Abstract
OBJECTIVE
Recent studies using untargeted metabolomics approaches have suggested that plasma branched-chain amino acids (BCAAs) are associated with incident diabetes. However, little is known about the role of plasma BCAAs in metabolic abnormalities underlying diabetes and whether these relationships are consistent across ethnic populations at high risk for diabetes. We investigated the associations of BCAAs with insulin sensitivity (SI), acute insulin response (AIR), and metabolic clearance of insulin (MCRI) in a multiethnic cohort.
RESEARCH DESIGN AND METHODS
In 685 participants without diabetes of the Insulin Resistance Atherosclerosis Study (IRAS) (290 Caucasians, 165 African Americans, and 230 Hispanics), we measured plasma BCAAs (sum of valine, leucine, and isoleucine) by mass spectrometry and SI, AIR, and MCRI by frequently sampled intravenous glucose tolerance tests.
RESULTS
Elevated plasma BCAAs were inversely associated with SI and MCRI and positively associated with fasting insulin in regression models adjusted for potential confounders (β = −0.0012 [95% CI −0.0018, −0.00059], P < 0.001 for SI; β = −0.0013 [95% CI −0.0018, −0.00082], P < 0.001 for MCRI; and β = 0.0015 [95% CI 0.0008, 0.0023], P < 0.001 for fasting insulin). The association of BCAA with SI was significantly modified by ethnicity, with the association only being significant in Caucasians and Hispanics. Elevated plasma BCAAs were associated with incident diabetes in Caucasians and Hispanics (multivariable-adjusted odds ratio per 1-SD increase in plasma BCAAs: 1.67 [95% CI 1.21, 2.29], P = 0.002) but not in African Americans. Plasma BCAAs were not associated with SI-adjusted AIR.
CONCLUSIONS
Plasma BCAAs are associated with incident diabetes and underlying metabolic abnormalities, although the associations were generally stronger in Caucasians and Hispanics.
Introduction
Over the past decade, emerging technologies for comprehensive metabolic profiling have made it possible to assess the impact on disease outcomes of a large number of substrates and products representing different metabolic pathways. These approaches are of particular relevance to the study of type 2 diabetes etiology because the condition is characterized by a protracted period of progressive declines in insulin sensitivity and/or insulin secretion before the clinical onset of the overt disease (1). A number of recent epidemiological studies using metabolomic approaches have suggested that elevated levels of plasma branched-chain amino acids (BCAAs), including valine, leucine, and isoleucine, are associated with insulin resistance (2,3) and predict the onset of type 2 diabetes (4–6) in European whites, Chinese, and South Asians through possible mechanisms involving decreased expression of BCAA catabolic enzymes and increased muscle protein degradation (7,8). In addition to its effect on insulin sensitivity, elevated plasma BCAAs coupled with hyperinsulinemia exert substantial secretory pressure on the pancreatic β-cells, eventually contributing to β-cell dysfunction (9).
Insulin sensitivity, secretion, and clearance are integrated physiological processes. Insulin clearance declines in the wake of insulin resistance to conserve circulating insulin concentrations, thus preserving β-cell viability in prediabetes states (10). Recent evidence has suggested a link between plasma BCAAs and insulin clearance. In a 4-week dietary interventional study, there was a significant inverse correlation between plasma BCAAs and insulin clearance in overweight persons who were randomized to a diet high in saturated fat (11). Although perturbation in amino acid homeostasis is closely related to insulin sensitivity and β-cell function, and abnormal BCAA metabolism precedes hyperglycemia (12), relatively little is known about how BCAAs are linked to insulin clearance in high-risk populations, such as Hispanics and African Americans. Exploring these associations could provide insight into potential mechanisms in the pathogenesis of type 2 diabetes. Therefore, we aimed to examine the association of plasma BCAAs with metabolic clearance rate of insulin (MCRI), acute insulin response (AIR), and insulin sensitivity (SI) derived from frequently sampled intravenous glucose tolerance tests (FSIGTT), as well as incident diabetes, in the Caucasian, African American, and Hispanic populations of the Insulin Resistance Atherosclerosis Study (IRAS).
