responses to the 2-mg and 3-mg intranasal doses of intranasal glucagon were similar. Moreover, the adverse effects of the two intranasal glucagon levels occurred at similar frequencies and were transient. Nausea with or without vomiting tended to be less frequent with the two intranasal doses than with weight-adjusted doses of intramuscular glucagon.
Hypoglycemia was not intentionally induced in the current study for ethical reasons in the pediatric population, which may be viewed as a limitation in study design. Yet, the demonstration that the patterns of plasma glucose responses to intranasal and intramuscular glucagon were similar over the first 20 min and that the peak increment in plasma glucose levels was on average 126 mg/dL above nadir values in the 58 successful intranasal glucagon dosing visits supports the contention that these results can be extrapolated to what would be observed in treating severe hypoglycemia in an outpatient setting. Importantly, participants were maintained on their usual basal insulin once the target glucose of <80 mg/dL was reached, allowing us to more closely approximate what may happen in a real-life, outpatient setting during a severe hypoglycemic episode where oftentimes basal insulin delivery is not interrupted after glucagon administration. It is also noteworthy that the subcutaneous route of insulin administration in insulin pump patients did not mitigate against our ability to detect a rise in glucose with intranasal glucagon, even though the pharmacodynamic effects of the increased basal rates would have continued after basal rates were returned to their normal setting.
All of the investigators involved in this study believed that it would be unrealistic and too much of a burden to require each subject in the two younger age-groups to undergo three separate studies. Therefore, subjects in the 4- to <8- and 8- to <12-year old cohorts were randomized to either receive one weight-adjusted dose of intramuscular glucagon or two visits using both the 2-mg and 3-mg intranasal glucagon doses. This precluded direct comparison of the intramuscular glucagon to the intranasal glucagon formulation in each individual studied and could be seen as a limitation of the current study. However, paired studies comparing the responses to two different doses of intranasal glucagon in the same subject provided greater power to assess whether weight- or age-adjusted dosing would be required with the intranasal formulation.
Since this study was carried out in controlled research center settings and both intramuscular and intranasal glucagon were administered by trained professionals, it remains to be determined whether similar results will be seen in the outpatient setting in a combative child or one who is seizing. On the other hand, recent studies have demonstrated the difficulty with administration of currently available injectable preparations. In one study, 94 of 136 parents had difficulty handling the kit, 8 aborted the injection entirely, and 5 injected only air or diluent (22). Another study compared the ability of trained caregivers of insulin-using persons with diabetes to use intramuscular and intranasal devices to treat a simulated episode of severe hypoglycemia (23). Intranasal glucagon was successfully administered by 15 of 16 caregivers (average time 16 s), while injectable glucagon was only administered by 8 of 16 caregivers (average time >7 times that required for intranasal administration). Importantly, only two caregivers were able to correctly administer the full injectable dose, while two of the caregivers mistakenly injected insulin rather than glucagon, highlighting the potential benefit of a glucagon rescue therapy that does not have the same mode of delivery as insulin. Indeed, the first step to ensure glucagon can be administered is to assure that those who may need it have it readily available. In a survey conducted by Glu, the T1D Exchange patient/caregiver online community (myglu.org), nearly 75% of participants (n = 277) stated they never or rarely carried a glucagon emergency kit (24).
Administration of intramuscular glucagon is thought to be so complex that many states allow only nurses or other trained health professionals to give glucagon injections while youngsters with T1D are in school. Consequently, if clinicians submit school orders for intramuscular glucagon, youth with T1D may be barred from school trips or after-school activities in some states because of the absence of adequately trained volunteers. Even in states permissive to nonmedical personnel providing assistance to children during an episode of severe hypoglycemia, only 25% of parents surveyed felt such individuals had been identified and adequately trained (25).
The problems in preparing and injecting the current intramuscular glucagon emergency kits stand in stark contrast to the ease of using the single-dose, needle-free intranasal glucagon-dispensing device used in these studies. This study’s data indicate that a single 3-mg intranasal dose of glucagon can be safely used in pediatric patients 4 to <18 years of age. Indeed, we also have shown that the 3-mg intranasal dose of glucagon is as effective and safe as the administration of a 1-mg intramuscular dose in adults with T1D, which will simplify prescribing this medication across the entire age range of patients with T1D 4 years of age or older.
