Abstract
OBJECTIVE
We compared levels and associations of traditional (fasting glucose, HbA1c) and nontraditional (fructosamine, glycated albumin, and 1,5-anhydroglucitol [1,5-AG]) biomarkers of hyperglycemia with incident cardiovascular disease (CVD), incident end-stage renal disease (ESRD), and prevalent retinopathy in black and white adults.
RESEARCH DESIGN AND METHODS
We included 10,373 participants without (8,096 white, 2,277 black) and 727 with diagnosed diabetes (425 white, 302 black) from the Atherosclerosis Risk in Communities (ARIC) Study. We used Cox proportional hazards models to compare hazards ratios of CVD and ESRD among blacks and whites from baseline (1990–1992) through 2012. We compared the odds ratios (from logistic regression) of retinopathy among blacks and whites. We tested for the interaction of each biomarker with race.
RESULTS
Median values of biomarkers were higher among blacks versus whites (all P < 0.001). Relative risks for each biomarker with incident CVD and ESRD, and odds ratios for each biomarker with prevalent retinopathy, were similar by race (all P values for interaction by race >0.10).
CONCLUSIONS
The prognostic value of HbA1c, fructosamine, glycated albumin, and 1,5-AG with incident CVD, incident ESRD, and prevalent retinopathy were similar by race. Our results support similar interpretation of HbA1c and nontraditional biomarkers of hyperglycemia among black and whites with respect to long-term complications.
Introduction
Nontraditional biomarkers of hyperglycemia (fructosamine, glycated albumin, and 1,5-anhydroglucitol [1,5-AG]) have emerged as possible adjuncts to the traditional biomarkers, fasting glucose and HbA1c (1,2). Fasting glucose is an acute measure of current hyperglycemia. HbA1c is the proportion of hemoglobin in red blood cells bound to glucose (specifically, glucose that is bound to the N-terminal valine of the β-chain of hemoglobin), and measures average glycemia over 2–3 months, based on red blood cell turnover (3,4). Fructosamine and glycated albumin are markers of glucose bound to serum proteins and estimate average glycemia over 2–4 weeks (5). 1,5-AG is a monosaccharide, mainly derived from the diet, that is normally almost completely reabsorbed by the kidney. In states of hyperglycemia (>180 mg/dL), glucose in the renal tubular lumen inhibits tubular reabsorption of 1,5-AG, and more 1,5-AG is excreted, resulting in lower serum 1,5-AG levels. Levels of 1,5-AG are inversely associated with average glycemia over the past 2–14 days (6–10).
Higher levels of biomarkers of hyperglycemia have been associated with increased risk of microvascular and macrovascular complications in both people with and people without diabetes (11–17). However, prospective associations of nontraditional serum biomarkers of hyperglycemia with microvascular and macrovascular complications according to race have not been characterized and could shed further light on the debate over racial differences in HbA1c. Whereas HbA1c is a measure of intracellular hyperglycemia and can be affected by nonglycemic factors, such as hemoglobin characteristics or alterations in red cell turnover, fructosamine, glycated albumin, and 1,5-AG are serum measures of extracellular hyperglycemia and therefore are not affected by these nonglycemic factors. Comparing associations of each of these biomarkers in blacks and whites could provide insight into whether racial differences in levels of biomarkers may be attributed to glycemic or nonglycemic factors.
Our objective was to assess the associations of fasting glucose, HbA1c, and nontraditional serum biomarkers of hyperglycemia (fructosamine, glycated albumin, and 1,5-AG) with prevalent retinopathy and incident cardiovascular disease (CVD) and end-stage renal disease (ESRD), and to evaluate differential associations between blacks and whites.
Research Design and Methods
Setting and Participants
We conducted a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) Study, a community-based cohort of 15,792 middle-aged adults recruited in 1987–1989 from four field centers in the United States: Forsyth County, North Carolina; Jackson, Mississippi; suburban Minneapolis, Minnesota; and Washington County, Maryland (18). Follow-up visits 2 through 5 took place during 1990–1992, 1993–1995, 1996–1998, and 2011–2013, respectively. We restricted our study population to ARIC participants who attended visit 2 (n = 14,348) because this was the earliest visit at which all biomarkers of hyperglycemia were measured. We excluded participants who did not have values for all biomarkers of hyperglycemia (n = 1,159) or were missing key covariates (n = 379). We further excluded a small number of nonblack and nonwhite participants (n = 38), as well as people who were not fasting for ≥8 h (n = 396). Last, we excluded 1,276 people with prevalent CVD or preexisting ESRD based on linkage with the U.S. Renal Data System (USRDS) at visit 2. There were 11,100 participants included in our final study population of incident CVD and ESRD. Retinal photographs were obtained only at visit 3 in 1993–1995 (3 years after visit 2); analyses of prevalent retinopathy were restricted further to those participants who attended visit 3 and had valid retinal photographs (n = 8,615).