Research Design and Methods
Study Population
This study used data from the IRAS, which recruited 1,625 participants from four clinical centers located in San Antonio, TX; San Luis Valley, CO; Oakland, CA; and Los Angeles, CA, between October 1992 and April 1994. Details of the study population and research methods have previously been published (13). The institutional review boards at each study site approved the study protocol, and all participants provided written informed consent. The sample size of the current report was 685 participants after exclusion of those with prevalent diabetes at baseline (n = 537), those who did not return for follow-up (n = 347), and those with missing values of plasma BCAAs, SI, AIR, or MCRI (n = 56).
Data Collection
Participants were asked to fast for 12 h, to abstain from alcohol and heavy exercise for 24 h, and to abstain from smoking on the morning of the baseline and 5-year follow-up examinations. Height and weight were measured to the nearest 0.5 cm and 0.1 kg, respectively. BMI was calculated as weight in kilograms divided by the square of height in meters. Waist and hip circumferences were measured to the nearest 0.5 cm using a steel tape. All measurements were taken in duplicate following standardized procedures, and the averages of these measurements were used in the analyses. Blood pressure was measured using a standard mercury sphygmomanometer after participants were rested for 5 min. The average of the second and the third readings was used for analysis. Demographic and socioeconomic information (e.g., age, sex, ethnicity), as well as lifestyle factors (e.g., smoking, alcohol consumption), were collected on standardized questionnaires by self-report (13). Energy expenditure was estimated from information on physical activity in home, workplace, and leisure environments collected from a validated questionnaire (13). Centrally trained interviewers conducted interviews to complete a semiquantitative food frequency questionnaire (FFQ), which was designed to assess usual dietary intake in the past year. The 114-item FFQ was adapted from the National Cancer Institute Health Habits and History Questionnaire. The food items were expanded to reflect the dietary intake of the multiethnic IRAS populations. The FFQ was validated against eight 24-h dietary recalls. Nutrient intakes from the FFQ were analyzed by the HHHQ-DietSys analysis software (version 3.0, 1993; National Cancer Institute, Bethesda, MD) (14).
Biochemical Analyses
Participants provided a fasting blood draw at each examination. Plasma BCAAs were measured by mass spectrometry (Lipomics Technologies, Inc., West Sacramento, CA) in blood samples collected at baseline (1992–1994) that had been stored at −80°C for 18–20 years. Specifically, stored plasma samples were mixed with deuterium-labeled internal standards. The mixture was derivatized with Tri-Sil and propyl chloroformate in n-propanol and then extracted into chloroform under nitrogen and reconstituted in iso-octane:chloroform. With use of helium as the carrier gas, this solution was injected onto a 7890/5975 gas chromatography–mass spectrometry with a ZB-50 column. Each amino acid was quantified by mass spectrometry in the single-ion monitoring mode with electron ionization. Plasma glucose was measured using the glucose oxidase technique on an autoanalyzer. Plasma insulin level was determined with the dextran-charcoal radioimmunoassay (13). Incident type 2 diabetes was determined by 75-g oral glucose tolerance test using World Health Organization criteria (15).
Measurement of Insulin Sensitivity, Insulin Secretion, and Insulin Clearance
Insulin sensitivity, β-cell function, and insulin clearance were measured using an FSIGTT (16), with two modifications to the original protocol: 1) insulin, instead of tolbutamide, was injected at 20 min to ensure adequate levels of plasma insulin to calculate insulin sensitivity accurately across a broad range of glucose tolerance (17), and 2) a reduced sampling protocol, using 12 instead of 30 samples, was used because of the large number of participants (18). Insulin resistance, expressed as SI, was calculated using minimal-model analysis (MINMOD, version 3.0) (19). A higher value of SI indicates an increase in insulin sensitivity.
Insulin secretion was measured by AIR defined as the average increase in plasma insulin at time points 2 and 4 min after infusing glucose (20). A higher value of AIR indicates increased insulin secretion. MCRI was calculated as the ratio of the insulin dose over the incremental area under the curve of insulin from 20 min to infinity (21) using the following equation:
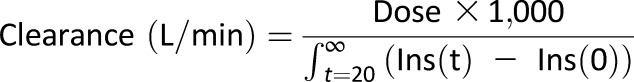 |
where Dose represents the amount of insulin injected at 20 min, Ins(t) the plasma insulin concentration in standard units at each FSIGTT sampling point, and Ins(0) the fasting plasma insulin concentration determined before injection of glucose in the FSIGTT. A higher value of MCRI indicates an increase in insulin clearance.