Now that we have demonstrated that needle-free intranasal administration of glucagon is a promising alternative to currently available injected glucagon in youth with T1D, the next step will be to determine its effectiveness when used in the treatment of real-life outpatient hypoglycemic events in youth with T1D.
Supplementary Material
Article Information
Funding and Duality of Interest. Funding was provided by a grant from the Leona M. and Harry B. Helmsley Charitable Trust and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grants UL1TR000003 (University of Pennsylvania), UL1TR000064 (University of Florida), UL1TR001108 (Indiana University), and Locemia Solutions. Studies at the Barbara Davis Center for Childhood Diabetes were performed in their infusion center and not the hospital Clinical and Translational Research Center. Locemia Solutions provided the intranasal glucagon product. The nonprofit employer of J.L.S. has received grants for support of supplies for investigator-initiated studies from Medtronic. C.A.P. is an employee and member of the board for Locemia Solutions, one of the entities that provided financial support for the conduct of this study. H.D. is an employee for Locemia Solutions. M.R.R. has received consultancy payments from Longevity Biotech, Janssen Research & Development, and Semma Therapeutics and research support from Merck. The nonprofit employer of W.V.T. has received consulting fees from Novo Nordisk and Locemia (Locemia consultancy for protocol development). R.P.W. reports receiving research support from Novo Nordisk and serves as a consultant for Novo Nordisk and Medtronic. E.R. and L.M. received payment for pharmacokinetic/pharmacodynamic analysis. The nonprofit employer of R.W.B. has received consultant payments on his behalf from Sanofi and Animas and a research grant from Novo Nordisk, with no personal compensation to R.W.B. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. J.L.S. researched data and wrote and edited the manuscript. K.J.R. researched data and wrote and edited the manuscript. N.C.F., E.R., and L.M. researched data, performed statistical analyses, and wrote and edited the manuscript. C.A.P., H.D., M.R.R., W.V.T., K.E.B., L.A.D., L.A.F., R.P.W., D.A.S., B.M.N., S.M.M., and R.W.B. researched data and reviewed and edited the manuscript. N.C.F. and R.W.B. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT01997411, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc15-1606/-/DC1.
A complete list of participating investigators and coordinators is provided in the Supplementary Data online.
A slide set summarizing this article is available online.
References
- 1.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Control and Complications Trial Research Group Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr 1994;125:177–188 [DOI] [PubMed] [Google Scholar]
- 3.Campbell MS, Schatz DA, Chen V, et al.; T1D Exchange Clinic Network . A contrast between children and adolescents with excellent and poor control: the T1D Exchange clinic registry experience. Pediatr Diabetes 2014;15:110–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke WL, Gonder-Frederick A, Snyder AL, Cox DJ. Maternal fear of hypoglycemia in their children with insulin dependent diabetes mellitus. J Pediatr Endocrinol Metab 1998;11(Suppl. 1):189–194 [DOI] [PubMed] [Google Scholar]
- 5.Patton SR, Dolan LM, Henry R, Powers SW. Parental fear of hypoglycemia: young children treated with continuous subcutaneous insulin infusion. Pediatr Diabetes 2007;8:362–368 [DOI] [PubMed] [Google Scholar]