Biomarkers of Hyperglycemia
All biomarkers of hyperglycemia were measured using blood specimens from visit 2. Fasting glucose was measured using a hexokinase method. HbA1c was measured using high-performance liquid chromatography with Tosoh A1c 2.2 Plus Glycohemoglobin and Tosoh G7 Analyzers (Tosoh Bioscience, South San Francisco, CA), and was standardized to the Diabetes Control and Complications Trial assay (19). All HbA1c values were calibrated to account for the changes in methods (19). Fructosamine, glycated albumin, and 1,5-AG in stored serum samples were measured in 2012–2013 using a Roche Modular P800 analyzer (Roche Diagnostics, Indianapolis, IN). Fructosamine was measured using a colorimetric method (Roche Diagnostics). Glycated albumin (Lucica GA-L; Asahi Kasei Pharma Corp., Tokyo, Japan) and 1,5-AG (GlycoMark, New York, NY) were measured using enzymatic methods. Glycated albumin was expressed as a percentage of total albumin, calculated using the following equation derived by the manufacturer: ([glycated albumin concentration in g/dL ÷ serum albumin concentration in g/dL] ÷ 1.14 × 100) + 2.9. The interassay coefficients of variation were 3% for fructosamine, 1.8% for glycated albumin, and 5% for 1,5-AG. Previous studies from our group and others have demonstrated the stability and reliability of these biomarkers measured in samples stored for long periods (13,20–23).
Covariates
We defined diagnosed diabetes as a self-reported physician diagnosis of diabetes or self-reported use of glucose-lowering medication at visit 1 or visit 2. The following covariates were self-reported by participants as responses to questionnaires at visit 2, unless otherwise specified: age, sex, race (visit 1), education level (visit 1), alcohol consumption, smoking status, and physical activity (Baecke sport activity index at visit 1) (24). Antihypertensive medication use was obtained via self-report or medication inventory. Cholesterol-lowering medication use was obtained via medication inventory.
Diastolic and systolic blood pressures were measured using a random zero sphygmomanometer and recorded as the mean of the second and third readings. BMI was calculated as measured weight (in kilograms) divided by measured height (in meters) squared. Total cholesterol, HDL cholesterol (HDL-c), LDL cholesterol (LDL-c), and triglycerides were measured in stored plasma using the Roche Cobas Bio (Roche Diagnostics). Total cholesterol and triglycerides were measured using an enzymatic method, and HDL-c was measured using a precipitation method. LDL-c was calculated from measured total cholesterol, HDL-c, and triglycerides using the Friedewald equation. Creatinine was measured using the Jaffe method with a Coulter DACOS analyzer. We used the Chronic Kidney Disease Epidemiology Collaboration equation to calculate the estimated glomerular filtration rate (eGFR) using serum creatinine, age, sex, and race (25).
Outcomes and Follow-up
We used a composite CVD end point defined as a first coronary heart disease (CHD), stroke, or heart failure event, based on standard ARIC definitions: first occurrence of definite or probable hospitalized myocardial infarction or death caused by CHD (26); definite or probable hospitalized stroke or death caused by stroke (27); or hospitalization or death caused by heart failure, based on International Classification of Diseases, 9th Revision, code 428 or 10th Revision code I50 (28). All cardiovascular events through 31 December 2012 were ascertained via continuous surveillance of hospitalizations and death certificates, annual telephone follow-up with the participant or a proxy, and linkage to the National Death Index. CHD and stroke events were adjudicated over the entire follow-up; heart failure events were adjudicated beginning in 2005. We conducted secondary analyses with CHD, stroke, and heart failure as separate end points.
For analyses of incident ESRD, we identified treated cases through linkage with the USRDS national registry through 30 September 2011, since that was the most recent available linkage to the USRDS. The Centers for Medicare and Medicaid Services report any people receiving renal replacement therapy to the USRDS within 45 days of initiation of treatment, which includes people undergoing dialysis or a kidney transplant.
Prevalent retinopathy was measured at visit 3 (1993–1995) using fundus photography (29,30). It was defined as a score of ≥20 on the Early Treatment Diabetic Retinopathy Study scale (31).
Statistical Analysis
We calculated descriptive statistics for demographic and clinical characteristics, stratified by diagnosed diabetes status and race. We compared median levels of HbA1c, fructosamine, glycated albumin, and 1,5-AG within clinical categories of fasting glucose among blacks and whites separately, and we tested for differences using the Wilcoxon rank sum test.
We created a five-level variable for each biomarker based on diagnosed diabetes status and biomarker level. In people without diagnosed diabetes, we used clinical cut points recommended by the American Diabetes Association to categorize fasting glucose (<100, 100–125, ≥126 mg/dL) and HbA1c (<5.7, 5.7–6.4, ≥6.5%) (1). We categorized people with diagnosed diabetes based on the clinically recommended cut point of <7 vs. ≥7% for HbA1c. Since clinical cut points have not been established for fructosamine or glycated albumin, we used cut points pegged to the clinically relevant HbA1c values of 5.7% (the 75th percentile) and 6.5% (the 96.5th percentile) in people without diagnosed diabetes and HbA1c of 7% (the 40th percentile) in people with diagnosed diabetes (1). Because of the inverse association of 1,5-AG with hyperglycemia, we used cut points at the 25th and 3.5th percentiles in people without diabetes and the 60th percentile in people with diagnosed diabetes.
We used Cox proportional hazards regression models to assess associations with incident CVD and ESRD. Follow-up for CVD and ESRD began at the visit 2 exam date (1990–1992) and continued until the time of the event, last date of follow-up, or 31 December 2012 (30 September 2011 for ESRD analyses), whichever occurred first. We used logistic regression to evaluate the associations of biomarkers of hyperglycemia with prevalent retinopathy in blacks and whites. Models included age, sex, BMI, BMI2, LDL-c, HDL-c, triglycerides, cholesterol-lowering medication use, systolic blood pressure, antihypertensive medication use, eGFR, family history of diabetes, education level, alcohol consumption, cigarette smoking status, and physical activity level. We also adjusted for fasting glucose and HbA1c in sensitivity analyses. To test for a linear trend among whites and blacks separately, we included the categorical biomarker variable as a continuous variable and conducted a Wald test for the coefficient. We used likelihood ratio tests to evaluate interactions with race. P values <0.05 were considered statistically significant. We verified that the proportional hazards assumption was met using likelihood ratio tests (with P > 0.05 indicating no violation of the assumption).