Statistical Analyses
We summarized the characteristics of participants by thirds of the distribution of plasma BCAAs and SI using median and interquartile range for continuous variables and percentages for categorical variables. We used ANOVA, Kruskal-Wallis tests, and χ2 tests to determine whether continuous and categorical variables differed across the distribution of plasma BCAAs. We also presented the descriptive statistics of exposure variables (total and each individual BCAA) and outcome variables (fasting insulin, SI, AIR, and MCRI) for each ethnicity. We used Spearman correlation analyses to explore, first, the relationship among the individual plasma BCAAs and, second, the relationship of plasma BCAAs with measures of insulin sensitivity, secretion, and clearance in the whole population and within ethnic groups. In light of similar directions of association between BCAAs and outcomes in the Caucasian and Hispanic groups and the lack of statistical interaction between these two ethnic groups on associations of plasma BCAAs and various outcomes (P = 0.21), we present data with Caucasians and Hispanics combined. Separate results for Caucasians and Hispanics are presented in the Supplementary Data. Because of the strong correlation between individual plasma BCAAs, we elected a priori to model the sum of all three plasma BCAAs as the primary exposure.
Since the distribution of MCRI was skewed, we natural log transformed this variable to achieve normality for subsequent analyses. Because of the presence of 0 values for SI, we added a constant of 1 to all values of SI before the log transformation. We used unadjusted and multivariable-adjusted linear regression to explore the association of plasma BCAAs as continuous exposures and measures of SI, SI-adjusted AIR, and MCRI as continuous outcomes. We used unadjusted and multivariable-adjusted logistic regression to explore the association between plasma BCAAs and incident type 2 diabetes.
We presented the regression coefficients (β) for linear regression and odds ratio for logical regression along with their 95% CIs per 1-SD increase in total plasma BCAAs. We included covariates in multivariable-adjusted models if they were associated with both the exposure and the outcome, or if they were of a priori clinical relevance. Potential confounders included age, sex, ethnicity, smoking, alcohol consumption, family history of diabetes, caloric expenditure, energy intake, dietary protein intake, fasting blood glucose, and BMI. We also additionally adjusted for SI and MCRI in fully adjusted logistic regression models to test whether these two underlying pathophysiological traits mediated the association between plasma BCAAs and incident diabetes. We examined the interactions of plasma BCAAs with sex, ethnicity, obesity, and glucose tolerance status on all outcome measures in both unadjusted and fully adjusted models.
In sensitivity analyses, we modeled waist circumference, a component of metabolic syndrome, instead of BMI in regression models with MCRI as an outcome to investigate the link between BCAA, metabolic syndrome, and insulin clearance. To examine potential dose-response relationships between exposures and outcomes by ethnic groups, we stratified plasma BCAAs into fifths of its distribution and plotted SI and MCRI levels across these categories. For the logistic regression analysis, we stratified plasma BCAAs by thirds of its distribution and conducted the logistic regression models for incident diabetes to determine whether there was any dose-response relationship. We calculated areas under the receiver operating characteristic curve (aROC) for traditional diabetes risk factors (metabolic syndrome, impaired fasting glucose, and BMI) with or without BCAAs in the model to assess the additional predictive value of BCAAs on top of these well-documented risk factors (adjusted for age, sex, and ethnicity) and determined whether the aROCs were significantly different between the models with or without BCAAs. All statistical analyses were performed using STATA 12.0 (StataCorp, College Station, TX).
Results
Of the 685 participants without diabetes in this analysis, 40% were Caucasian (n = 290), 24% were African American (n = 165), and 34% were Hispanic (n = 230). Among them, 55% were women and 39% reported to have a family history of diabetes. The median age of the study population was 54 years (range 47–62), and 34% had impaired glucose tolerance. The median concentration of plasma BCCAs was 327 µmol/L (interquartile range 287–363) for the entire cohort, 331 µmol/L (interquartile range 294–372) for Caucasians, 315 µmol/L (interquartile range 277–355) for African Americans, and 329 µmol/L (interquartile range 287–361) for Hispanics.
Participants who had higher plasma BCAA concentrations were more likely to be male, have a higher BMI and waist-to-hip ratio, and have a higher dietary intake of energy and protein (Table 1). Total and individual plasma BCAAs were not different by ethnicity. SI and MCRI were significantly lower in African Americans and Hispanics compared with Caucasians. However, fasting insulin and AIR were significantly higher in African Americans and Hispanics compared with Caucasians (Supplementary Table 1). Participants who had higher SI were more likely to have lower plasma BCAA, lower BMI, and a lower incidence of diabetes (Supplementary Table 2).
