Abstract
OBJECTIVE
Type 2 diabetes (T2D) is associated with increased mortality in ethnically diverse populations, although the extent to which this association is genetically determined is unknown. We sought to determine whether T2D-related genetic variants predicted all-cause mortality, even after accounting for BMI, in the Third National Health and Nutrition Examination Survey.
RESEARCH DESIGN AND METHODS
We modeled mortality risk using a genetic risk score (GRS) from a weighted sum of risk alleles at 38 T2D-related single nucleotide polymorphisms. In age-, sex-, and BMI-adjusted logistic regression models, accounting for the complex survey design, we tested the association with mortality in 6,501 participants. We repeated the analysis within ethnicities (2,528 non-Hispanic white [NHW], 1,979 non-Hispanic black [NHB], and 1,994 Mexican American [MA]) and within BMI categories (<25, 25–30, and ≥30 kg/m2). Significance was set at P < 0.05.
RESULTS
Over 17 years, 1,556 participants died. GRS was associated with mortality risk (OR 1.04 [95% CI 1.00–1.07] per T2D-associated risk allele, P = 0.05). Within ethnicities, GRS was positively associated with mortality risk in NHW and NHB, but not in MA (0.95 [0.90–1.01], P = 0.07). The negative trend in MA was largely driven by those with BMI <25 kg/m2 (0.91 [0.82–1.00]). In NHW, the positive association was strongest among those with BMI ≥30 kg/m2 (1.07 [1.02–1.12]).
CONCLUSIONS
In the U.S., a higher T2D genetic risk was associated with increased mortality risk, especially among obese NHW. The underlying genetic basis for mortality likely involves complex interactions with factors related to ethnicity, T2D, and body weight.
Introduction
The escalating type 2 diabetes (T2D) epidemic is a major public health concern (1). In the U.S., the rate of increase in T2D is projected to be disproportionately higher among certain ethnic minorities (2,3). Epidemiologic studies (3–6) have shown that T2D is associated with increased all-cause mortality risk. Given that T2D is partly genetically determined (7,8), genetic factors that increase T2D susceptibility may also raise mortality risk through T2D or its related complications.
The T2D epidemic parallels the rising prevalence of obesity, on a genetic background of varying permissibility. Responsible for this coepidemic are social-behavioral influences (e.g., urbanization, efficient transportation networks, increase in sedentary work, advent of modern technology, reliance on electronic transactions and Internet-based social connections) (9–11), coupled with the growing consumption of conveniently prepared and readily available food and beverages rich in poorly satiating calories (9,10,12). As social, behavioral, and lifestyle factors are associated with both T2D and mortality (13–17), these modifiable risk factors may partly explain the T2D-mortality relationship. Although genetic factors are fixed from birth and potentially exert their effects throughout life, modifiable factors can act as environmental exposures that amplify or nullify these genetic effects. Obesity, reflecting energy balance and capturing these modifiable factors, is a fundamental effect modifier in any genetic study of T2D risk (18) that has to be accounted for in our increasingly obesogenic environment (19). Here, we tested the hypothesis that carrying a higher aggregate genetic burden of T2D risk, modeled using a genetic risk score (GRS), predicted all-cause mortality independently of BMI, the principal physical reflection of this obesogenic environment, in the Third National Health and Nutrition Examination Survey (NHANES III).
Investigations have also suggested an “obesity paradox,” where a higher BMI is associated with lower mortality risk in individuals with T2D or other chronic conditions (20,21). It has also been shown that genetic effects on T2D susceptibility are stronger in leaner individuals than in heavier counterparts (22,23); such findings may be particularly relevant to individuals of certain nonwhite ethnicities in whom normal-weight T2D is more likely to develop (24). Conversely, individuals with a higher T2D genetic predisposition may be more susceptible to the metabolic derangements and health consequences of obesity. Thus, in secondary analyses we tested whether T2D genetic-mortality associations differed by BMI category (normal weight, overweight, and obese), and whether obesity-associated mortality risk differed by T2D genetic risk.
Research Design and Methods
Study Population
NHANES III was a nationally representative sample of the noninstitutionalized civilian U.S. population collected using stratified multistage probability sampling. The study sample was restricted to participants ≥20 years of age with ≥8 h fasting blood sampling who underwent a phlebotomy, household interview, and physical examination (25). DNA extraction was generated from Epstein-Barr transformed lymphocyte cell lines of participants in NHANES III Phase II (1991–1994). NHANES III phenotypic data were merged with mortality data accrued over 17 years. Of the 7,170 participants with phenotype-genotype data, we excluded 347 who were not non-Hispanic black (NHB), Mexican American (MA), or non-Hispanic white (NHW), and 322 participants with inadequate genotyping (>5 single nucleotide polymorphisms [SNPs] missing from GRS), leaving 6,501 participants in the main analysis. Written informed consent was obtained from all subjects. This study was approved by the National Center for Health Statistics Ethics Review Board.
Genotyping and GRSs
We modeled T2D genetic risk using an additive GRS comprising T2D-associated SNPs. We first consulted the largest T2D meta-analysis genome-wide association study (GWAS), DIAbetes Genetics Replication and Meta-analysis (DIAGRAM) (26), and successfully genotyped 38 T2D-related SNPs or proxies that had estimated odds ratios (ORs) of ≥1.05 for T2D. Genotyping was performed using the Sequenom iPLEX platform. Genotypes with call rates <95% were removed. Allele frequencies of all SNPs were in Hardy-Weinberg equilibrium based on National Center for Health Statistics standards (Hardy-Weinberg equilibrium rejected if P < 0.01 in two or fewer ethnicities). To create a weighted GRS that was scaled to increments per risk allele, we summed the number of risk alleles (0, 1, or 2) at each SNP multiplied by their published trans-ethnic β-coefficients for T2D in DIAGRAM version 4, multiplied by the number of SNPs in the GRS, divided by the sum of β-coefficients (27). We imputed missing SNPs using dosage estimates (continuous values between 0 and 2) from risk allele frequency for each ethnicity. We initially attempted to account for ethnic-specific differences in SNP weights using β-coefficients from GWAS on people of African ancestry and Mexican ancestry; however, given that ethnic-specific β-coefficients were not reported for a number of these variants in meta-analysis GWAS (26,28), we opted to apply the same weights derived from trans-ethnic β-coefficients for all participants. In a sensitivity analysis, we repeated the analysis using an unweighted GRS (i.e., a simple sum of risk alleles carried by individuals at each SNP) to determine whether the application of SNP weights impacted risk estimates. Risk allele frequencies, β-coefficients for T2D, and the proportion missing for each SNP by ethnicity are displayed in Supplementary Table 1.