In supplemental analyses, we first accounted for participants who did not have diabetes at baseline but developed it at some point during follow-up. For analyses of incident CVD and incident ESRD, we censored at the time of diabetes development people who developed diabetes after visit 2 if they either did not experience the event of interest or developed diabetes before experiencing the event of interest. For analyses of prevalent retinopathy, we excluded all people who developed diabetes during follow-up (n = 2,672). Second, we used Poisson regression to calculate age- and sex-adjusted race-specific incidence rates of CVD and ESRD separately in people with and without diagnosed diabetes. We included an offset term of the natural log of person-years to account for differences in follow-up time. We obtained predictive margins from logistic regression models to calculate the race-specific prevalence of retinopathy (also adjusted for age and sex). We used the Wald test to test differences in incidence rates and prevalence, comparing whites and blacks. All statistical analyses were conducted using Stata version 14.0 (StataCorp, College Station, TX).
Results
Our study population included 10,373 participants without diagnosed diabetes (8,096 white and 2,277 black) and 727 participants with diagnosed diabetes (425 white and 302 black). Baseline characteristics varied between those with and without diagnosed diabetes and according to race (Table 1). Even at similar levels of fasting glucose, blacks had higher levels of HbA1c, fructosamine, and glycated albumin (P < 0.001 for all) and marginally statistically significant lower levels of 1,5-AG (P < 0.07 for all) compared with whites (Table 2). The magnitudes of these black/white differences were particularly large among people with diagnosed diabetes (Table 2). Among people with and without diabetes, we observed statistically significantly higher age- and sex-adjusted incidence rates of CVD and ESRD among blacks compared with whites, and the prevalence of retinopathy was higher among blacks compared with whites (P < 0.05 for all) (Supplementary Fig. 1).
Table 1.
Characteristics of the study population by diabetes status and race, ARIC visit 2 (1990–1992)
| No diagnosed diabetes |
Diagnosed diabetes |
|||||
|---|---|---|---|---|---|---|
| White (n = 8,096) | Black (n = 2,277) | P value* | White (n = 425) | Black (n = 302) | P value* | |
| Age, years | 57.3 (5.6) | 56.2 (5.7) | <0.001 | 58.7 (5.7) | 57.6 (5.7) | 0.013 |
| Male sex | 43.9% | 35.8% | <0.001 | 48.0% | 29.5% | <0.001 |
| Less than HS education | 13.9% | 35.0% | <0.001 | 24.2% | 44.0% | <0.001 |
| Obese (BMI ≥ 30 kg/m2) | 21.7% | 41.5% | <0.001 | 47.5% | 55.0% | 0.048 |
| Current smoking | 20.5% | 24.5% | <0.001 | 17.9% | 21.2% | 0.265 |
| Current drinking | 66.2% | 36.8% | <0.001 | 48.5% | 21.5% | <0.001 |
| Family history of diabetes | 21.9% | 25.0% | 0.002 | 41.9% | 41.1% | 0.824 |
| Hypertension† | 29.4% | 51.4% | <0.001 | 51.3% | 64.9% | <0.001 |
| Antihypertensive medication use | 21.7% | 40.3% | <0.001 | 42.6% | 57.0% | <0.001 |
| Systolic BP, mmHg | 118.8 (17.4) | 125.5 (19.9) | <0.001 | 125.2 (17.2) | 127.9 (20.1) | 0.052 |
| Systolic BP ≥140 mmHg | 11.7% | 21.0% | <0.001 | 19.8% | 22.2% | 0.428 |
| Diastolic BP, mmHg | 71.2 (9.7) | 75.7 (10.8) | <0.001 | 70.8 (9.7) | 73.0 (9.7) | 0.003 |
| Diastolic BP ≥90 mmHg | 3.8% | 10.1% | <0.001 | 3.3% | 4.3% | 0.478 |
| HDL-c, mg/dL | 50.1 (16.6) | 54.5 (17.0) | <0.001 | 42.1 (12.8) | 49.1 (13.6) | <0.001 |
| Total cholesterol, mg/dL | 209.0 (37.4) | 208.9 (40.0) | 0.921 | 208.7 (39.2) | 217.5 (45.6) | 0.005 |
| LDL-c, mg/dL | 132.8 (35.6) | 133.7 (38.3) | 0.281 | 132.6 (35.2) | 141.4 (42.7) | 0.003 |
| Triglycerides, mg/dL | 130.7 (64.5) | 103.5 (50.0) | <0.001 | 169.5 (75.3) | 134.8 (68.3) | <0.001 |
| Cholesterol-lowering medication use | 5.5% | 2.8% | <0.001 | 12.9% | 5.0% | <0.001 |
| Glucose-lowering medications‡ | — | — | — | 61.5% | 76.5% | <0.001 |
| eGFR <60 mL/min/1.73 m2 | 1.2% | 1.1% | 0.670 | 2.4% | 4.6% | 0.090 |
| Prevalent retinopathy§ | 1.6% | 3.3% | <0.001 | 19.6% | 30.7% | 0.005 |
| Baecke sport index‖ | 2.5 (0.8) | 2.2 (0.7) | <0.001 | 2.4 (0.8) | 2.1 (0.6) | <0.001 |
Data are mean (SD) or percentages.