Table 1.
Baseline characteristics of participants without diabetes in the IRAS by distribution of total plasma BCAAs
| Distribution of plasma BCAAs | ||||
|---|---|---|---|---|
| Lowest third | Middle third | Upper third | P | |
| BCAA (µmol/L) | 262 ± 34.0 | 327 ± 14.3 | 394 ± 37.8 | <0.0001 |
| Age (years) | 55.1 ± 8.7 | 55.0 ± 8.5 | 53.7 ± 8.3 | 0.148 |
| Male sex | 22.7 | 44.7 | 67.1 | <0.001 |
| Ethnicity | ||||
| Caucasian | 38.9 | 42.1 | 46.1 | |
| African American | 28.4 | 22.4 | 21.5 | 0.347 |
| Hispanic | 32.8 | 35.5 | 32.5 | |
| Current smoker | 17.0 | 12.7 | 11.4 | 0.316 |
| Current drinker | 69.9 | 71.9 | 79.4 | 0.065 |
| Energy expenditure (kcal/kg/day) | 38.5 (36.0–42.6) | 37.9 (35.4–43.5) | 39.0 (35.8–44.9) | 0.288 |
| Family history of diabetes | 38.0 | 39.5 | 40.5 | 0.873 |
| Impaired glucose tolerance | 25.3 | 35.5 | 39.9 | 0.03 |
| BMI (kg/m2) | 26.9 ± 5.0 | 28.5 ± 5.6 | 29.7 ± 5.4 | <0.0001 |
| Waist-to-hip ratio | 0.82 ± 0.079 | 0.87 ± 0.083 | 0.89 ± 0.075 | <0.0001 |
| Energy intake (kcal/day) | 1,687 ± 693 | 1,886 ± 749 | 1,994 ± 802 | <0.0001 |
| Protein intake (g/day) | 69.8 ± 30.5 | 77.6 ± 32.9 | 82.7 ± 33.5 | <0.0001 |
| Incident diabetes | 9.6 | 18.9 | 19.7 | 0.005 |
| Insulin sensitivity (×10−4 min−1 [μU/L]−1) | 2.19 (1.35–3.66) | 1.64 (0.90–2.70) | 1.27 (0.74–2.26) | <0.0001 |
| Fasting insulin (pmol/L) | 11 (7–15.5) | 13 (9–18) | 15 (10.5–21) | <0.0001 |
| AIR (μU/mL) | 48 (28.5–79) | 50.25 (29.3–89) | 54.5 (28.8–85.3) | 0.39 |
| MCRI (L/min) | 6.28 (4.67–8.21) | 5.40 (3.88–6.64) | 4.47 (3.47–5.90) | <0.0001 |
Data are mean ± SD, percent, or median (interquartile range).
All individual BCAAs were highly significantly correlated with each other (Supplementary Table 3). Fasting insulin was positively correlated with plasma BCAAs overall and within ethnic subgroups, whereas SI and MCRI were negatively correlated with plasma BCAAs in the whole population and in Caucasian and Hispanic subgroups (all P < 0.0001). Plasma BCAAs were only positively correlated with AIR in Caucasians (P < 0.05) (Supplementary Table 4). All individual BCAAs were highly significantly correlated with fasting insulin, SI, and MCRI; however, only valine was significantly correlated with AIR (Supplementary Table 5). With stratification by ethnicity, the significant correlations of individual BCAAs with MCRI existed in all three ethnic subgroups. However, the correlations with fasting insulin and SI were only observed in Caucasians and Hispanics—not in African Americans.
In unadjusted linear regression models, plasma BCAAs were positively associated with fasting insulin (Table 2). The significant association remained after adjustment for age, sex, ethnicity, study center, smoking, alcohol consumption, energy expenditure, family history of diabetes, BMI, fasting blood glucose, energy intake, and dietary protein intake. Plasma BCAAs were inversely associated with SI and MCRI in both unadjusted and multivariable-adjusted regression models (Table 2). We found a significant interaction of plasma BCAAs with ethnicity on insulin sensitivity in both unadjusted and multivariable-adjusted models. In stratified analyses, the significant inverse association between plasma BCAAs and insulin sensitivity was only present in Caucasians and the combined group of Caucasians and Hispanics (Table 3 and Supplementary Table 6). Plasma BCAAs were not associated with SI-adjusted AIR in unadjusted or multivariable-adjusted regression models (Table 2). In multivariable-adjusted logistic regression models, plasma BCAAs were associated with the risk of developing type 2 diabetes in Caucasians and in the combined Caucasian and Hispanic group but not in Hispanics alone or in African Americans (Fig. 1 and Supplementary Table 7). The results of linear and logistic regressions were similar when individual plasma BCAAs were modeled as exposures (data not shown).