- 6.Tsalikian E, Fox L, Weinzimer S, et al.; Diabetes Research in Children Network Study Group . Feasibility of prolonged continuous glucose monitoring in toddlers with type 1 diabetes. Pediatr Diabetes 2012;13:301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mauras N, Beck R, Xing D, et al.; Diabetes Research in Children Network (DirecNet) Study Group . A randomized clinical trial to assess the efficacy and safety of real-time continuous glucose monitoring in the management of type 1 diabetes in young children aged 4 to <10 years. Diabetes Care 2012;35:204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pedersen JS. The nature of amyloid-like glucagon fibrils. J Diabetes Sci Technol 2010;4:1357–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pontiroli AE, Alberetto M, Pozza G. Intranasal glucagon raises blood glucose concentrations in healthy volunteers. Br Med J (Clin Res Ed) 1983;287:462–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pontiroli AE, Calderara A, Pajetta E, Alberetto M, Pozza G. Intranasal glucagon as remedy for hypoglycemia. Studies in healthy subjects and type I diabetic patients. Diabetes Care 1989;12:604–608 [DOI] [PubMed] [Google Scholar]
- 11.Freychet L, Rizkalla SW, Desplanque N, et al. Effect of intranasal glucagon on blood glucose levels in healthy subjects and hypoglycaemic patients with insulin-dependent diabetes. Lancet 1988;1:1364–1366 [DOI] [PubMed] [Google Scholar]
- 12.Slama G, Reach G, Cahane M, Quetin C, Villanove-Robin F. Intranasal glucagon in the treatment of hypoglycaemic attacks in children: experience at a summer camp. Diabetologia 1992;35:398. [DOI] [PubMed] [Google Scholar]
- 13.Hvidberg A, Djurup R, Hilsted J. Glucose recovery after intranasal glucagon during hypoglycaemia in man. Eur J Clin Pharmacol 1994;46:15–17 [DOI] [PubMed] [Google Scholar]
- 14.Boido A, Ceriani V, Pontiroli AE. Glucagon for hypoglycemic episodes in insulin-treated diabetic patients: a systematic review and meta-analysis with a comparison of glucagon with dextrose and of different glucagon formulations. Acta Diabetol 2015;52:405–412 [DOI] [PubMed] [Google Scholar]
- 15.Rosenfalck AM, Bendtson I, Jørgensen S, Binder C. Nasal glucagon in the treatment of hypoglycaemia in type 1 (insulin-dependent) diabetic patients. Diabetes Res Clin Pract 1992;17:43–50 [DOI] [PubMed] [Google Scholar]
- 16.Rickels MR, Ruedy KJ, Foster NC, et al.; T1D Exchange Intranasal Glucagon Investigators . Intranasal glucagon for treatment of insulin induced hypoglycemia in adults with type 1 diabetes: a randomized, cross-over non-inferiority study. Diabetes Care 2016;39:264–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, DuBose SN, Hall CA; T1D Exchange Clinic Network . The T1D Exchange clinic registry. J Clin Endocrinol Metab 2012;97:4383–4389 [DOI] [PubMed] [Google Scholar]
- 18.Novo Nordisk. GlucaGen prescribing information [Internet], 2014. Available from http://www.novonordiskmedicalinformation.com//file_upload/GlucaGen%20HypoKit%20Prescribing%20Information,April%202014%20.pdf. Accessed 16 December 2015
- 19.Locemia Solutions ULC. Safety and efficacy of a novel glucagon formulation in type 1 diabetic patients following insulin-induced hypoglycemia (AMG102) [Internet], 2014. Available from https://clinicaltrials.gov/ct2/show/NCT01556594?term=amg+medical&rank=1. Accessed 16 December 2015
- 20.Tsalikian E, Tamborlane W, Xing D, et al.; Diabetes Research in Children Network (DirecNet) Study Group . Blunted counterregulatory hormone responses to hypoglycemia in young children and adolescents with well-controlled type 1 diabetes. Diabetes Care 2009;32:1954–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lily. Glucagon for injection prescribing information [Internet], 2015. Available from http://pi.lilly.com/us/rglucagon-pi.pdf. Accessed 16 December 2015
- 22.Harris G, Diment A, Sulway M, Wilkinson M. Glucagon administration-underevaluated and undertaught. Pract Diabetes Int 2001;18:22–25 [Google Scholar]
- 23.Yale JF, Piche C, Lafontaine M, et al. Needlefree nasal delivery of glucagon is superior to injectable delivery in simulated hypoglycaemia rescue [Internet], 2015. Available from http://www.easdvirtualmeeting.org/resources/needle-free-nasal-delivery-of-glucagon-is-superior-to-injectable-delivery-in-simulated-hypoglycaemia-rescue–3. Accessed 16 December 2015
- 24.Glu. Frequency of carrying glucagon [Internet], 2015. Available from https://myglu.org/polls/1303. Accessed 16 December 2015
- 25.Driscoll KA, Volkening LK, Haro H, et al. Are children with type 1 diabetes safe at school? Examining parent perceptions. Pediatr Diabetes 2015;16:613–620 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.