Baseline Characteristics
Clinically measured variables included T2D, defined as a fasting glucose concentration of ≥7.0 mmol/L; report of a diabetes diagnosis or antidiabetic medication use; hypertension, defined as systolic blood pressure of ≥140 mmHg, diastolic blood pressure of ≥90 mmHg, or antihypertensive medication use; BMI (normal weight [<25 kg/m2], overweight [25 to <30 kg/m2], and obese [≥30 kg/m2]); total cholesterol (TC) level; HDL cholesterol level; and waist circumference (WC). Self-reported characteristics included ethnicity (NHW, NHB, or MA), family history (one or more first-degree relatives with diabetes), educational attainment (<12 years, 12 years, or >12 years of education), insurance (covered vs. not covered), smoking status (current, former, or never), alcohol consumption (nondrinker, average of >0–3 drinks/week, average of ≥3 drinks/week in the prior year), physical activity (PA) level (no activity, average of >0–5 sessions/week, or average of ≥5 exercise sessions/week of moderate to vigorous activities [i.e., dancing, calisthenics, gardening or yard work, walking a mile without stopping, jogging/running, biking, or swimming]), and the Healthy Eating Index (HEI) (a measure of diet quality based on a 100-point scale, where higher scores indicated greater conformity to guidelines on 10 dietary components). We had no missing values for age, sex, GRS, T2D, BMI, and death during follow-up. Complete data for other covariates were available for 79% of the data set; the proportion of missing values was <15% for any single covariate. To impute missing values, we used multiple imputation implemented by “PROC MI,” which was a multivariate imputation by fully conditional specification methods that accommodated arbitrary missing-value patterns. For prediction equations, we used logistic regression for categorical variables and predictive mean matching for continuous variables to generate 10 imputation sets.
GRS Association With Baseline T2D and Other Risk Factors
To determine whether GRS was associated with baseline T2D, we tested the association of GRS with baseline T2D in age- and sex-adjusted logistic models. To assess pleiotropic associations with clinical risk factors, we used age- and sex-adjusted regression models to test the association of GRS with TC, HDL, WC, hypertension, family history, educational attainment, insurance coverage, HEI, PA, smoking, and alcohol consumption.
Statistical Analyses
First, in age-, sex-, and BMI-adjusted logistic models, we estimated mortality risk per T2D GRS risk allele. Second, to determine whether associations were independent of modifiable and nonmodifiable risk factors, we estimated mortality risk per T2D GRS risk allele in models that additionally included family history, educational attainment, insurance coverage, HEI, PA, smoking, alcohol consumption, TC, HDL, WC, and hypertension.
As genetic effects may differ by BMI, we performed interaction analyses between BMI and GRS as categorical variables (GRS greater than or equal to the ethnic-specific median vs. less than the ethnic-specific median; and obese [BMI ≥ 30 kg/m2], overweight [BMI 25 to <30 kg/m2], or normal weight [BMI <25 kg/m2]) to guide stratified analyses where an interaction P value of <0.1 would motivate stratified analyses. We conducted stratified analyses in two ways. First, we estimated the mortality risk per allele within BMI categories. Second, we estimated the association of obesity versus normal weight with mortality risk, within T2D genetic risk groups (GRS greater than or equal to or less than the ethnic-specific median).
We used SAS (version 9.2 or 9.3; SAS Institute, Cary, NC) for all analyses and applied procedures to account for NHANES III sampling probabilities and complex sampling design in all models. We considered a P value of ≤0.05 to be statistically significant for the analysis that tested our primary hypothesis.
Results
Baseline Characteristics
Participants’ baseline characteristics are summarized in Table 1. After accounting for sampling weights and the complex survey design, 81.1% of the cohort was NHW, 12.7% was NHB, and 6.2% was MA. T2D baseline prevalence was similar across all ethnicities (8–11%). Over 17 years, 1,556 participants (19.1%) died. Cardiovascular disease (CVD) was the leading cause of death (39.2%), followed by cancer (21.2%). The death rate was higher among those with BMI ≥30 kg/m2 (15.7 deaths per 1,000 person-years [95% CI 13.4–18.0]) compared to those with BMI <25 kg/m2 (8.1 deaths per 1,000 person-years [95% CI 6.7–9.7]) (Table 2).
Table 1.