Table 2.
Estimated regression coefficients (95% CI) on the association between plasma BCAAs and insulin metabolism in IRAS participants without diabetes
| β-Coefficients (95% CI) | P | |
|---|---|---|
| Log fasting insulin | ||
| Unadjusted | 0.0026 (0.0019, 0.0034) | <0.001 |
| Model 1 | 0.0032 (0.0024, 0.0040) | <0.001 |
| Model 2 | 0.0023 (0.0016, 0.0031) | <0.001 |
| Model 3 | 0.0015 (0.0008, 0.0023) | <0.001 |
| Log insulin sensitivity* | ||
| Unadjusted | −0.0022 (−0.0029, −0.0016) | <0.001 |
| Model 1 | −0.0026 (−0.0033, −0.0019) | <0.001 |
| Model 2 | −0.0021 (−0.0027, −0.0014) | <0.001 |
| Model 3 | −0.0012 (−0.0018, −0.00059) | <0.001 |
| Log SI-adjusted AIR | ||
| Unadjusted | −0.00017 (−0.0011, 0.00075) | 0.721 |
| Model 1 | −0.00012 (−0.0011, 0.00084) | 0.804 |
| Model 2 | 0.00029 (−0.00061, 0.0012) | 0.528 |
| Model 3 | 0.00011 (−0.00080, 0.0010) | 0.811 |
| Log MCRI | ||
| Unadjusted | −0.0021 (−0.0027, −0.0016) | <0.001 |
| Model 1 | −0.0025 (−0.0030, −0.0019) | <0.001 |
| Model 2 | −0.0021 (−0.0027, −0.0016) | <0.001 |
| Model 3 | −0.0013 (−0.0018, −0.00082) | <0.001 |
Model 1 adjusted for age, sex, ethnicity, and study center. Model 2: model 1 adjustments plus smoking, alcohol consumption, caloric expenditure, family history of diabetes, dietary kilocalories, dietary protein, and fasting blood glucose. Model 3: model 2 adjustments plus BMI.
*Significant interaction with ethnicity.
Table 3.
Estimated regression coefficients (95% CI) on the association between plasma BCAAs and log-transformed SI, stratified by ethnicity
| β-Coefficients (95% CI) | P | |
|---|---|---|
| Caucasians plus Hispanics | ||
| Unadjusted | −0.0027 (−0.0035, −0.0020) | <0.001 |
| Model 1 | −0.0031 (−0.0039, −0.0023) | <0.001 |
| Model 2 | −0.0024 (−0.0031, −0.0016) | <0.001 |
| Model 3 | −0.0015 (−0.0022, −0.00078) | <0.001 |
| African Americans | ||
| Unadjusted | −0.00081 (−0.0020, 0.00042) | 0.193 |
| Model 1 | −0.0013 (−0.0026, 0.000067) | 0.063 |
| Model 2 | −0.0011 (−0.0024, 0.00025) | 0.109 |
| Model 3 | −0.00053 (−0.0017, 0.00068) | 0.387 |
Model 1 adjusted for age, sex, ethnicity, and study center. Model 2: model 1 adjustments plus smoking, alcohol consumption, caloric expenditure, family history of diabetes, dietary kilocalories, dietary protein, and fasting blood glucose. Model 3: model 2 adjustments plus BMI.
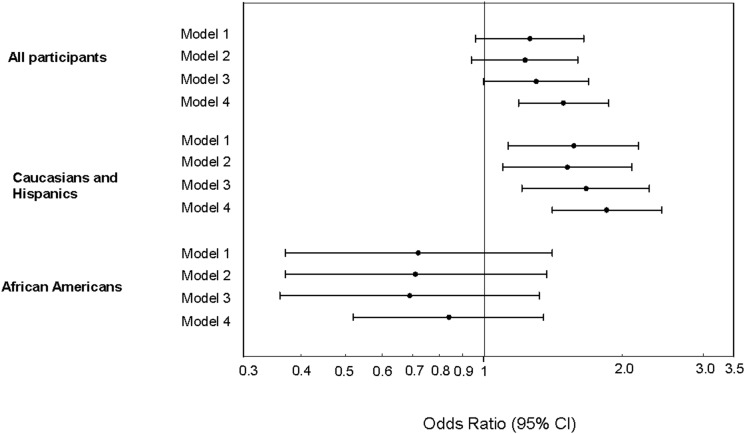
Figure 1.