BP, blood pressure; HS, high school; IQR, interquartile range.
*Two-sided P values calculated using the Student t test for continuous variables and the χ2 test for categorical variables.
†Hypertension defined as diastolic BP ≥90 mmHg or systolic BP ≥140 mmHg or antihypertensive medication use.
‡Among persons with diabetes, 13 black and 22 white participants are missing a response to self-reported use of glucose-lowering medication.
§Retinopathy was assessed at visit 3 (1993–1995) and therefore was available for only a subset of the main study population (176 blacks and 317 whites among participants with diagnosed diabetes; 1,516 blacks and 6,561 whites among participants without diagnosed diabetes).
‖The Baecke sport index is a score of sport index during leisure time, with values ranging from 1 to 5, based on intensity (light, moderate, heavy); time (hours per week); and proportion (months per year) of activity. Higher values indicate higher levels of physical activity.
Table 2.
Baseline levels of biomarkers of hyperglycemia by diabetes status and race
| Median (25th, 75th percentile) | Median (25th, 75th percentile) | P value* | |
|---|---|---|---|
| Among people without diagnosed diabetes | White (n = 8,096) | Black (n = 2,277) | |
| FG, mg/dL | 101 (95, 108) | 104 (97, 113) | <0.001 |
| FG <100 mg/dL | 94 (90, 97) | 94 (90, 97) | 0.189 |
| FG 100–125 mg/dL | 106 (102, 111) | 108 (103, 114) | <0.001 |
| FG ≥126 mg/dL | 136 (129, 151) | 138 (131, 154) | 0.150 |
| HbA1c, % | 5.4 (5.1, 5.6) | 5.7 (5.4, 6.0) | <0.001 |
| FG <100 mg/dL | 5.3 (5.1, 5.4) | 5.5 (5.2, 5.7) | <0.001 |
| FG 100–125 mg/dL | 5.4 (5.2, 5.7) | 5.7 (5.4, 6.0) | <0.001 |
| FG ≥126 mg/dL | 6.2 (5.8, 6.8) | 6.6 (6.2, 7.2) | <0.001 |
| Fructosamine, μmol/L | 225 (214, 237) | 234 (220, 250) | <0.001 |
| FG <100 mg/dL | 224 (212, 234) | 230 (217, 242) | <0.001 |
| FG 100–125 mg/dL | 226 (214, 237) | 234 (220, 249) | <0.001 |
| FG ≥126 mg/dL | 247 (229, 268) | 260 (236, 285) | <0.001 |
| Glycated albumin, % | 12.5 (11.7, 13.3) | 13.3 (12.4, 14.3) | <0.001 |
| FG <100 mg/dL | 12.4 (11.7, 13.2) | 13.1 (12.3, 13.9) | <0.001 |
| FG 100–125 mg/dL | 12.4 (11.7, 13.2) | 13.3 (12.4, 14.3) | <0.001 |
| FG ≥126 mg/dL | 14.1 (12.8, 15.8) | 15.2 (13.6, 17.3) | <0.001 |
| 1,5-AG, μg/mL | 18.9 (15.3, 22.5) | 17.3 (13.9, 21.0) | <0.001 |
| FG <100 mg/dL | 18.6 (15.2, 22.1) | 17.3 (14.2, 21.1) | <0.001 |
| FG 100–125 mg/dL | 19.4 (15.8, 22.9) | 17.8 (14.3, 21.2) | <0.001 |
| FG ≥126 mg/dL | 15.5 (10.0, 20.4) | 14.6 (8.2, 18.7) | 0.055 |
| Among people with diagnosed diabetes | White (n = 425) | Black (n = 302) | |
| FG, mg/dL | 158 (122, 216) | 194 (136, 271) | <0.001 |
| FG <149 mg/dL | 117 (103, 133) | 120 (103, 135) | 0.517 |
| FG ≥149 mg/dL | 209 (176, 256) | 245 (191, 296) | <0.001 |
| HbA1c, % | 7.1 (5.9, 8.6) | 8.4 (6.7, 10.6) | <0.001 |
| FG <149 mg/dL | 5.8 (5.4, 6.5) | 6.4 (5.9, 7.1) | <0.001 |
| FG ≥149 mg/dL | 8.4 (7.4, 9.8) | 9.7 (8.0, 11.4) | <0.001 |
| Fructosamine, μmol/L | 280 (241, 358) | 331 (267, 423) | <0.001 |
| FG <149 mg/dL | 240 (221, 260) | 254 (237, 281) | <0.001 |
| FG ≥149 mg/dL | 341 (290, 404) | 378 (316, 465) | <0.001 |
| Glycated albumin, % | 16.6 (13.3, 22.4) | 21.7 (16.0, 29.0) | <0.001 |
| FG <149 mg/dL | 13.1 (12.1, 14.9) | 15.0 (13.4, 16.4) | <0.001 |
| FG ≥149 mg/dL | 21.1 (17.4, 27.1) | 25.0 (21.1, 32.7) | <0.001 |
| 1,5-AG, μg/mL | 7.7 (2.4, 15.4) | 4.0 (1.5, 12.1) | <0.001 |
| FG <149 mg/dL | 15.1 (10.2, 20.1) | 13.3 (9.5, 17.3) | 0.040 |
| FG ≥149 mg/dL | 2.8 (1.4, 7.1) | 2.1 (1.2, 5.3) | 0.065 |
Among people without diagnosed diabetes, there were 3,573 whites and 772 blacks with FG <100 mg/dL; 4,133 whites and 1,266 blacks with FG 100–125 mg/dL; and 390 whites and 239 blacks with FG ≥126 mg/dL. Among people with diagnosed diabetes, there were 193 whites and 95 blacks with FG <149 mg/dL and 232 whites and 207 blacks with FG ≥149 mg/dL. FG, fasting glucose.