Baseline characteristics accounting for sampling weights and complex survey design by ethnicity in the NHANES III Survey DNA Bank (NHANES III, 1991–1994)
| All ethnicities (N = 6,501) | NHW (N = 2,528) | NHB (N = 1,979) | MA (N = 1,994) | |
|---|---|---|---|---|
| Ethnicity, % | — | 81.1 (77.3–84.9) | 12.7 (9.3–16.1) | 6.2 (4.4–8.1) |
| Age, years | 41.2 (39.8–42.7) | 42.5 (40.6–44.4) | 37.2 (35.6–38.8) | 33.3 (32.1–34.5) |
| ≥45 years of age, % | 37.4 (33.3–41.6) | 40.3 (35.0–45.5) | 27.9 (23.7–32.0) | 20.3 (17.7–23.0) |
| 20–44 years of age, % | 62.6 (58.4–66.7) | 59.7 (54.5–65.0) | 72.1 (68.0–76.3) | 79.7 (77.0–82.3) |
| Women, % | 51.5 (49.9–53.1) | 51.2 (49.3–53.2) | 54.8 (52.2–57.4) | 48.5 (46.8–50.2) |
| Unweighted GRS | 37.8 (37.6–38.0) | 37.9 (37.7–38.2) | 36.2 (36.1–36.4) | 39.3 (39.0–39.6) |
| Weighted GRS | 37.6 (37.4–37.8) | 37.5 (37.3–37.8) | 37.7 (37.5–37.8) | 39.0 (38.7–39.3) |
| BMI, kg/m2 | 26.3 (26.0–26.7) | 26.0 (25.6–26.4) | 27.7 (27.1–28.3) | 27.5 (27.1–27.8) |
| BMI categories, % | ||||
| <25 kg/m2 | 46.8 (43.9–49.7) | 48.4 (45.0–51.8) | 39.6 (36.4–42.8) | 39.6 (37.1–42.2) |
| 25–30 kg/m2 | 30.8 (28.8–32.9) | 30.4 (28.0–32.9) | 31.2 (29.5–32.8) | 35.4 (32.2–38.5) |
| ≥30 kg/m2 | 22.4 (20.2–24.6) | 21.1 (18.6–23.7) | 29.3 (26.5–32.0) | 25.0 (23.5–36.5) |
| Educational attainment, % | ||||
| <12 years | 30.2 (26.8–33.6) | 25.9 (22.6–29.2) | 41.8 (37.1–46.5) | 62.5 (57.5–67.5) |
| 12 years | 31.4 (29.1–33.7) | 31.7 (28.7–34.8) | 33.5 (30.4–36.6) | 23.1 (20.3–25.8) |
| >12 years | 38.4 (33.4–43.4) | 42.4 (37.0–47.7) | 24.7 (20.2–29.2) | 14.4 (11.4–17.5) |
| Insurance coverage, % | 86.6 (84.6–88.6) | 89.4 (87.1–91.7) | 81.0 (77.2–84.8) | 58.6 (53.3–63.8) |
| Family history, % | 19.5 (17.5–21.6) | 18.2 (16.0–20.4) | 25.3 (22.8–27.7) | 25.5 (22.9–28.1) |
| Smoking, % | ||||
| Never | 26.2 (23.2–29.3) | 26.0 (22.3–29.7) | 30.4 (28.3–32.4) | 21.5 (19.1–23.9) |
| Former | 25.0 (22.9–27.1) | 27.1 (24.6–29.5) | 13.87 (11.8–15.9) | 19.9 (17.4–22.3) |
| Current | 48.7 (45.9–51.5) | 46.9 (43.5–50.5) | 55.8 (52.5–59.1) | 58.6 (55.4–61.9) |
| Alcohol consumption, % | ||||
| Nondrinker | 47.2 (43.6–50.8) | 45.8 (41.73–49.81) | 55.4 (52.1–58.7) | 50.5 (48.5–52.5) |
| >0 to <3 drinks/week | 24.9 (22.8–27.1) | 25.9 (23.35–28.41) | 19.2 (16.9–21.4) | 23.1 (20.2–26.0) |
| ≥3 drinks/week | 27.9 (25.3–30.4) | 28.4 (25.23–31.47) | 25.4 (22.9–28.0) | 26.4 (23.6–29.1) |
| PA level, % | ||||
| No activity | 27.4 (24.1–30.8) | 26.3 (22.6–30.1) | 31.2 (27.9–34.2) | 35.0 (32.1–38.0) |
| >0 to <5 times/week | 44.5 (40.9–48.1) | 45.9 (41.9–50.0) | 38.1 (34.6–41.6) | 38.1 (35.6–40.9) |
| ≥5 times/week | 28.1 (25.5–30.7) | 27.8 (24.8–30.7) | 30.9 (27.6–34.2) | 26.9 (23.8–30.0) |
| HEI | 64.3 (63.4–65.1) | 64.8 (63.8–65.7) | 60.3 (59.5–61.1) | 65.9 (64.5–67.4) |
| Diabetes, % | 8.8 (7.4–10.3) | 8.5 (7.0–10.1) | 10.8 (8.6–13.1) | 8.7 (7.6–9.8) |
| Hypertension, % | 21.1 (18.7–23.6) | 21.1 (18.1–24.1) | 26.0 (22.9–29.0) | 12.5 (10.1–14.8) |
| WC (cm) | 90.3 (89.5–91.1) | 89.8 (88.9–90.7) | 92.0 (90.7–93.4) | 92.7 (92.0–93.4) |
| TC (mmol/L) | 5.13 (5.08–5.17) | 5.12 (5.07–5.17) | 5.11 (5.03–5.19) | 5.17 (5.11–5.24) |
| HDL (mmol/L) | 1.30 (1.28–1.33) | 1.29 (1.26–1.32) | 1.41 (1.38–1.44) | 1.26 (1.23–1.29) |
Values are reported as the mean (95% CI). For all variables, procedures to account for sampling probabilities and complex sampling design were applied. Continuous variables were adjusted for age and sex.
Table 2.
Death rates by BMI categories, T2D genetic risk groups, and ethnicity, and causes of death by ethnicity in the NHANES III DNA Bank (NHANES III, 1991–1994), accounting for sampling weights and complex survey design
| All ethnicities (N = 6,501) | NHW (N = 2,528) | NHB (N = 1,979) | MA (N = 1,994) | |
|---|---|---|---|---|
| Death rate, per 1,000 person-years (95% CI) | 11.2 (9.5–12.9) | 11.7 (9.6–13.7) | 10.9 (9.3–12.6) | 6.2 (5.1–7.2) |
| BMI categories, per 1,000 person-years (95% CI) | ||||
| <25 kg/m2 | 8.1 (6.7–9.7) | 8.3 (6.5–10.1) | 9.0 (6.7–11.4) | 3.9 (3.1–4.8) |
| 25–30 kg/m2 | 12.8 (10.5–15.1) | 13.4 (10.6–16.3) | 12.3 (10.2–14.4) | 7.0 (5.6–8.5) |
| ≥30 kg/m2 | 15.7 (13.4–18.0) | 17.3 (14.4–20.2) | 11.7 (8.8–14.7) | 8.6 (6.0–11.1) |
| T2D genetic risk groups, per 1,000 person-years (95% CI) | ||||
| GRS < ethnic-specific median | 10.6 (8.3–12.8) | 11.0 (8.2–13.8) | 9.6 (7.4–11.8) | 6.7 (5.4–8.0) |
| GRS ≥ ethnic-specific median | 11.9 (10.0–13.7) | 12.3 (10.0–14.6) | 12.2 (10.7–13.8) | 5.6 (4.1–7.2) |
| Cause of death, % (95% CI) | ||||
| CVD | 39.2 (35.5–42.9) | 39.8 (35.5–44.1) | 35.4 (32.5–38.2) | 37.0 (29.5–44.6) |
| Cancer | 21.2 (17.8–24.6) | 20.9 (16.9–24.8) | 24.5 (20.5–28.5) | 17.9 (13.0–22.9) |
| External | 2.2 (1.6–2.9) | 1.8 (1.2–2.5) | 3.8 (1.3–6.4) | 6.3 (1.0–11.5) |
| Other | 37.4 (33.2–41.6) | 37.5 (32.5–42.5) | 36.3 (32.1–40.6) | 38.8 (32.2–45.4) |
Cause-specific death was ascertained for CVD (ICD-9 codes 390–434 and 436–459; ICD-10 codes I00–I99), cancer (ICD-9 codes 140–208; ICD-10 codes C00–C97), and external (ICD-9 codes E800–E999.9, ICD-10 codes V, W, X, and Y).