Estimated odds ratio (95% CI) per 1-SD increase in BCAAs on the association between plasma BCAAs and incident diabetes in the whole population and by ethnic subgroups. Model 1 adjusted for age, sex, ethnicity (in all participants), and study center. Model 2: model 1 adjustments plus smoking, alcohol consumption, caloric expenditure, family history of diabetes, energy intake, dietary protein intake, fasting blood glucose, and BMI. Model 3: model 2 adjustments plus SI. Model 4: model 3 adjustments plus MCRI.
There were no interactions of plasma BCAAs and sex, obesity, and glucose tolerance status with any other outcomes in either linear or logistic regression models. In sensitivity analyses, the magnitude of associations did not change considerably after replacement of BMI by waist circumference in regression models with MCRI as the outcome variable (data not shown). We observed that the significant associations between plasma BCAAs and incident diabetes in Caucasians combined with Hispanics were only partially mediated by SI and/or MCRI (Fig. 1, models 3 and 4). There were significant linear trends in MCRI and SI across quintiles of plasma BCAAs, although these trends were stronger in Caucasians and Hispanics compared with African Americans (Supplementary Figs. 1 and 2). When BCAAs were added to models containing metabolic syndrome, impaired fasting glucose, and BMI (adjusted for age, sex, and ethnicity), aROCs for each model improved (0.62–0.66, 0.72–0.74, and 0.68–0.69, respectively), although the differences were not statistically significant (P values 0.10, 0.25, and 0.34, respectively) (Supplementary Figs. 3–5).
Conclusions
In IRAS participants without diabetes, higher plasma BCAAs were associated with lower SI and MCRI and higher fasting insulin concentrations. These significant associations remained after adjustment for demographic, lifestyle, and dietary factors, as well as established risk factors for diabetes. In addition, plasma BCAAs were associated with incident type 2 diabetes at 5-year follow-up. In stratified analysis by ethnicity, the significant associations with SI and incident diabetes were only present in the combined Caucasian and Hispanic group. A recent article by our group demonstrated that plasma BCAAs were positively associated with insulin resistance and incident type 2 diabetes in a subsample of IRAS participants selected from the extremes of the SI distribution (n = 196), although this report did not investigate insulin clearance or incident diabetes by subgroups of ethnicity (22). In addition, the present analysis included the full distributional range of SI in the IRAS cohort, and the larger sample size allowed us to examine the association in ethnic groups of Caucasians, Hispanics, and African Americans. Our study extends the existing literature on the topic of plasma BCAAs in diabetes etiology by describing the relationship with insulin clearance and by carefully analyzing the impact of plasma BCAAs within ethnic subgroups. The addition of BCAAs to models that included traditional risk factors for type 2 diabetes (i.e., metabolic syndrome, impaired fasting glucose, and BMI) resulted in improved incident type 2 diabetes–predictive capacity according to aROC testing, although differences were not statistically significant, possibly due a relatively modest number of incident cases of type 2 diabetes in this cohort.
Consistent with previous studies (4,6), we observed that higher plasma BCAAs were associated with incident diabetes in the combined group of Caucasians and Hispanics. However, this association did not exist in African Americans. Existing evidence suggests that the magnitude of the BCAA-diabetes association may be ethnicity specific. In the SABRE (Southall And Brent REvisited) study, the adverse association between plasma BCAAs and incident diabetes was stronger in South Asian men than in European men (6). The null associations of BCAAs with incident diabetes in African Americans documented in the present study require confirmation in future studies. Although potential mechanisms underlying this null association have not been elucidated, the documented high heritability of BCAA concentrations (23) may offer one possible explanation.