*P values were calculated using the Wilcoxon rank sum (Mann-Whitney U) test.
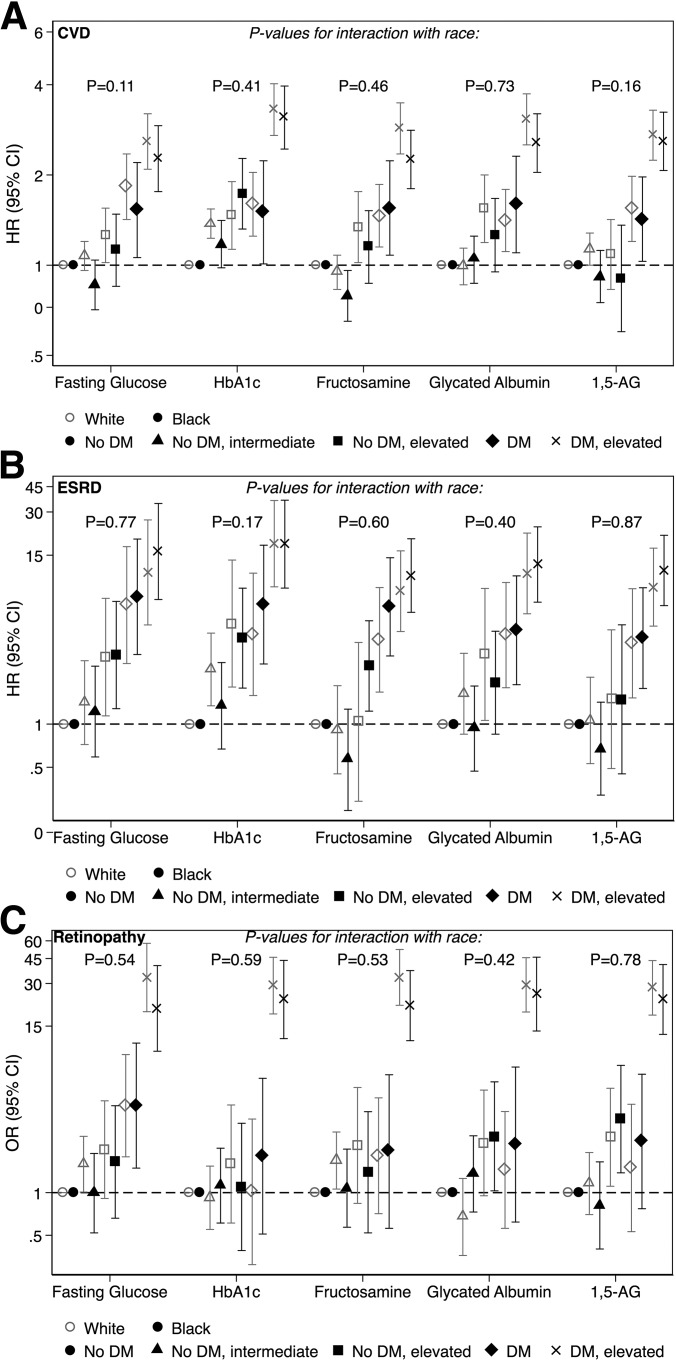
Among 11,100 participants who were free of CVD and ESRD at baseline, there were 2,642 incident cases of CVD and 170 cases of ESRD during approximately 20 years of follow-up. Magnitudes of association were greatest for HbA1c compared with other biomarkers (Fig. 1 and Supplementary Table 1). The associations followed a linear trend among both whites and blacks (Ptrend < 0.001 for all). These associations of biomarkers with incident CVD were similar among blacks and whites (P values for interaction were >0.10 for all biomarkers) (Fig. 1 and Supplementary Table 1). Patterns and magnitudes of associations of biomarkers of hyperglycemia with individual CVD outcomes (CHD, stroke, and heart failure) were similar to those of the composite CVD outcome, with no evidence of a race interaction (P values for interaction were >0.10 for all biomarkers and all individual outcomes) (Supplementary Tables 2–4).
Figure 1.