GRS Association With Baseline T2D and Other Risk Factors
The GRS per risk allele was associated with T2D at baseline (OR 1.05 [95% CI 1.02–1.08], P = 0.003); this association was consistent in NHW (OR 1.05 [95% CI 1.01–1.10], P = 0.01) and MA (OR 1.05 [95% CI 1.02–1.09], P = 0.01), but not in NHB (OR 0.99 [95% CI 0.95–1.03], P = 0.71). In the entire cohort, GRS per allele was associated with T2D family history (OR 1.04 [95% CI 1.01–1.06], P = 0.004), BMI (in kilograms per square meter; −0.05 [95% CI −0.08 to −0.01], P = 0.02), and WC (in centimeters; −0.15 [95% CI −0.27 to −0.03], P = 0.02); GRS was not associated with age, sex, HEI, PA, alcohol consumption, smoking, or educational attainment. GRS per allele was associated with insurance coverage (0.96 [95% CI 0.93–1.00], P = 0.03), but not after adjusting for ethnicity (0.97 [95% CI 0.94–1.01], P = 0.17) or within ethnicities (Supplementary Table 2).
Mortality Risk per T2D-Weighted GRS Risk Allele
Baseline T2D status was associated with increased mortality risk over the time of follow-up (OR 1.69 [95% CI 1.21–2.36], P = 0.002; Table 3 and Supplementary Table 3). The BMI-adjusted mortality risk per T2D GRS risk allele was 1.04 (95% CI 1.00–1.07; P = 0.05). Effect estimates were consistent in NHW and NHB but not in MA. Adjusting for T2D at baseline did not greatly influence the effect estimates (Supplementary Table 4). The negative trend in MA remained after adjusting for family history, educational attainment, insurance coverage, HEI, PA, smoking, alcohol consumption, TC, HDL, hypertension, and WC. We repeated the analysis using Cox regression and showed that results were generally similar to those obtained from logistic regression (Supplementary Table 5). In a sensitivity analysis, we repeated the analysis using an unweighted GRS (i.e., a simple sum of risk alleles); effect estimates were similar to those of the weighted GRS, although CIs were wider (Supplementary Table 6).
Table 3.
Estimated mortality risk per T2D risk allele by ethnicity in the NHANES III DNA Bank (NHANES III, 1991–1994), accounting for sampling weights and complex survey design
| All ethnicities (N = 6,501) | NHW (N = 2,528) | NHB (N = 1,979) | MA (N = 1,994) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | |
| Unadjusted for BMI | 1.04 | (1.00–1.07) | 0.07 | 1.04 | (0.99–1.09) | 0.09 | 1.04 | (1.01–1.06) | 0.002 | 0.95 | (0.90–1.00) | 0.07 |
| Adjusted for BMI | 1.04 | (1.00–1.07) | 0.05 | 1.04 | (1.00–1.09) | 0.08 | 1.04 | (1.01–1.06) | 0.002 | 0.95 | (0.90–1.01) | 0.07 |
| Fully adjusted model* | 1.04 | (1.00–1.08) | 0.05 | 1.04 | (1.00–1.10) | 0.06 | 1.03 | (1.01–1.06) | 0.007 | 0.95 | (0.89–1.01) | 0.10 |
All models were adjusted for age, sex, and ethnicity. BMI, BMI categories (normal weight, BMI <25 kg/m2; overweight, BMI 25–30 kg/m2; obese, BMI ≥30 kg/m2).
*Fully adjusted model includes BMI, family history, educational attainment, insurance coverage, PA, HEI, smoking status, alcohol consumption, TC, HDL cholesterol level, WC, and hypertension.
In the interaction analysis, we observed a possible interaction between obesity and normal weight with GRS (P = 0.07) in the entire cohort. When BMI was modeled as a continuous variable, we observed a possible interaction with GRS in MA (P = 0.03) (Supplementary Table 7).
Stratified Analyses
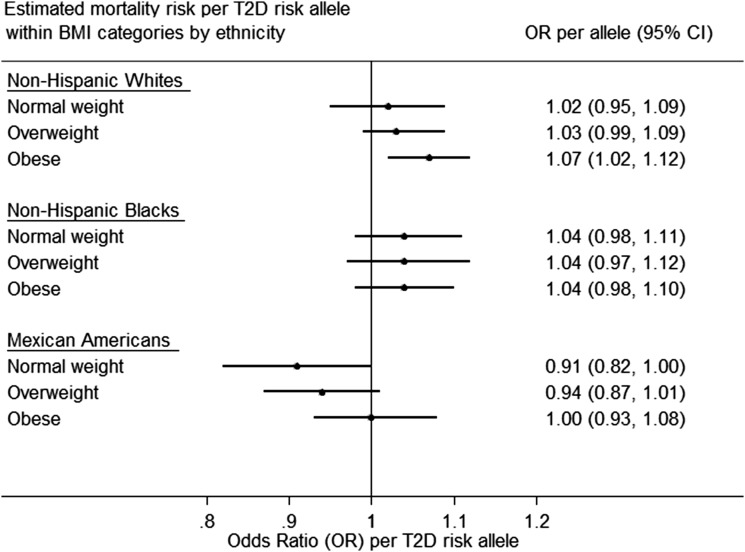
In BMI-stratified analyses, the estimated mortality risk per allele was slightly higher among obese NHW (OR 1.07 [95% CI 1.02–1.12]) compared with normal-weight NHW. A negative trend was observed between GRS per allele and mortality risk among normal-weight MA (OR 0.91 [95% CI 0.82–1.00]) (Fig. 1 and Supplementary Tables 8 and 9).