Reduced insulin clearance is thought to be a compensatory mechanism to preserve β-cell function and to maintain peripheral insulin levels in insulin resistant states (10,24). Alternatively, reduced insulin clearance has been proposed as a marker of liver dysfunction (25). The inverse association between plasma BCAAs and insulin clearance could be linked through metabolic syndrome. Previous studies have shown that metabolic syndrome is associated with reduced insulin clearance (26,27) and, further, that the gene expression of BCAA catabolic enzymes is reduced in individuals with metabolic syndrome (28). Although the significant association between BCAAs and insulin clearance remained after additional adjustment for waist circumference, HDL cholesterol, systolic blood pressure, and plasma triglycerides, the magnitude of the association was attenuated, suggesting that these components of metabolic syndrome may, in part, link plasma BCAA to insulin clearance. Alternatively, it is possible that the elevated BCAAs may have a direct detrimental impact on hepatic insulin clearance. Although literature examining this pathway is sparse, previous studies (7,29) have reported decreases in BCAAs in states of decreased liver function. Notably, however, these studies were conducted among individuals in advanced stages of liver disease, including cirrhosis and liver failure.
The significant positive association of plasma BCAAs with fasting insulin is consistent with our results for SI and MCRI, since elevated fasting insulin is a result of underlying insulin resistance and compensatory decreases in insulin clearance. Although elevated plasma BCAAs could lead to decreased insulin secretion through mechanisms involving insulin resistance and hyperinsulinemia (7), we did not observe a significant association between plasma BCAAs and AIR in the IRAS cohort.
The strengths of this study include the well-characterized multiethnic population, the availability of detailed measurements of insulin clearance and sensitivity, and the comprehensive dietary assessment. Several limitations should also be considered. A frequently sampled oral glucose tolerance test was not conducted as part of this study protocol, and thus we do not have metabolic measures that take into account contributions of the incretin system. The plasma samples used to determine BCAA concentrations had been frozen at −80°C for 18–20 years before assaying. It has previously been reported that concentrations of plasma amino acids are stable at −50°C, without discernable impact from freezing and thawing (30). In addition, our findings are likely generalizable only to individuals with similar demographic and ethnic characteristics.
In conclusion, we observed significant associations of plasma BCAAs with diabetes incidence, insulin sensitivity, and insulin clearance, although these relationships differed across ethnic groups. These findings extend the scientific literature on the role of plasma BCAAs in the etiology of type 2 diabetes and highlight the need for additional studies in well-characterized multiethnic cohorts.
Supplementary Material
Article Information
Funding. IRAS was supported by grants U01-HL-47892, U01-HL-47902, DK-29867, R01-58329, and DK-079888 from the National Heart, Lung, and Blood Institute and grant M01-RR-43 from the National Institutes of Health. A.J.H. holds a Tier II Canada Research Chair in the Epidemiology of Type 2 Diabetes.
Duality of Interest. S.M.W. is an employee of Metabolon, Inc., which provides metabolomics services and sells diagnostics for the management of metabolic disorders. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. C.C.L. and A.J.H. wrote the manuscript, reviewed and edited the manuscript, and contributed to the discussion. S.M.W., C.L., D.I., and Y.-D.I.C. reviewed and edited the manuscript and contributed to the discussion. L.E.W. and S.M.H. researched data, reviewed and edited the manuscript, and contributed to the discussion. A.J.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 13–17 June 2014.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc15-2284/-/DC1.