Adjusted associations of hyperglycemia with incident CVD, incident ESRD, and prevalent retinopathy by race. Hazard ratios (HRs) for CVD and ESRD were obtained using separate Cox proportional hazards regression models for white and black participants. Odds ratios for prevalent retinopathy were obtained using separate logistic regression models for white and black participants. In models that included both white and black participants, P values for interactions were calculated by conducting a likelihood ratio test to compare models with and without terms for the interaction between race and hyperglycemia. Models included adjustment for age; sex (male, female); BMI; BMI2; LDL-c; HDL-c; triglycerides; cholesterol-lowering medication use (yes, no); systolic blood pressure; antihypertensive medication use (yes, no); eGFR; family history of diabetes (yes, no); education level (less than high school, high school or some college, college or more); alcohol consumption (current, former, never); cigarette smoking status (current, former, never); and physical activity level. Categories of diabetes—no diabetes; no diabetes, intermediate levels; no diabetes, elevated levels; diabetes; and diabetes, elevated levels—were defined using the following levels of each biomarker, respectively: fasting glucose: <100, 100–125, ≥126, <149, ≥149 mg/dL; HbA1c: <5.7, 5.7–6.4, ≥6.5, <7.0, ≥7.0%; fructosamine: <239.8, 239.8–268.6, ≥268.7, <275.7, ≥275.7 mg/dL; glycated albumin: <13.52, 13.52–15.55, ≥15.56, <16.46, ≥16.46%; 1,5-AG: ≥15.0, 7.9–14.9, <7.9, >9.2, ≤9.2 μg/mL. Gray symbols indicate results for white participants. Black symbols indicate results for black participants. DM, diabetes.
Patterns of associations of biomarkers of hyperglycemia with ESRD were similar to those for CVD, but the risk of ESRD and strength of association with the biomarkers were substantially higher (Fig. 1 and Supplementary Table 5). The associations followed a linear trend among both whites and blacks (Ptrend < 0.001 for all). Associations of biomarkers of hyperglycemia with incident ESRD were similar among blacks and whites (P values for interaction were >0.15 for all biomarkers) (Fig. 1 and Supplementary Table 5).
Associations of biomarkers of hyperglycemia with prevalent retinopathy were of the particularly largest magnitude among people with the highest levels of hyperglycemia and diagnosed diabetes (Fig. 1 and Supplementary Table 6). Similar to CVD and ESRD, associations of biomarkers of hyperglycemia with retinopathy were similar among blacks and whites (P values for interaction were >0.40 for all biomarkers) (Fig. 1 and Supplementary Table 6).
Additional adjustment for continuous fasting glucose or HbA1c substantially attenuated the associations with outcomes, but inferences about racial comparisons of associations were similar (Supplementary Tables 1–6). Results of analyses that censored or excluded participants who developed diabetes after visit 2 were similar to those of the main analyses, with conclusions unchanged (Supplementary Table 7).
Conclusions
We found the relative associations of both traditional and nontraditional biomarkers of hyperglycemia with CVD, ESRD, and retinopathy to be similar by race, although blacks had higher levels of hyperglycemia, higher absolute risks of CVD and ESRD, and a higher burden of retinopathy than whites. Our results suggest that the prognostic utility of HbA1c and nontraditional serum biomarkers of hyperglycemia, particularly at diabetic levels, is similar among both black and white adults.
Our findings support previous studies that have shown similar associations of HbA1c with microvascular and macrovascular disease in blacks and whites (14,15,32,33) and that have recommended using the same HbA1c diagnostic cut points across races/ethnicities (34,35). To our knowledge, this is the first prospective study to conduct head-to-head comparisons of traditional and nontraditional biomarkers of hyperglycemia with major diabetic complications. The similar associations of HbA1c and nontraditional biomarkers with prevalent retinopathy are particularly relevant, because diagnostic cut points for diabetes are largely based on established associations with prevalent retinopathy (36).
Our study supports the idea that racial differences in biomarkers of hyperglycemia may be the result of real differences in glycemia (rather than differences in the behavior of the biomarkers studied), perhaps due to disparities in environmental factors, stress, behavior, diet, physical activity, and lifestyle factors that contribute to differences in circulating nonfasting hyperglycemia in blacks compared with whites (37). In particular, differences in postprandial glucose levels and insulin deficiency may play a role in racial differences in the levels of these biomarkers. Even after controlling for fasting glucose concentrations, we observed here that blacks had higher levels of hyperglycemia compared with whites, as indicated by higher levels of HbA1c, fructosamine, and glycated albumin, and lower concentrations of 1,5-AG. As mentioned earlier, fructosamine, glycated albumin, and 1,5-AG are independent of the red blood cells or hemoglobin. Therefore, the observation that these nontraditional serum biomarkers of hyperglycemia exhibit a pattern of racial differences similar to that of HbA1c provides evidence that nonglycemic factors, such as hemoglobin glycation or red cell turnover, may not explain observed racial disparities in the overall population (38). We cannot rule out the possibility that nonglycemic determinants may be important in a subset of the population.
An important consideration in the interpretation of racial differences in biomarker levels is that blacks are at higher risk of diabetes and diabetes-related complications than whites (39,40). We confirm here that blacks had higher incidence rates of CVD and ESRD and a higher prevalence of retinopathy compared with whites in the ARIC Study. It should be noted that some of the racial differences in absolute risk of diabetes complications may be due to differences in both sociodemographics and environmental and lifestyle exposures between blacks and whites, some of which may not be readily measured in epidemiologic studies.