Figure 1.
Estimated mortality risk per T2D risk allele within BMI categories (obese, overweight, and normal weight) by ethnicity in the NHANES III DNA Bank (NHANES III, 1991–1994), accounting for sampling weights and the complex survey design.
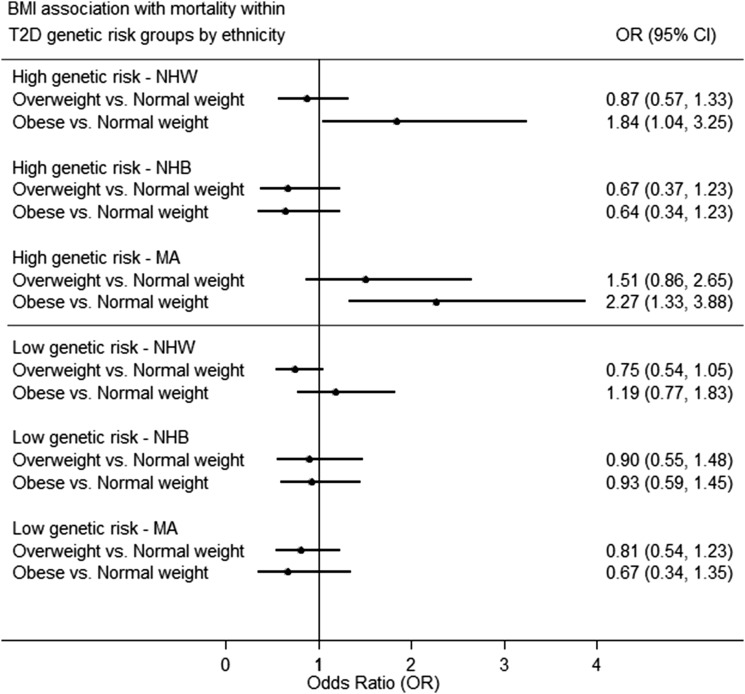
Obesity was associated with increased mortality risk (OR 1.37 [95% CI 1.04–1.81]). Adjusting for family history, educational attainment, insurance coverage, HEI, PA, smoking, alcohol consumption, T2D status, TC level, HDL level, hypertension, and WC weakened the association (OR 1.25 [95% CI 0.58–2.72]) (Supplementary Table 10). In stratified analysis by T2D genetic risk, no association between obesity and mortality risk was found among participants with low T2D genetic risk. Among those participants with high T2D genetic risk, obesity was associated with higher mortality risk in MA (OR 2.27 [95% CI 1.33–3.88]) (Fig. 2). This association attenuated with adjustment for other modifiable risk factors (OR 1.30 [95% CI 0.55–3.07]) (Supplementary Tables 11 and 12).
Figure 2.
Association of BMI categories with mortality risk within T2D genetic risk groups by ethnicity in the NHANES III DNA Bank (NHANES III, 1991–1994), accounting for sampling weights and the complex survey design.
Conclusions
Epidemiologic studies have reported that T2D patients have a 15% increased risk of all-cause mortality compared with the general population (29), and an approximately twofold risk in younger adults (3,29,30). This relationship may be partly explained by modifiable risk factors that predispose individuals to both T2D and mortality risk. Here, in a nationally representative sample of the U.S., we demonstrated that mortality risk increased by 4% per T2D-related risk allele (17% per 4 alleles, the SD of the GRS in NHANES III), independently of BMI and other social, lifestyle, and clinical risk factors tested, indicating that a higher aggregate genetic burden of T2D risk, carried throughout one’s life, may increase earlier mortality risk.
While previous investigations (31) have shown that T2D-related GRSs predict incident T2D in the general population, their predictive performance for incident CVD has been mixed. Our group has previously shown that a T2D-related GRS was not associated with markers of subclinical atherosclerosis, coronary artery calcium score, carotid artery intima-media thickness, and ankle-brachial index (32). A Danish study by Borglykke et al. (33) reported that a T2D-related GRS per allele was associated with a composite end point of fatal and nonfatal CVD (hazard ratio [OR] 1.02 [95% CI 1.01–1.03], P = 0.004). A previous study (34) by our group of 3,041 NHANES III participants reported that a glycated hemoglobin (HbA1c)–related GRS did not predict all-cause mortality risk despite epidemiologic evidence supporting an association between measured HbA1c and mortality risk. Notably, in this present study, we examined a larger sample of NHANES III participants (N = 6,501) and tested the association between T2D-related, and not HbA1c-related, genetic variants with all-cause mortality.
Two recent Mendelian randomization studies (35,36) reported that genetically determined T2D and fasting glucose levels were associated with CVD, supporting a causal relationship between genetically determined hyperglycemia and CVD. The principle of Mendelian randomization is based on the random allocation of alleles at meiosis, so alleles are distributed in the population independently of socioenvironmental and behavioral factors that often confound observational associations (37). Such an approach strengthens or refutes causality in disease etiology through the use of genetic instruments of modifiable risk factors in an instrumental variable analysis (38). In this study, incident T2D during follow-up was unavailable for analysis; thus, we have not performed a complete two-step instrumental variable analysis, which would have allowed us to determine whether genetically increased T2D risk raises mortality risk to the same extent observed in epidemiologic studies of T2D on mortality. Nevertheless, our group has previously demonstrated in a longitudinal examination of NHW and NHB in the CARDIA Study that a T2D-related GRS predicted incident T2D in participants of both ethnicities (NHW: HR 1.08 [95% CI 1.04–1.12], N = 1,650; NHB: HR 1.05 [95% CI 1.01–1.09], N = 820) (31), and so it is likely that the T2D-related GRS would predict incident T2D in NHANES III participants if such data were available for analysis. The positive association between the T2D-related GRS and mortality risk from reduced-form estimates reported herein supported the notion that genetically increased T2D risk was associated with higher all-cause mortality among NHW and NHB residing in the U.S.