References
- 1.Bain JR, Stevens RD, Wenner BR, Ilkayeva O, Muoio DM, Newgard CB. Metabolomics applied to diabetes research: moving from information to knowledge. Diabetes 2009;58:2429–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tai ES, Tan ML, Stevens RD, et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 2010;53:757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huffman KM, Shah SH, Stevens RD, et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care 2009;32:1678–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Floegel A, Stefan N, Yu Z, et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 2013;62:639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tillin T, Hughes AD, Wang Q, et al. Diabetes risk and amino acid profiles: cross-sectional and prospective analyses of ethnicity, amino acids and diabetes in a South Asian and European cohort from the SABRE (Southall And Brent REvisited) Study. Diabetologia 2015;58:968–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adeva MM, Calviño J, Souto G, Donapetry C. Insulin resistance and the metabolism of branched-chain amino acids in humans. Amino Acids 2012;43:171–181 [DOI] [PubMed] [Google Scholar]
- 8.Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol 2014;10:723–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol 2008;9:193–205 [DOI] [PubMed] [Google Scholar]
- 10.Mittelman SD, Van Citters GW, Kim SP, et al. Longitudinal compensation for fat-induced insulin resistance includes reduced insulin clearance and enhanced beta-cell response. Diabetes 2000;49:2116–2125 [DOI] [PubMed] [Google Scholar]
- 11.Chiu S, Williams PT, Dawson T, et al. Diets high in protein or saturated fat do not affect insulin sensitivity or plasma concentrations of lipids and lipoproteins in overweight and obese adults. J Nutr 2014;144:1753–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Würtz P, Tiainen M, Mäkinen VP, et al. Circulating metabolite predictors of glycemia in middle-aged men and women. Diabetes Care 2012;35:1749–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagenknecht LE, Mayer EJ, Rewers M, et al. The Insulin Resistance Atherosclerosis Study (IRAS) objectives, design, and recruitment results. Ann Epidemiol 1995;5:464–472 [DOI] [PubMed] [Google Scholar]
- 14.Mayer-Davis EJ, Vitolins MZ, Carmichael SL, et al. Validity and reproducibility of a food frequency interview in a Multi-Cultural Epidemiology Study. Ann Epidemiol 1999;9:314–324 [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva, World Health Org., 1999 (publ. no. WHO/NCD/NCS/99.2) [Google Scholar]
- 16.Bergman RN, Finegood DT, Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev 1985;6:45–86 [DOI] [PubMed] [Google Scholar]
- 17.Welch S, Gebhart SS, Bergman RN, Phillips LS. Minimal model analysis of intravenous glucose tolerance test-derived insulin sensitivity in diabetic subjects. J Clin Endocrinol Metab 1990;71:1508–1518 [DOI] [PubMed] [Google Scholar]
- 18.Steil GM, Volund A, Kahn SE, Bergman RN. Reduced sample number for calculation of insulin sensitivity and glucose effectiveness from the minimal model. Suitability for use in population studies. Diabetes 1993;42:250–256 [DOI] [PubMed] [Google Scholar]
- 19.Pacini G, Bergman RN. MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput Methods Programs Biomed 1986;23:113–122 [DOI] [PubMed] [Google Scholar]
- 20.Lorenzo C, Wagenknecht LE, D’Agostino RB Jr, Rewers MJ, Karter AJ, Haffner SM. Insulin resistance, beta-cell dysfunction, and conversion to type 2 diabetes in a multiethnic population: the Insulin Resistance Atherosclerosis Study. Diabetes Care 2010;33:67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polonsky KS, Pugh W, Jaspan JB, et al. C-peptide and insulin secretion. Relationship between peripheral concentrations of C-peptide and insulin and their secretion rates in the dog. J Clin Invest 1984;74:1821–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer ND, Stevens RD, Antinozzi PA, et al. Metabolomic profile associated with insulin resistance and conversion to diabetes in the Insulin Resistance Atherosclerosis Study. J Clin Endocrinol Metab 2015;100:E463–E468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah SH, Hauser ER, Bain JR, et al. High heritability of metabolomic profiles in families burdened with premature cardiovascular disease. Mol Syst Biol 2009;5:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SP, Ellmerer M, Kirkman EL, Bergman RN. Beta-cell “rest” accompanies reduced first-pass hepatic insulin extraction in the insulin-resistant, fat-fed canine model. Am J Physiol Endocrinol Metab 2007;292:E1581–E1589 [DOI] [PubMed] [Google Scholar]
- 25.Kotronen A, Vehkavaara S, Seppälä-Lindroos A, Bergholm R, Yki-Järvinen H. Effect of liver fat on insulin clearance. Am J Physiol Endocrinol Metab 2007;293:E1709–E1715 [DOI] [PubMed] [Google Scholar]
- 26.Pivovarova O, Bernigau W, Bobbert T, et al. Hepatic insulin clearance is closely related to metabolic syndrome components. Diabetes Care 2013;36:3779–3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee CC, Lorenzo C, Haffner SM, et al. Components of metabolic syndrome and 5-year change in insulin clearance - the Insulin Resistance Atherosclerosis Study. Diabetes Obes Metab 2013;15:441–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lackey DE, Lynch CJ, Olson KC, et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am J Physiol Endocrinol Metab 2013;304:E1175–E1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tajiri K, Shimizu Y. Branched-chain amino acids in liver diseases. World J Gastroenterol 2013;19:7620–7629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kornhuber ME, Balabanova S, Heiligensetzer GV, Kornhuber C, Zettlmeissl H, Kornhuber AW. Stability of human blood serum aminoacids after storage at different pH and temperature conditions. Clin Chim Acta 1991;197:189–200 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.