There were several limitations of this study. We had only single measurements of biomarkers of hyperglycemia at baseline, and 2-h glucose measurements were not conducted. There is a lack of evidence regarding the long-term stability of 1,5-AG, although measurements from stored samples were strongly associated with diabetes and HbA1c in the diabetic range (consistent with other studies using fresh samples [41]), and 1,5-AG measured from these stored samples in the ARIC Study was strongly associated with diabetes and its complications, demonstrating construct validity (12,23,42). Age at diabetes diagnosis was not collected at the first or second ARIC examinations, and we therefore were unable to account for potential racial differences in duration of diabetes. However, among participants who had ever reported a diagnosis of diabetes at visit 2 (the baseline examination for this study), 31% (33% of blacks and 30% of whites) did not report diabetes at visit 1, 3 years earlier, and could be considered to have new-onset diabetes. We also cannot rule out the possibility that differences in levels of biomarkers of hyperglycemia by race may be the result of cultural differences by geography, rather than race alone. In the ARIC Study, black participants were recruited almost exclusively from two of the four field centers (Jackson, Mississippi, and Forsyth County, North Carolina). Although this was one of the largest studies to address this research question, it is possible that we may have been underpowered to detect moderate but statistically significant differences in the associations of biomarkers with outcomes in whites versus blacks. However, it is important to point out that comparing the magnitudes of associations in blacks and whites (regardless of statistical significance) is also important when assessing whether there are racial differences in the prognostic value of these biomarkers.
Our study had several important strengths. This is the largest prospective study to rigorously compare associations of HbA1c and nontraditional serum biomarkers of hyperglycemia with long-term complications of diabetes. We also leveraged the large numbers of both black and white participants in the cohort to assess the prognostic implications of racial differences in biomarkers of hyperglycemia. The ARIC Study included rigorous assessment of retinopathy and surveillance of both CVD and ESRD over two decades.
Characterizing associations of these biomarkers of hyperglycemia with hard clinical end points is of the utmost importance because the goal of early diagnosis and improved disease management is prevention of microvascular and macrovascular complications in people with and at risk for diabetes. We found that biomarkers of hyperglycemia similarly reflect a risk of clinical outcomes in both blacks and whites. Our results suggest similar prognostic utility of HbA1c, fructosamine, glycated albumin, and 1,5-AG in black and white adults.
Supplementary Material
Article Information
Acknowledgments. The authors thank the staff and participants of the ARIC Study for their important contributions.
Funding. C.M.P. is supported by NIH/NHLBI Cardiovascular Epidemiology training grant T32HL007024. This research was supported by NIH/NIDDK grants R01DK089174 and K24DK106414 to E.S. The ARIC Study is carried out as a collaborative study supported by NIH/NHLBI contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Reagents for the fructosamine assays were donated by Roche Diagnostics. Reagents for the glycated albumin assays were donated by the Asahi Kasei Pharma Corporation. Reagents for the 1,5-anhydroglucitol assays were donated by the GlycoMark Corporation.
Duality of Interest. R.M.B. is a past volunteer of the American Diabetes Association. No other potential conflicts of interest relevant to this article were reported.
Some of the data reported here were supplied by the U.S. Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the U.S. government.
Author Contributions. C.M.P. designed the study, interpreted and analyzed the data, and wrote the manuscript. A.R.S., N.M.M., R.M.B., M.E.G., and J.C. interpreted the data and reviewed and edited the manuscript. E.S. designed the study, interpreted the data, and reviewed and edited the manuscript. E.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This work was presented at the American Heart Association Epidemiology and Prevention and Lifestyle and Cardiometabolic Health 2015 Scientific Sessions, Baltimore, MD, 3–6 March 2015.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc15-1360/-/DC1.
References
- 1.American Diabetes Association Classification and diagnosis of diabetes. Diabetes Care 2015;38(Suppl. 1):S8–S16 [DOI] [PubMed] [Google Scholar]
- 2.Parrinello CM, Selvin E. Beyond HbA1c and glucose: the role of nontraditional glycemic markers in diabetes diagnosis, prognosis, and management. Curr Diab Rep 2014;14:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldstein DE, Little RR, Lorenz RA, et al. Tests of glycemia in diabetes. Diabetes Care 2004;27:1761–1773 [DOI] [PubMed] [Google Scholar]
- 4.Sacks DB. A1C versus glucose testing: a comparison. Diabetes Care 2011;34:518–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armbruster DA. Fructosamine: structure, analysis, and clinical usefulness. Clin Chem 1987;33:2153–2163 [PubMed] [Google Scholar]
- 6.Buse JB, Freeman JLR, Edelman SV, Jovanovic L, McGill JB. Serum 1,5-anhydroglucitol (GlycoMark ): a short-term glycemic marker. Diabetes Technol Ther 2003;5:355–363 [DOI] [PubMed] [Google Scholar]
- 7.Dungan KM. 1,5-anhydroglucitol (GlycoMark) as a marker of short-term glycemic control and glycemic excursions. Expert Rev Mol Diagn 2008;8:9–19 [DOI] [PubMed] [Google Scholar]
- 8.Kim WJ, Park C-Y. 1,5-Anhydroglucitol in diabetes mellitus. Endocrine 2013;43:33–40 [DOI] [PubMed] [Google Scholar]
- 9.Yamanouchi T, Akanuma Y. Serum 1,5-anhydroglucitol (1,5 AG): new clinical marker for glycemic control. Diabetes Res Clin Pract 1994;24(Suppl.):S261–S268 [DOI] [PubMed] [Google Scholar]