As T2D-related genetic variants may have effects on other metabolic traits (BMI, WC, TC level, and blood pressure) independent of T2D, it is possible that these pleiotropic effects may partly account for the association between T2D-related genetic variants and mortality risk. In our study, it is noteworthy that a higher T2D-related GRS was associated with lower BMI and smaller WC. Certain T2D risk-raising alleles contributing to the GRS have known associations with lower BMI and WC. For instance, the T allele at rs7903146 (TCF7L2 locus) is associated with increased T2D risk in the DIAGRAM consortium (26), but lower BMI (P = 1.1 × 10−12) and smaller WC (P = 1.7 × 10−9) in a large meta-analysis GWAS by the Genetic Investigation of Anthropometric Traits (GIANT) consortium (39,40). Nevertheless, adjusting for BMI, WC, TC level, and hypertension status did not attenuate the association of T2D GRS with mortality risk, suggesting that it is unlikely that these metabolic traits lie on the causal pathway from T2D-related genetic variants to mortality.
A Mendelian randomization study showed that BMI-related genetic variants did not increase the CVD or stroke risk despite being associated with multiple cardiometabolic traits, including T2D (41). A previous study (42) of 2,607 NHANES III participants with T2D showed that BMI and measures of adiposity did not predict mortality over 6.5 years. While it remains unclear whether BMI increases mortality risk, the results herein indicated that T2D-related GRS may be more strongly associated with mortality risk among obese participants compared with normal-weight participants, suggesting that today’s obesogenic environment may be particularly detrimental for individuals with a higher genetic predisposition for T2D.
A longitudinal cohort study of 637 T2D participants ≥66 years of age by Murphy et al. (43) reported that normal-weight participants compared with overweight participants had higher mortality. This paradoxical relationship was reported to be partly mediated by muscle size (43). Conversely, our stratified analysis revealed that, among those participants with high T2D genetic risk, obesity may be associated with an increased risk of mortality. Adjusting for modifiable behavioral and clinical risk factors attenuated the association, suggesting that these factors may be mediators of the obesity-mortality relationship. These findings did not support a genetic etiology for the obesity paradox, where one would expect that, among those participants with a high T2D genetic risk, lighter individuals would have higher mortality risk than their heavier counterparts. Others have argued (44,45) that the obesity paradox was observed only in the setting of uncontrolled confounding factors (e.g., comorbidities, smoking, and intentional weight loss around the time of T2D diagnosis), selection bias (e.g., better detection of outcomes among sicker individuals), or noncoinciding start of follow-up and exposure.
BMI-stratified analyses showed a paradoxical negative trend between T2D-related GRS and mortality risk among normal-weight MA. Notably, MA were, on average, 9 years younger than NHW, and only 1 in 10 died during follow-up. A longer follow-up period to accrue deaths might render ethnic groups more comparable. Restricting the analysis to MA, ≥45 years of age, still showed a negative trend between T2D-related GRS and mortality (Supplementary Table 12). The Hispanic epidemiologic paradox is an observation that Hispanic Americans compared with NHW have similar or lower mortality rates despite having a lower socioeconomic status (46,47) and a higher prevalence of cardiometabolic risk factors (48). It has been proposed that population-specific genetic factors and gene-environmental interactions may partly account for this phenomenon (49,50), although sociobehavioral factors (e.g., salmon bias [some sick immigrants may return to their country of origin before death]) (51) and healthy-immigrant bias (immigrants may be generally healthy or have more healthful behaviors) (51) are relevant considerations. In this study, adjusting for educational attainment and insurance coverage did not impact effect estimates. The trend toward a mortality advantage among normal-weight MA carrying more T2D-related risk alleles warrants replication in larger population-based cohorts consisting of persons of Mexican ancestry with thorough longitudinal follow-up for clinical end points.
We acknowledge several limitations. We were not able to distinguish type 1 diabetes and T2D, though the overwhelming majority of persons in the general population likely have T2D. Our study was underpowered to demonstrate an association between GRS and specific causes of death. Nevertheless, CVD was the most common cause and likely one of several pathways through which genetically determined T2D influenced mortality risk. Our results cannot be generalized to nonfatal events or other T2D-related complications because participants were not followed for these outcomes. Because we did not observe participants for future hyperglycemia or incident T2D, we were unable to determine whether associations were mediated through hyperglycemia that developed during the follow-up period. As ethnicity was ascertained by self-report and not by ancestry markers, we were unable to account for genetic admixture. Although we were able to adjust for multiple risk factors ascertained at baseline, we recognize that we were unable to account for time-dependent covariates or the moderating effects of glucose-lowering medications.
Our exploratory stratified analyses had small sample sizes, and thus the results ought to be interpreted with caution; for instance, the paradoxical negative trend between GRS and mortality risk in MA may be a chance finding. Wide CIs may be partly attributed to the impact of the complex survey design on variance estimates. The GRS was associated with T2D at baseline in NHW and MA, but not in NHB; inconsistencies across ethnicities may be due to T2D-related genetic variants that were discovered mainly through GWAS in European populations, which may have markedly different allele frequencies in non-European ancestries. This underscores the need to extend discovery GWAS and genetic association studies to diverse populations. Uncovering ethnic-specific genetic variants may enable us to better characterize the role of T2D genetic risk in ethnic disparities in T2D-related outcomes (52).
This study has important strengths. This longitudinal examination of a large multiethnic representative sample of the U.S. population has provided insights into the genetic underpinnings of mortality, and demonstrated important interactions between T2D genetic risk and BMI for mortality risk. We applied sampling weights and accounted for the complex survey design to obtain estimates that enabled population-level inferences. We adjusted our analyses for well-characterized sociodemographic, clinical, and behavioral modifiable factors associated with metabolic health. To better understand the implications of the T2D and obesity coepidemic in ethnically diverse populations, we took advantage of the available multiethnic samples to perform stratified analyses by ethnicity and BMI. Uncovering heterogeneous genetic effects and higher-risk subgroups may partly explain ethnic disparities in T2D-related outcomes, which is pertinent for precision medicine. Lifestyle intervention studies targeting weight loss could consider the influence of genetic variation on clinical response and clinical outcomes. Future genetic-environment interaction studies may clarify the mechanisms underlying the heterogeneous effects of T2D-related genetic variants on mortality by ethnicity and BMI, and inform lifestyle intervention strategies directed at those with stronger genetic susceptibility to T2D-related mortality.