- 10.Yamanouchi T, Ogata N, Tagaya T. Clinical usefulness of serum 1,5-anhydroglucitol in monitoring glycaemic control. Lancet 1996;347:1514–1518 [DOI] [PubMed] [Google Scholar]
- 11.Selvin E, Rawlings AM, Grams M, et al. Fructosamine and glycated albumin for risk stratification and prediction of incident diabetes and microvascular complications: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol 2014;2:279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Selvin E, Rawlings AM, Grams M, Klein R, Steffes M, Coresh J. Association of 1,5-anhydroglucitol with diabetes and microvascular conditions. Clin Chem 2014;60:1409–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selvin E, Francis LM, Ballantyne CM, et al. Nontraditional markers of glycemia: associations with microvascular conditions. Diabetes Care 2011;34:960–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selvin E, Ning Y, Steffes MW, et al. Glycated hemoglobin and the risk of kidney disease and retinopathy in adults with and without diabetes. Diabetes 2011;60:298–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010;362:800–811; author reply 2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe M, Kokubo Y, Higashiyama A, Ono Y, Miyamoto Y, Okamura T. Serum 1,5-anhydro-D-glucitol levels predict first-ever cardiovascular disease: an 11-year population-based cohort study in Japan, the Suita study. Atherosclerosis 2011;216:477–483 [DOI] [PubMed] [Google Scholar]
- 17.Selvin E, Rawlings AM, Lutsey PL, et al. Fructosamine and glycated albumin and the risk of cardiovascular outcomes and death. Circulation 2015;132:269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol 1989;129:687–702 [PubMed] [Google Scholar]
- 19.Selvin E, Coresh J, Zhu H, Folsom A, Steffes MW. Measurement of HbA1c from stored whole blood samples in the Atherosclerosis Risk in Communities study. J Diabetes 2010;2:118–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nathan DM, Steffes MW, Sun W, Rynders GP, Lachin JM. Determining stability of stored samples retrospectively: the validation of glycated albumin. Clin Chem 2011;57:286–290 [DOI] [PubMed] [Google Scholar]
- 21.Juraschek SP, Steffes MW, Selvin E. Associations of alternative markers of glycemia with hemoglobin A(1c) and fasting glucose. Clin Chem 2012;58:1648–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shafi T, Sozio SM, Plantinga LC, et al. Serum fructosamine and glycated albumin and risk of mortality and clinical outcomes in hemodialysis patients. Diabetes Care 2013;36:1522–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juraschek SP, Steffes MW, Miller ER 3rd, Selvin E. Alternative markers of hyperglycemia and risk of diabetes. Diabetes Care 2012;35:2265–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baecke JAH, Burema J, Frijters JER. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 1982;36:936–942 [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White AD, Folsom AR, Chambless LE, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol 1996;49:223–233 [DOI] [PubMed] [Google Scholar]
- 27.Rosamond WD, Folsom AR, Chambless LE, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke 1999;30:736–743 [DOI] [PubMed] [Google Scholar]
- 28.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol 2008;101:1016–1022 [DOI] [PubMed] [Google Scholar]
- 29.Hubbard LD, Brothers RJ, King WN, et al. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology 1999;106:2269–2280 [DOI] [PubMed] [Google Scholar]
- 30.Couper DJ, Klein R, Hubbard LD, et al. Reliability of retinal photography in the assessment of retinal microvascular characteristics: the Atherosclerosis Risk in Communities Study. Am J Ophthalmol 2002;133:78–88 [DOI] [PubMed] [Google Scholar]
- 31.Klein R, Sharrett AR, Klein BE, et al.; ARIC Group . The association of atherosclerosis, vascular risk factors, and retinopathy in adults with diabetes : the Atherosclerosis Risk in Communities study. Ophthalmology 2002;109:1225–1234 [DOI] [PubMed] [Google Scholar]
- 32.Bower JK, Brancati FL, Selvin E. No ethnic differences in the association of glycated hemoglobin with retinopathy: the national health and nutrition examination survey 2005-2008. Diabetes Care 2013;36:569–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Selvin E, Rawlings AM, Bergenstal RM, Coresh J, Brancati FL. No racial differences in the association of glycated hemoglobin with kidney disease and cardiovascular outcomes. Diabetes Care 2013;36:2995–3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsugawa Y, Mukamal KJ, Davis RB, Taylor WC, Wee CC. Should the hemoglobin A1c diagnostic cutoff differ between blacks and whites? A cross-sectional study. Ann Intern Med 2012;157:153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabanayagam C, Khoo EY, Lye WK, et al. Diagnosis of diabetes mellitus using HbA1c in Asians: relationship between HbA1c and retinopathy in a multiethnic Asian population. J Clin Endocrinol Metab 2015;100:689–696 [DOI] [PubMed] [Google Scholar]
- 36.International Expert Committee International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selvin E, Brancati FL. A conundrum addressed: the prognostic value of HbA1c. Nat Rev Endocrinol 2011;7:c1; author reply c2. [DOI] [PubMed] [Google Scholar]
- 38.Selvin E, Steffes MW, Ballantyne CM, Hoogeveen RC, Coresh J, Brancati FL. Racial differences in glycemic markers: a cross-sectional analysis of community-based data. Ann Intern Med 2011;154:303–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chow EA, Foster H, Gonzalez V, McIver L. The disparate impact of diabetes on racial/ethnic minority populations. Clin Diabetes 2012;30:130–133 [Google Scholar]
- 40.Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA 2000;283:2253–2259 [DOI] [PubMed] [Google Scholar]
- 41.Kim WJ, Park CY, Park SE, et al. Serum 1,5-anhydroglucitol is associated with diabetic retinopathy in Type 2 diabetes. Diabet Med 2012;29:1184–1190 [DOI] [PubMed] [Google Scholar]
- 42.Selvin E, Rawlings A, Lutsey P, et al. Association of 1,5-anhydroglucitol with cardiovascular disease and mortality. Diabetes 2016;65:201–208 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.