In sum, in the U.S., carriers of more T2D risk-raising alleles have a higher mortality risk than noncarriers, suggesting that having a higher genetic burden for the development of T2D may increase the mortality risk. The underlying genetic basis of mortality likely involves complex interactions with nongenetic factors related to ethnicity, T2D, or body weight. In the midst of a T2D and obesity coepidemic from an increasingly obesogenic environment, maintaining a normal body weight may be especially important for lowering mortality risk in individuals with a high genetic predisposition to T2D.
Supplementary Material
Article Information
Acknowledgments. The authors thank Carolyn S. Neal, PhD, Research Analyst at the Centers for Disease Control and Prevention, for her assistance in proposal submission preparation, use of restricted data, merging of the relevant data sets, and data analysis.
Funding. A.L. is supported by a Canadian Diabetes Association postdoctoral research fellowship. J.D., J.C.F., and J.B.M. are supported by National Institutes of Health grant R01-DK-078616. J.C.F. is a Massachusetts General Hospital Research Scholar. J.B.M. is supported in part by National Institute of Diabetes and Digestive and Kidney Diseases grant K24-DK-080140.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.L. developed the study design and analysis plan, performed the analysis, and drafted the article. B.P. performed the analysis and contributed to the study design and the analysis plan. J.D., J.C.F., and J.B.M. contributed to the study design and analysis plan and reviewed and edited all aspects of the article (Introduction, Research Design and Methods, Results, and Conclusions). All authors approved the final version of the article. J.B.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 75th Scientific Sessions of the American Diabetes Association, Boston, MA, 5–9 June 2015.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc15-2080/-/DC1.
References
- 1.Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: U.S., 2005-2050. Diabetes Care 2006;29:2114–2116 [DOI] [PubMed] [Google Scholar]
- 2.Mainous AG 3rd, Baker R, Koopman RJ, et al. Impact of the population at risk of diabetes on projections of diabetes burden in the United States: an epidemic on the way. Diabetologia 2007;50:934–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971-1993. Diabetes Care 1998;21:1138–1145 [DOI] [PubMed] [Google Scholar]
- 4.Morgan CL, Currie CJ, Peters JR. Relationship between diabetes and mortality: a population study using record linkage. Diabetes Care 2000;23:1103–1107 [DOI] [PubMed] [Google Scholar]
- 5.Franco OH, Steyerberg EW, Hu FB, Mackenbach J, Nusselder W. Associations of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. Arch Intern Med 2007;167:1145–1151 [DOI] [PubMed] [Google Scholar]
- 6.Saydah SH, Eberhardt MS, Loria CM, Brancati FL. Age and the burden of death attributable to diabetes in the United States. Am J Epidemiol 2002;156:714–719 [DOI] [PubMed] [Google Scholar]
- 7.Almgren P, Lehtovirta M, Isomaa B, et al. Botnia Study G: heritability and familiality of type 2 diabetes and related quantitative traits in the Botnia Study. Diabetologia 2011;54:2811–2819 [DOI] [PubMed] [Google Scholar]
- 8.Poulsen P, Kyvik KO, Vaag A, Beck-Nielsen H. Heritability of type II (non-insulin-dependent) diabetes mellitus and abnormal glucose tolerance—a population-based twin study. Diabetologia 1999;42:139–145 [DOI] [PubMed] [Google Scholar]
- 9.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science 2003;299:853–855 [DOI] [PubMed] [Google Scholar]
- 10.James WP. The epidemiology of obesity: the size of the problem. J Intern Med 2008;263:336–352 [DOI] [PubMed] [Google Scholar]
- 11.Colley RC, Garriguet D, Janssen I, Craig CL, Clarke J, Tremblay MS. Physical activity of Canadian adults: accelerometer results from the 2007 to 2009 Canadian Health Measures Survey. Health Rep 2011;22:7–14 [PubMed] [Google Scholar]
- 12.Siraj ES, Williams KJ. Another agent for obesity—will this time be different? N Engl J Med 2015;373:82–83 [DOI] [PubMed] [Google Scholar]
- 13.Baer HJ, Glynn RJ, Hu FB, et al. Risk factors for mortality in the nurses’ health study: a competing risks analysis. Am J Epidemiol 2011;173:319–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tice JA, Kanaya A, Hue T, et al. Risk factors for mortality in middle-aged women. Arch Intern Med 2006;166:2469–2477 [DOI] [PubMed] [Google Scholar]
- 15.Ikeda N, Inoue M, Iso H, et al. Adult mortality attributable to preventable risk factors for non-communicable diseases and injuries in Japan: a comparative risk assessment. PLoS Med 2012;9:e1001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirko KA, Kantor ED, Cohen SS, Blot WJ, Stampfer MJ, Signorello LB. Body mass index in young adulthood, obesity trajectory, and premature mortality. Am J Epidemiol 2015;182:441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White IR, Altmann DR, Nanchahal K. Alcohol consumption and mortality: modelling risks for men and women at different ages. BMJ 2002;325:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knowler WC, Pettitt DJ, Savage PJ, Bennett PH. Diabetes incidence in Pima indians: contributions of obesity and parental diabetes. Am J Epidemiol 1981;113:144–156 [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. New diabetes atlas [article online], 2015. Available from http://www.cdc.gov/diabetes/statistics. Accessed 12 November 2015
- 20.Hainer V, Aldhoon-Hainerova I. Obesity paradox does exist. Diabetes Care 2013;36(Suppl. 2):S276–S281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doehner W, Erdmann E, Cairns R, et al. Inverse relation of body weight and weight change with mortality and morbidity in patients with type 2 diabetes and cardiovascular co-morbidity: an analysis of the PROactive study population. Int J Cardiol 2012;162:20–26 [DOI] [PubMed] [Google Scholar]
- 22.Perry JR, Voight BF, Yengo L, et al.; Magic; DIAGRAM Consortium; GIANT Consortium . Stratifying type 2 diabetes cases by BMI identifies genetic risk variants in LAMA1 and enrichment for risk variants in lean compared to obese cases. PLoS Genet 2012;8:e1002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langenberg C, Sharp SJ, Franks PW, et al. Gene-lifestyle interaction and type 2 diabetes: the EPIC interact case-cohort study. PLoS Med 2014;11:e1001647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carnethon MR, De Chavez PJ, Biggs ML, et al. Association of weight status with mortality in adults with incident diabetes. JAMA 2012;308:581–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94. Series 1: programs and collection procedures. Vital Health Stat 1 1994:1–407 [PubMed] [Google Scholar]
- 26.Mahajan A, Go MJ, Zhang W, et al.; Diabetes Genetics Replication and Meta-analysis (DIAGRAM) Consortium; Asian Genetic Epidemiology Network Type 2 Diabetes (AGEN-T2D) Consortium; South Asian Type 2 Diabetes (SAT2D) Consortium; Mexican American Type 2 Diabetes (MAT2D) Consortium; Type 2 Diabetes Genetic Exploration by Nex-generation sequencing in multi-Ethnic Samples (T2D-GENES) Consortium . Genome-wide trans-ancestry meta-analysis provides insight into the genetic architecture of type 2 diabetes susceptibility. Nat Genet 2014;46:234–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cornelis MC, Qi L, Zhang C, et al. Joint effects of common genetic variants on the risk for type 2 diabetes in U.S. men and women of European ancestry. Ann Intern Med 2009;150:541–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng MC, Shriner D, Chen BH, et al.; FIND Consortium; eMERGE Consortium; DIAGRAM Consortium; MuTHER Consortium; MEta-analysis of type 2 DIabetes in African Americans Consortium . Meta-analysis of genome-wide association studies in African Americans provides insights into the genetic architecture of type 2 diabetes. PLoS Genet 2014;10:e1004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tancredi M, Rosengren A, Svensson AM, et al. Excess mortality among persons with type 2 diabetes. N Engl J Med 2015;373:1720–1732 [DOI] [PubMed] [Google Scholar]
- 30.Lind M, Garcia-Rodriguez LA, Booth GL, et al. Mortality trends in patients with and without diabetes in Ontario, Canada and the UK from 1996 to 2009: a population-based study. Diabetologia 2013;56:2601–2608 [DOI] [PubMed] [Google Scholar]
- 31.Vassy JL, Hivert MF, Porneala B, et al. Polygenic type 2 diabetes prediction at the limit of common variant detection. Diabetes 2014;63:2172–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dauriz M, Porneala BC, Guo X, et al. Association of a 62 variant type 2 diabetes genetic risk score with markers of subclinical atherosclerosis: a transethnic, multicenter study. Circ Cardiovasc Genet 2015;8:507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borglykke A, Grarup N, Sparso T, et al. Genetic variant SLC2A2 is associated with risk of cardiovascular disease—assessing the individual and cumulative effect of 46 type 2 diabetes related genetic variants. PLoS One 2012;7:e50418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grimsby JL, Porneala BC, Vassy JL, et al. Race-ethnic differences in the association of genetic loci with HbA1c levels and mortality in U.S. adults: the third National Health and Nutrition Examination Survey (NHANES III). BMC Med Genet 2012;13:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmad OS, Morris JA, Mujammami M, et al. A Mendelian randomization study of the effect of type-2 diabetes on coronary heart disease. Nat Commun 2015;6:7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ross S, Gerstein HC, Eikelboom J, Anand SS, Yusuf S, Pare G. Mendelian randomization analysis supports the causal role of dysglycaemia and diabetes in the risk of coronary artery disease. Eur Heart J 2015;36:1454–1462 [DOI] [PubMed] [Google Scholar]
- 37.Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 2008;27:1133–1163 [DOI] [PubMed] [Google Scholar]
- 38.Palmer TM, Lawlor DA, Harbord RM, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res 2012;21:223–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Locke AE, Kahali B, Berndt SI, et al.; ADIPOGen Consortium; AGEN-BMI Working Group; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GLGC; ICBP; MAGIC Investigators; MuTHER Consortium; MIGen Consortium; PAGE Consortium; ReproGen Consortium; GENIE Consortium; International Endogene Consortium.et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015;518:197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shungin D, Winkler TW, Croteau-Chonka DC, et al.; ADIPOGen Consortium; CARDIOGRAMplusC4D Consortium; CKDGen Consortium; GEFOS Consortium; GENIE Consortium; GLGC; ICBP; International Endogene Consortium; LifeLines Cohort Study; MAGIC Investigators; MuTHER Consortium; PAGE Consortium; ReproGen Consortium.et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature 2015;518:187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holmes MV, Lange LA, Palmer T, et al. Causal effects of body mass index on cardiometabolic traits and events: a Mendelian randomization analysis. Am J Hum Genet 2014;94:198–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menke A, Casagrande SS, Cowie CC. The relationship of adiposity and mortality among people with diabetes in the US general population: a prospective cohort study. BMJ Open 2014;4:e005671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy RA, Reinders I, Garcia ME, et al. Adipose tissue, muscle, and function: potential mediators of associations between body weight and mortality in older adults with type 2 diabetes. Diabetes Care 2014;37:3213–3219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lajous M, Banack HR, Kaufman JS, Hernan MA. Should patients with chronic disease be told to gain weight? The obesity paradox and selection bias. Am J Med 2015;128:334–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tobias D, Pan A, Hu FB. BMI and mortality among adults with incident type 2 diabetes. N Engl J Med 2014;370:1363–1364 [DOI] [PubMed] [Google Scholar]
- 46.Franzini L, Ribble JC, Keddie AM. Understanding the Hispanic paradox. Ethn Dis 2001;11:496–518 [PubMed] [Google Scholar]
- 47.Markides KS, Coreil J. The health of Hispanics in the southwestern United States: an epidemiologic paradox. Public Health Rep 1986;101:253–265 [PMC free article] [PubMed] [Google Scholar]
- 48.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med 2003;163:427–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Medina-Inojosa J, Jean N, Cortes-Bergoderi M, Lopez-Jimenez F. The Hispanic paradox in cardiovascular disease and total mortality. Prog Cardiovasc Dis 2014;57:286–292 [DOI] [PubMed] [Google Scholar]
- 50.Qi L, Campos H. Genetic predictors for cardiovascular disease in Hispanics. Trends Cardiovasc Med 2011;21:15–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turra CM, Elo IT. The impact of salmon bias on the hispanic mortality advantage: new evidence from Social Security data. Popul Res Policy Rev 2008;27:515–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rotimi CN, Jorde LB. Ancestry and disease in the age of genomic medicine. N Engl J Med 2010;363:1551–1558 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.